HPMC- and PLGA-Based Nanoparticles for the Mucoadhesive Delivery of Sitagliptin: Optimization and In Vivo Evaluation in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Optimization and Formulation of Nanoparticles
2.3. Nanoparticle Yield
2.4. Drug Content
2.5. Scanning Electron Microscopy (SEM)
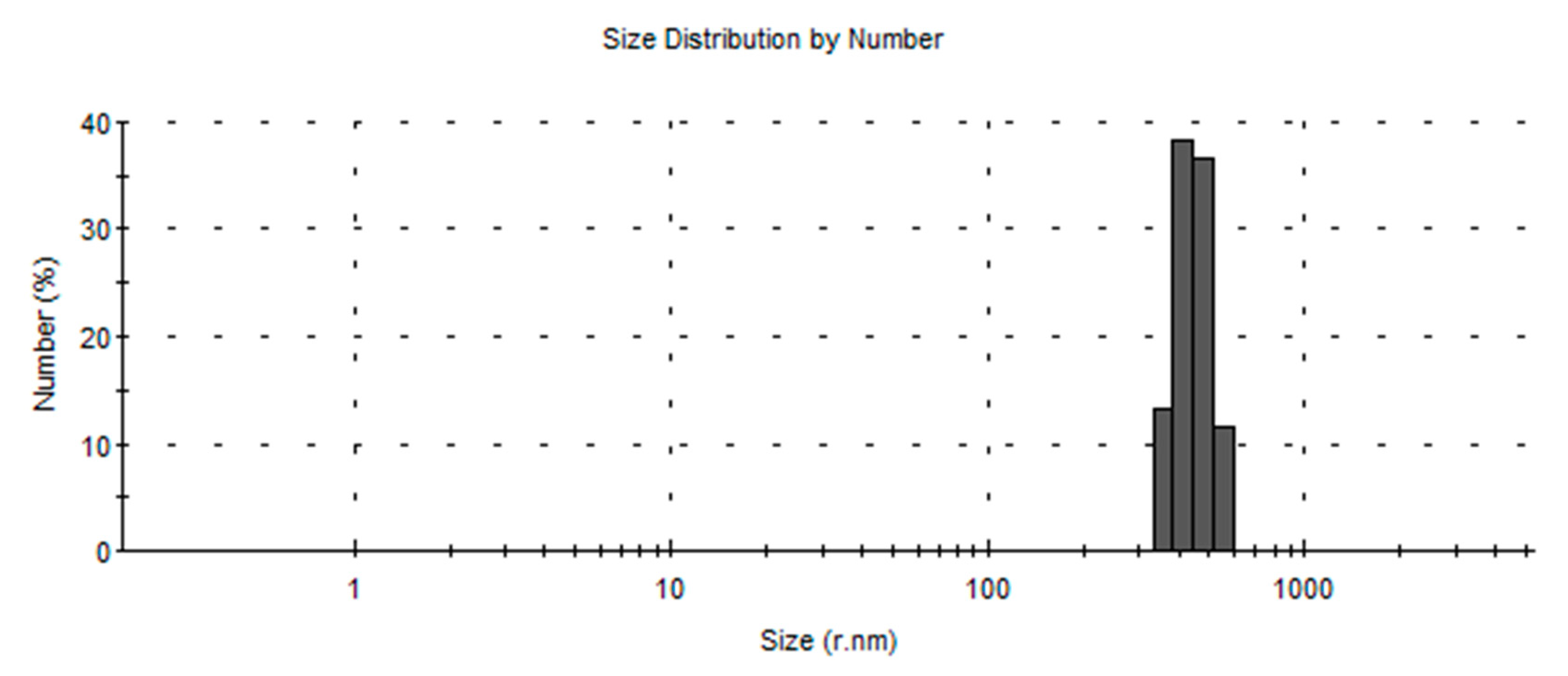
2.6. Particle Size Distribution
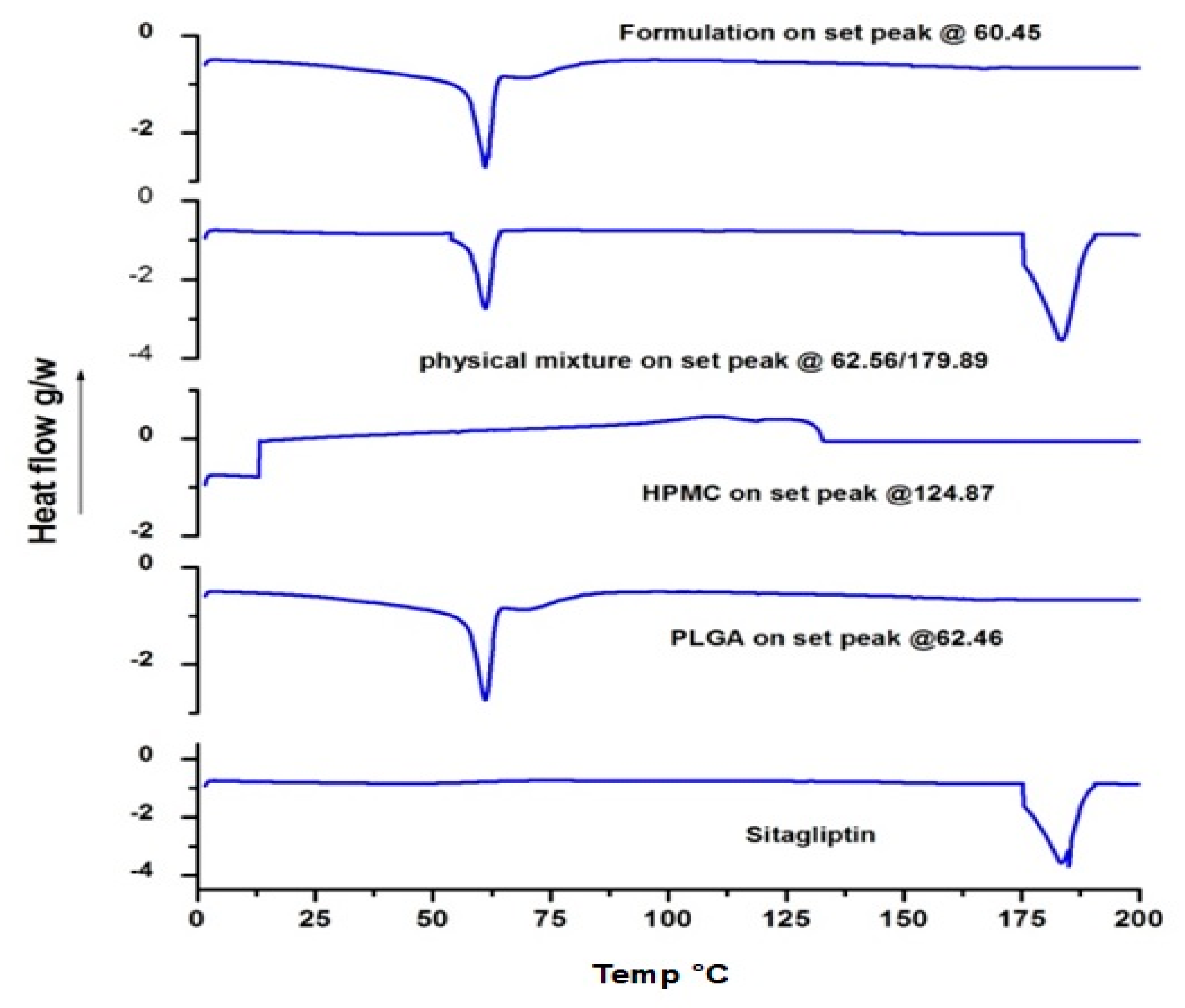
