Synthesis, Characterization, and Modification of Alumina Nanoparticles for Cationic Dye Removal
Abstract
1. Introduction
2. Materials and Methods
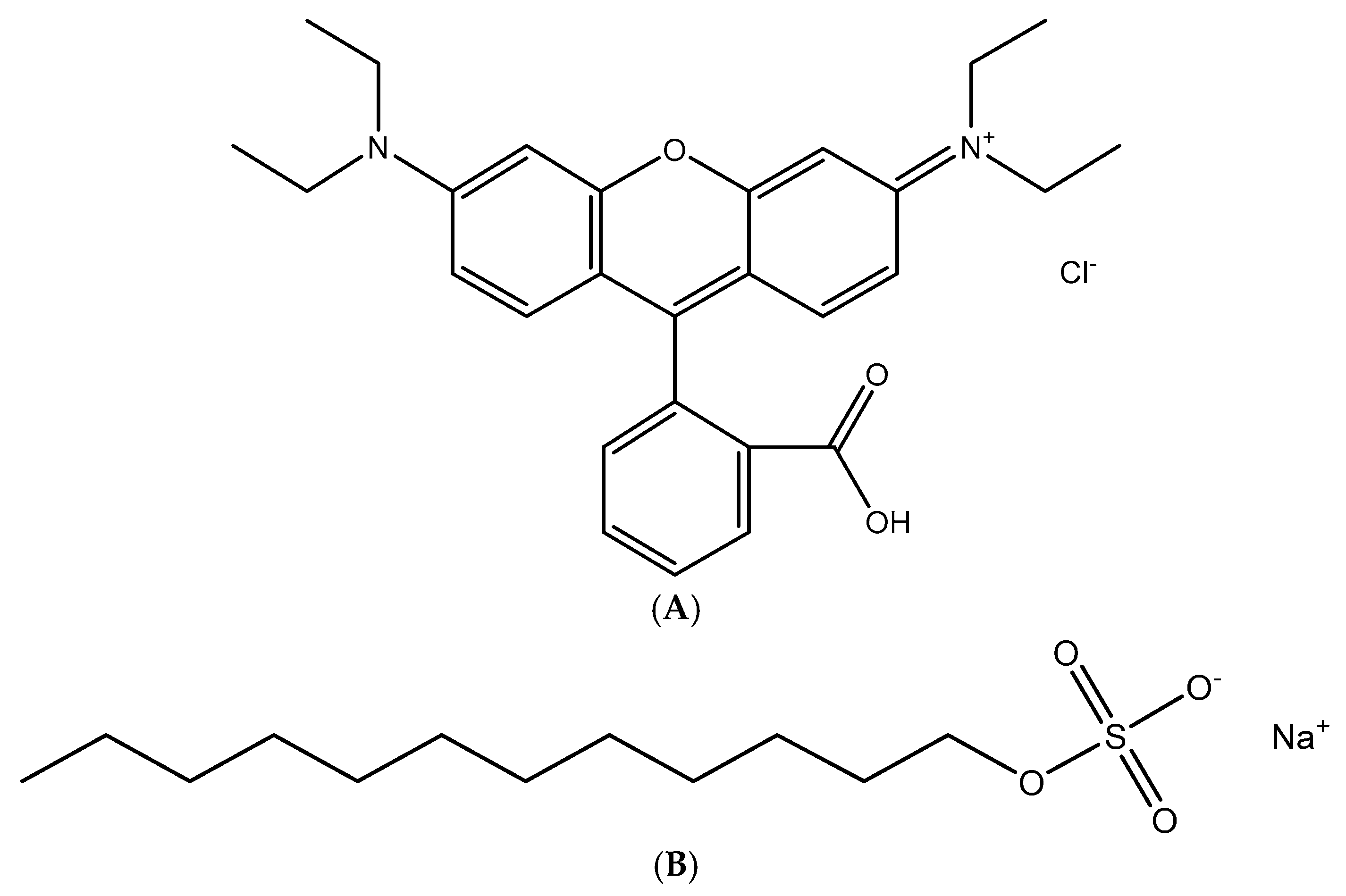
2.1. Materials
2.2. Fabrication of Alumina Nanoparticles
2.3. Characterization Methods
2.4. Adsorption Studies
3. Results and Discussion
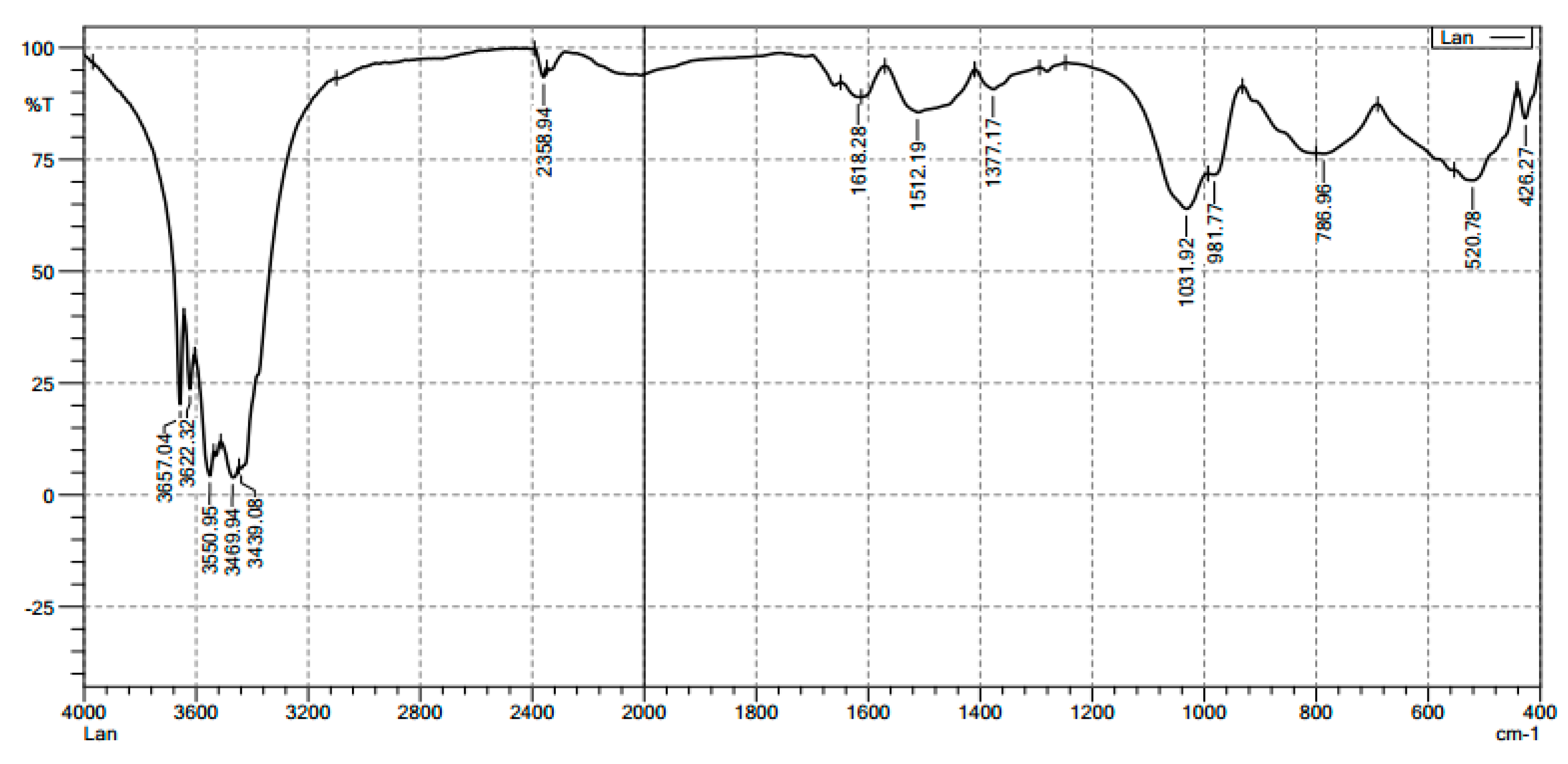
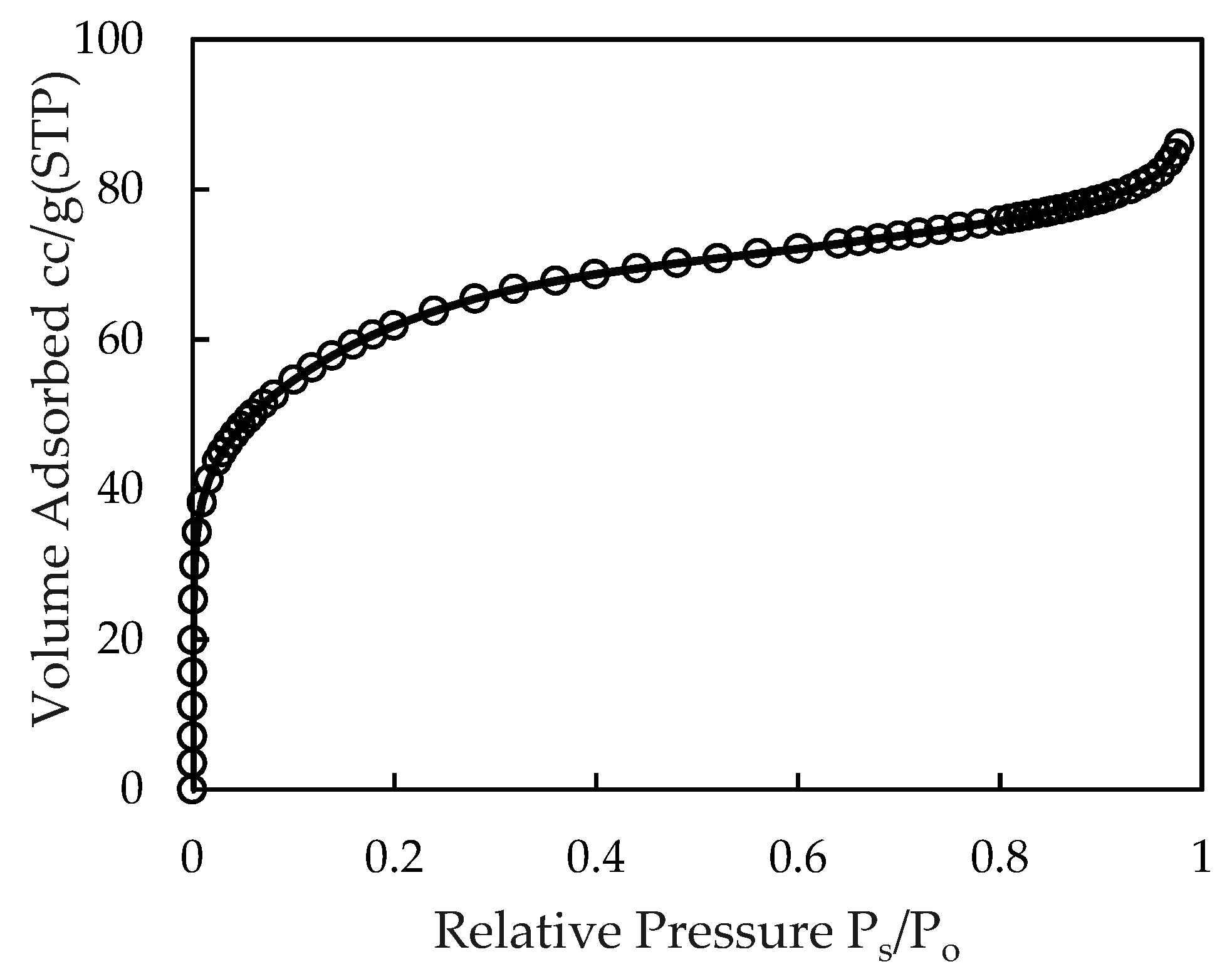
3.1. Characterization of Synthesized Alumina Nanoparticles
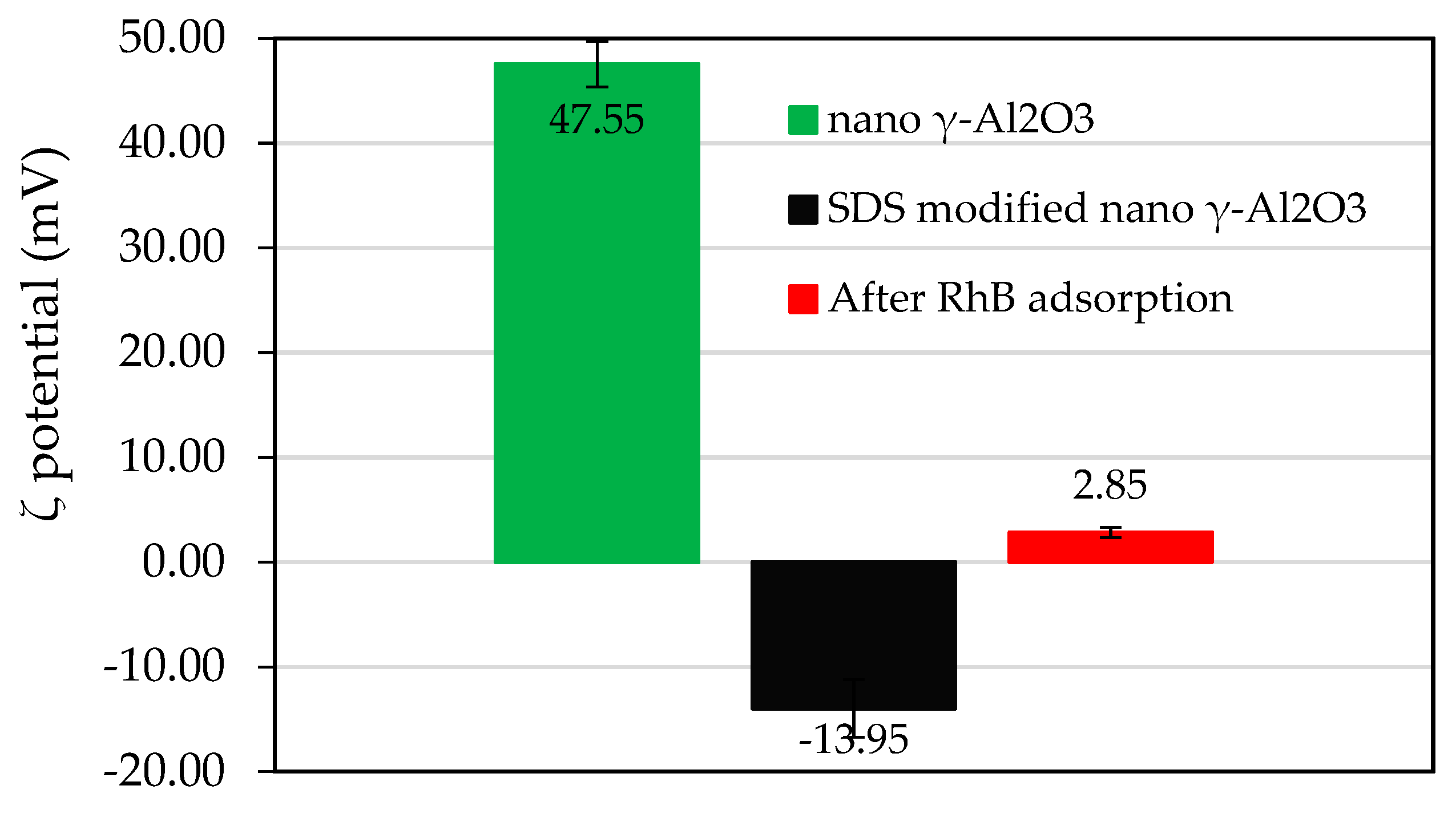
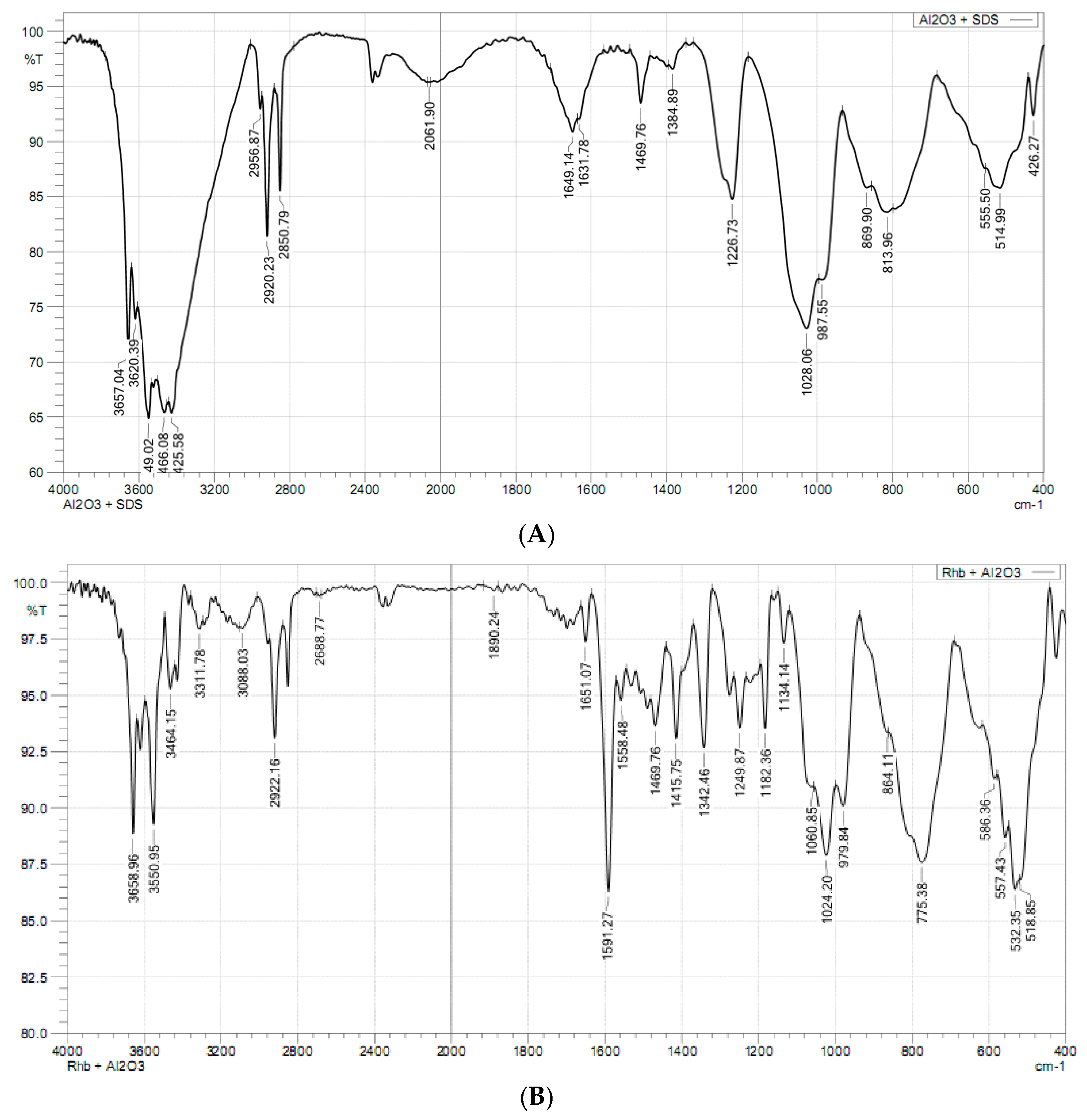
3.2. Modification of Synthesized Nano-Alumina by SDS Adsorption
3.3. Adsorptive Removal of RhB Using Synthesized Nano-Alumina (NA) without and with SDS Modification
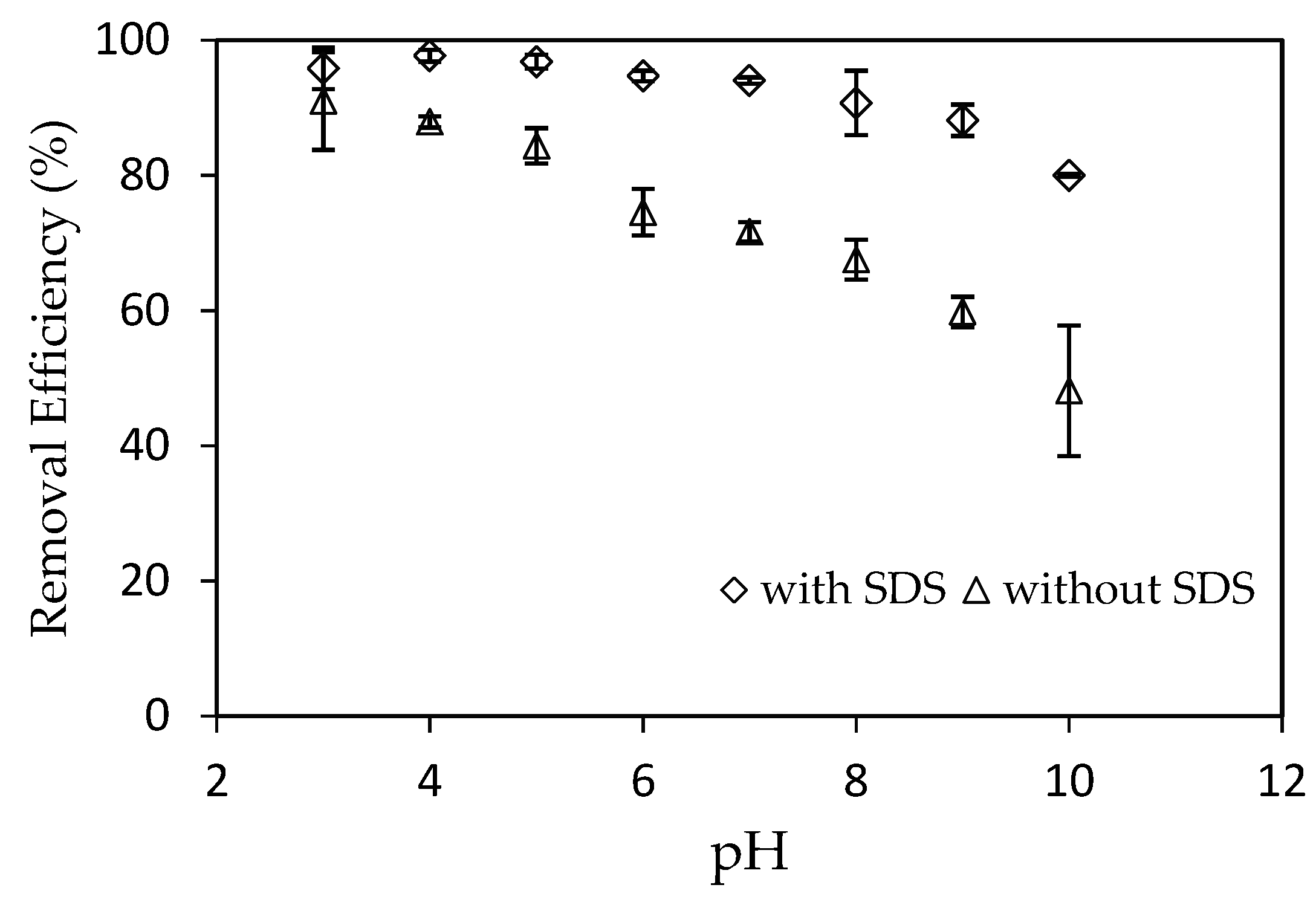
3.3.1. Effect of pH
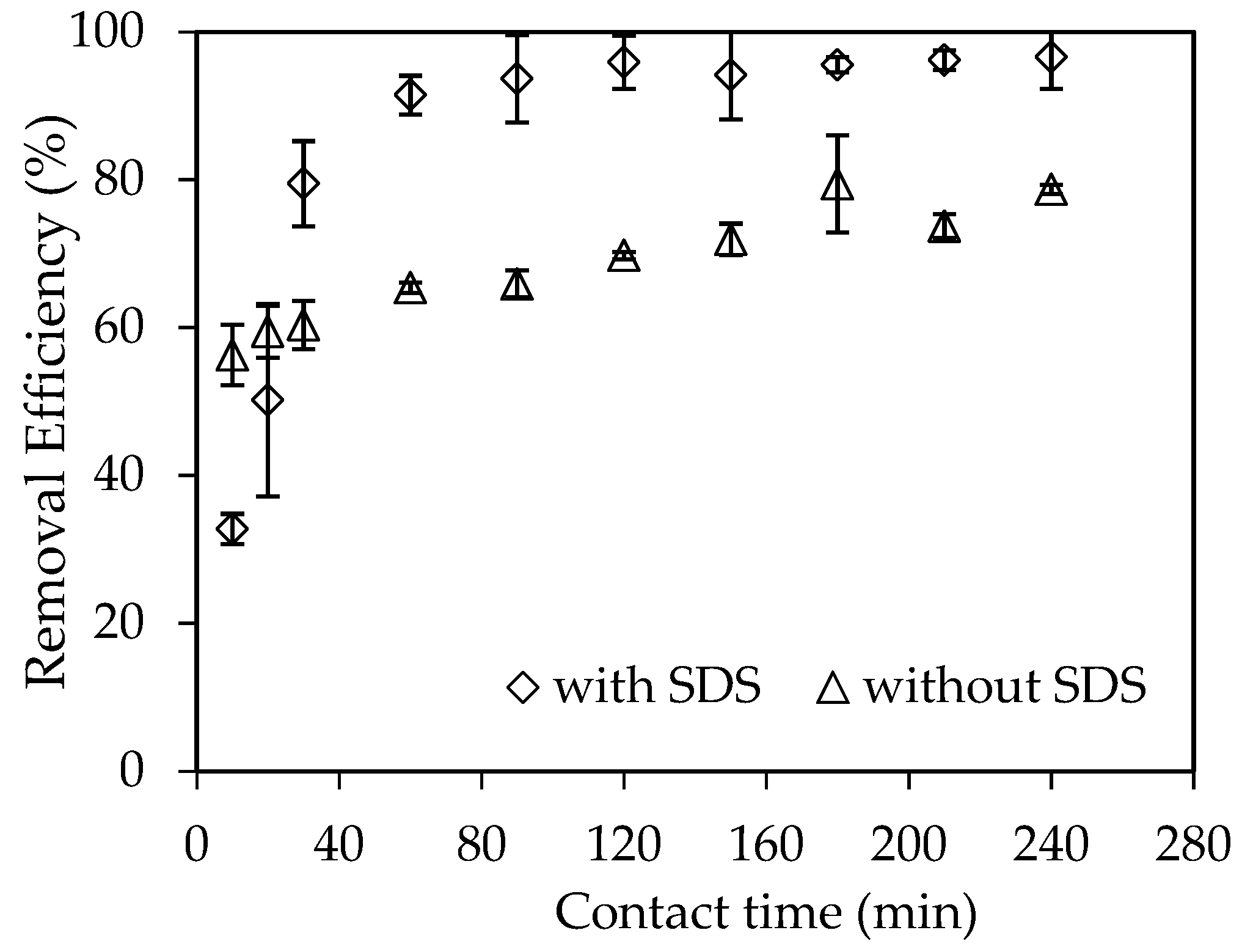
3.3.2. Effect of Adsorption Time
3.3.3. The Effect of Adsorbent Dosage
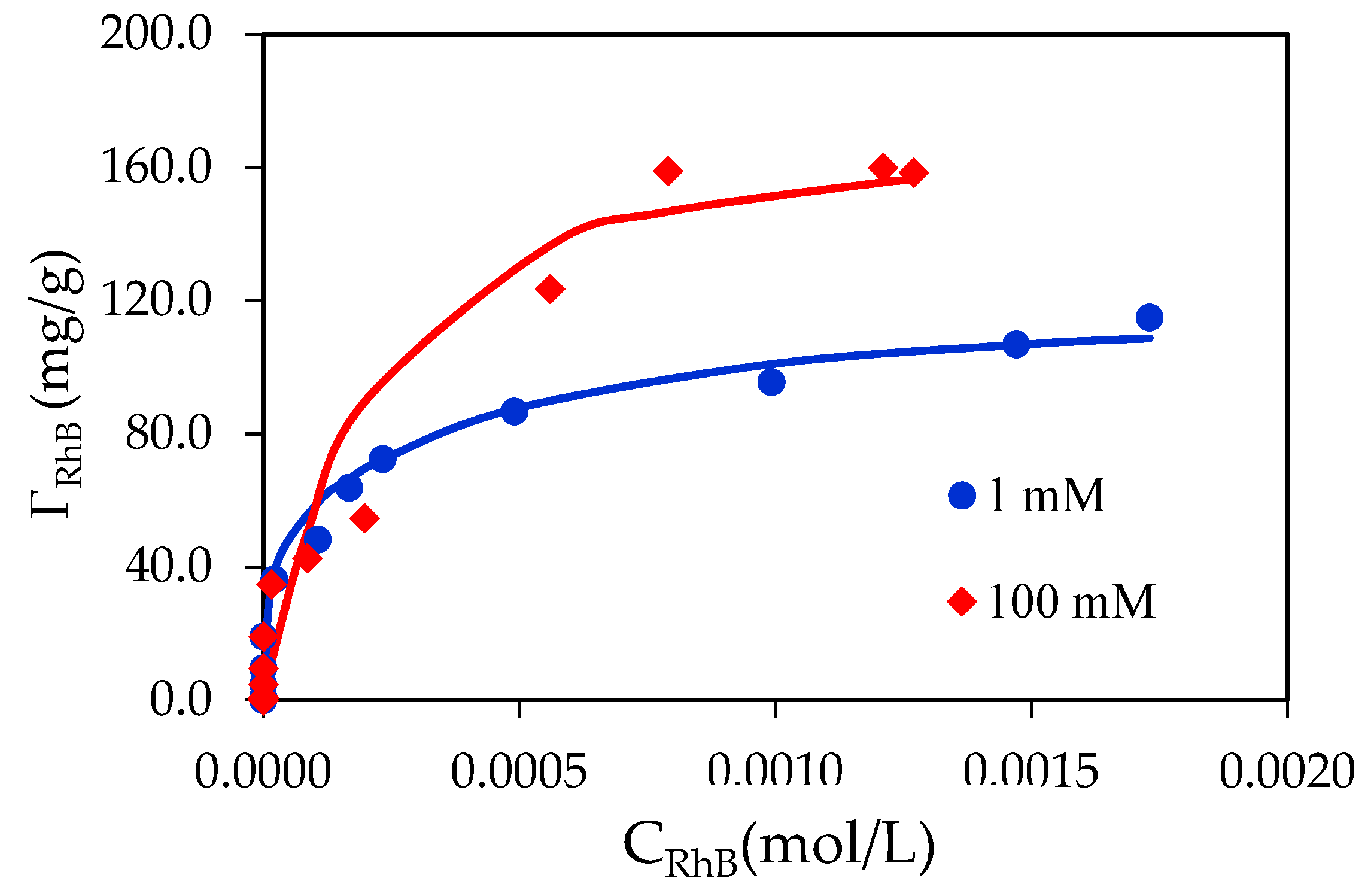
3.4. Adsorption Isotherms of RhB on SDS Modified Nano-Alumina (SMNA)
3.5. Adsorption Mechanisms of RhB onto SDS Modified Nano γ-Al2O3 (SMNA)
3.6. Comparison of Effectiveness of Surfactant-Modified Nano γ-Al2O3 (SMNA) and Other Adsorbents for RhB Removal and Other Nano γ-Al2O3
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Adak, A.; Bandyopadhyay, M.; Pal, A. Removal of crystal violet dye from wastewater by surfactant-modified alumina. Sep. Purif. Technol. 2005, 44, 139–144. [Google Scholar] [CrossRef]
- Wong, Y.C.; Szeto, Y.S.; Cheung, W.H.; McKay, G. Equilibrium Studies for Acid Dye Adsorption onto Chitosan. Langmuir 2003, 19, 7888–7894. [Google Scholar] [CrossRef]
