Improved Self-Cleaning Properties of Photocatalytic Gypsum Plaster Enriched with Glass Fiber
Abstract
:1. Introduction
2. Materials and Methods
- (1)
- The mechanical mixing the pure gypsum plaster with different amounts of TiO2/N photocatalyst (1 wt.% or 3 wt.%) or the reference P25 photocatalyst (1 wt.%);
- (2)
- The blending of the homogenous powders with distilled water in an appropriate water/binder ratio;
- (3)
- The addition of glass fiber to the pastes of the selected samples (from 0.1 wt.% to 0.5 wt.%);
- (4)
- The pouring of the obtained pastes into silicone moulds (20 mm × 20 mm × 6 mm);
- (5)
- The demolding of plates after 1 day of drying at room temperature;
- (6)
- The drying of plates at 40 °C to dry matter.
3. Results and Discussion
3.1. Colour Variation of Plates before Photocatalytic Processes
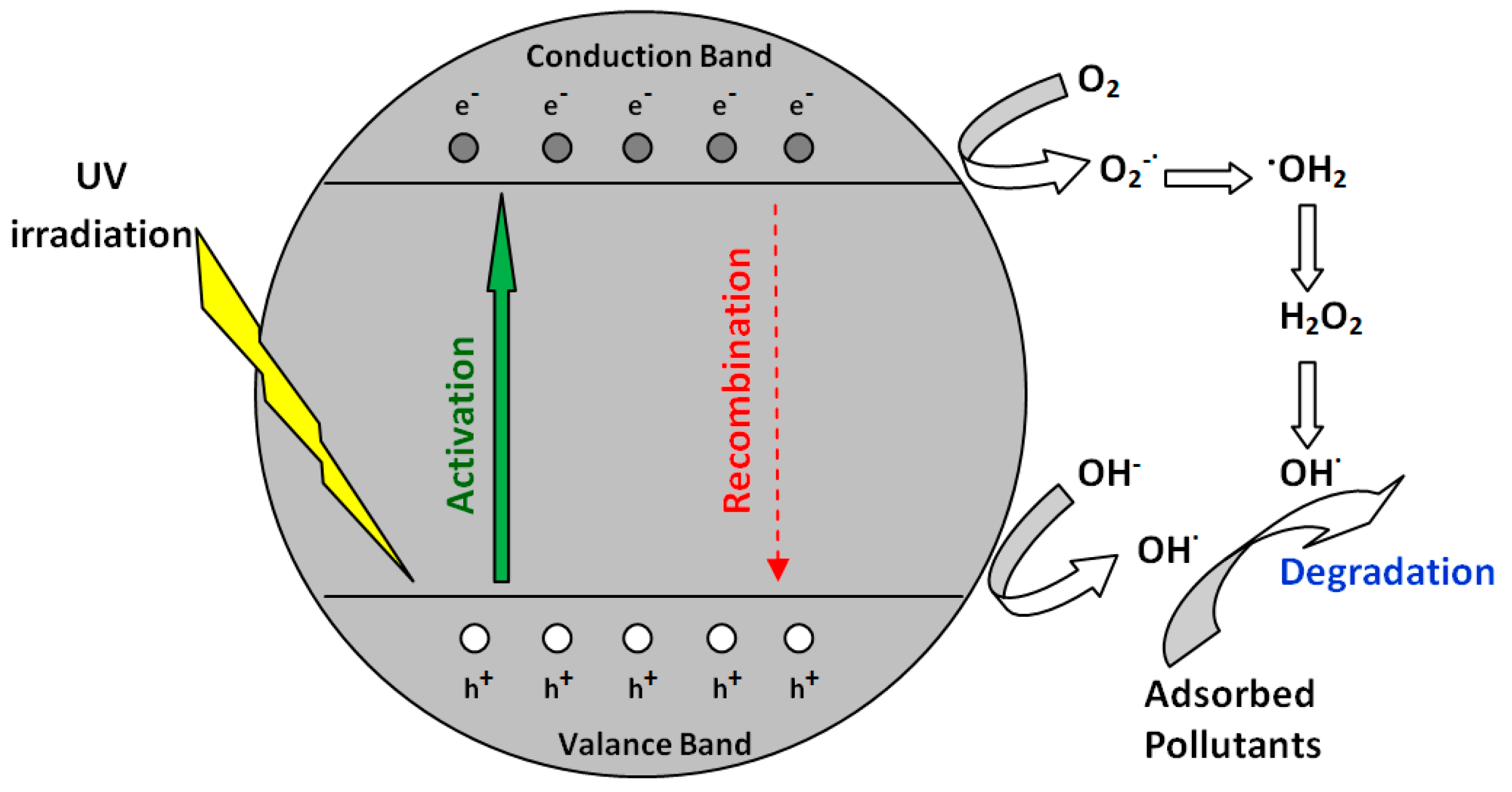
3.2. Photocatalytic Properties of Gypsum Plates under UV Irradiation
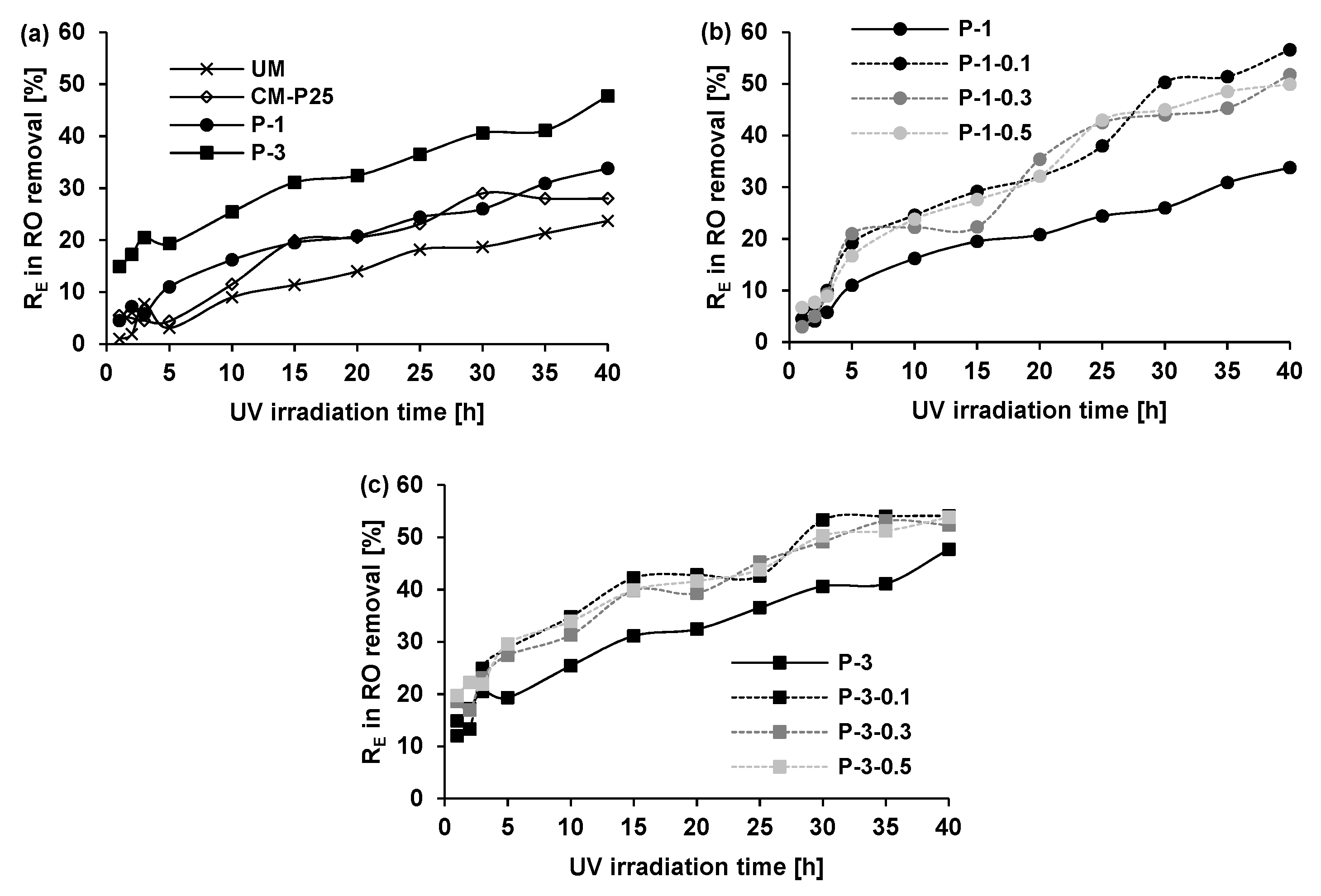
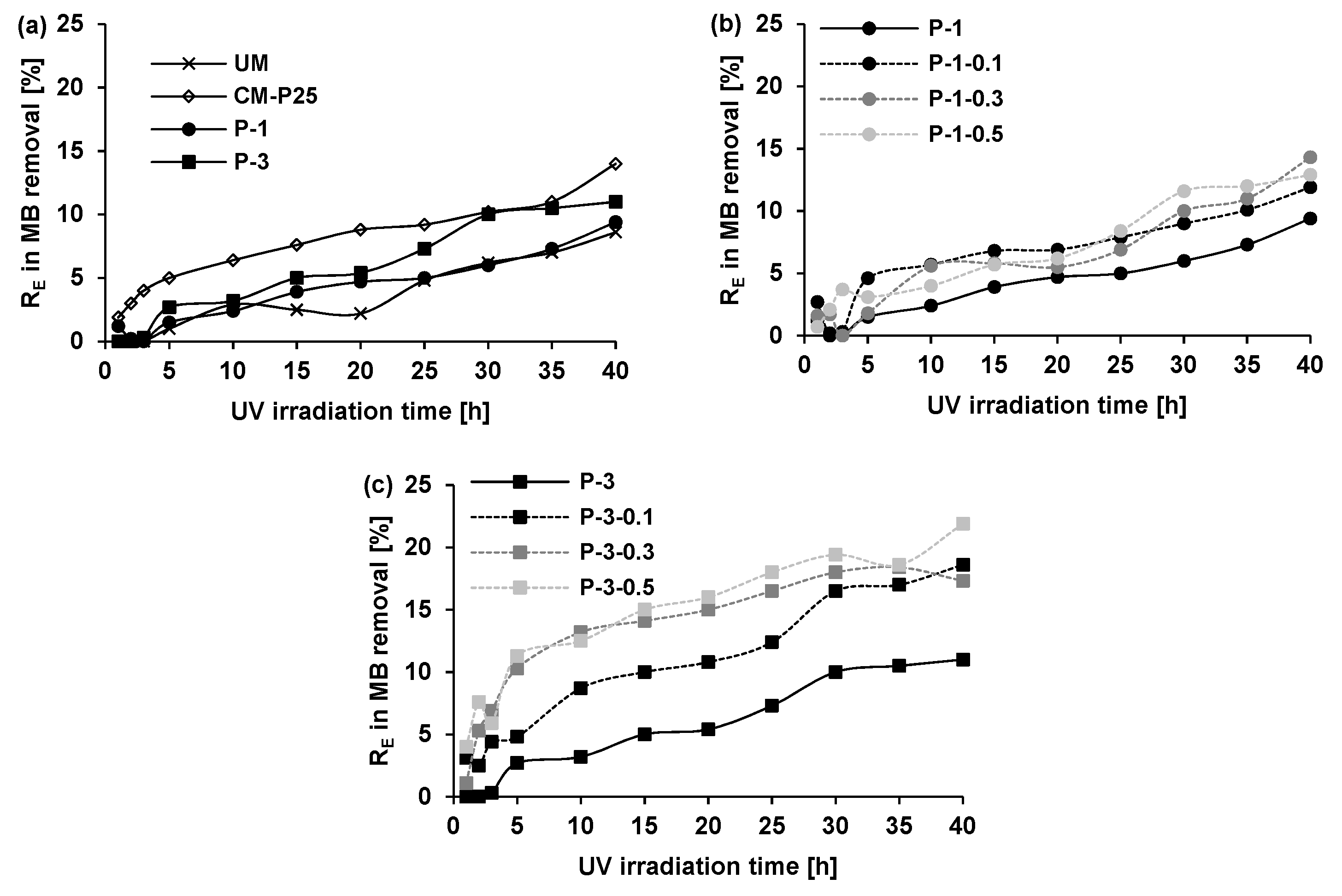
