Studies on Cell Compatibility, Antibacterial Behavior, and Zeta Potential of Ag-Containing Polydopamine-Coated Bioactive Glass-Ceramic
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of the Samples and Structural Characterization
2.2. Surface Zeta Potential
2.3. Preconditioning of the Samples
2.4. Cell Biology Studies
2.4.1. Cell Seeding and Culture
2.4.2. Cell Viability
2.4.3. Cell Morphology
2.5. Statistical Analysis
2.6. Antibacterial Tests
3. Results and Discussion
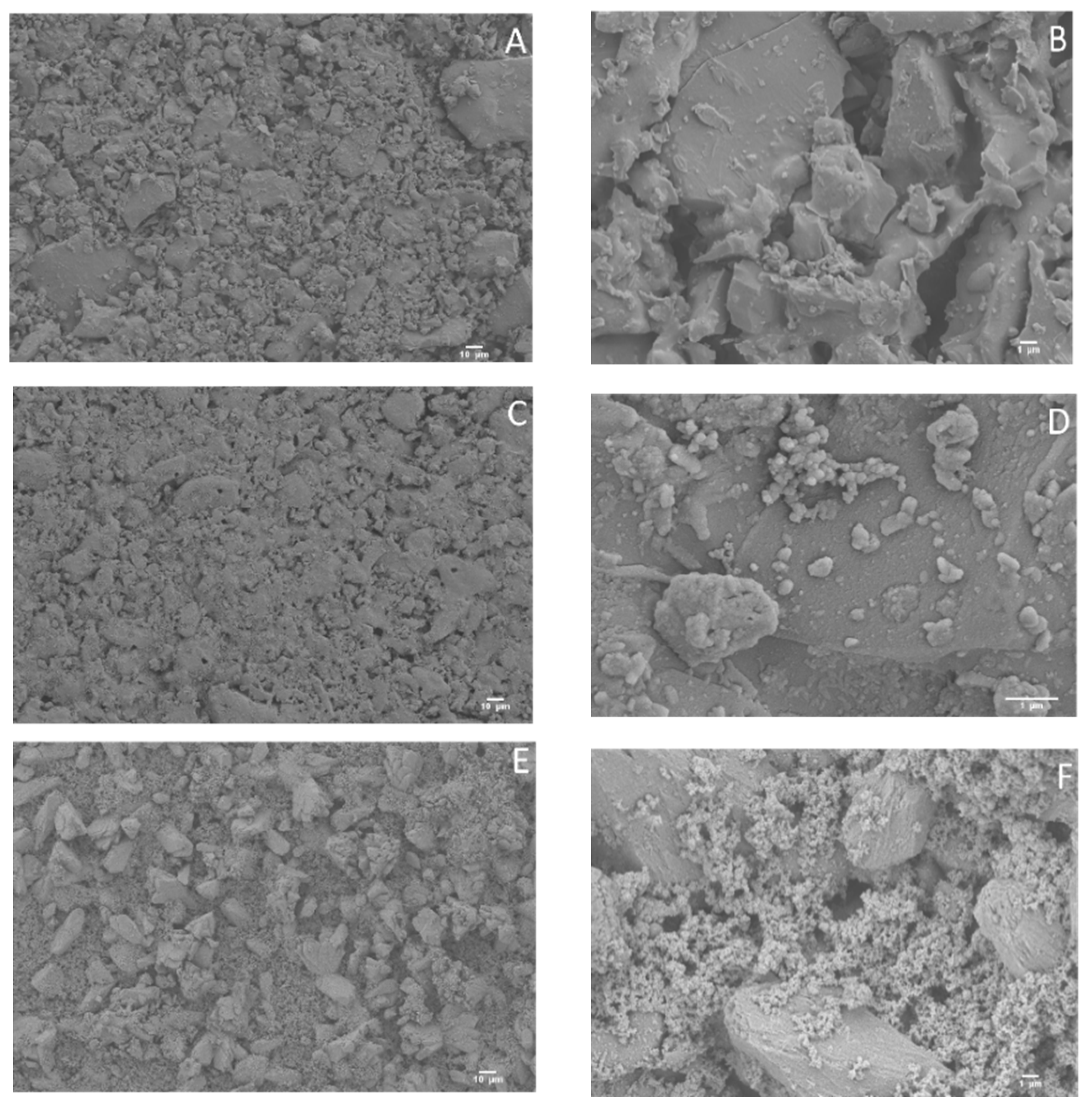
3.1. Structural Characterization
3.2. Surface Zeta Potential
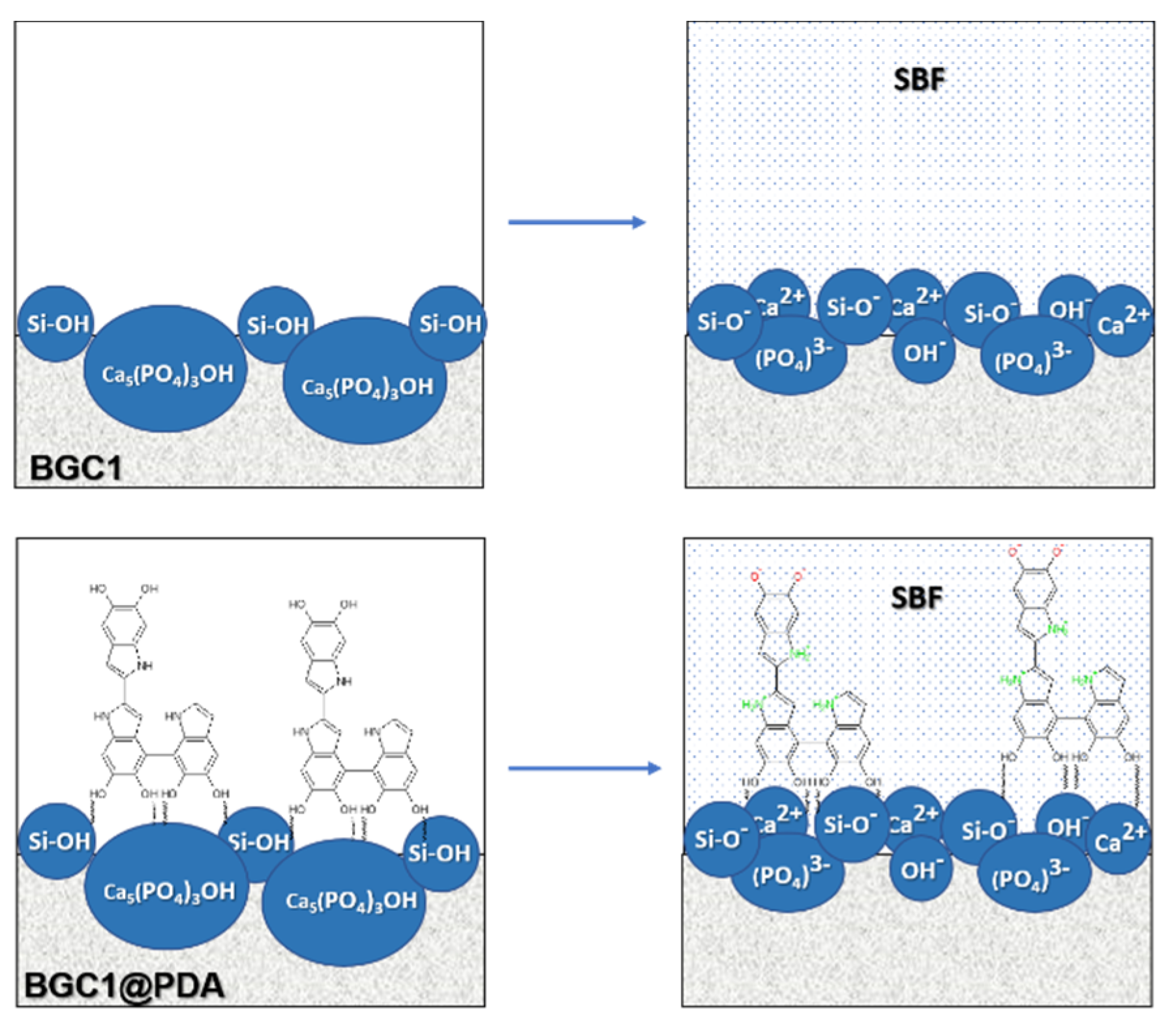
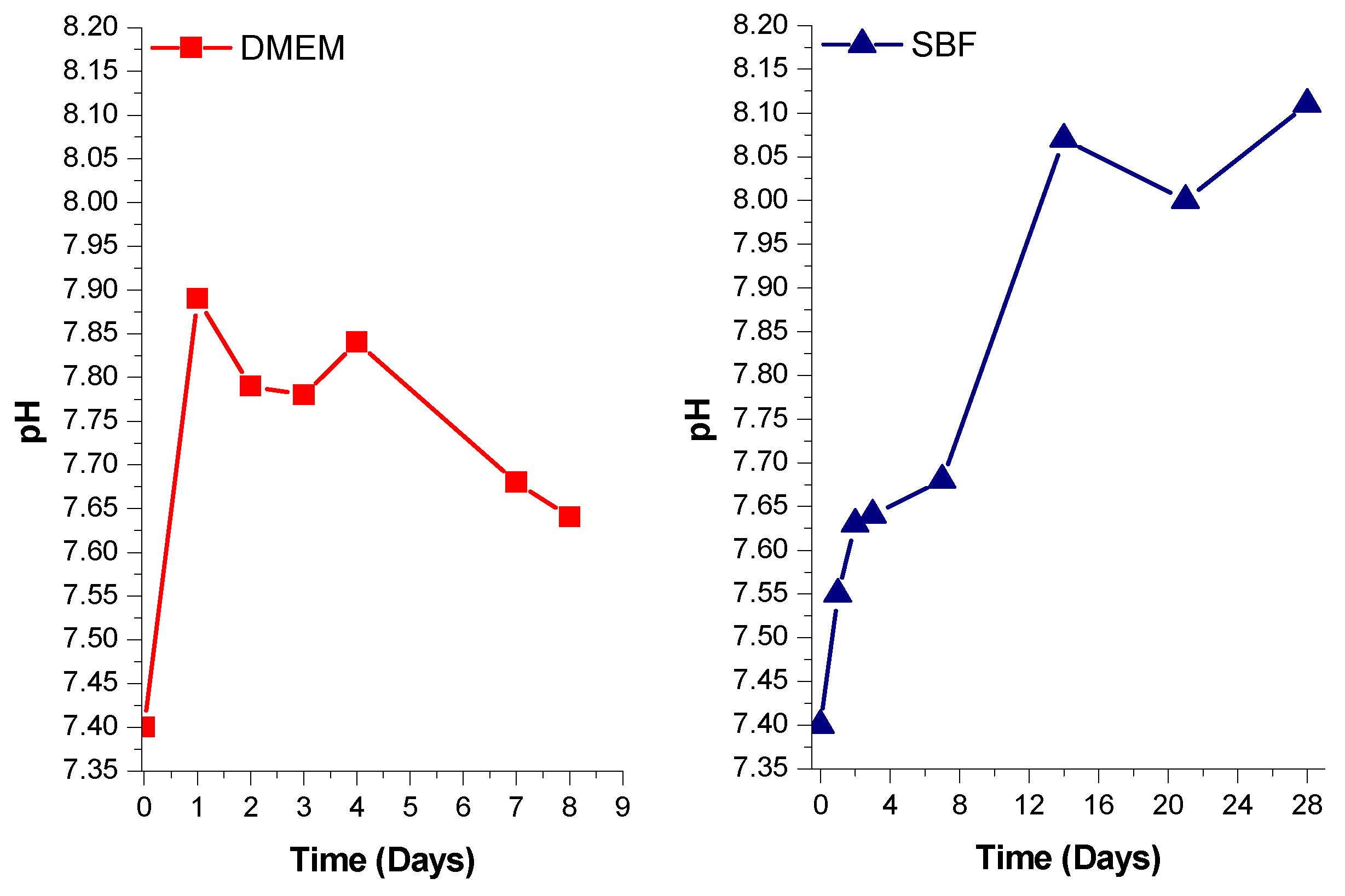
3.3. Preconditioning of the Samples
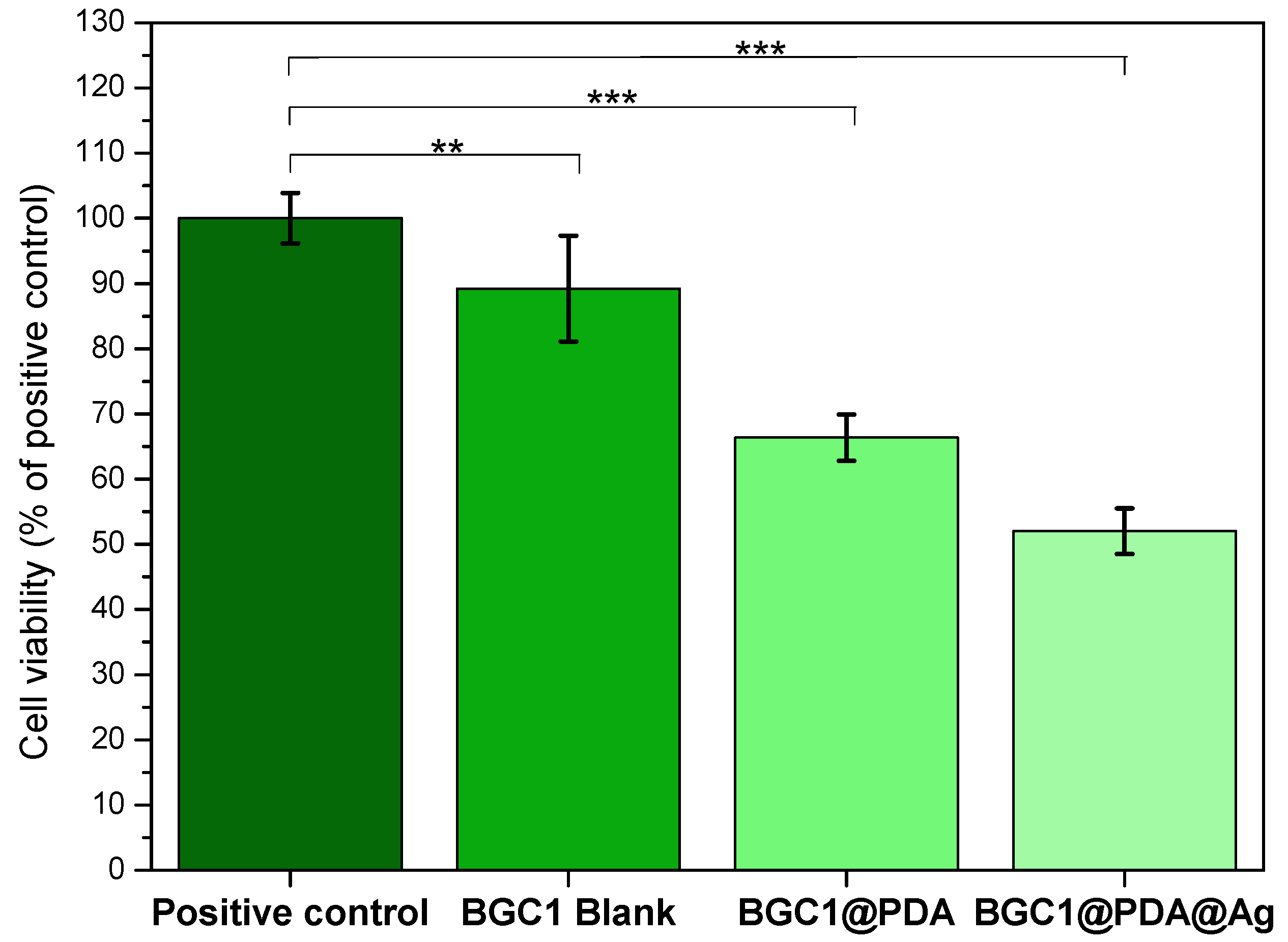
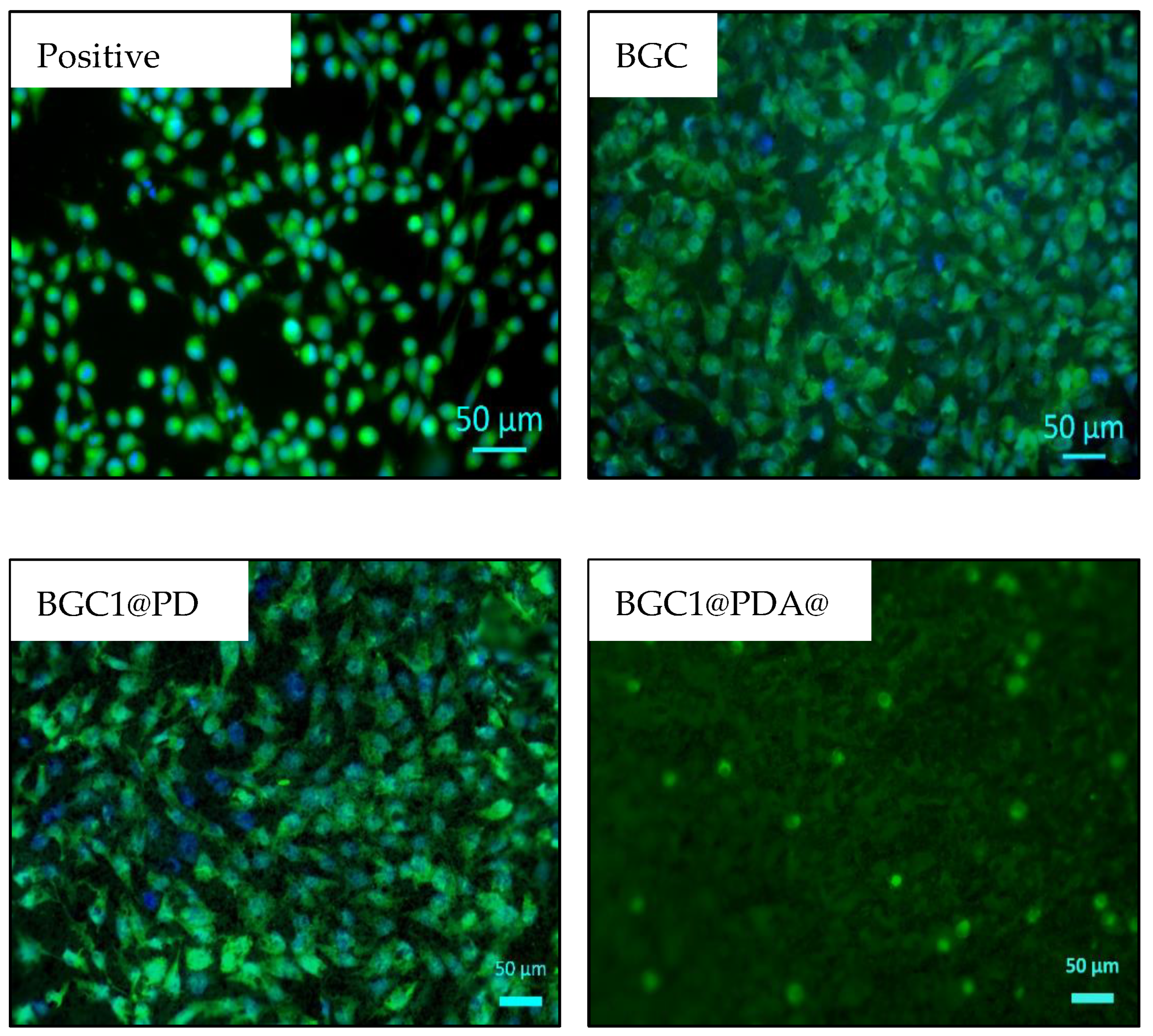
3.4. Cell Biology Studies
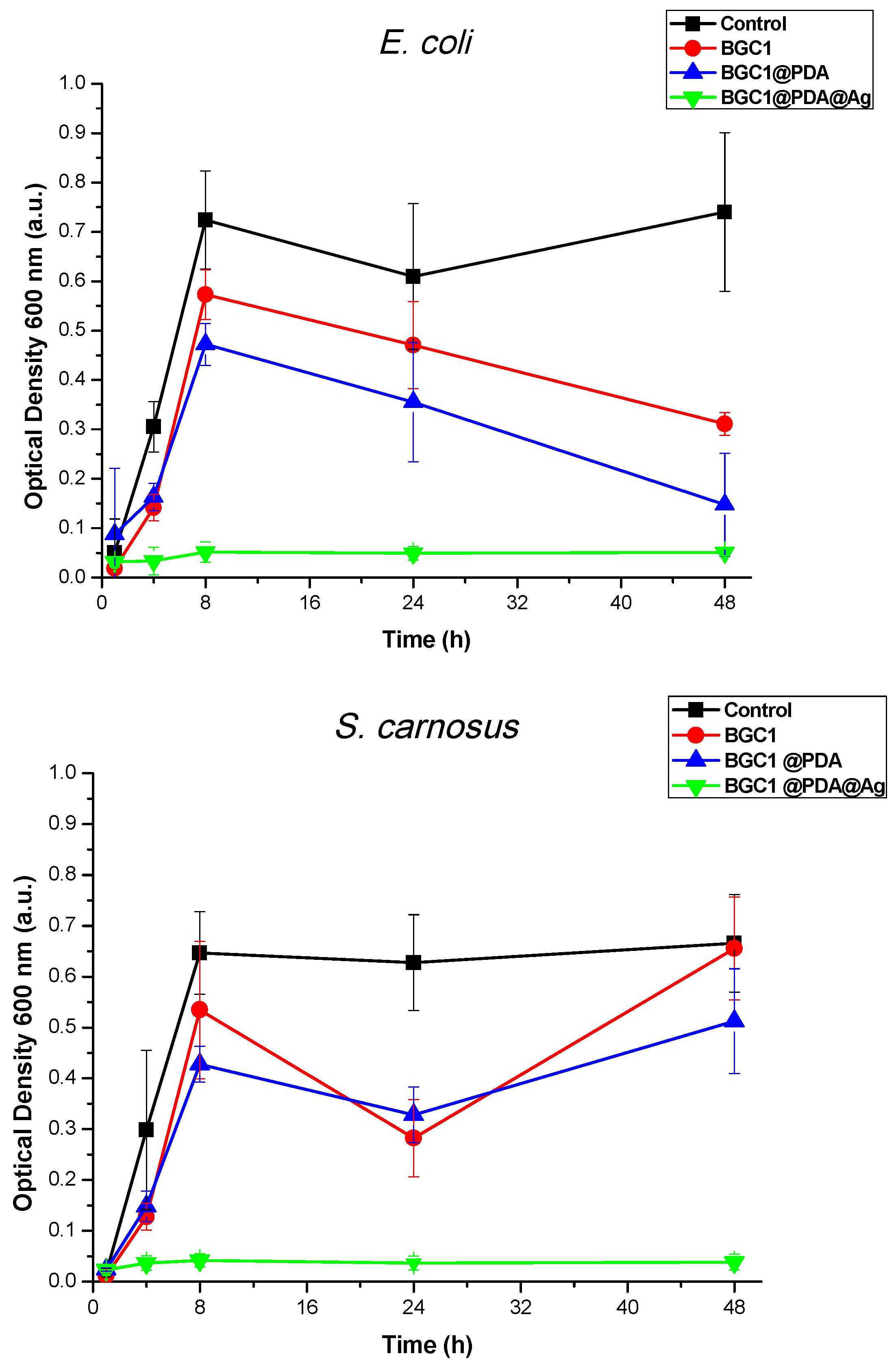
3.5. Antibacterial Test
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mantripragada, V.P.; Lecka-Czernik, B.; Ebraheim, N.A.; Jayasuriya, A.C. An overview of recent advances in designing orthopedic and craniofacial implants. J. Biomed. Mater. Res. Part A 2013, 101, 3349–3364. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control; European Medicines Agency. The Bacterial Challenge: Time to React; European Centre for Disease Prevention and Control: Stockholm, Sweden, 2009. [Google Scholar] [CrossRef]
- World Bank Group. Drug-Resistant Infections: A Threat to Our Economic Future; World Bank Group: Washington, DC, USA, 2017. [Google Scholar] [CrossRef]
- Kokubo, T. Bioactive glass ceramics: Properties and applications. Biomaterials 1991, 12, 155–163. [Google Scholar] [CrossRef]
- Peitl, O.; Zanotto, E.D.; Hench, L.L. Highly bioactive P2O5–Na2O–CaO–SiO2 glass-ceramics. J. Non Cryst. Solids 2001, 292, 115–126. [Google Scholar] [CrossRef]