- Almeida, M.R.; Stephani, R.; Dos Santos, H.F.; de Oliveira, L.F.C. Spectroscopic and Theoretical Study of the “Azo”-Dye E124 in Condensate Phase: Evidence of a Dominant Hydrazo Form. J. Phys. Chem. A 2009, 114, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Doğan, M.; Alkan, M.; Türkyilmaz, A.; Özdemir, Y. Kinetics and mechanism of removal of methylene blue by adsorption onto perlite. J. Hazard. Mater. 2004, 109, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Hameed, B.H.; El-Khaiary, M.I. Removal of basic dye from aqueous medium using a novel agricultural waste material: Pumpkin seed hull. J. Hazard. Mater. 2008, 155, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.D.; Kobayashi, M.; Adachi, Y. Adsorption characteristics of anionic azo dye onto large α-alumina beads. Colloid Polym. Sci. 2015, 293, 1877–1886. [Google Scholar] [CrossRef]
- Al-Momani, F.; Touraud, E.; Degorce-Dumas, J.R.; Roussy, J.; Thomas, O. Biodegradability enhancement of textile dyes and textile wastewater by VUV photolysis. J. Photochem. Photobiol. A Chem. 2002, 153, 191–197. [Google Scholar] [CrossRef]
- Kang, S.-F.; Liao, C.-H.; Po, S.-T. Decolorization of textile wastewater by photo-fenton oxidation technology. Chemosphere 2000, 41, 1287–1294. [Google Scholar] [CrossRef]
- Mohan, N.; Balasubramanian, N.; Basha, C.A. Electrochemical oxidation of textile wastewater and its reuse. J. Hazard. Mater. 2007, 147, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Vlyssides, A.G.; Loizidou, M.; Karlis, P.K.; Zorpas, A.A.; Papaioannou, D. Electrochemical oxidation of a textile dye wastewater using a Pt/Ti electrode. J. Hazard. Mater. 1999, 70, 41–52. [Google Scholar] [CrossRef]
- Papić, S.; Koprivanac, N.; Lončarić Božić, A.; Meteš, A. Removal of some reactive dyes from synthetic wastewater by combined Al(III) coagulation/carbon adsorption process. Dyes Pigments 2004, 62, 291–298. [Google Scholar] [CrossRef]