3.2.1. Effect of Photocatalyst Type and Model Dye Structure
3.2.2. Effect of Photocatalyst Type and Model Dye Structure
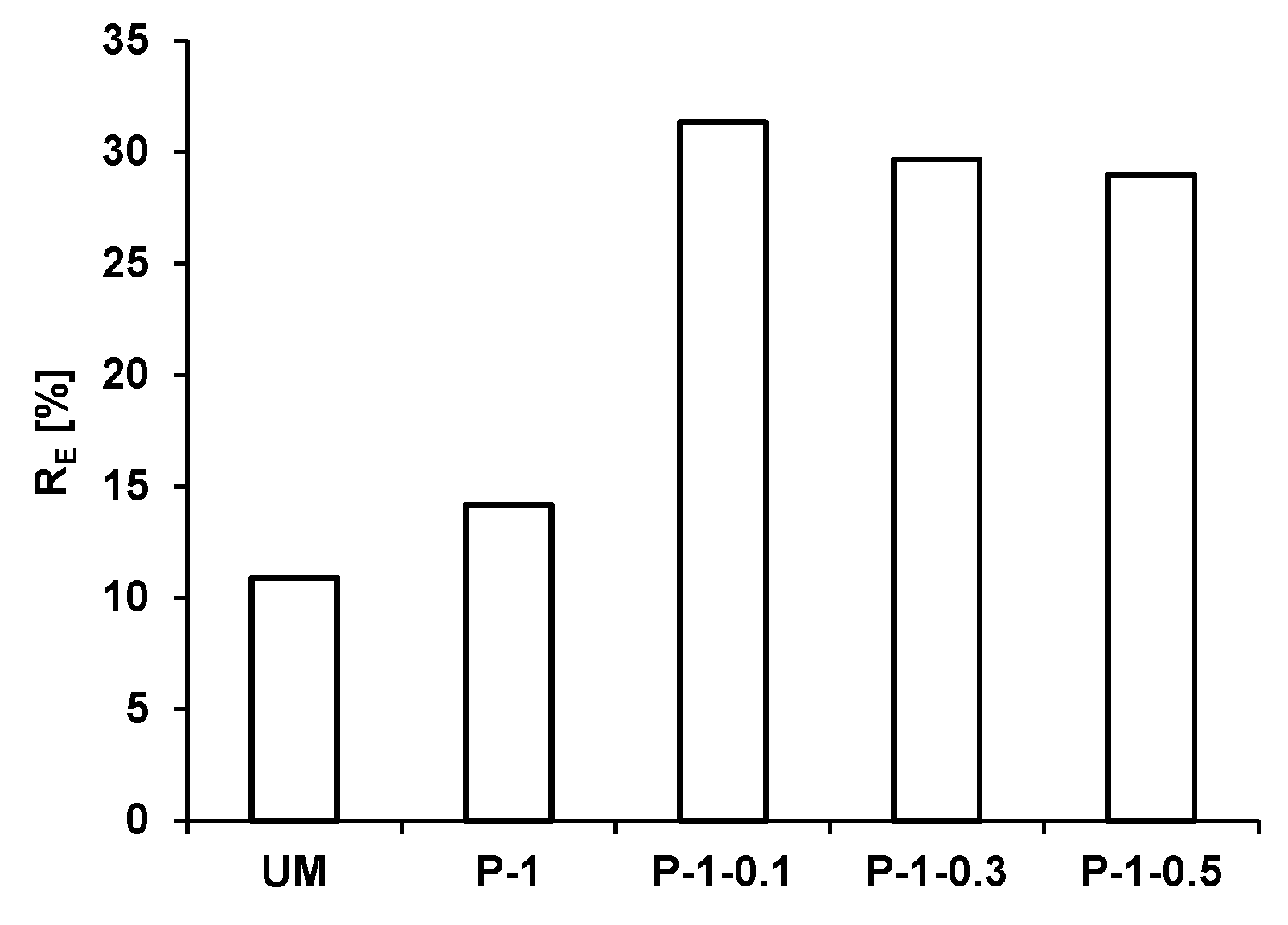
3.2.3. Effect of Glass Fiber Presence
- (1)
- As the increase of available surface of photocatalyst increased it allowed an increase in adsorption of pollutants molecules [38];
- (2)
- As the increase of amount of surface adsorbed water and hydroxyl groups, which are necessary in photocatalytic processes [39];
- (3)
- As the increase of dispersion of TiO2 particles [40].
