Hydration and Microstructure of Cement Pastes with Calcined Hwangtoh Clay
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Materials
2.2. Mix Design Information
2.3. Methods
3. Results and Discussion
3.1. Characterization of the Calcined HT
3.2. Compressive Strength
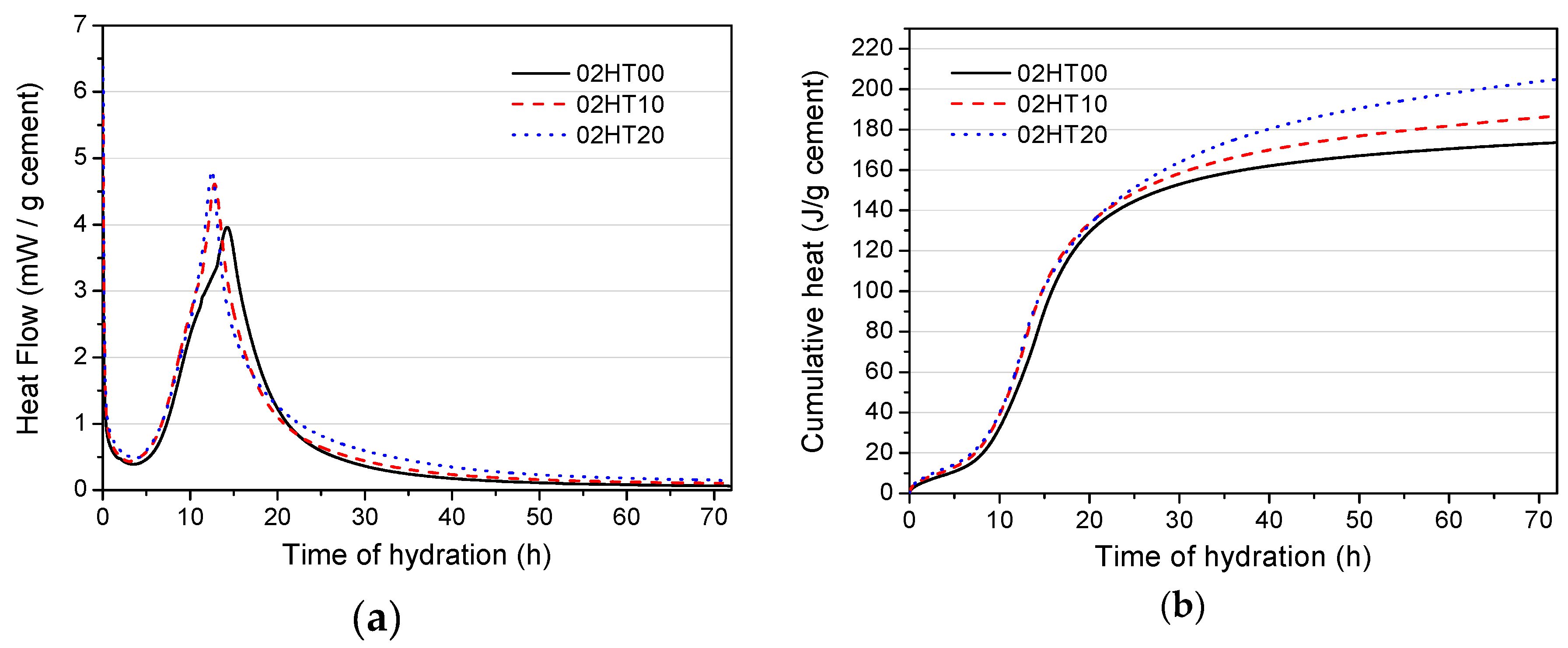
3.3. Hydration Heat
3.4. Aluminum Ion Concentration
3.5. Paste Microstructure
3.6. Thermogravimetric Analysis
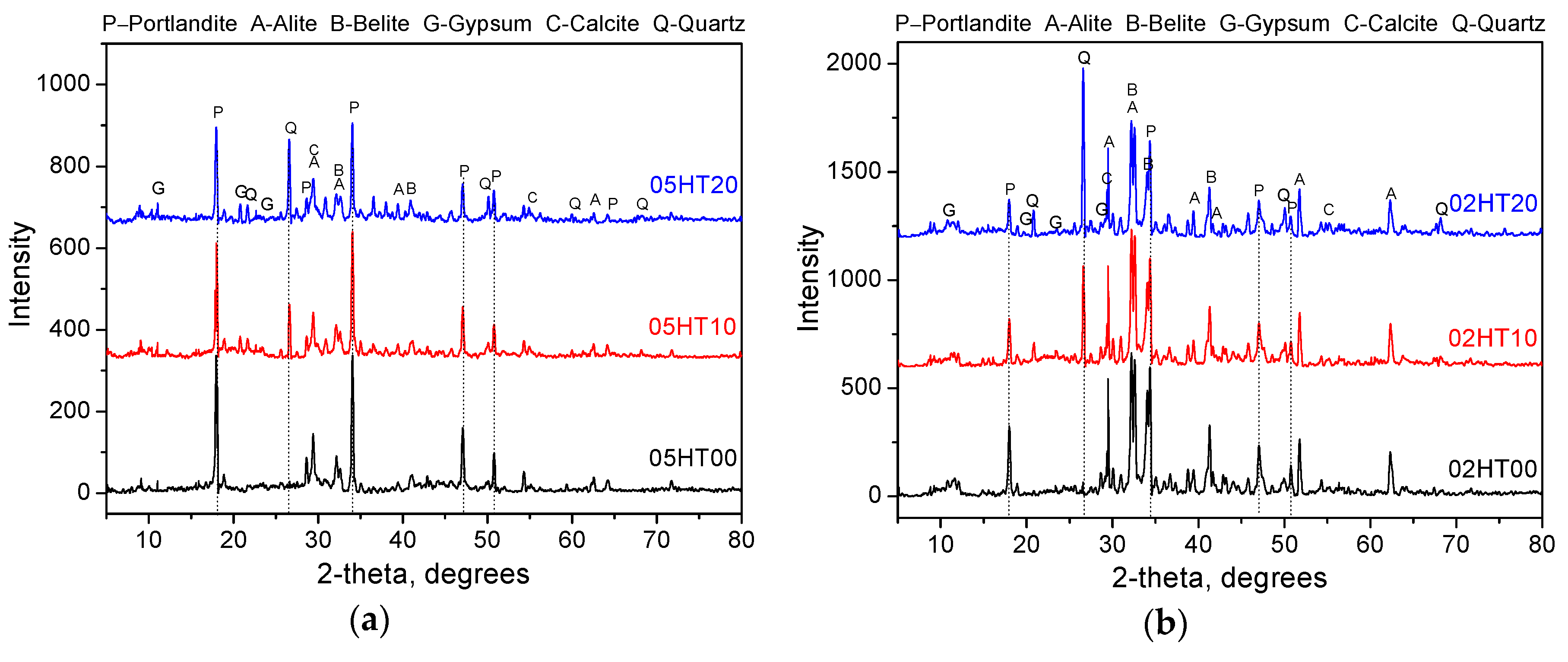
3.7. X-Ray Powder Diffraction
3.8. ATR-FTIR
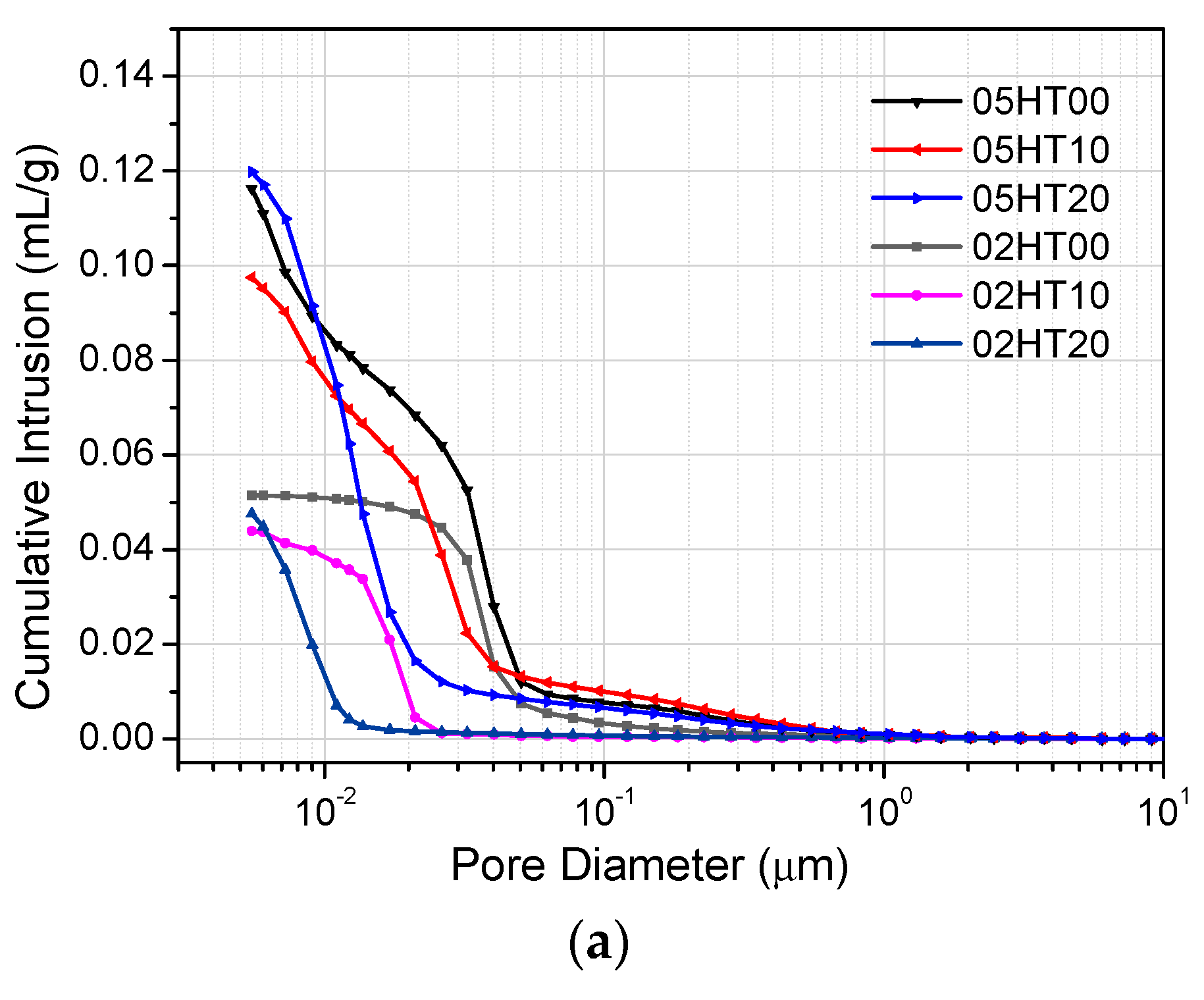
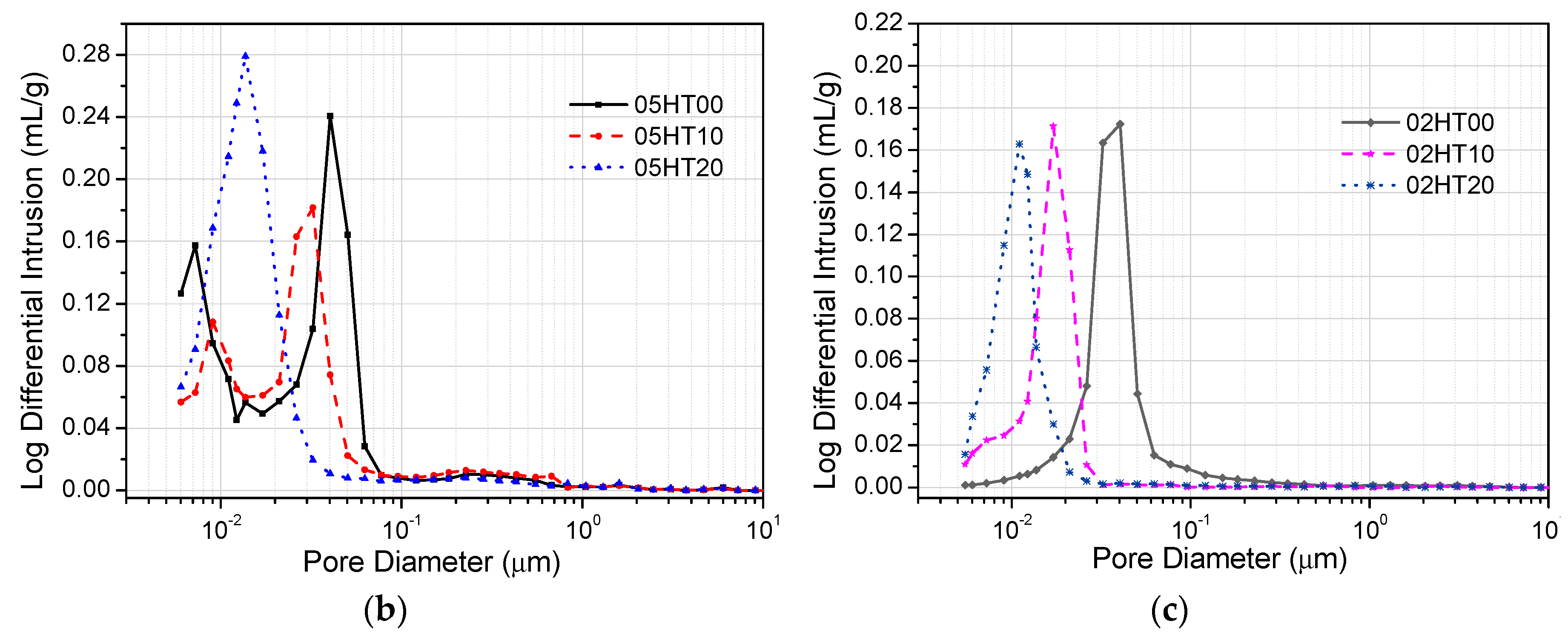
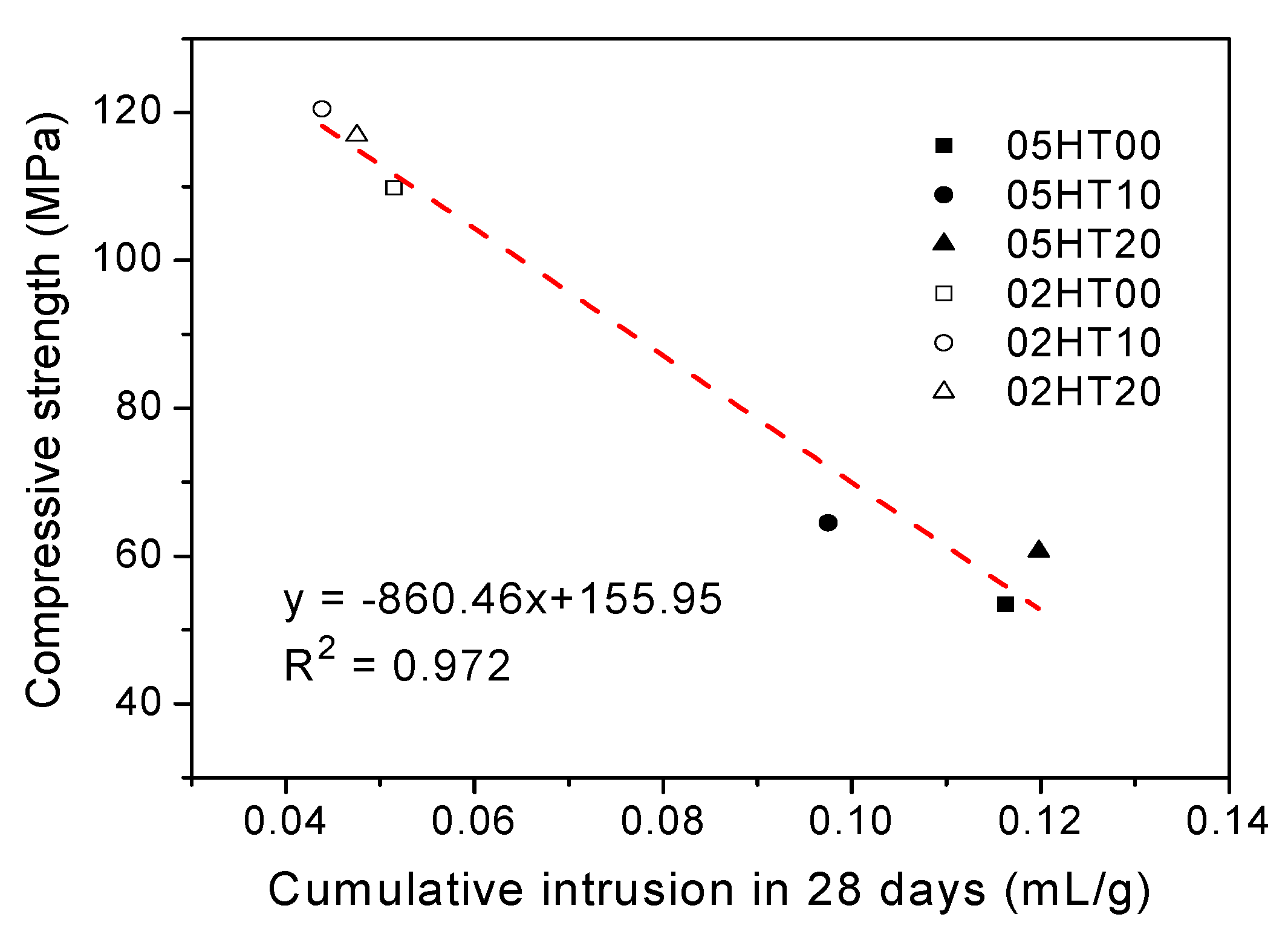
3.9. Mercury Intrusion Porosimetry
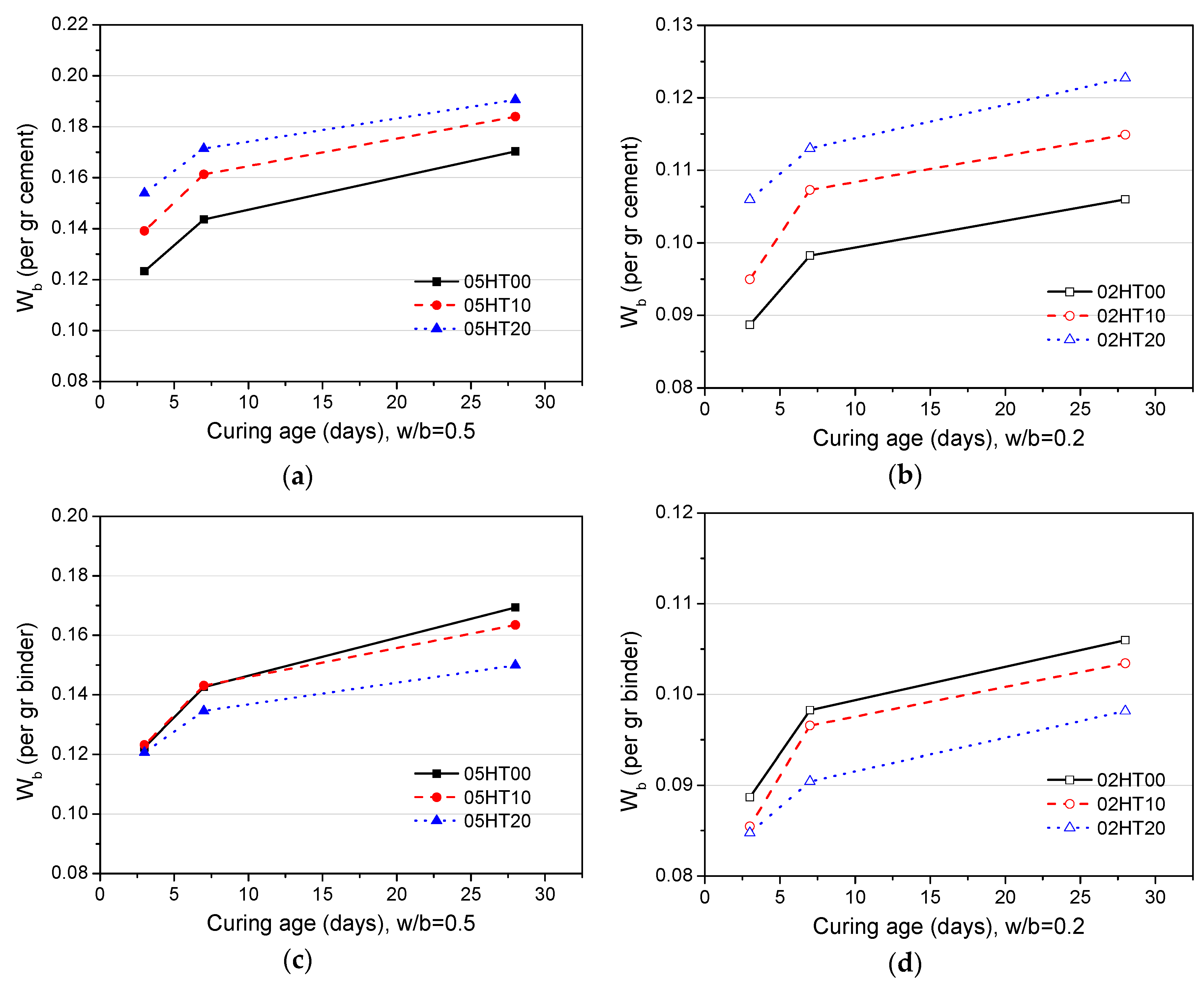
3.10. Comparison Between Cumulative Heat of Hydration and Wb
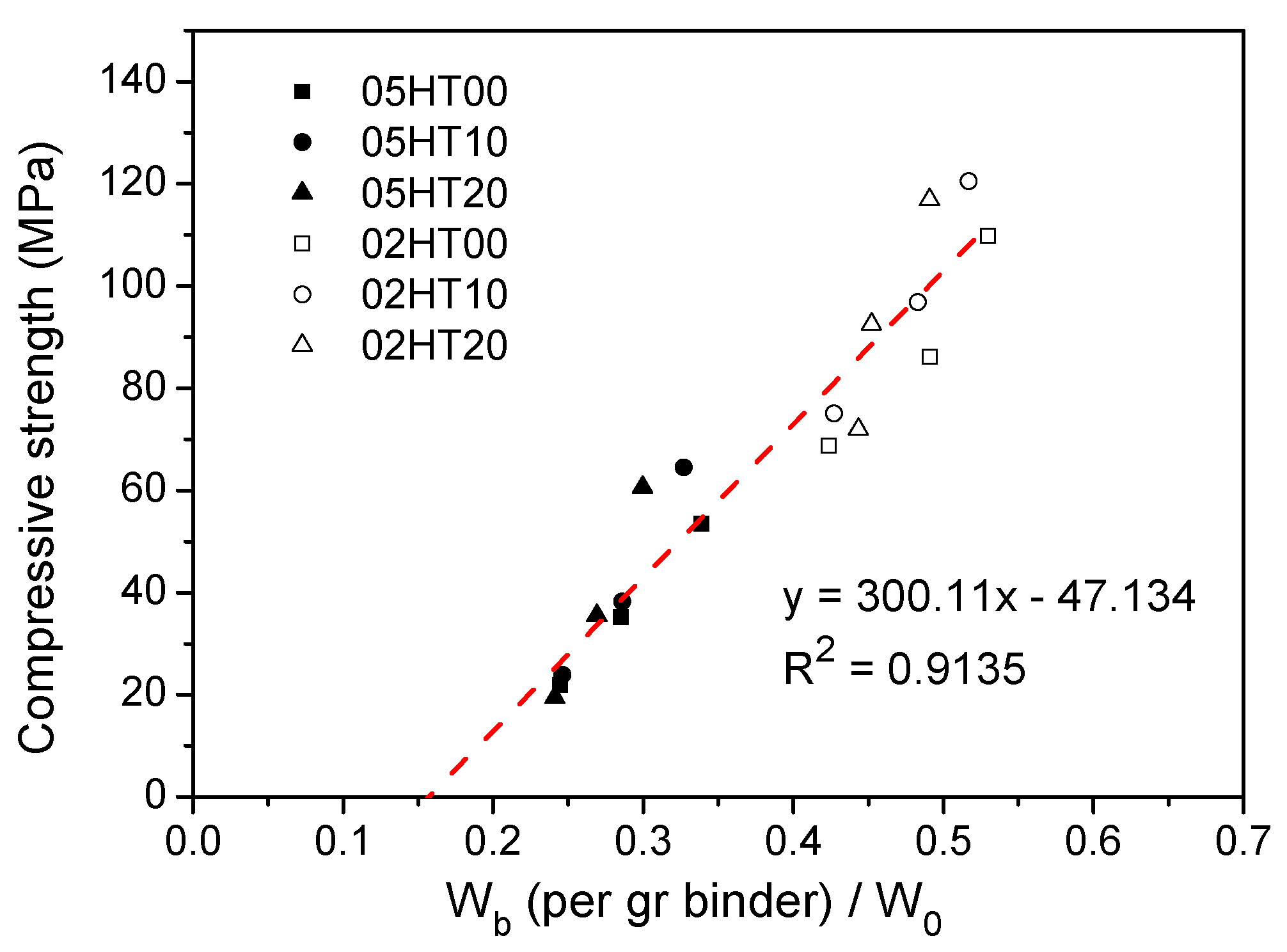
3.11. Comparison Between Compressive Strength and Wb/W0.
4. Further Research Focuses
5. Conclusions
- Calcined HT can be used to produce concrete. When the replacement ratio of HT powder is 10%, HT-blended cement paste has the highest compressive strength after three, seven, and twenty-eight days. When the replacement rate of HT is 20%, the compressive strength is higher than that of the control paste after curing for twenty-eight days, regardless of the w/b ratio. However, the strength of samples with 20% HT is reduced after three days, regardless of the w/b ratio. Moreover, the HT-blended cement pastes with a w/b ratio of 0.2 show improvements in compressive strength sooner than the pastes with a w/b of 0.5.
- The HT powder enhances the peak of hydration heat as the amount of HT added increases. Compared with pure cement, HT-containing paste has a reduced cumulative heat, which is normalized per gram of binder. The cumulative hydration heat value at a w/b of 0.5 is higher than at a w/b of 0.2.
- The Al3+ ion concentration in the HT-blended cement paste is higher than that of the OPC in the early stage, thus promoting the formation of ettringite.