- Miguez-Pacheco, V.; Hench, L.L.; Boccaccini, A.R. Bioactive glasses beyond bone and teeth: Emerging applications in contact with soft tissues. Acta Biomater. 2015, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Tallawi, M.; Rosellini, E.; Barbani, N.; Cascone, M.G.; Rai, R.; Saint-Pierre, G.; Boccaccini, A.R. Strategies for the chemical and biological functionalization of scaffolds for cardiac tissue engineering: A review. J. R. Soc. Interface 2015, 12, 20150254. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and its derivative materials: Synthesis and promising applications in energy, environmental, and biomedical fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.C.; Ding, S.J. Structure, properties and applications of mussel-inspired polydopamine. J. Biomed. Nanotechnol. 2014, 10, 3063–3084. [Google Scholar] [CrossRef] [PubMed]
- Mondin, G.; Wisser, F.M.; Leifert, A.; Mohamed-Noriega, N.; Grothe, J.; Dörfler, S.; Kaskel, S. Metal deposition by electroless plating on polydopamine functionalized micro- and nanoparticles. J. Colloid Interface Sci. 2013, 411, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Schaubroeck, D.; Mader, L.; Dubruel, P.; Vanfleteren, J. Surface modification of an epoxy resin with polyamines and polydopamine: Adhesion toward electroless deposited copper. Appl. Surf. Sci. 2015, 353, 238–244. [Google Scholar] [CrossRef]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef] [PubMed]
- Lara, H.H.; Ayala-Núñez, N.V.; del Turrent, L.C.I.; Padilla, C.R. Bactericidal effect of silver nanoparticles against multidrug-resistant bacteria. World J. Microbiol. Biotechnol. 2010, 26, 615–621. [Google Scholar] [CrossRef]
- Panácek, A.; Smékalová, M.; Kilianová, M.; Prucek, R.; Bogdanová, K.; Věcěrová, R.; Kolár, M.; Havrdová, M.; Płaza, G.A.; Chojniak, J.; et al. Strong and nonspecific synergistic antibacterial efficiency of antibiotics combined with silver nanoparticles at very low concentrations showing no cytotoxic effect. Molecules 2016, 21, 26. [Google Scholar] [CrossRef] [PubMed]
- Blaker, J.J.; Nazhat, S.N.; Boccaccini, A.R. Development and characterisation of silver-doped bioactive glass-coated sutures for tissue engineering and wound healing applications. Biomaterials 2004, 25, 1319–1329. [Google Scholar] [CrossRef] [PubMed]
