Two-Stage Plasma-Thermal Nitridation Processes for the Production of Aluminum Nitride Powders from Aluminum Powders
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Effects of NH4Cl and Temperature on the Size and Dispersibility of Products by the Thermal Nitridation Process
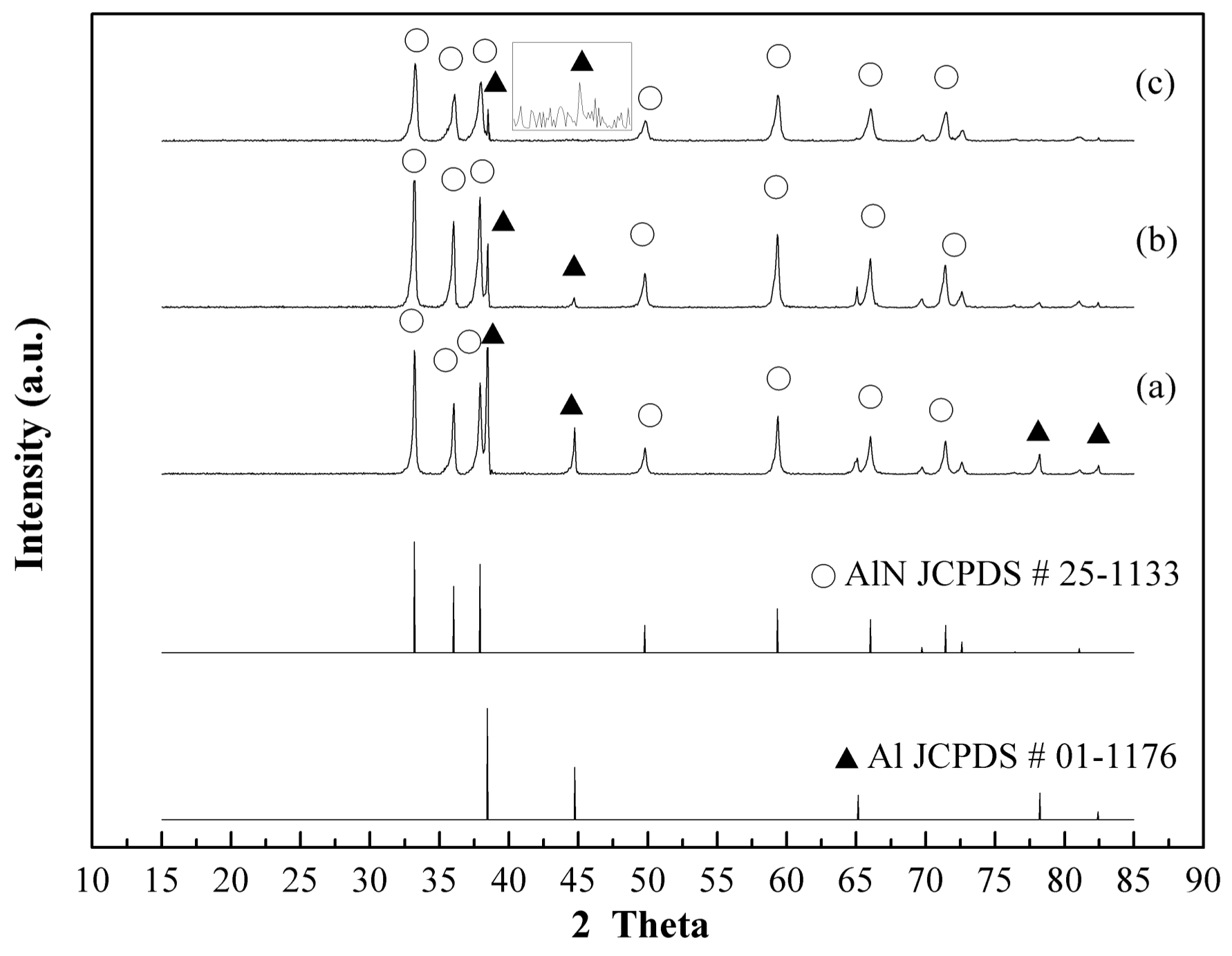
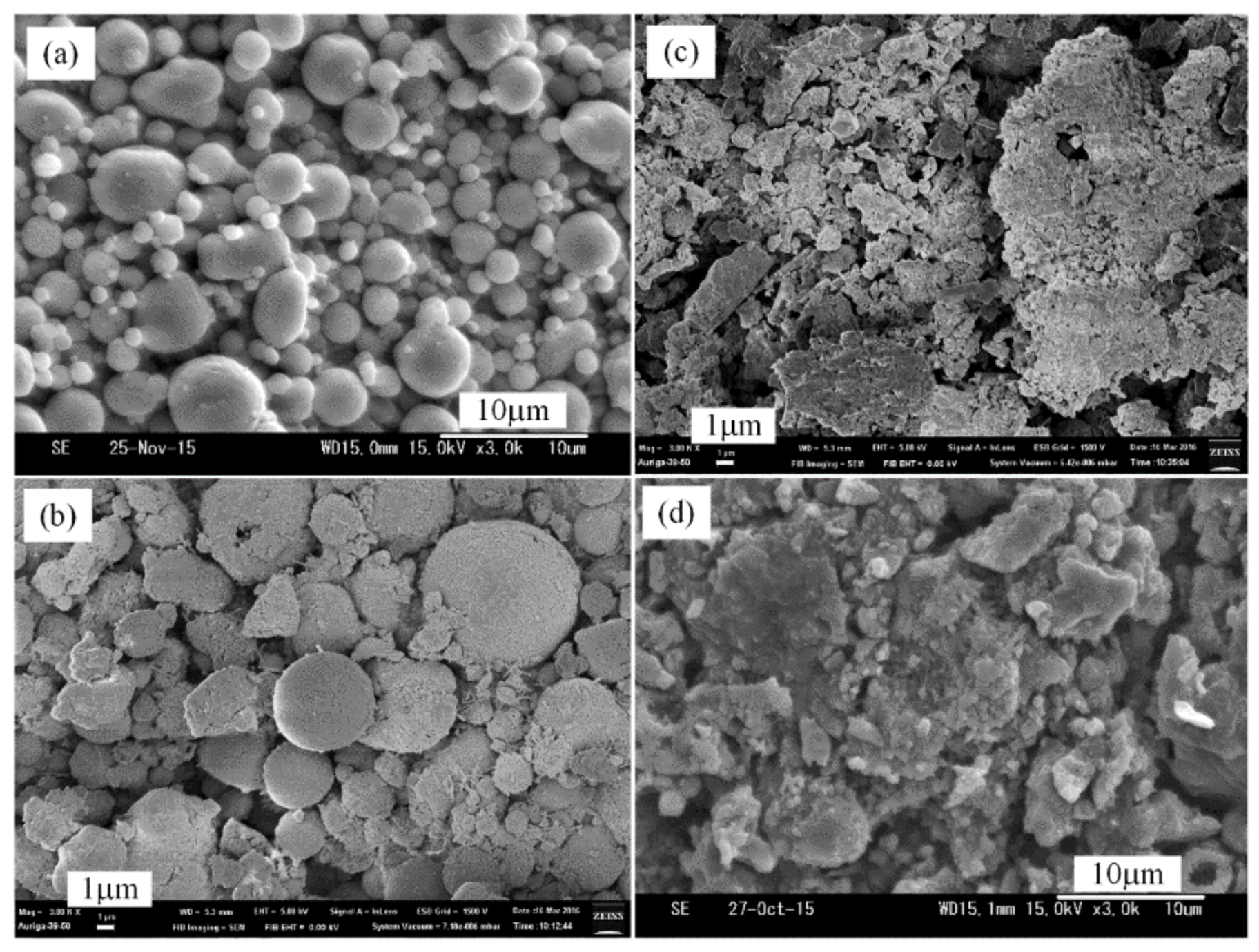
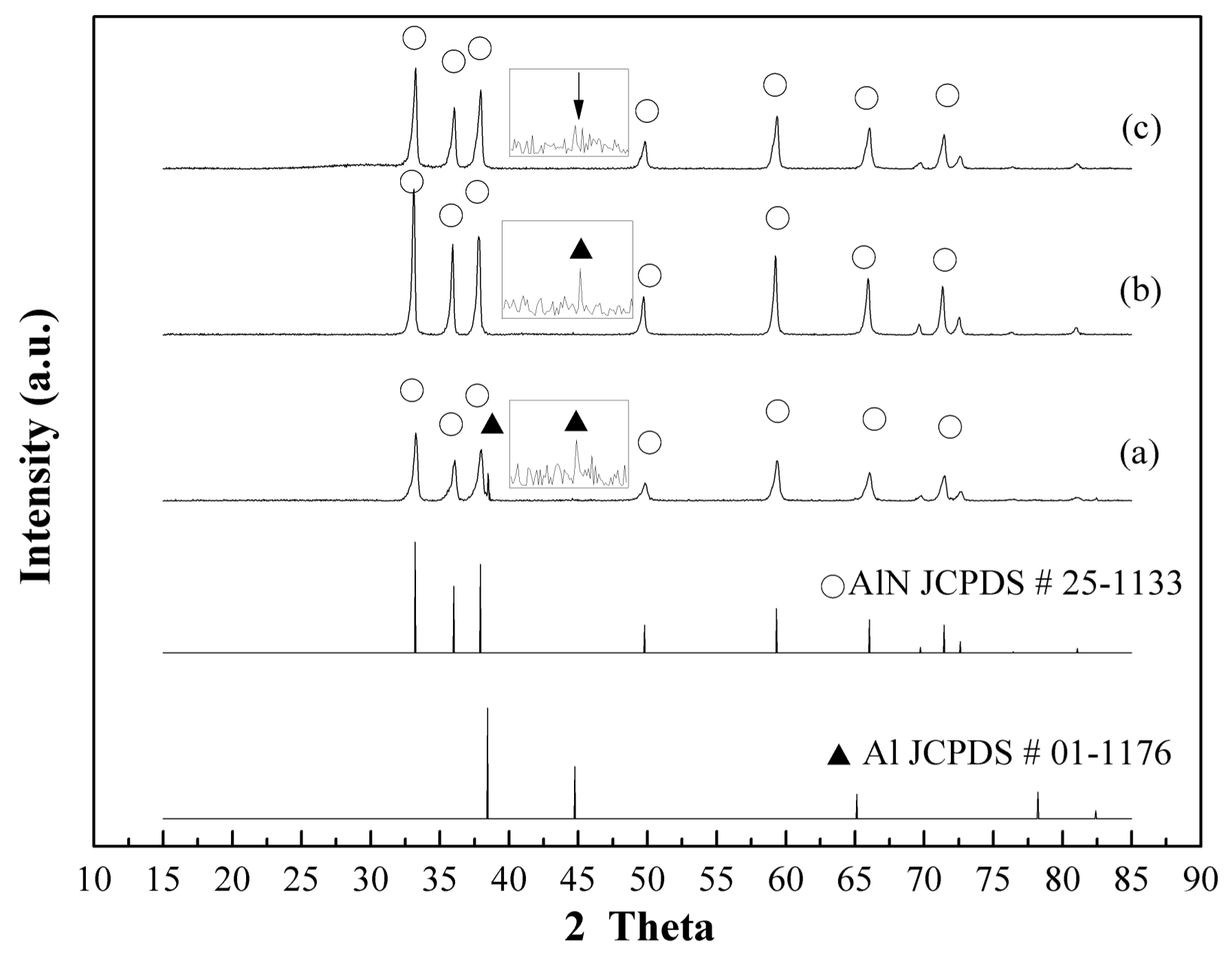
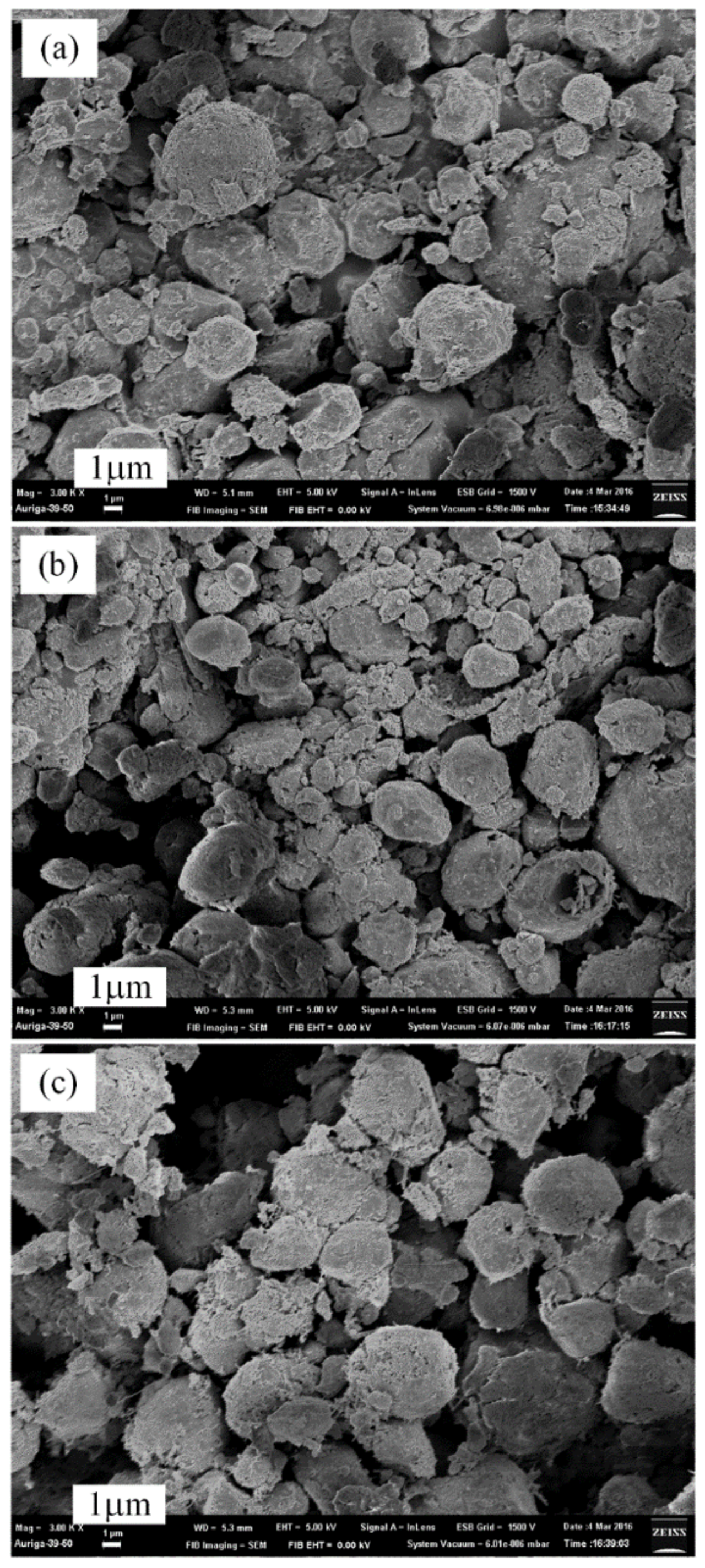
3.2. Plasma Nitridation in the First Stage for Pre-Synthesizing AlN/Al Powders
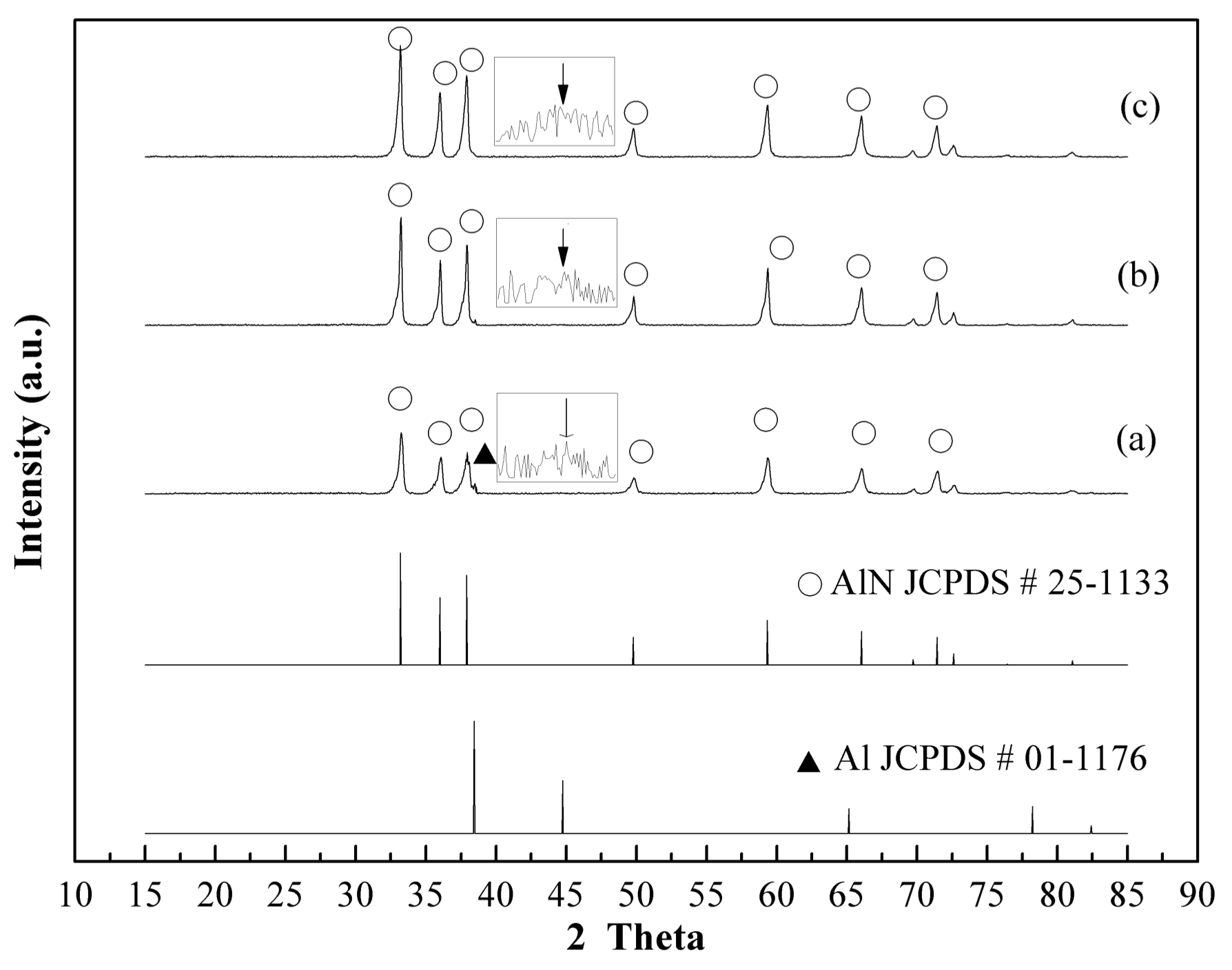
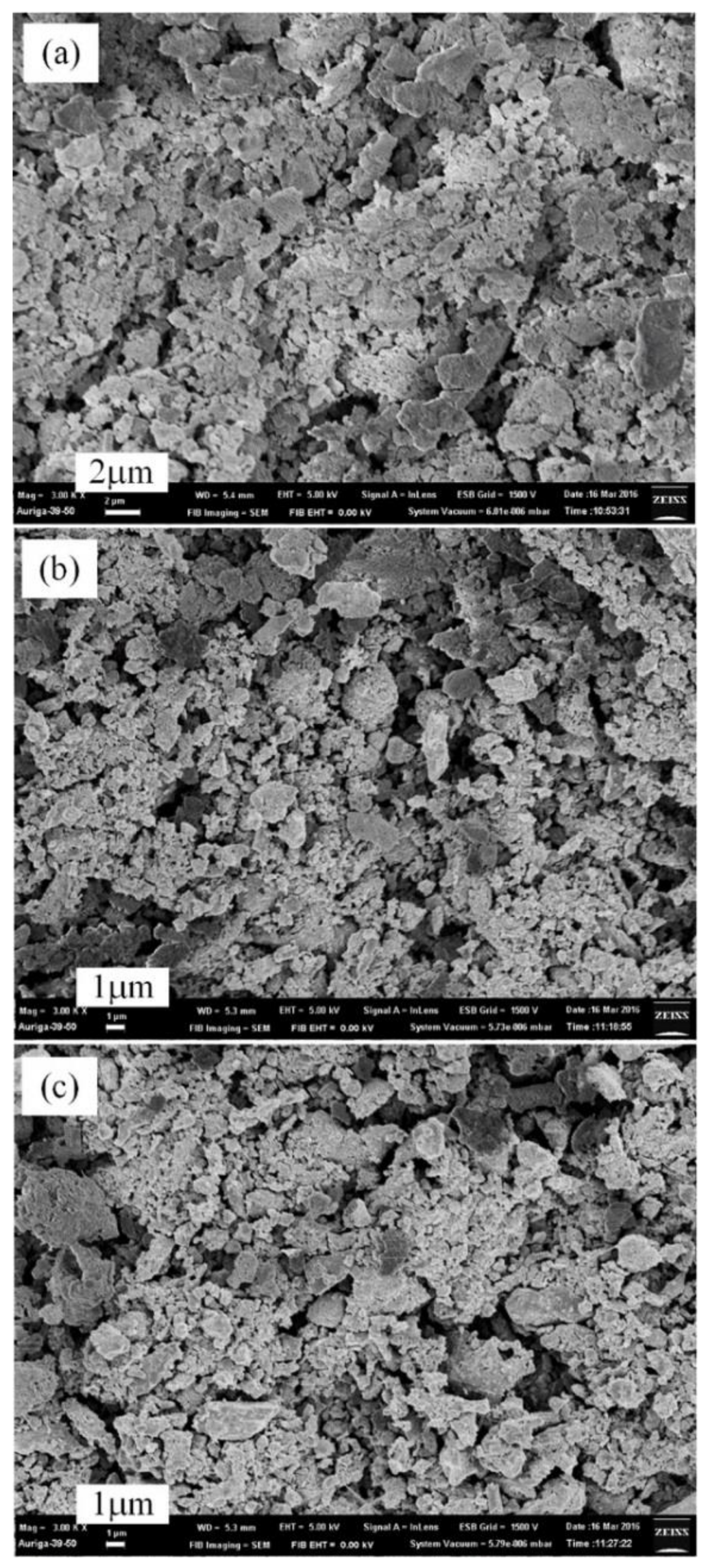
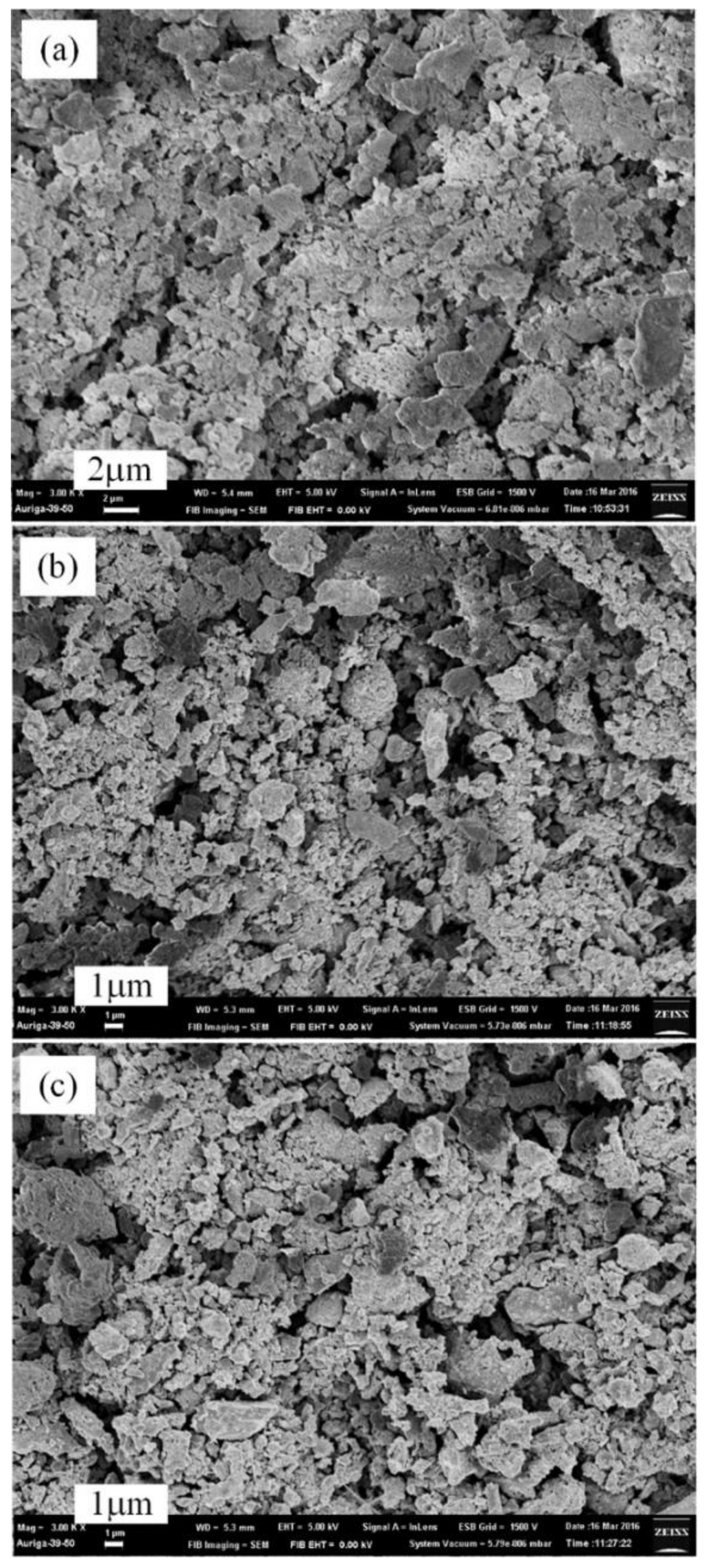
3.3. Thermal Nitridation in Second Stage (after Plasma Nitridation) for Synthesizing AlN Powders
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kim, K. Plasma synthesis and characterization of nanocrystalline aluminum nitride particles by aluminum plasma jet discharge. J. Cryst. Growth 2005, 283, 540–546. [Google Scholar] [CrossRef]