- As the replacement ratio of HT increases, chemically bound water and calcium hydroxide decrease. As the w/b ratio decreases from 0.5 to 0.2, chemically bound water and calcium hydroxide also decrease. In addition, the cumulative heat is directly proportional to the amount of chemically bound water. The compressive strength has a linear relationship with Wb/W0.
- ATR-FTIR shows that the structure of C-(A)-S-H gels changes in samples with the addition of the HT powder. According to the MIP experimental analysis, the pore structure of HT-blended cement paste is more refined.
Author Contributions
Funding
Conflicts of Interest
References
- Malhotra, V.M. Introduction: Sustainable Development and Concrete. Concr. Int. 2002, 24, 22. [Google Scholar]
- Worrell, E.; Price, L.; Martin, N.; Hendriks, C.; Meida, L.O. Carbon dioxide emissions from the global cement industry. Annu. Rev. Energy Env. 2001, 26, 303–329. [Google Scholar] [CrossRef]
- Golewski, G.L. An assessment of microcracks in the Interfacial Transition Zone of durable concrete composites with fly ash additives. Compos. Struct. 2018, 200, 515–520. [Google Scholar] [CrossRef]
- Golewski, G.L. Generalized fracture toughness and compressive strength of sustainable concrete including low calcium fly ash. Materials 2017, 10, 1393. [Google Scholar] [CrossRef] [PubMed]
- Golewski, G.L. Green concrete composite incorporating fly ash with high strength and fracture toughness. J. Clean. Prod. 2018, 172, 218–226. [Google Scholar] [CrossRef]
- Lothenbach, B.; Scrivener, K.; Hooton, R.D. Supplementary cementitious materials. Cem. Concr. Res. 2011, 41, 1244–1256. [Google Scholar] [CrossRef]
- UN Environment; Scrivener, K.L.; John, V.M.; Gartner, E.M. Eco-efficient cements: Potential economically viable solutions for a low-CO2cement-based materials industry. Cem. Concr. Res. 2018. [Google Scholar] [CrossRef]
- Long, G.; Gao, Y.; Xie, Y. Designing more sustainable and greener self-compacting concrete. Constr. Build. Mater. 2015, 84, 301–306. [Google Scholar] [CrossRef]