- Miola, M.; Ferraris, S.; di Nunzio, S.; Robotti, P.F.; Bianchi, G.; Fucale, G.; Maina, G.; Cannas, M.; Gatti, S.; Massé, A.; et al. Surface silver-doping of biocompatible glasses to induce antibacterial properties. Part II: Plasma sprayed glass-coatings. J. Mater. Sci. Mater. Med. 2009, 20, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Ciraldo, F.E.; Liverani, L.; Gritsch, L.; Goldmann, W.H.; Boccaccini, A.R. Synthesis and characterization of silver-doped mesoporous bioactive glass and its applications in conjunction with electrospinning. Materials 2018, 11, 692. [Google Scholar] [CrossRef] [PubMed]
- Kawashita, M.; Tsuneyama, S.; Miyaji, F.; Kokubo, T.; Kozuka, H.; Yamamoto, K. Antibacterial silver-containing silica glass prepared by sol-gel method. Biomaterials 2000, 21, 393–398. [Google Scholar] [CrossRef]
- Di Nunzio, S.; Brovarone, C.V.; Spriano, S.; Milanese, D.; Verné, E.; Bergo, V.; Maina, G.; Spinelli, P. Silver containing bioactive glasses prepared by molten salt ion-exchange. J. Eur. Ceram. Soc. 2004, 24, 2935–2942. [Google Scholar] [CrossRef]
- Saidin, S.; Chevallier, P.; Kadir, M.R.A.; Hermawan, H.; Mantovani, D. Polydopamine as an intermediate layer for silver and hydroxyapatite immobilisation on metallic biomaterials surface. Mater. Sci. Eng. C 2013, 33, 4715–4724. [Google Scholar] [CrossRef]
- Li, M.; Liu, Q.; Jia, Z.; Xu, X.; Shi, Y.; Cheng, Y.; Zheng, Y. Polydopamine-induced nanocomposite Ag/CaP coatings on the surface of titania nanotubes for antibacterial and osteointegration functions. J. Mater. Chem. B 2015, 3, 8796–8805. [Google Scholar] [CrossRef]
- Wang, Z.; Ou, J.; Wang, Y.; Xue, M.; Wang, F.; Pan, B.; Li, C.; Li, W. Anti-bacterial superhydrophobic silver on diverse substrates based on the mussel-inspired polydopamine. Surf. Coat. Technol. 2015, 280, 378–383. [Google Scholar] [CrossRef]