- Paul, R.K.; Song, H.Y.; Lee, K.H.; Lee, B.T. Formation of AlN nanowires using Al powder. Mater. Chem. Phys. 2008, 112, 562–565. [Google Scholar] [CrossRef]
- Selvaduray, G.; Sheet, L. Aluminum nitride: Review of synthesis methods. Mater. Sci. Tech. Ser. 1993, 9, 463–473. [Google Scholar] [CrossRef]
- Fu, R.; Zhou, H.; Chen, L.; Wu, Y. Synthesis of aluminum nitride fibres from aluminum silicate fibres by carbothermal reduction method. J. Mater. Sci. 1999, 34, 3605–3608. [Google Scholar] [CrossRef]
- Wang, M.C.; Tsai, M.S.; Wu, N.C. Effect of heat treatment on phase transformation of aluminum nitride ultrafine powder prepared by chemical vapor deposition. J. Cryst. Growth 2000, 210, 487–495. [Google Scholar] [CrossRef]
- Radwan, M.; Bahgat, M. A modified direct nitridation method for formation of nano-AlN whiskers. J. Mater. Process. Tech. 2007, 181, 99–105. [Google Scholar] [CrossRef]
- Chung, S.L.; Yu, W.L.; Liu, C.N. A self-propagating high temperature synthesis method for synthesis of AlN powder. J. Mater. Res. 1999, 14, 1928–1933. [Google Scholar] [CrossRef]
- Lin, C.N.; Hsieh, C.Y.; Chung, S.L.; Cheng, J.; Agrawal, D.K. Combustion synthesis of AlN powder and its sintering properties. Int. J. Self-Propag. High-Temp. Synth. 2004, 13, 93–106. [Google Scholar]
- Yu, S.; Li, D.; Sun, H.; Li, H.; Yang, H.; Zou, G. Microanalysis of single-phase AlN nanocrystals and AlN-Al nanocomposites prepared by DC arc-discharge. J. Cryst. Growth 1998, 183, 284–288. [Google Scholar] [CrossRef]
- Renevier, N.; Czerwiec, T.; Billard, A.; Stebut, J.V.; Michel, H. A way to decrease the nitriding temperature of aluminum: The low-pressure arc-assisted nitriding process. Surf. Coat. Tech. 1999, 116, 380–385. [Google Scholar] [CrossRef]