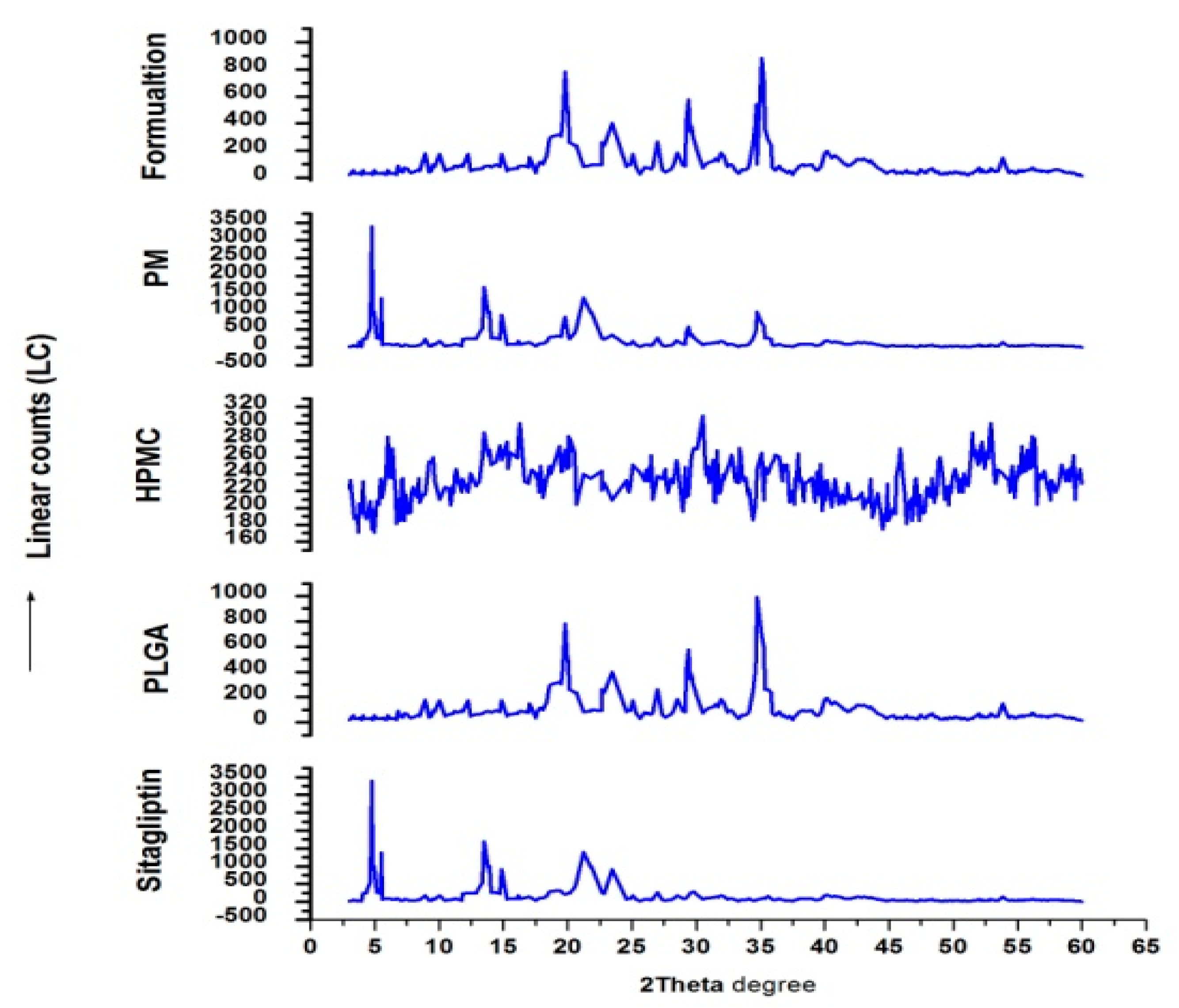
2.7. Differential Scanning Calorimetry (DSC) and X-ray Diffraction (XRD)
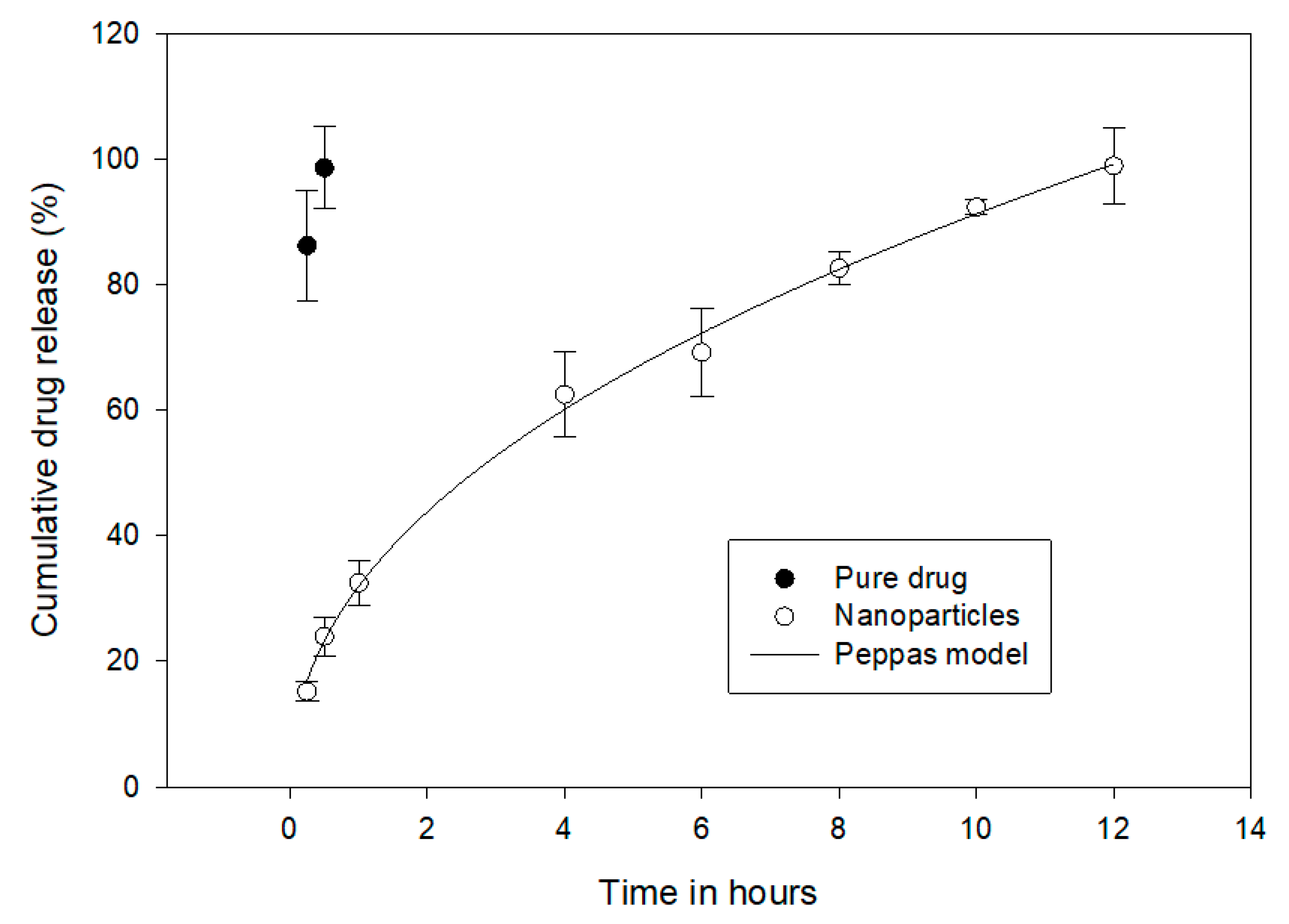
2.8. Drug Release Pattern and Kinetics