- Forte, L.; Torricelli, P.; Bonvicini, F.; Boanini, E.; Gentilomi, G.A.; Lusvardi, G.; della Bella, E.; Fini, M.; Nepita, E.V.; Bigi, A. Biomimetic fabrication of antibacterial calcium phosphates mediated by polydopamine. J. Inorg. Biochem. 2018, 178, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Sileika, T.S.; Kim, H.D.; Maniak, P.; Messersmith, P.B. Antibacterial performance of polydopamine-modified polymer surfaces containing passive and active components. ACS Appl. Mater. Interfaces 2011, 3, 4602–4610. [Google Scholar] [CrossRef] [PubMed]
- Tejido-Rastrilla, R.; Baldi, G.; Boccaccini, A.R. Ag containing polydopamine coating on a melt-derived bioactive glass-ceramic: Effect on surface reactivity. Ceram. Int. 2018, 44, 16083–16087. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Zangmeister, R.A.; Morris, T.A.; Tarlov, M.J. Characterization of polydopamine thin films deposited at short times by autoxidation of dopamine. Langmuir 2013, 29, 8619–8628. [Google Scholar] [CrossRef] [PubMed]
- Spriano, S.; Chandra, V.S.; Cochis, A.; Uberti, F.; Rimondini, L.; Bertone, E.; Vitale, A.; Scolaro, C.; Ferrari, M.; Cirisano, F.; et al. How do wettability, zeta potential and hydroxylation degree affect the biological response of biomaterials? Mater. Sci. Eng. C 2017, 74, 542–555. [Google Scholar] [CrossRef] [PubMed]
- Bernsmann, F.; Frisch, B.; Ringwald, C.; Ball, V. Protein adsorption on dopamine-melanin films: Role of electrostatic interactions inferred from ζ-potential measurements versus chemisorption. J. Colloid Interface Sci. 2010, 344, 54–60. [Google Scholar] [CrossRef]
- Fu, J.; Chen, Z.; Wang, M.; Liu, S.; Zhang, J.; Zhang, J.; Han, R.; Xu, Q. Adsorption of methylene blue by a high-efficiency adsorbent (Polydopamine microspheres): Kinetics, isotherm, thermodynamics and mechanism analysis. Chem. Eng. J. 2015, 259, 53–61. [Google Scholar] [CrossRef]
- Su, L.; Yu, Y.; Zhao, Y.; Liang, F.; Zhang, X. Strong Antibacterial Polydopamine Coatings Prepared by a Shaking-assisted Method. Sci. Rep. 2016, 6, 24420. [Google Scholar] [CrossRef] [PubMed]