3.3. Photocatalytic Properties of Gypsum Plates under Vis Light
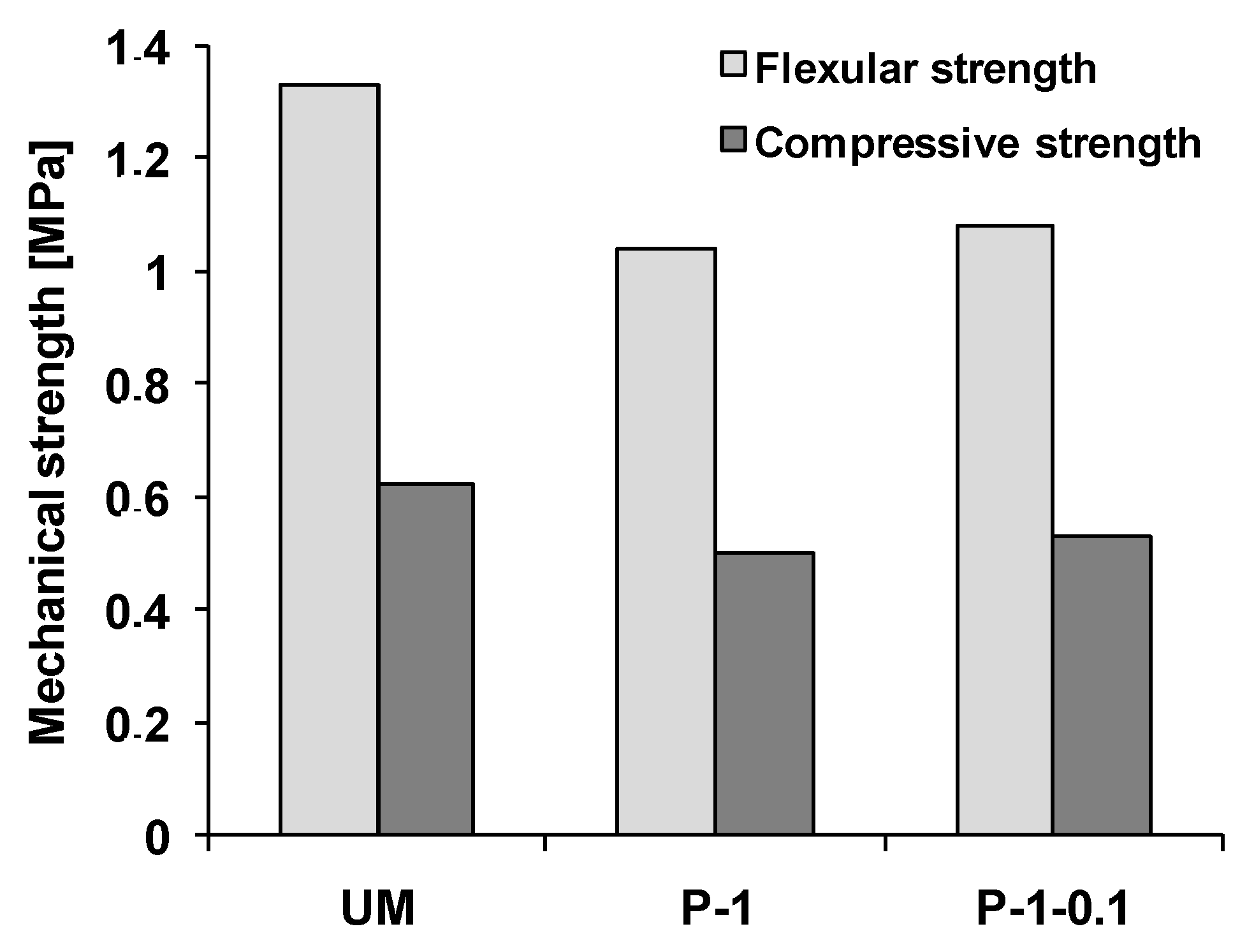
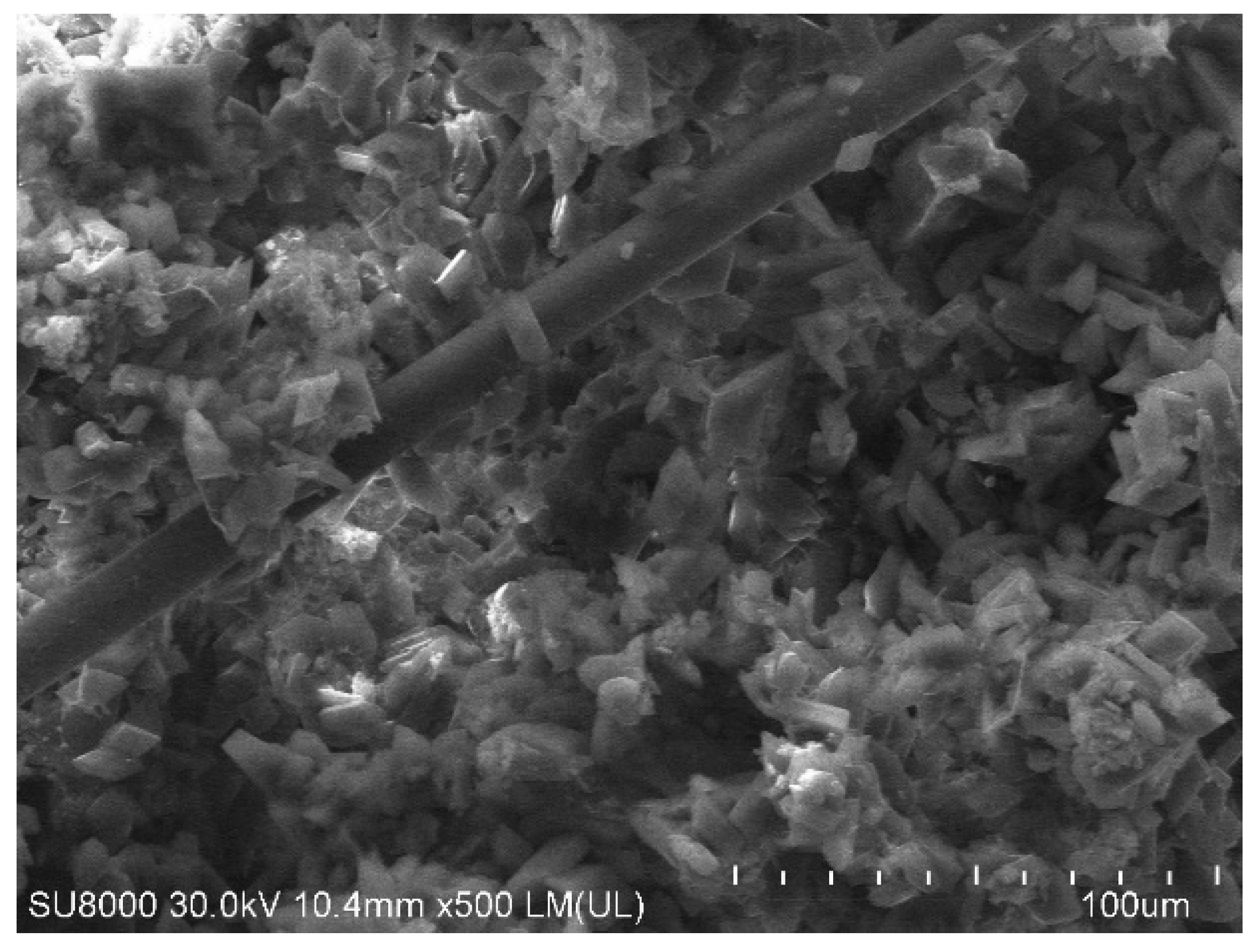
3.4. Mechanical Properties and Microstructure of Modified Gypsum Plaster
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mamaghani, A.H.; Haghighat, C.S. Photocatalytic oxidation technology for indoor environment air purification: The state-of-the-art. Appl. Catal. B 2017, 203, 247–269. [Google Scholar] [CrossRef]
- Mishra, A.; Mehta, A.; Basu, S. Clay supported TiO2 nanoparticles for photocatalytic degradation of environmental pollutants: A review. J. Environ. Chem. Eng. 2018, 6, 6088–6107. [Google Scholar] [CrossRef]
- Binas, V.; Venieri, D.; Kotzias, D.; Kiriakidis, G. Modified TiO2 based photocatalysts for improved air and health quality. J. Mater. 2017, 3, 3–16. [Google Scholar]
- Dong, H.; Zeng, G.; Tang, L.; Fan, C.; Zhang, C.; He, X.; He, Y. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Res. 2015, 79, 128–146. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Huang, Z.-H.; Xu, Y.; Kang, F.-Y. Carbon—Coated TiO2 composites for the photocatalytic degradation of low concentration benzene. New Carbon Mater. 2011, 26, 63–70. [Google Scholar]