- Sabir, B.B.; Wild, S.; Bai, J. Metakaolin and Clacined Clays as Pozzolans for Concrete: A Review. Cem. Concr. Compos. 2001, 23. [Google Scholar] [CrossRef]
- Danner, T.; Norden, G.; Justnes, H. Characterisation of calcined raw clays suitable as supplementary cementitious materials. Appl. Clay Sci. 2018, 162, 391–402. [Google Scholar] [CrossRef]
- Scrivener, K.; Martirena, F.; Bishnoi, S.; Maity, S. Calcined clay limestone cements (LC3). Cem. Concr. Res. 2017, 114, 49–56. [Google Scholar] [CrossRef]
- Yang, K.H.; Hwang, H.Z.; Kim, S.Y.; Song, J.K. Development of a cementless mortar using hwangtoh binder. Build. Environ. 2007, 42, 3717–3725. [Google Scholar] [CrossRef]
- Koo, B.M.; Kim, J.H.J.; Kim, T.K.; Kim, B.Y. Material performance and animal clinical studies on performance-optimized Hwangtoh mixed mortar and concrete to evaluate their mechanical properties and health benefits. Materials 2015, 8, 6257–6276. [Google Scholar] [CrossRef] [PubMed]
- Koo, B.M.; Kim, J.H.J.; Kim, S.B.; Mun, S. Material and Structural performance evaluations of Hwangtoh admixtures and recycled PET fiber-added eco-friendly concrete for CO2 emission reduction. Materials 2014, 7, 5959–5981. [Google Scholar] [CrossRef] [PubMed]
- Go, S.S.; Lee, H.C.; Lee, J.Y.; Kim, J.H.; Chung, C.W. Experimental investigation of mortars using activated Hwangtoh. Constr. Build. Mater. 2009, 23, 1438–1445. [Google Scholar] [CrossRef]
- Go, S.S.; Chung, C.W.; Struble, L.J.; Lee, H.C. Pozzolanic activity of Hwangtoh clay. Constr. Build. Mater. 2010, 24, 2638–2645. [Google Scholar] [CrossRef]
- Frías, M.; De Rojas, M.I.S.; Cabrera, J. Effect that the pozzolanic reaction of metakaolin has on the heat evolution in metakaolin-cement mortars. Cem. Concr. Res. 2000, 30, 209–216. [Google Scholar] [CrossRef]
- Kantro, D.L. Influence of Water-Reducing Admixtures on Properties of Cement Paste—A Miniature Slump Test. Cem. Concr. Aggreg. 1980, 2, 95–102. [Google Scholar] [CrossRef]
- Li, Z.; Ding, Z. Property improvement of Portland cement by incorporating with metakaolin and slag. Cem. Concr. Res. 2003, 33, 579–584. [Google Scholar] [CrossRef]