- Ledakowicz, S.; Solecka, M.; Zylla, R. Biodegradation, decolourisation and detoxification of textile wastewater enhanced by advanced oxidation processes. J. Biotechnol. 2001, 89, 175–184. [Google Scholar] [CrossRef]
- Gupta, V.K.; Carrott, P.J.M.; Ribeiro Carrott, M.M.L.; Suhas. Low-Cost Adsorbents: Growing Approach to Wastewater Treatment—A Review. Crit. Rev. Environ. Sci. Technol. 2009, 39, 783–842. [Google Scholar] [CrossRef]
- Gupta, V.K.; Suhas. Application of low-cost adsorbents for dye removal—A review. J. Environ. Manag. 2009, 90, 2313–2342. [Google Scholar] [CrossRef] [PubMed]
- Kasprzyk-Hordern, B. Chemistry of alumina, reactions in aqueous solution and its application in water treatment. Adv. Colloid Interface Sci. 2004, 110, 19–48. [Google Scholar] [CrossRef] [PubMed]
- Franks, G.V.; Gan, Y. Charging Behavior at the Alumina–Water Interface and Implications for Ceramic Processing. J. Am. Ceram. Soc. 2007, 90, 3373–3388. [Google Scholar] [CrossRef]
- Adak, A.; Pal, A.; Bandyopadhyay, M. Removal of phenol from water environment by surfactant-modified alumina through adsolubilization. Colloids Surfaces A 2006, 277, 63. [Google Scholar] [CrossRef]
- Khobragade, M.U.; Pal, A. Fixed-Bed Column Study on Removal of Mn(II), Ni(II) and Cu(II) from Aqueous Solution by Surfactant Bilayer Supported Alumina. Sep. Sci. Technol. 2016, 51, 1287–1298. [Google Scholar] [CrossRef]
- Khobragade, M.U.; Pal, A. Adsorptive removal of Mn(II) from water and wastewater by surfactant-modified alumina. Desalin. Water Treat. 2016, 57, 2775–2786. [Google Scholar] [CrossRef]
- Khobragade, M.U.; Nayak, A.K.; Pal, A. Solid-Phase Extraction of Cu(II) from Aqueous Solution Using Surfactant-Modified Alumina. J. Hazard. Toxic Radioact. Waste 2017, 21, 04016017. [Google Scholar] [CrossRef]