- Yue, Y.; Li, Y.; Bridges, C.A.; Rother, G.; Zhang, J.; Chen, J.; Hensley, D.K.; Kidder, M.K.; Richardson, B.C.; Paranthaman, M.P.; et al. Hierarchically superstructured metal sulfides: Facile perturbation—Assisted nanofusion synthesis and visible light photocatalytic characterization. ChemNanoMat 2016, 2, 1104–1110. [Google Scholar] [CrossRef]
- Laurier, K.G.M.; Vermoortele, F.; Ameloot, R.; Vos, D.E.; Hofkens, J.; Roeffaers, M.B.J. Iron(III)-based metal-organic frameworks as visible light photocatalysts. J. Am. Chem. Soc. 2013, 135, 14488–14491. [Google Scholar] [CrossRef]
- Chen, J.; Poon, C.S. Photocatalytic construction and building materials: From fundamentals to applications. Build. Environ. 2009, 44, 1899–1906. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.T. Titanium dioxide photocatalysis: Present situation and future approaches. Comp. Rend. Chim. 2006, 9, 750–760. [Google Scholar] [CrossRef]
- Photocatalyst Market Analysis by Material, by Application, by Region and Segment Forecasts, 2014–2025. Available online: https://www.researchandmarkets.com/research/2h77tx/global?w=5 (accessed on 15 November 2018).