- Maekawa, K.; Ishida, T.; Kishi, T. Multi-Scale Modeling of Structural Concrete; Taylor & Francis: New York, NY, USA, 2009; ISBN 9780415465540. [Google Scholar]
- Ščančar, J.; Milačič, R.; Séby, F.; Donard, O.F.X. Determination of hexavalent chromium in cement by the use of HPLC-ICP-MS, FPLC-ETAAS, spectrophotometry and selective extraction techniques. J. Anal. At. Spectrom. 2005, 20, 871–875. [Google Scholar] [CrossRef]
- Scrivener, K.; Snellings, R.; Lothenbach, B. A Practical Guide to Microstructural Analysis of Cementitious Materials; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Sedaghat, A.; Zayed, A.; Sandberg, P. Measurement and Prediction of Heat of Hydration of Portland Cement Using Isothermal Conduction Calorimetry. J. Test. Eval. 2013, 41, 20120272. [Google Scholar] [CrossRef]
- Lam, L.; Wong, Y.L.; Poon, C.S. Degree of hydration and gel/space ratio of high-volume fly ash/cement systems. Cem. Concr. Res. 2000, 30, 747–756. [Google Scholar] [CrossRef]
- Ylmén, R.; Jäglid, U.; Steenari, B.M.; Panas, I. Early hydration and setting of Portland cement monitored by IR, SEM and Vicat techniques. Cem. Concr. Res. 2009, 39, 433–439. [Google Scholar] [CrossRef]
- Zhou, J.; Ye, G.; van Breugel, K. Characterization of pore structure in cement-based materials using pressurization-depressurization cycling mercury intrusion porosimetry (PDC-MIP). Cem. Concr. Res. 2010, 40, 1120–1128. [Google Scholar] [CrossRef]
- Lin, R.-S.; Wang, X.-Y.; Zhang, G.-Y. Effects of quartz powder on the microstructure and key properties of cement paste. Sustainability 2018, 10, 3369. [Google Scholar] [CrossRef]
- Stark, J.; Möser, B.; Bellmann, F. Nucleation and growth of C-S-H phases on mineral admixtures. Adv. Constr. Mater. 2007, 531–538. [Google Scholar] [CrossRef]
- Paper, T. Nucleation and Pozzolanic Factors in Strength Development. Mater. J. 1993, 90, 117–121. [Google Scholar]
- Garrault-Gauffinet, S.; Nonat, A. Experimental investigation of calcium silicate hydrate (C-S-H) nucleation. J. Cryst. Growth 1999, 200, 565–574. [Google Scholar] [CrossRef]