- Bao, C.; Xiong, X.; Gong, W.; Feng, D.; Xian, M.; Ge, Z.; Xu, N. Removal of rhodamine B by ozone-based advanced oxidation process. Desalination 2011, 278, 84–90. [Google Scholar]
- Saleh, T.A.; Gupta, V.K. Functionalization of tungsten oxide into MWCNT and its application for sunlight-induced degradation of rhodamine B. J. Colloid Interface Sci. 2011, 362, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Fu, J.; Zhang, Y.; Chen, Z.; Wang, M.; Zhu, J.; Cui, W.; Zhang, J.; Xu, Q. Removal of Rhodamine B, a Cationic Dye From Aqueous Solution Using Poly(cyclotriphosphazene-co-4,4′-sulfonyldiphenol) Nanotubes. J. Macromol. Sci. Part A 2015, 52, 105–113. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the Adsorption in Solution. J. Phys. Chem. 1906, 57, 385–470. [Google Scholar]
- Langmuir, I. The Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Zhu, B.-Y.; Gu, T. Surfactant adsorption at solid-liquid interfaces. Adv. Colloid Interface Sci. 1991, 37, 1–32. [Google Scholar] [CrossRef]
- Hoffmann, I.; Oppel, C.; Gernert, U.; Barreleiro, P.; von Rybinski, W.; Gradzielski, M. Adsorption Isotherms of Cellulose-Based Polymers onto Cotton Fibers Determined by Means of a Direct Method of Fluorescence Spectroscopy. Langmuir 2012, 28, 7695–7703. [Google Scholar] [CrossRef] [PubMed]
- Ndong, R.; Russel, W. Linear viscoelasticity of ZrO2 nanoparticle dispersions with associative polymers. Rheol. Acta 2012, 51, 771–782. [Google Scholar] [CrossRef]
- Pham, T.D.; Kobayashi, M.; Adachi, Y. Adsorption of Polyanion onto Large Alpha Alumina Beads with Variably Charged Surface. Adv. Phys. Chem. 2014, 2014, 460942. [Google Scholar] [CrossRef]