- Guo, M.Z.; Chen, J.; Xia, M.; Wang, T.; Poon, C.S. Pathways of conversion of nitro gen oxides by nano TiO2 incorporated in cement-based materials. Build. Environ. 2018, 144, 412–418. [Google Scholar] [CrossRef]
- Iucolano, F.; Liguori, B.; Aprea, P.; Caputo, D. Thermo-mechanical behaviour of hemp fibers-reinforced gypsum plasters. Constr. Build. Mater. 2018, 185, 256–263. [Google Scholar] [CrossRef]
- Yildizel, S.A. Mechanical performance of glass fiber reinforced composites made with gypsum, expanded perlite and silica sand. Roman J. Mater. 2018, 48, 229–235. [Google Scholar]
- Singh, M.; Garg, M. Fibre reinforced gypsum binder composite, its microstructure and durability. Mater. Struct. 2000, 33, 525–528. [Google Scholar] [CrossRef]
- Chinta, S.K.; Katkar, P.M.; Mirji, M.J. Natural fibres reinforced gypsum composites. Int. J. Eng. Manag. Sci. 2013, 4, 318–325. [Google Scholar]
- Salimian, A.; Hadizadeh, M.; Zeini, M. Investigations on the reinforcement of mechanical properties of gypsum composities containing E-glass woven fabrics. J. Text. Polym. 2016, 4, 20–26. [Google Scholar]
- Wu, Y.F. The structural behavior and design methodology for a new building system consisting of glass fiber reinforced gypsum panels. Constr. Build. Mater. 2009, 23, 2905–2913. [Google Scholar] [CrossRef]
- Wu, Y.F. The effect of longitudinal reinforcement on the cyclic shear behavior of glass fiber reinforced gypsum wall panels: Tests. Eng. Struct. 2004, 26, 1633–1646. [Google Scholar] [CrossRef]
- Martias, C.; Joliff, Y.; Favotto, C. Effects of the addition of glass fibers, mica and vermiculite on the mechanical properties of a gypsum-based composites at room temperature and during a fire test. Comp. B Eng. 2014, 62, 37–53. [Google Scholar] [CrossRef]
- Zhou, K.; Hu, X.Y.; Chen, B.Y.; Hsueh, C.C.; Zhang, Q.; Wang, J.; Lin, Y.J.; Chang, C.T. Synthesized TiO2/ZSM-5 composites used for the photocatalytic degradation of azo dye: Intermediates, reaction pathway, mechanism and bio-toxicity. Appl. Surf. Sci. 2016, 383, 300–309. [Google Scholar] [CrossRef]
- Lučić, M.; Milosavljević, N.; Radetić, M.; Šaponjić, Z.; Radoičić, M.; Krušić, M.K. The potential application of TiO2/hydrogel nanocomposite for removal of various textile azo dyes. Separ. Purif. Technol. 2014, 122, 206–216. [Google Scholar] [CrossRef]
- Ding, X.; Pan, S.; Lu, C.; Guan, H.; Yu, X.; Tong, Y. Hydrophobic photocatalytic composite coatings based on nano-TiO2 hydrosol and aminopropyl terminated polydimethylsiloxane prepared by a facile approach. Mater. Lett. 2018, 228, 5–8. [Google Scholar] [CrossRef]
- Bubacz, K.; Choina, J.; Dolat, D.; Borowiak-Paleń, E.; Moszyński, D.; Morawski, A.W. Studies on nitrogen modified TiO2 photocatalyst prepared in different conditions. Mater. Res. Bull. 2010, 45, 1085–1091. [Google Scholar] [CrossRef]
- Munafò, P.; Goffredo, G.B.; Quagliarini, E. TiO2-based nanocoatings for preserving architectural stone surfaces: An overview. Constr. Build. Mater. 2015, 84, 201–218. [Google Scholar] [CrossRef]
- Graziani, L.; Quagliarini, E.; D’Orazio, M. The role of roughness and porosity on the self-cleaning and anti-biofouling efficiency of TiO2-Cu and TiO2-Ag nanocoatings applied on fired bricks. Constr. Build. Mater. 2016, 129, 116–124. [Google Scholar] [CrossRef]
- Pinho, L.; Mosquera, M.J. Photocatalytic activity of TiO2-SiO2 nanocomposites applied to buildings: Influence of particle size and loading. Appl. Catal. B Environ. 2013, 134–135, 205–221. [Google Scholar] [CrossRef]
- Graziani, L.; Quagliarini, E.; Bondioli, F.; D’Orazio, M. Durability of self-cleaning TiO2 coatings on fired clay brick façades: Effects of UV exposure and wet & dry cycles. Build. Environ. 2017, 71, 193–203. [Google Scholar]