- Ball, V. Impedance spectroscopy and zeta potential titration of dopa-melanin films produced by oxidation of dopamine. Colloids Surf. A Phys. Eng. Asp. 2010, 363, 92–97. [Google Scholar] [CrossRef]
- Cazzola, M.; Corazzari, I.; Prenesti, E.; Bertone, E.; Vernè, E.; Ferraris, S. Bioactive glass coupling with natural polyphenols: Surface modification, bioactivity and anti-oxidant ability. Appl. Surf. Sci. 2016, 367, 237–248. [Google Scholar] [CrossRef]
- Suzuki, T.; Nishizawa, K.; Yokogawa, Y.; Nagata, F.; Kawamoto, Y.; Kameyama, T. Time-dependent variation of the surface structure of bioceramics in tissue culture medium and the effect on adhesiveness of cells. J. Ferment. Bioeng. 1996, 81, 226–232. [Google Scholar] [CrossRef]
- Ryu, J.; Ku, S.H.; Lee, H.; Park, C.B. Mussel-inspired polydopamine coating as a universal route to hydroxyapatite crystallization. Adv. Funct. Mater. 2010, 20, 2132–2139. [Google Scholar] [CrossRef]
- El-Ghannam, A.; Hamazawy, E.; Yehia, A. Effect of thermal treatment on bioactive glass microstructure, corrosion behavior ζ potential, and protein adsorption. J. Biomed. Mater. Res. 2001, 55, 387–395. [Google Scholar] [CrossRef]
- Lu, H.H.; Pollack, S.R.; Ducheyne, P. 45S5 Bioactive glass surface charge variations and the formation of a surface calcium phosphate layer in a solution containing fibronectin. J. Biomed. Mater. Res. 2001, 54, 454–461. [Google Scholar] [CrossRef]
- Zhang, C.; Gong, L.; Xiang, L.; Du, Y.; Hu, W.; Zeng, H.; Xu, Z.K. Deposition and Adhesion of Polydopamine on the Surfaces of Varying Wettability. ACS Appl. Mater. Interfaces 2017, 9, 30943–30950. [Google Scholar] [CrossRef]
- Sathishkumar, M.; Sneha, K.; Won, S.W.; Cho, C.W.; Kim, S.; Yun, Y.S. Cinnamon zeylanicum bark extract and powder mediated green synthesis of nano-crystalline silver particles and its bactericidal activity. Colloids Surf. B Biointerfaces 2009, 73, 332–338. [Google Scholar] [CrossRef]
- Roy, A.K.; Park, B.; Lee, K.S.; Park, S.Y.; In, I. Boron nitride nanosheets decorated with silver nanoparticles through mussel-inspired chemistry of dopamine. Nanotechnology 2014, 25, 445603. [Google Scholar] [CrossRef]