2.9. Histopathological Examination
2.10. Drug Retention in GIT
2.11. Stability Studies
3. Result and Discussion
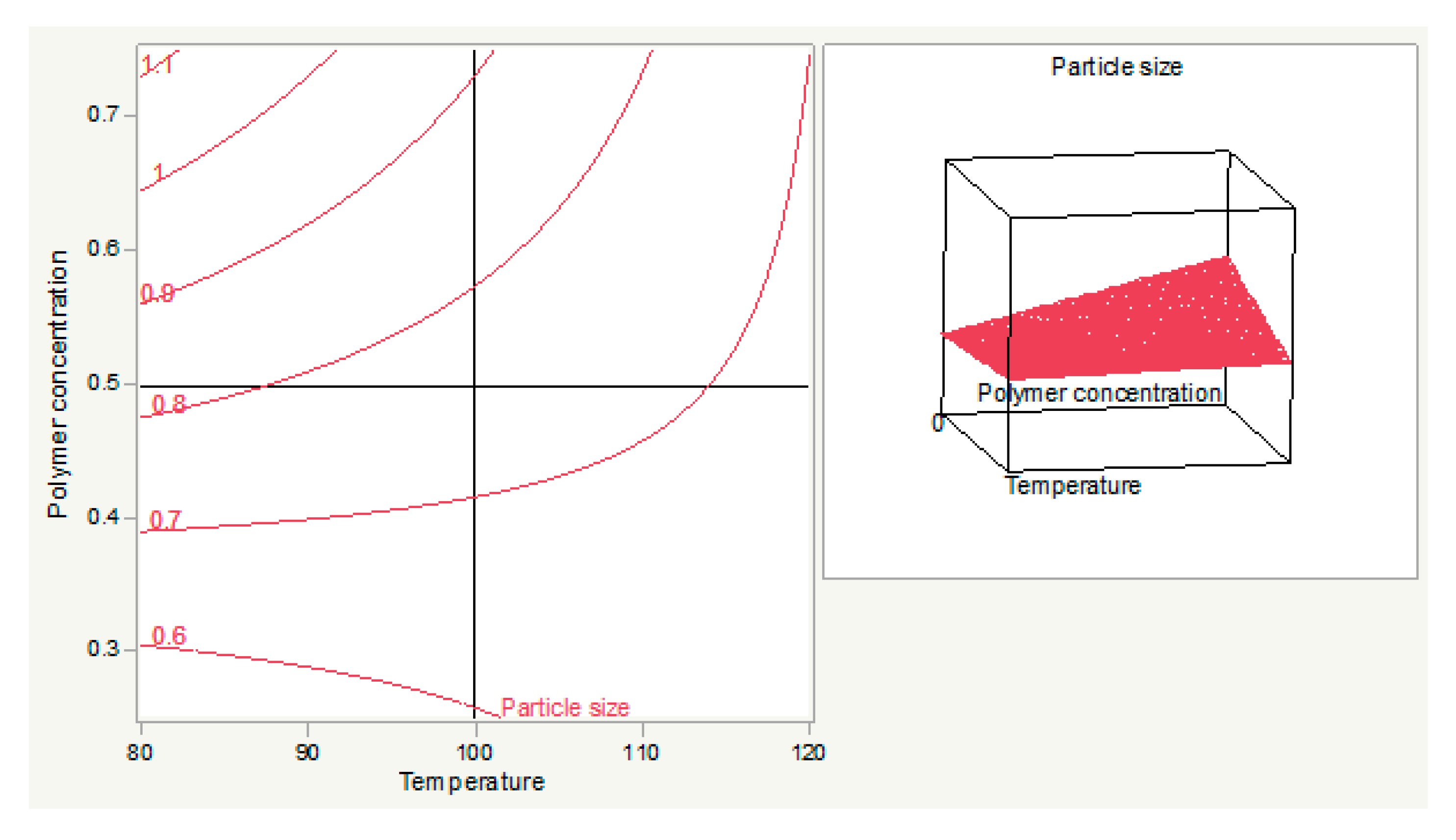
3.1. Optimization and Formulation
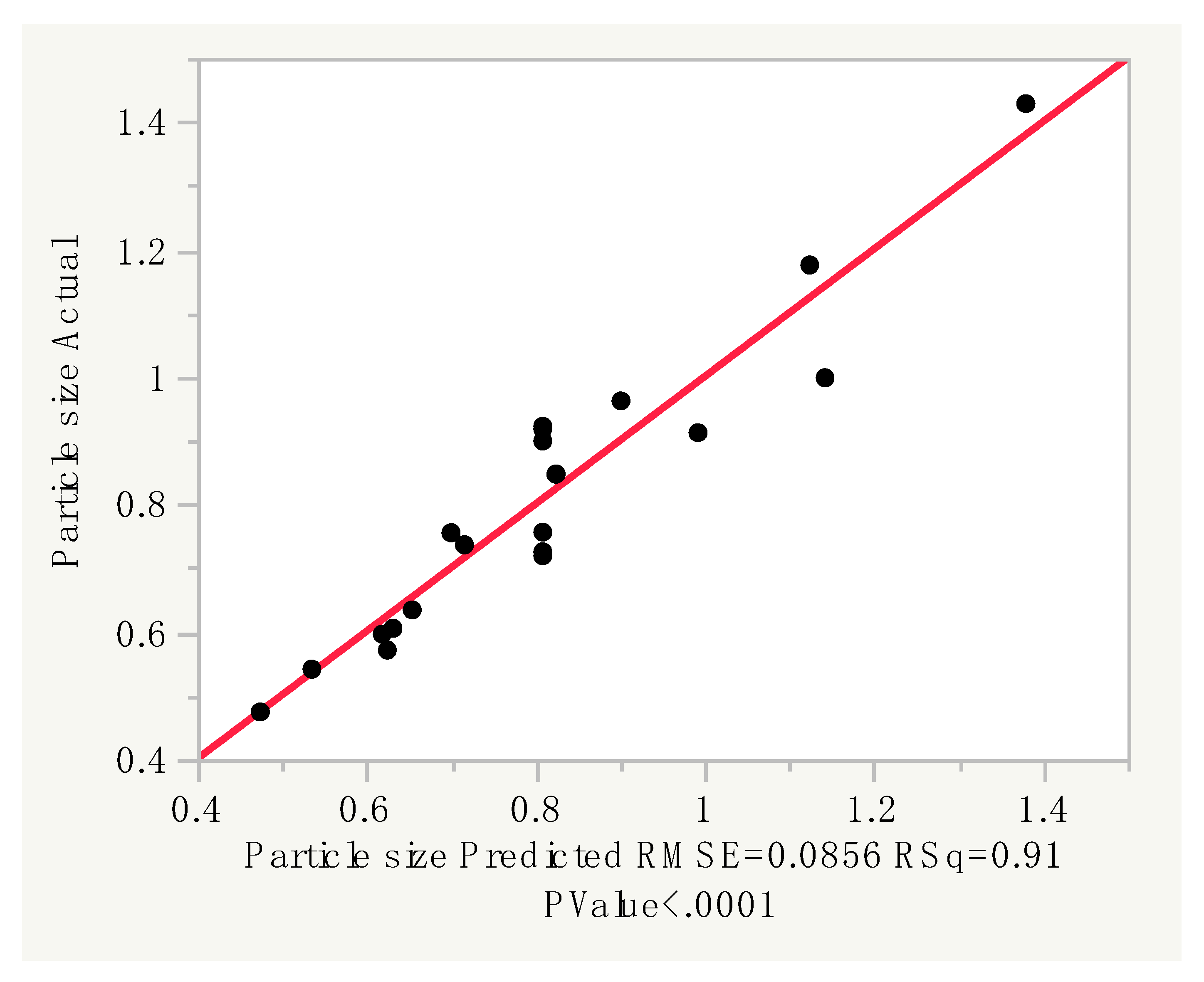