- Rahhal, V.; Bonavetti, V.; Trusilewicz, L.; Pedrajas, C.; Talero, R. Role of the filler on Portland cement hydration at early ages. Constr. Build. Mater. 2012, 27, 82–90. [Google Scholar] [CrossRef]
- Avet, F.; Boehm-Courjault, E.; Scrivener, K. Investigation of C-A-S-H composition, morphology and density in Limestone Calcined Clay Cement (LC3). Cem. Concr. Res. 2019, 115, 70–79. [Google Scholar] [CrossRef]
- Gutteridge, W.A.; Dalziel, J.A. Filler cement: The effect of the secondary component on the hydration of Portland cement: Part I. A fine non-hydraulic filler. Cem. Concr. Res. 1990, 20, 778–782. [Google Scholar] [CrossRef]
- Marchon, D.; Flatt, R.J. Mechanisms of cement hydration. Sci. Technol. Concr. Admix. 2015, 41, 129–145. [Google Scholar] [CrossRef]
- Quennoz, A.; Scrivener, K.L. Interactions between alite and C3A-gypsum hydrations in model cements. Cem. Concr. Res. 2013, 44, 46–54. [Google Scholar] [CrossRef]
- Hu, J.; Ge, Z.; Wang, K. Influence of cement fineness and water-to-cement ratio on mortar early-age heat of hydration and set times. Constr. Build. Mater. 2014, 50, 657–663. [Google Scholar] [CrossRef]
- Bentz, D.P.; Peltz, M.A.; Winpigler, J. Early-Age Properties of Cement-Based Materials. II: Influence of Water-to-Cement Ratio. J. Mater. Civ. Eng. 2009, 21, 512–517. [Google Scholar] [CrossRef]
- Sakai, E.; Kasuga, T.; Sugiyama, T.; Asaga, K.; Daimon, M. Influence of superplasticizers on the hydration of cement and the pore structure of hardened cement. Cem. Concr. Res. 2006, 36, 2049–2053. [Google Scholar] [CrossRef]
- Richardson, I.G.; Groves, G.W. The Incorporation of Minor and Trace Elements into Calcium Silicate Hydrate (C-S-H) Gel in Hardened Cement Pastes. Cem. Concr. Res. 1993, 23, 131–138. [Google Scholar] [CrossRef]
- Dharmawardhana, C.C.; Misra, A.; Ching, W.Y. Theoretical investigation of C-(A)-S-H(I) cement hydrates. Constr. Build. Mater. 2018, 184, 536–548. [Google Scholar] [CrossRef]