- Pham, T.D.; Kobayashi, M.; Adachi, Y. Adsorption of anionic surfactant sodium dodecyl sulfate onto alpha alumina with small surface area. Colloid Polym. Sci. 2015, 293, 217–227. [Google Scholar] [CrossRef]
- Pham, T.D.; Bui, T.T.; Nguyen, V.T.; Bui, T.K.V.; Tran, T.T.; Phan, Q.C.; Pham, T.D.; Hoang, T.H. Adsorption of Polyelectrolyte onto Nanosilica Synthesized from Rice Husk: Characteristics, Mechanisms, and Application for Antibiotic Removal. Polymers 2018, 10, 220. [Google Scholar] [CrossRef]
- Pham, T.D.; Do, T.T.; Ha, V.L.; Doan, T.H.Y.; Nguyen, T.A.H.; Mai, T.D.; Kobayashi, M.; Adachi, Y. Adsorptive removal of ammonium ion from aqueous solution using surfactant-modified alumina. Environ. Chem. 2017, 14, 327–337. [Google Scholar] [CrossRef]
- Hiemstra, T.; Yong, H.; Van Riemsdijk, W.H. Interfacial Charging Phenomena of Aluminum (Hydr)oxides. Langmuir 1999, 15, 5942–5955. [Google Scholar]
- Delgado, A.V.; González-Caballero, F.; Hunter, R.J.; Koopal, L.K.; Lyklema, J. Measurement and interpretation of electrokinetic phenomena. J. Colloid Interface Sci. 2007, 309, 194–224. [Google Scholar] [CrossRef]
- Renuka, N.K.; Shijina, A.V.; Praveen, A.K. Mesoporous γ-alumina nanoparticles: Synthesis, characterization and dye removal efficiency. Mater. Lett. 2012, 82, 42–44. [Google Scholar] [CrossRef]
- Del Nero, M.; Galindo, C.; Barillon, R.; Halter, E.; Madé, B. Surface reactivity of α-Al2O3 and mechanisms of phosphate sorption: In situ ATR-FTIR spectroscopy and ζ potential studies. J. Colloid Interface Sci. 2010, 342, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Khataee, A.R.; Kasiri, M.B. Photocatalytic degradation of organic dyes in the presence of nanostructured titanium dioxide: Influence of the chemical structure of dyes. J. Mol. Catal. A Chem. 2010, 328, 8–26. [Google Scholar] [CrossRef]