- Wang, J.; Rahman, M.F.; Duhart, H.M.; Newport, G.D.; Patterson, T.A.; Murdock, R.C.; Hussain, S.M.; Schlager, J.J.; Ali, S.F. Expression changes of dopaminergic system-related genes in PC12 cells induced by manganese, silver, or copper nanoparticles. Neurotoxicology 2009, 30, 926–933. [Google Scholar] [CrossRef]
- Rehman, M.A.U.; Ferraris, S.; Goldmann, W.H.; Perero, S.; Bastan, F.E.; Nawaz, Q.; di Confiengo, G.G.; Ferraris, M.; Boccaccini, A.R. Antibacterial and Bioactive Coatings Based on Radio Frequency Co-Sputtering of Silver Nanocluster-Silica Coatings on PEEK/Bioactive Glass Layers Obtained by Electrophoretic Deposition. ACS Appl. Mater. Interfaces 2017, 9, 32489–32497. [Google Scholar] [CrossRef] [PubMed]
- Verné, E.; Bretcanu, O.; Balagna, C.; Bianchi, C.L.; Cannas, M.; Gatti, S.; Vitale-Brovarone, C. Early stage reactivity and in vitro behavior of silica-based bioactive glasses and glass-ceramics. J. Mater. Sci. Mater. Med. 2009, 20, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Vitale-Brovarone, C.; Verné, E.; Robiglio, L.; Martinasso, G.; Canuto, R.A.; Muzio, G. Biocompatible glass-ceramic materials for bone substitution. J. Mater. Sci. Mater. Med. 2008, 19, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Ciraldo, F.E.; Boccardi, E.; Melli, V.; Westhauser, F.; Boccaccini, A.R. Tackling bioactive glass excessive in vitro bioreactivity: Preconditioning approaches for cell culture tests. Acta Biomater. 2018, 75, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Yamamuro, T.; Higashi, S.; Kokubo, T.; Itoo, S. A new glass-ceramic for bone replacement: Evaluation of its bonding to bone tissue. J. Biomed. Mater. Res. 1985, 19, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, S.; Kasugai, S. Evaluation of implant materials (hydroxyapatite, glass-ceramics, titanium) in rat bone marrow stromal cell culture. Biomaterials 1996, 17, 23–29. [Google Scholar] [CrossRef]