- Bishnoi, S.; Scrivener, K.L. Studying nucleation and growth kinetics of alite hydration using μic. Cem. Concr. Res. 2009, 39, 849–860. [Google Scholar] [CrossRef]
- Dunster, A.M.; Parsonage, J.R.; Thomas, M.J.K. The pozzolanic reaction of metakaolinite and its effects on Portland cement hydration. J. Mater. Sci. 1993, 28, 1345–1350. [Google Scholar] [CrossRef]
- Barnett, S.J.; Macphee, D.E.; Lachowski, E.E.; Crammond, N.J. XRD, EDX and IR analysis of solid solutions between thaumasite and ettringite. Cem. Concr. Res. 2002, 32, 719–730. [Google Scholar] [CrossRef]
- Hanna, R.A.; Barrie, P.J.; Cheeseman, C.R.; Hills, C.D.; Buchler, P.M.; Perry, R. Solid state 29Si and 27Al NMR and FTIR study of cement pastes containing industrial wastes and organics. Cem. Concr. Res. 1995, 25, 1435–1444. [Google Scholar] [CrossRef]
- Mendes, A.; Gates, W.P.; Sanjayan, J.G.; Collins, F. NMR, XRD, IR and synchrotron NEXAFS spectroscopic studies of OPC and OPC/slag cement paste hydrates. Mater. Struct. 2011, 44, 1773–1791. [Google Scholar] [CrossRef]
- Gao, X.F.; Lo, Y.; Tam, C.M.; Chung, C.Y. Analysis of the infrared spectrum and microstructure of hardened cement paste. Cem. Concr. Res. 1999, 29, 805–812. [Google Scholar] [CrossRef]
- Mollah, M.Y.A.; Yu, W.; Schennach, R.; Cocke, D.L. A Fourier transform infrared spectroscopic investigation of the early hydration of Portland cement and the influence of sodium lignosulfonate. Cem. Concr. Res. 2000, 30, 267–273. [Google Scholar] [CrossRef]
- Yu, P.; Kirkpatrick, R.J.; Poe, B.; McMillan, P.F.; Cong, X. Structure of Calcium Silicate Hydrate (C-S-H): Near-, Mid-, and Far-Infrared Spectroscopy. J. Am. Ceram. Soc. 1999, 82, 742–748. [Google Scholar] [CrossRef]
- Mollah, M.Y.A.; Kesmez, M.; Cocke, D.L. An X-ray diffraction (XRD) and Fourier transform infrared spectroscopic (FT-IR) investigation of the long-term effect on the solidification/ stabilization (S/S) of arsenic(V) in Portland cement type-V. Sci. Total Environ. 2004, 325, 255–262. [Google Scholar] [CrossRef]