- Pham, T.D.; Kobayashi, M.; Adachi, Y. Interfacial characterization of α-alumina with small surface area by streaming potential and chromatography. Colloids Surf. A 2013, 436, 148–157. [Google Scholar] [CrossRef]
- Nguyen, T.M.T.; Do, T.P.T.; Hoang, T.S.; Nguyen, N.V.; Pham, H.D.; Nguyen, T.D.; Pham, T.N.M.; Le, T.S.; Pham, T.D. Adsorption of Anionic Surfactants onto Alumina: Characteristics, Mechanisms, and Application for Heavy Metal Removal. Int. J. Polym. Sci. 2018, 2018, 11. [Google Scholar] [CrossRef]
- Esumi, K.; Yamanaka, Y. Interaction between Sodium Dodecyl Poly(oxyethylene) Sulfate and Alumina Surface in Aqueous Solution. J. Colloid Interface Sci. 1995, 172, 116–120. [Google Scholar] [CrossRef]
- Lefèvre, G.; Duc, M.; Fédoroff, M. Effect of solubility on the determination of the protonable surface site density of oxyhydroxides. J. Colloid Interface Sci. 2004, 269, 274–282. [Google Scholar] [CrossRef]
- Kadirvelu, K.; Karthika, C.; Vennilamani, N.; Pattabhi, S. Activated carbon from industrial solid waste as an adsorbent for the removal of Rhodamine-B from aqueous solution: Kinetic and equilibrium studies. Chemosphere 2005, 60, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Mazloomi, F.; Jalali, M. Ammonium removal from aqueous solutions by natural Iranian zeolite in the presence of organic acids, cations and anions. J. Environ. Chem. Eng. 2016, 4, 1664–1673. [Google Scholar] [CrossRef]
- Hind, A.R.; Bhargava, S.K.; McKinnon, A. At the solid/liquid interface: FTIR/ATR—The tool of choice. Adv. Colloid Interface Sci. 2001, 93, 91–114. [Google Scholar] [CrossRef]