- Zheng, X.; Ren, Z.; Li, X.; Wang, Y. Microstructural characterization and mechanical properties of nitrided layers on aluminum substrate prepared by nitrogen arc. Appl. Surf. Sci. 2012, 259, 508–514. [Google Scholar] [CrossRef]
- Chazels, C.; Coudert, J.F.; Jarrige, J.; Fauchais, P. Synthesis of ultra fine particles by plasma transferred arc: Influence of anode material on particle properties. J. Eur. Ceram. Soc. 2006, 26, 3499–3507. [Google Scholar] [CrossRef]
- Iwata, M.; Adachi, K.; Furukawa, S.; Amakawa, T. Synthesis of purified AlN nano powder by transferred type arc plasma. J. Phys. D Appl. Phys. 2004, 37, 1041–1047. [Google Scholar] [CrossRef]
- Cheng, H.; Sun, Y.; Hing, P. The influence of deposition conditions on structure and morphology of aluminum nitride films deposited by radio frequency reactive sputtering. Thin Solid Films 2003, 434, 112–120. [Google Scholar] [CrossRef]
- Galca, A.C.; Stan, G.E.; Trinca, L.M.; Negrila, C.C.; Nistor, L.C. Structural and optical properties of c-axis oriented aluminum nitride thin films prepared at low temperature by reactive radio-frequency magnetron sputtering. Thin Solid Films 2012, 524, 328–333. [Google Scholar] [CrossRef]
- Park, J.R.; Rhee, S.W.; Lee, K.H. Gas-phase synthesis of AlN powders from AlCl3-NH3-N2. J. Mater. Sci. 1993, 28, 57–64. [Google Scholar] [CrossRef]
- Kinemuchi, Y.; Murai, K.; Sangurai, C.; Cho, C.H.; Suematsu, H.; Jiang, W.; Yatsui, K. Nanosize powders of aluminum nitride synthesized by pulsed wire discharge. J. Am. Ceram. Soc. 2003, 86, 420–424. [Google Scholar] [CrossRef]
- Ishihara, S.; Suematsu, H.; Nakayama, T.; Suzuki, T.; Niihara, K. Synthesis of nanosized alumina powders by pulsed wire discharge in air flow atmosphere. Ceram. Int. 2012, 38, 4477–4484. [Google Scholar] [CrossRef]
- Li, C.H.; Kao, L.H.; Chen, M.J.; Wang, Y.F.; Tsai, C.H. Rapid preparation of aluminum nitride powders by using microwave plasma. J. Alloy. Compd. 2012, 542, 78–84. [Google Scholar] [CrossRef]