- Trezza, M.A.; Lavat, A.E. Analysis of the system 3CaO·Al2O3-CaSO4·2H2O-CaCO3-H2O by FT-IR spectroscopy. Cem. Concr. Res. 2001, 31, 869–872. [Google Scholar] [CrossRef]
- Richard, T.; Mercury, L.; Poulet, F.; D’Hendecourt, L. Diffuse reflectance infrared Fourier transform spectroscopy as a tool to characterise water in adsorption/confinement situations. J. Colloid Interface Sci. 2006, 304, 125–136. [Google Scholar] [CrossRef]
- Silva, D.A.; Roman, H.R.; Gleize, P.J.P. Evidences of chemical interaction between EVA and hydrating Portland cement. Cem. Concr. Res. 2002, 32, 1383–1390. [Google Scholar] [CrossRef]
- Okpala, D.C. Pore structure of hardened cement paste and mortar. Int. J. Cem. Compos. Lightweight Concr. 1989, 11, 245–254. [Google Scholar] [CrossRef]
- Kondraivendhan, B.; Bhattacharjee, B. Effect of age and water-cement ratio on size and dispersion of pores in ordinary portland cement paste. ACI Mater. J. 2010, 107, 147–154. [Google Scholar]
- Olek, J.; Cohen, M.; Lobo, C. Determination of Surface Area of Portland Cement and Silica Fume by Mercury Intrusion Porosimetry. ACI Mater. J. 1990, 87, 473–478. [Google Scholar]
- Poon, C.S.; Lam, L.; Kou, S.C.; Wong, Y.L.; Wong, R. Rate of pozzolanic reaction of metakaolin in high-performance cement pastes. Cem. Concr. Res. 2001, 31, 1301–1306. [Google Scholar] [CrossRef]
- Pane, I.; Hansen, W. Investigation of blended cement hydration by isothermal calorimetry and thermal analysis. Cem. Concr. Res. 2005, 35, 1155–1164. [Google Scholar] [CrossRef]
- Han, F.; Liu, J.; Yan, P. Comparative study of reaction degree of mineral admixture by selective dissolution and image analysis. Constr. Build. Mater. 2016, 114, 946–955. [Google Scholar] [CrossRef]

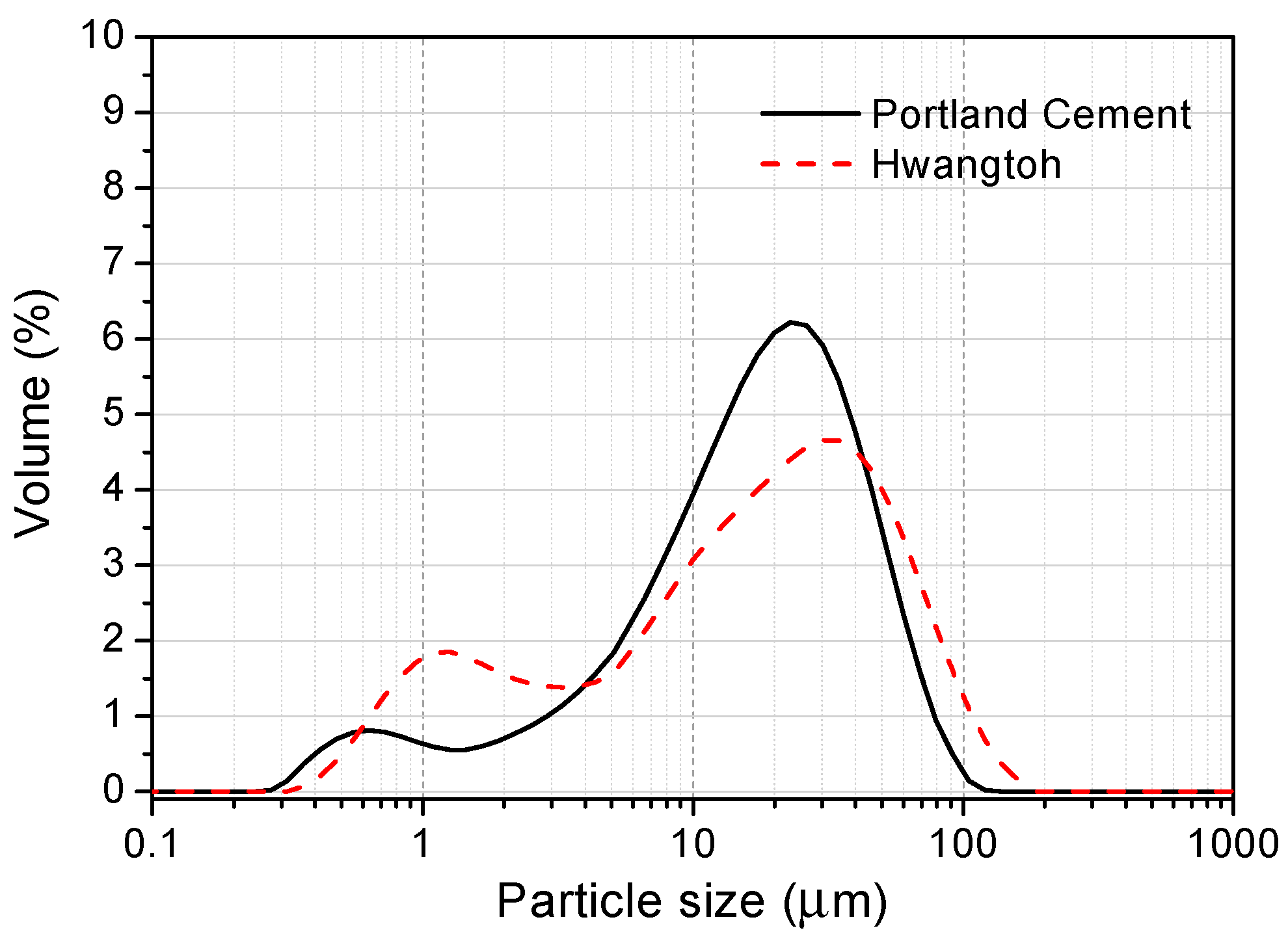

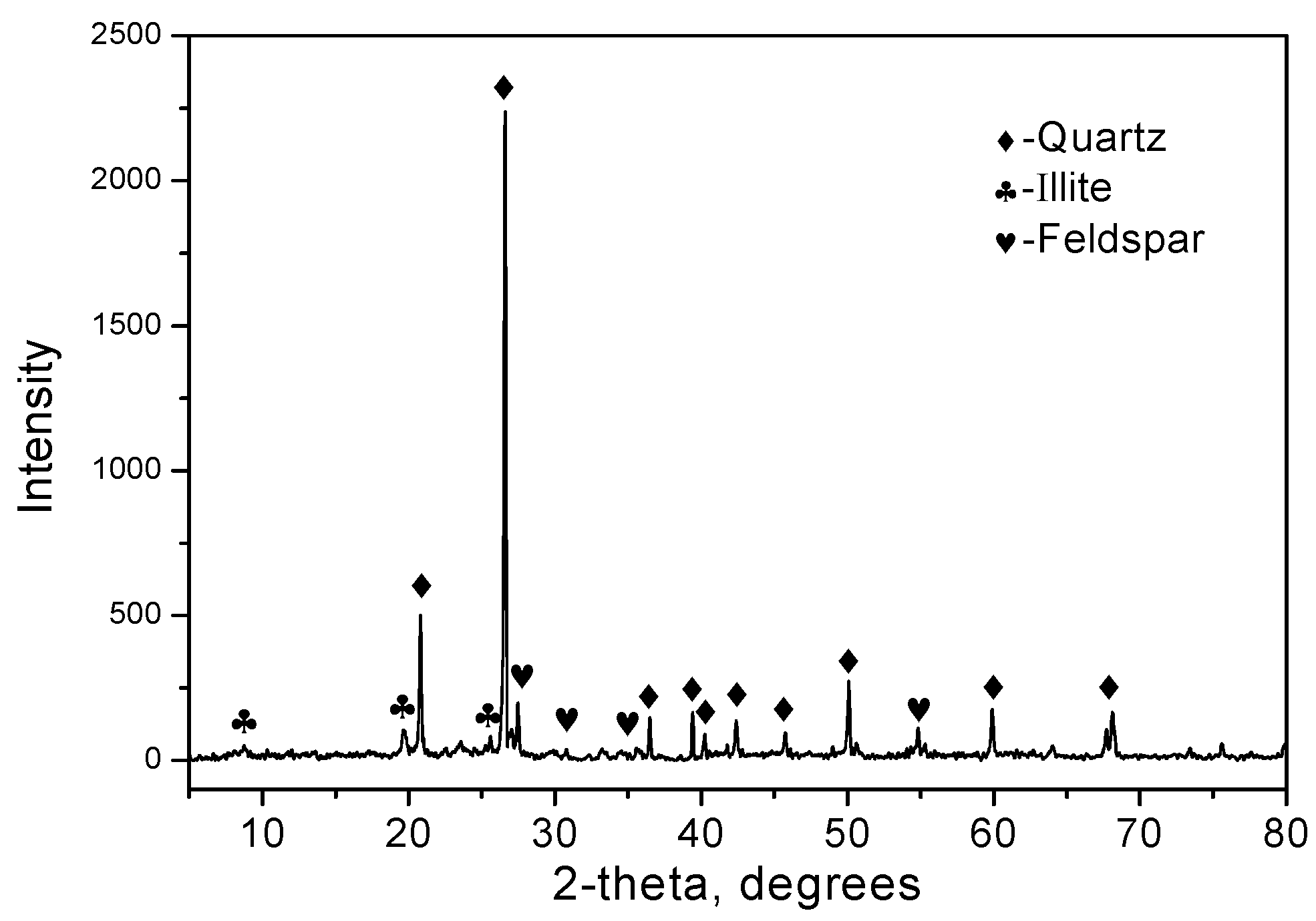
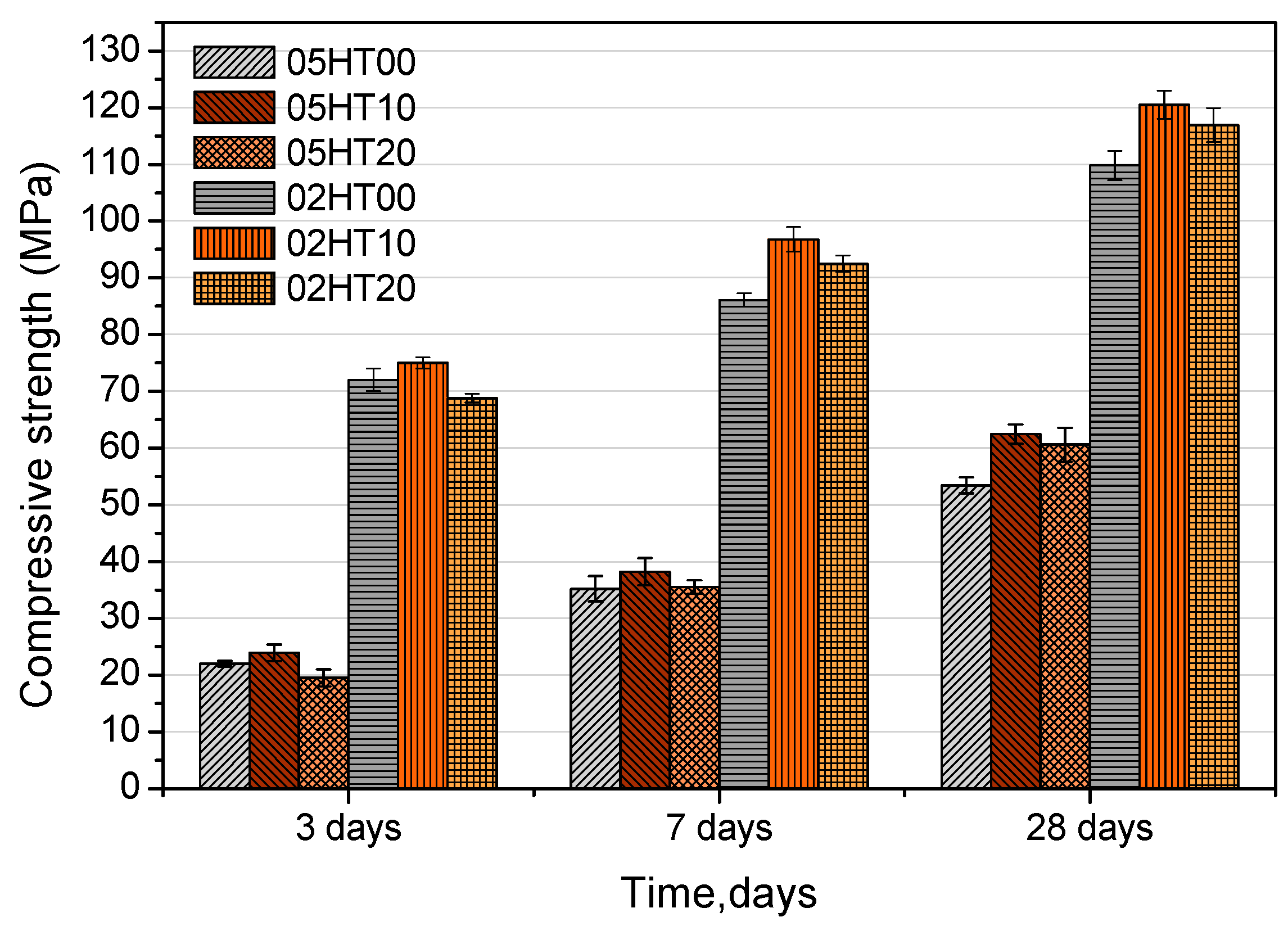
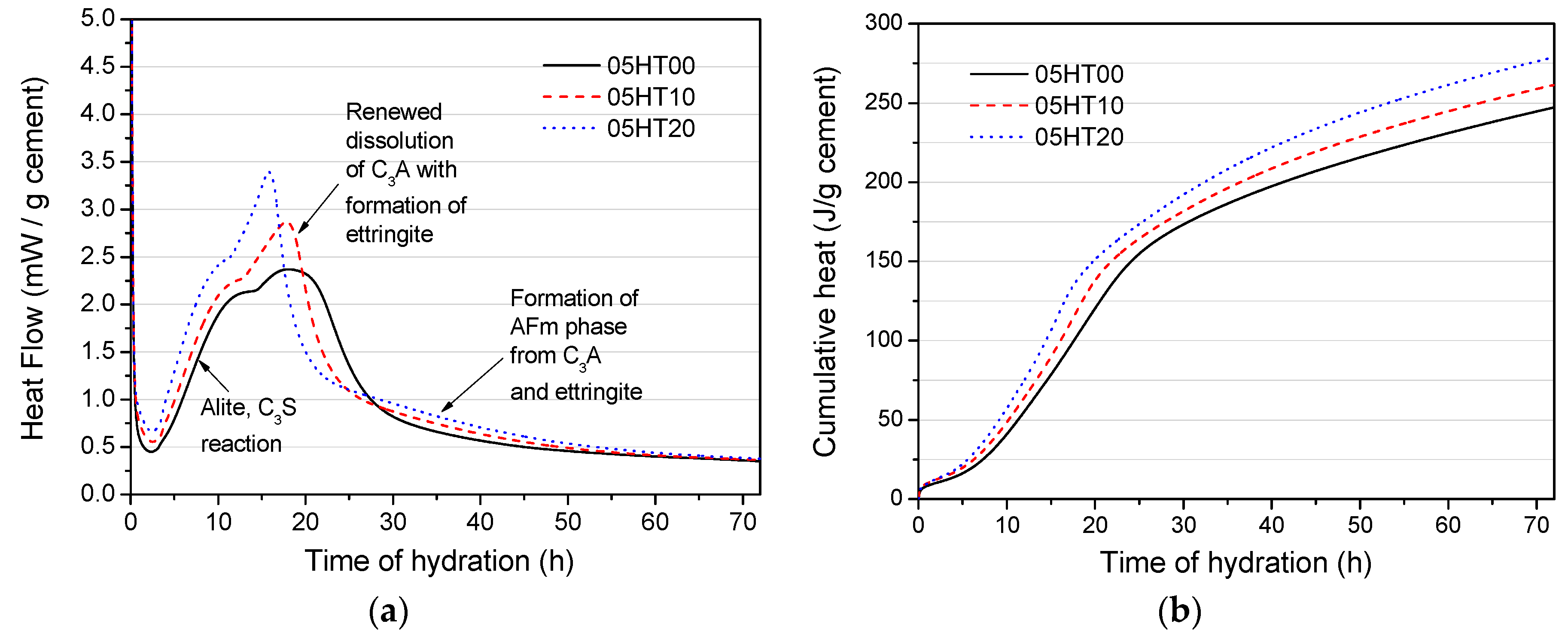





| Samples | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O | TiO2 | SO3 | ZnO | P2O5 | K2O | LOI 1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cement | 21.7 | 5.77 | 2.5 | 62.9 | 2.4 | 0.45 | 0.22 | 2.34 | 0.11 | 0.09 | 1.03 | 0.46 |