- Sperline, R.P.; Song, Y.; Freiser, H. Fourier transform infrared attenuated total reflection spectroscopy linear dichroism study of sodium dodecyl sulfate adsorption at the alumina/water interface using alumina-coated optics. Langmuir 1992, 8, 2183–2191. [Google Scholar] [CrossRef]
- Saleh, T.A.; Ali, I. Synthesis of polyamide grafted carbon microspheres for removal of rhodamine B dye and heavy metals. J. Environ. Chem. Eng. 2018, 6, 5361–5368. [Google Scholar] [CrossRef]
- Qin, P.; Yang, Y.; Zhang, X.; Niu, J.; Yang, H.; Tian, S.; Zhu, J.; Lu, M. Highly Efficient, Rapid, and Simultaneous Removal of Cationic Dyes from Aqueous Solution Using Monodispersed Mesoporous Silica Nanoparticles as the Adsorbent. Nanomaterials 2018, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.A.; Dahiya, S.; Ali, I. Use of kaolinite as adsorbent: Equilibrium, dynamics and thermodynamic studies on the adsorption of Rhodamine B from aqueous solution. Appl. Clay Sci. 2012, 69, 58–66. [Google Scholar] [CrossRef]
- Huang, J.-H.; Huang, K.-L.; Liu, S.-Q.; Wang, A.T.; Yan, C. Adsorption of Rhodamine B and, methyl orange on a hypercrosslinked polymeric adsorbent in aqueous solution. Colloids Surfaces A 2008, 330, 55–61. [Google Scholar] [CrossRef]
- Sureshkumar, M.V.; Namasivayam, C. Adsorption behavior of Direct Red 12B and Rhodamine B from water onto surfactant-modified coconut coir pith. Colloids Surf. A 2008, 317, 277–283. [Google Scholar] [CrossRef]
- Ali, S.; Abbas, Y.; Zuhra, Z.; Butler, I.S. Synthesis of γ-alumina (Al2O3) nanoparticles and their potential for use as an adsorbent in the removal of methylene blue dye from industrial wastewater. Nanoscale Adv. 2019, 1, 213–221. [Google Scholar] [CrossRef]




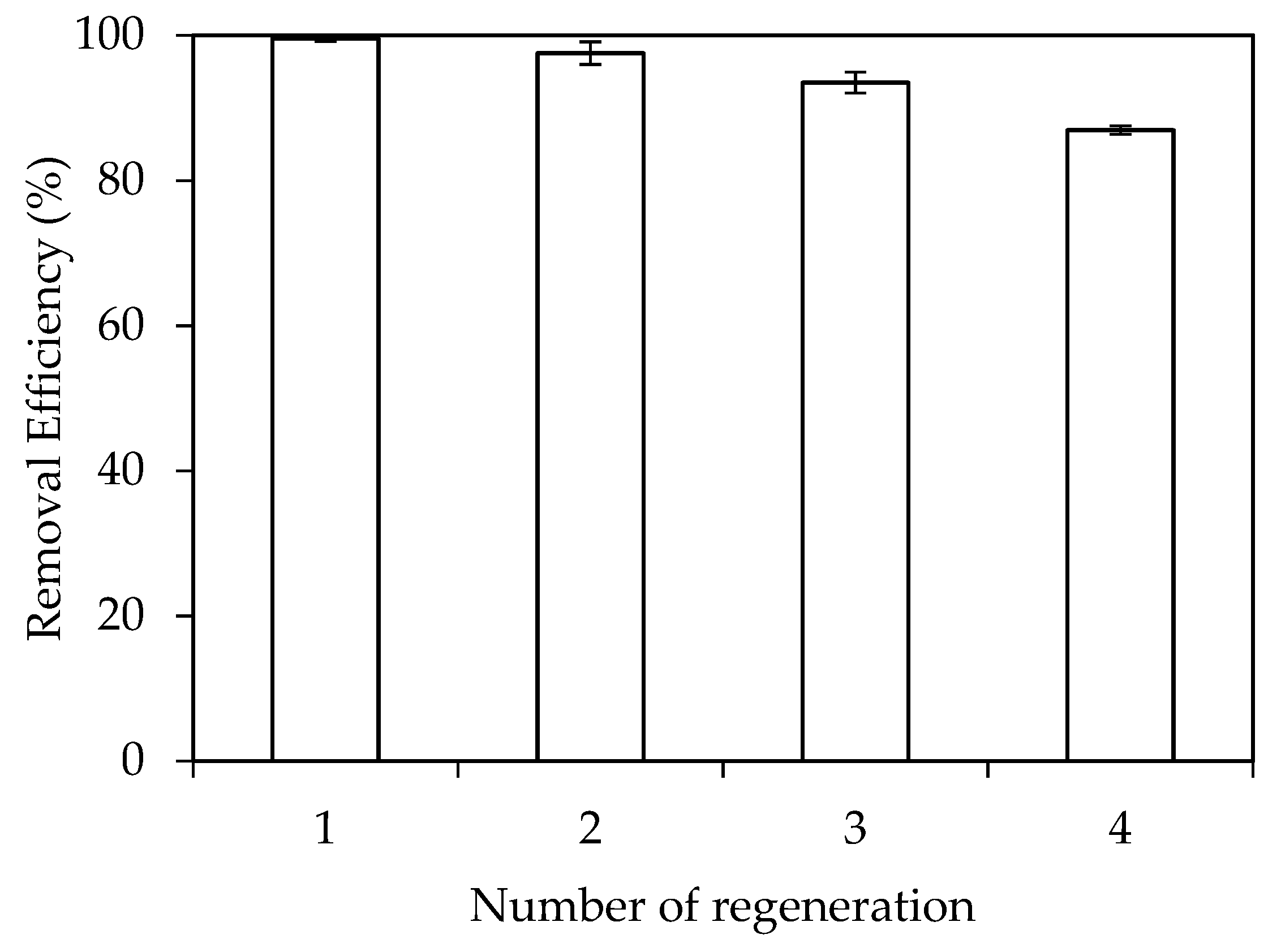
| CNaCl (mM) | Γ (mg/g) | k1 (104 g/mg) | k2 (103 g/mg)n−1 | n |
|---|---|---|---|---|
| 100 | 165 | 10 | 20 | 2.2 |