3.1.1. Prediction Expression
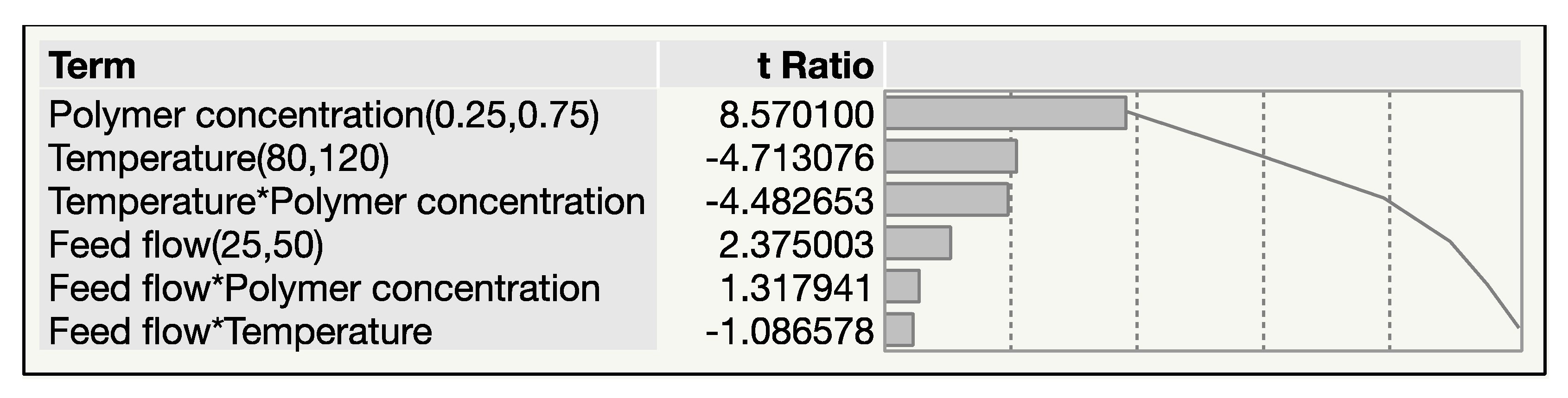
3.1.2. Pareto Plot of Estimates
3.2. Surface Morphology
3.3. Particle Size Distribution
3.4. DSC
3.5. XRD
3.6. Drug Release and Kinetics