| Hwangtoh | 63.9 | 24.4 | 6.13 | 0.16 | 0.67 | - | 0.78 | - | - | 0.09 | 3.06 | 0.83 |
| Mix No. | w/b | Cement | Hwangtoh | Water | Superplasticizer | Flow | |||
|---|---|---|---|---|---|---|---|---|---|
| wt.% of Binder | kg/m3 | wt.% of Binder | kg/m3 | kg/m3 | wt.% of Binder | kg/m3 | (mm) | ||
| 05HT00 | 0.5 | 100 | 1221.8 | 0 | 0 | 610.9 | 0 | 0 | 255 |
| 05HT10 | 0.5 | 90 | 1094.4 | 10 | 121.6 | 608.1 | 0 | 0 | 246 |
| 05HT20 | 0.5 | 80 | 968.3 | 20 | 242.1 | 605.2 | 0 | 0 | 231 |
| 02HT00 | 0.2 | 100 | 1910.3 | 0 | 0 | 382.1 | 0.6 | 11.5 | 221 |
| 02HT10 | 0.2 | 90 | 1696.0 | 10 | 188.4 | 376.9 | 1.0 | 18.8 | 220 |
| 02HT20 | 0.2 | 80 | 1487.3 | 20 | 371.8 | 371.8 | 1.4 | 26.0 | 216 |
| Mixtures | 100% OPC | 90% OPC + 10% HT | 80% OPC + 20% HT |
|---|---|---|---|
| Intensity | 41286.9 | 49538.0 | 61733.5 |
| Al3+ ion concentration at one day (μg/L) | 2.24 | 2.69 | 3.36 |
| Wavenumber (cm−1) | Functional Groups | Reference |
|---|---|---|
| 968–971 | Si–O–Si (Si–O–Al), υ3 | [43,44,45] |
| 1400–1500 | C–O (CO32−),υ3 | [25,46,47,48] |
| 1640–1650 | H2O, υ2 | [49,50] |
| 3390–3408 | H2O, υ1,υ3 | [48,51] |
| 3641–3644 | Ca(OH)2 | [25,52] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, R.-S.; Wang, X.-Y.; Lee, H.-S.; Cho, H.-K. Hydration and Microstructure of Cement Pastes with Calcined Hwangtoh Clay. Materials 2019, 12, 458. https://doi.org/10.3390/ma12030458
Lin R-S, Wang X-Y, Lee H-S, Cho H-K. Hydration and Microstructure of Cement Pastes with Calcined Hwangtoh Clay. Materials. 2019; 12(3):458. https://doi.org/10.3390/ma12030458
Chicago/Turabian StyleLin, Run-Sheng, Xiao-Yong Wang, Han-Seung Lee, and Hyeong-Kyu Cho. 2019. "Hydration and Microstructure of Cement Pastes with Calcined Hwangtoh Clay" Materials 12, no. 3: 458. https://doi.org/10.3390/ma12030458
APA StyleLin, R.-S., Wang, X.-Y., Lee, H.-S., & Cho, H.-K. (2019). Hydration and Microstructure of Cement Pastes with Calcined Hwangtoh Clay. Materials, 12(3), 458. https://doi.org/10.3390/ma12030458