- Hench, L.L.; Paschall, H.A. Direct chemical bond of bioactive glass-ceramic materials to bone and muscle. J. Biomed. Mater. Res. 1973, 7, 25–42. [Google Scholar] [CrossRef]
- Deligianni, D.D.; Katsala, N.D.; Koutsoukos, P.G.; Missirlis, Y.F. Effect of surface roughness of hydroxyapatite on human bone marrow cell adhesion, proliferation, differentiation and detachment strength. Biomaterials 2001, 22, 87–96. [Google Scholar] [CrossRef]
- Shin, Y.M.; Lee, Y.B.; Shin, H. Time-dependent mussel-inspired functionalization of poly(l-lactide-co-e{open}-caprolactone) substrates for tunable cell behaviors. Colloids Surf. B Biointerfaces 2011, 87, 79–87. [Google Scholar] [CrossRef]
- Lynge, M.E. Recent developments in poly (dopamine)—Based coatings for biomedical applications. Nanomedicine 2015, 10, 2725–2742. [Google Scholar] [CrossRef]
- Chien, H.W.; Tsai, W.B. Fabrication of tunable micropatterned substrates for cell patterning via microcontact printing of polydopamine with poly(ethylene imine)-grafted copolymers. Acta Biomater. 2012, 8, 3678–3686. [Google Scholar] [CrossRef]
- Iqbal, Z.; Lai, E.P.C.; Avis, T.J. Antimicrobial effect of polydopamine coating on Escherichia coli. J. Mater. Chem. 2012, 22, 21608–21612. [Google Scholar] [CrossRef]
- Lim, K.; Chua, R.R.Y.; Ho, B.; Tambyah, P.A.; Hadinoto, K.; Leong, S.S.J. Development of a catheter functionalized by a polydopamine peptide coating with antimicrobial and antibiofilm properties. Acta Biomater. 2015, 15, 127–138. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Zhou, P.; Wang, L.; Xiong, X.; Zhang, Y.; Deng, Y.; Wei, S. Antibiotic-decorated titanium with enhanced antibacterial activity through adhesive polydopamine for dental/bone implant Antibiotic-decorated titanium with enhanced antibacterial activity through adhesive polydopamine for dental/bone implant. J. R. Soc. Interface 2014, 11, 20140169. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Deng, Y.; Lyu, B.; Zhang, R.; Zhang, H.; Ma, H.; Lyu, Y.; Wei, S. Rapidly-Deposited Polydopamine Coating via High Temperature and Vigorous Stirring: Formation, Characterization and Biofunctional Evaluation. PLoS ONE 2014, 9, e113087. [Google Scholar] [CrossRef] [PubMed]