- Kuźmiński, K.; Morawski, A.W.; Janus, M. Adsorption and Photocatalytic Degradation of Anionic and Cationic Surfactants on Nitrogen-Modified TiO2. J. Surfact. Deterg. 2018, 21, 909–921. [Google Scholar] [CrossRef]
- Bubacz, K.; Tryba, B.; Morawski, A.W. The role of adsorption in decomposition of dyes on TiO2 and N-modified TiO2 photocatalysts under UV and visible light irradiations. Mater. Res. Bull. 2012, 47, 3697–3703. [Google Scholar] [CrossRef]
- Janus, M.; Bubacz, K.; Zatorska, J.; Kusiak-Nejman, E.; Czyżewski, A.; Morawski, A.W. Preliminary studiem of photocatalytic activity of gypsum plaster containing TiO2 co-modified with nitrogen and carbon. Pol. J. Chem. Technol. 2015, 17, 96–102. [Google Scholar] [CrossRef]
- Kerkez-Kuyumcu, Ö.; Kibar, E.; Dayioğlu, K.; Gedik, F.; Akin, A.N.; Özkara-Audinoğlu, Ş. A comparative study for removal of different dyes over M/TiO2 (M = Cu, Ni, Co, Fe, Mn, and Cr) photocatalysts under visible light irradiation. J. Photochem. Photobiol. A Chem. 2015, 311, 176–185. [Google Scholar] [CrossRef]
- Nguyen, C.H.; Fu, C.C.; Juang, R.S. Degradation of methylene blue and methyl orange by palladium-doped TiO2 photocatalysis for water reuse: Efficiency and degradation pathways. J. Clean. Prod. 2018, 202, 413–427. [Google Scholar] [CrossRef]
- Mugunthan, E.; Saidutta, M.B.; Jagadeeshbabu, P.E. Visible light assisted photocatalytic degradation of diclofenac using TiO2-WO3 mixed oxide catalysts. Environ. Nanotechnol. Monit. Manag. 2018, 10, 322–330. [Google Scholar]
- Jiang, C.; Lee, K.; Parlett, C.M.A.; Bayazit, M.K.; Lau, C.C.; Ruan, Q.; Moniz, S.J.A.; Lee, A.F.; Tang, J. Size-controlled TiO2 nanoparticles on porous hosts for enhanced photocatalytic hydrogen production. Appl. Catal. A 2016, 521, 133–139. [Google Scholar] [CrossRef]
- Yang, L.; Wang, F.; Shu, C.; Liu, P.; Zhang, W.; Hu, S. TiO2/porous cementitious composites: Influences of porosities and TiO2 loading levels on photocatalytic degradation of gaseous benzene. Constr. Build. Mater. 2017, 150, 774–780. [Google Scholar] [CrossRef]
- Pal, A.; Jana, T.K.; Chatterjee, K. Silica supported TiO2 nanostructures for highly efficient photocatalytic application under visible light irradiation. Mater. Res. Bull. 2016, 76, 353–357. [Google Scholar] [CrossRef]
- Chen, W.; Li, S.; Feizbakhshan, M.; Amdebrhan, B.T.; Shi, S.; Xin, W.; Nguyen, T.; Chen, M.; Zhou, X. TiO2-SiO2 nanocomposite aerogel loaded in melamine-impregnated paper for multi-functionalization: Formaldehyde degradation and smoke suppression. Constr. Build. Mater. 2018, 161, 381–388. [Google Scholar] [CrossRef]
- Cendrowski, K.; Chen, X.; Zielińska, B.; Kaleńczuk, R.J.; Rümmeli, M.H.; Büchner, B.; Klingeler, R.; Borowiak-Paleń, E. Synthesis, characterization, and photocatalytic properties of core/Shell masoporous silica nanospheres supporting nanocrystalline titania. J. Nanopart. Res. 2011, 13, 5899–5908. [Google Scholar] [CrossRef]
- Hirano, M.; Ota, K. Preparation of photoactive anatase-type TiO2/silica gel by direct loading anatase-type TiO2 nanoparticles in acidic aqueous solution by thermal hydrolysis. J. Mater. Sci. 2004, 39, 1841–1844. [Google Scholar] [CrossRef]
- Cheng, S.; Tsai, S.J.; Lee, Y.F. Photocatalytic decomposition of phenol over titanium oxide of various structures. Catal. Today 1995, 26, 87–96. [Google Scholar] [CrossRef]
- Tang, X.; Feng, Q.; Liu, K.; Tan, Y. Synthesis and characterization of a novel nanofibrous TiO2/SiO2 composite with enhanced photocatalytic activity. Mater. Lett. 2016, 183, 175–178. [Google Scholar] [CrossRef]