- Shahien, M.; Yamada, M.; Yasui, T.; Fukumoto, M. Influence of NH4Cl powder addition for fabrication of aluminum nitride coating in reactive atmospheric plasma spray process. J. Therm. Spray Technol. 2010, 20, 205–212. [Google Scholar] [CrossRef]
- Caballero, E.S.; Cintas, J.; Cuevas, F.G.; Martos, M.; Gallardo, J.M. A new method for synthetizing nanocrystalline aluminium nitride via a solid-gas direct reaction. Powder Technol. 2016, 287, 341–345. [Google Scholar] [CrossRef]
- Qiu, Y.; Gao, L. Nitridation reaction of aluminum powder in flowing ammonia. J. Eur. Ceram. Soc. 2003, 23, 2015–2022. [Google Scholar] [CrossRef]
- Shi, Z.; Radwan, M.; Kirihara, S.; Miyamoto, Y.; Jin, Z. Morphology-controlled synthesis of quasi-aligned AlN nanowhiskers by combustion method: Effect of NH4Cl additive. Ceram. Int. 2009, 35, 2727–2733. [Google Scholar] [CrossRef]
- Rosenband, V.; Gany, A. Activation of combustion synthesis of aluminum nitride powder. J. Mater. Process Technol. 2004, 147, 197–203. [Google Scholar] [CrossRef]
- Timmermans, E.A.H.; Jonkers, J.; Rodero, A.; Quintero, M.C.; Sola, A.; Gamero, A.; Schram, D.C.; Mullen van der, J.A.M. The behavior of molecules in microwave-induced plasma studies by optical emission spectroscopy. 2: Plasma at reduced pressure. Spectrochim. Acta B 1999, 54, 1085–1098. [Google Scholar] [CrossRef]
- Deng, X.T.; Shi, J.J.; Kong, M.G. Protein destruction by a helium atmospheric pressure glow discharge: Capability and mechanisms. J. Appl. Phys. 2007, 101, 074701. [Google Scholar] [CrossRef]
- Ageorges, H.; Megy, S.; Chang, K.; Baronnet, J.M.; Williams, J.K.; Chapman, C. Synthesis of aluminum nitride in transferred arc plasma furnaces. Plasma Chem. Plasma Process. 1992, 13, 613–632. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sung, M.-C.; Wang, Y.-F.; Chen, S.-C.; Tsai, C.-H. Two-Stage Plasma-Thermal Nitridation Processes for the Production of Aluminum Nitride Powders from Aluminum Powders. Materials 2019, 12, 359. https://doi.org/10.3390/ma12030359
Sung M-C, Wang Y-F, Chen S-C, Tsai C-H. Two-Stage Plasma-Thermal Nitridation Processes for the Production of Aluminum Nitride Powders from Aluminum Powders. Materials. 2019; 12(3):359. https://doi.org/10.3390/ma12030359
Chicago/Turabian StyleSung, Mei-Chen, Ya-Fen Wang, Shang-Che Chen, and Cheng-Hsien Tsai. 2019. "Two-Stage Plasma-Thermal Nitridation Processes for the Production of Aluminum Nitride Powders from Aluminum Powders" Materials 12, no. 3: 359. https://doi.org/10.3390/ma12030359
APA StyleSung, M.-C., Wang, Y.-F., Chen, S.-C., & Tsai, C.-H. (2019). Two-Stage Plasma-Thermal Nitridation Processes for the Production of Aluminum Nitride Powders from Aluminum Powders. Materials, 12(3), 359. https://doi.org/10.3390/ma12030359