| - | Ra (μm) | Rmax (μm) |
|---|---|---|
| Sintered ceramic-glass pellets, uncoated (BGC1) | 1.4 ± 0.3 | 12 ± 5 |
| BGC1 coated with polydopamine (BGC1@PDA) | 1.0 ± 0.2 | 8 ± 2 |
| BGC1 coated with polydopamine and Ag (BGC1@PDA@Ag) | 0.8 ± 0.1 | 6.1 ± 0.3 |
| - | Initial Conditions | Measurements in SBF | ||
|---|---|---|---|---|
| - | pH | Conductivity (mS·m−1) | pH | Z (mV) |
| BGC1 | 7.32 | 16.14 | 7.34 ± 0.01 | −120 ± 9 |
| BGC1@PDA | 7.33 | 16.84 | 7.33 ± 0.00 | −83 ± 1 |
| BGC1@PDA@Ag | 7.38 | 16.20 | 7.35 ± 0.00 | −98 ± 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tejido-Rastrilla, R.; Ferraris, S.; Goldmann, W.H.; Grünewald, A.; Detsch, R.; Baldi, G.; Spriano, S.; Boccaccini, A.R. Studies on Cell Compatibility, Antibacterial Behavior, and Zeta Potential of Ag-Containing Polydopamine-Coated Bioactive Glass-Ceramic. Materials 2019, 12, 500. https://doi.org/10.3390/ma12030500
Tejido-Rastrilla R, Ferraris S, Goldmann WH, Grünewald A, Detsch R, Baldi G, Spriano S, Boccaccini AR. Studies on Cell Compatibility, Antibacterial Behavior, and Zeta Potential of Ag-Containing Polydopamine-Coated Bioactive Glass-Ceramic. Materials. 2019; 12(3):500. https://doi.org/10.3390/ma12030500
Chicago/Turabian StyleTejido-Rastrilla, Rocío, Sara Ferraris, Wolfgang H. Goldmann, Alina Grünewald, Rainer Detsch, Giovanni Baldi, Silvia Spriano, and Aldo R. Boccaccini. 2019. "Studies on Cell Compatibility, Antibacterial Behavior, and Zeta Potential of Ag-Containing Polydopamine-Coated Bioactive Glass-Ceramic" Materials 12, no. 3: 500. https://doi.org/10.3390/ma12030500
APA StyleTejido-Rastrilla, R., Ferraris, S., Goldmann, W. H., Grünewald, A., Detsch, R., Baldi, G., Spriano, S., & Boccaccini, A. R. (2019). Studies on Cell Compatibility, Antibacterial Behavior, and Zeta Potential of Ag-Containing Polydopamine-Coated Bioactive Glass-Ceramic. Materials, 12(3), 500. https://doi.org/10.3390/ma12030500