| 1 | 120 | 100 | 20 | 2.2 |
| Adsorbent | Adsorption Capacity (mg/g) | Removal Efficiency (%) | References |
|---|---|---|---|
| Monodispersed mesoporous nanosilica | 23.0 | 96 | [47] |
| Polyamide grafted carbon microspheres | 19.9 | 100 | [46] |
| Polymeric nanotubes | 35.58 | 99 | [23] |
| Kaolinite | 46.1 | 83 | [48] |
| Sago waste activated carbon | 16.1 | 100 | [42] |
| Hypercross linked polymeric adsorbent | 25.0 | 97 | [49] |
| Surfactant-modified coconut coir pith | 14.9 | 97 | [50] |
| Surfactant-modified nano γ-Al2O3 (SMNA) | 165.0 | 100 | This study |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, T.P.M.; Nguyen, N.T.; Vu, T.L.; Dao, T.H.; Dinh, L.C.; Nguyen, H.L.; Hoang, T.H.; Le, T.S.; Pham, T.D. Synthesis, Characterization, and Modification of Alumina Nanoparticles for Cationic Dye Removal. Materials 2019, 12, 450. https://doi.org/10.3390/ma12030450
Chu TPM, Nguyen NT, Vu TL, Dao TH, Dinh LC, Nguyen HL, Hoang TH, Le TS, Pham TD. Synthesis, Characterization, and Modification of Alumina Nanoparticles for Cationic Dye Removal. Materials. 2019; 12(3):450. https://doi.org/10.3390/ma12030450
Chicago/Turabian StyleChu, Thi Phuong Minh, Ngoc Trung Nguyen, Thi Lan Vu, Thi Huong Dao, Lan Chi Dinh, Hai Long Nguyen, Thu Ha Hoang, Thanh Son Le, and Tien Duc Pham. 2019. "Synthesis, Characterization, and Modification of Alumina Nanoparticles for Cationic Dye Removal" Materials 12, no. 3: 450. https://doi.org/10.3390/ma12030450
APA StyleChu, T. P. M., Nguyen, N. T., Vu, T. L., Dao, T. H., Dinh, L. C., Nguyen, H. L., Hoang, T. H., Le, T. S., & Pham, T. D. (2019). Synthesis, Characterization, and Modification of Alumina Nanoparticles for Cationic Dye Removal. Materials, 12(3), 450. https://doi.org/10.3390/ma12030450