- Wu, L.; Zhou, Y.; Nie, W.; Song, L.; Chen, P. Synthesis of highly monodispersed teardrop-shaped core-shell SiO2/TiO2 nanoparticles and their photocatalytic activities. Appl. Surf. Sci. 2015, 351, 320–326. [Google Scholar] [CrossRef]
- Chen, J.; Qiu, F.; Xu, W.; Cao, S.; Zhu, H. Recent progress in enhancing photocatalytic efficiency of TiO2-based materials. Appl. Catal. A 2015, 495, 131–140. [Google Scholar] [CrossRef]
- Dong, R.; Na, C.; Zhang, H.; Chen, Z.; Jin, C. TiO2/SiO2 mesoporous microspheres with intelligently controlled texture. Mater. Des. 2016, 89, 830–838. [Google Scholar] [CrossRef]
- Jin, E.M.; Park, J.Y.; Hwang, K.J.; Gu, H.B.; Jeong, S.M. Biotemplated hybrid TiO2 nanoparticles and TiO2-SiO2 composites for dye-sensitized solar cells. Mater. Lett. 2014, 131, 190–193. [Google Scholar] [CrossRef]
- Son, H.J.; Wang, X.; Prasittichai, C.; Jeong, N.C.; Aaltonen, T.; Gordon, R.G.; Hupp, J.T. Glass-encapsulated light harvesters: More efficient dye-sensitized solar cells by deposition of self-aligned, conformal, and self-limited silica layers. J. Am. Chem. Soc. 2012, 134, 9537–9540. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.L.; Abdullah, A.Z. Comparative study on the process behavior and reaction kinetics in sonocatalytic degradation of organic dyes by powder and nanotubes TiO2. Ultras. Sonochem. 2012, 19, 642–651. [Google Scholar] [CrossRef]
- Grześkowiak, M.; Wróbel, R.J.; Moszyński, D.; Mozia, S.; Grzechulska-Damszel, J.; Morawski, A.W.; Przepiórski, J. TiO2 supported on quartz wool for photocatalytic oxidation by hydrogen sulphide. Adsorp. Sci. Technol. 2014, 32, 765–773. [Google Scholar] [CrossRef]
- Low, W.; Boonamnuayvitaya, V. Enhancing the photocatalytic activity of TiO2 co-doping of grapheme-Fe3+ ions for formaldehyde removal. J. Environ. Manag. 2013, 127, 142–149. [Google Scholar] [CrossRef]
| Dye | Charge in Dissociated Form | Chromophore Group | Chemical Structure |
|---|---|---|---|
| Reactive Orange 20 (RO) | anionic | azo | 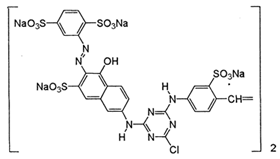 |
| Methylene Blue (MB) | cationic | thiazine | 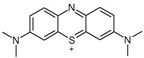 |
| Samples | Initial | After RO | After MB | |
|---|---|---|---|---|
| UM | L* | 81.71 ± 0.27 | 68.05 ± 0.15 | 62.26 ± 0.33 |
| a* | 1.96 ± 0.07 | 8.85 ± 0.5 | −8.80 ± 0.12 | |
| b* | 9.62 ± 0.41 | 30.36 ± 0.57 | −13.67 ± 0.46 | |
| ΔE | - | 25.78 ± 0.31 | 32.02 ± 0.53 | |
| P-1 | L* | 80.97 ± 0.93 | 70.52 ± 1.50 | 67.47 ± 0.15 |
| a* | 1.88 ± 0.22 | 8.47 ± 0.28 | −7.53 ± 0.13 | |
| b* | 9.86 ± 0.27 | 30.20 ± 0.19 | −10.99 ± 0.45 | |
| ΔE | - | 23.83 ± 0.45 | 26.53 ± 0.35 | |
| Sample | Initial Surface | Stained Surface | After 40 h of UV Irradiation |
|---|---|---|---|
| UM |  |  |  |
| P-1-0.1 |  |  |  |
| UM |  |  |  |
| P-3-0.1 |  |  |  |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zając, K.; Janus, M.; Morawski, A.W. Improved Self-Cleaning Properties of Photocatalytic Gypsum Plaster Enriched with Glass Fiber. Materials 2019, 12, 357. https://doi.org/10.3390/ma12030357
Zając K, Janus M, Morawski AW. Improved Self-Cleaning Properties of Photocatalytic Gypsum Plaster Enriched with Glass Fiber. Materials. 2019; 12(3):357. https://doi.org/10.3390/ma12030357
Chicago/Turabian StyleZając, Kamila, Magdalena Janus, and Antoni W. Morawski. 2019. "Improved Self-Cleaning Properties of Photocatalytic Gypsum Plaster Enriched with Glass Fiber" Materials 12, no. 3: 357. https://doi.org/10.3390/ma12030357
APA StyleZając, K., Janus, M., & Morawski, A. W. (2019). Improved Self-Cleaning Properties of Photocatalytic Gypsum Plaster Enriched with Glass Fiber. Materials, 12(3), 357. https://doi.org/10.3390/ma12030357