3.7. Swelling
3.8. Mucoadhesion
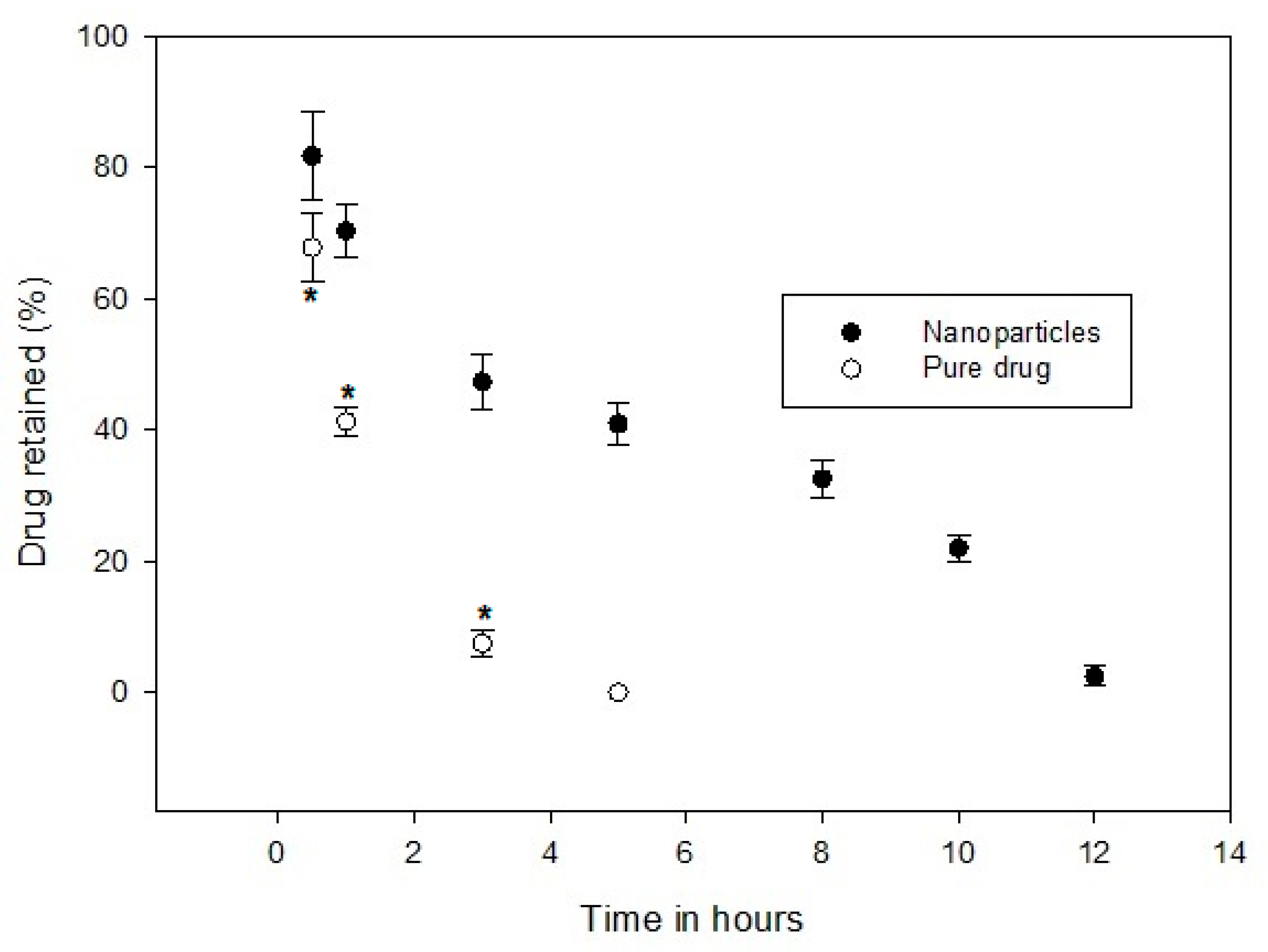
3.9. Drug Retention in GIT
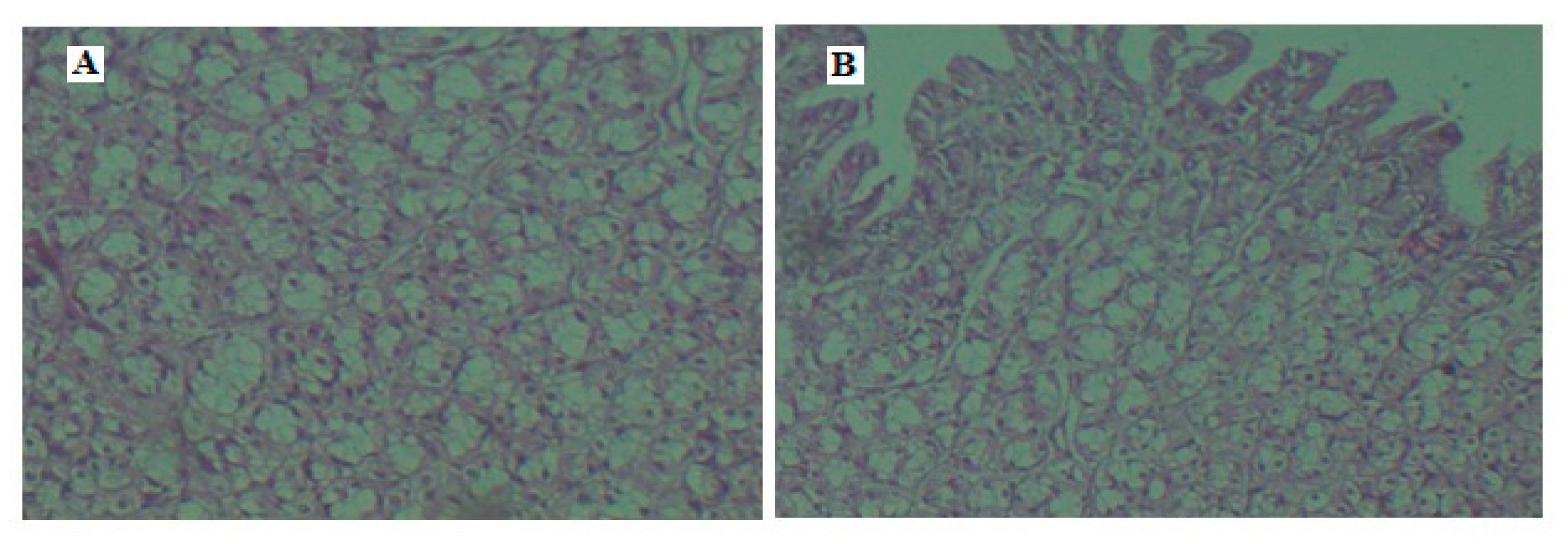
3.10. Histopathology
3.11. Stability
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. 2016. Available online: https://apps.who.int/iris/bitstream/handle/10665/204871/9789241565257_eng.pdf;jsessionid=4EBB5862DEF4FA1A37D4C6CE514D17FF?sequence=1 (accessed on 25 October 2019).
- Harsha, S.; Attimarad, M.; Khan, T.A.; Nair, A.B.; Aldhubiab, B.E.; Sangi, S.; Shariff, A. Design and formulation of mucoadhesive microspheres of sitagliptin. J. Microencapsul. 2013, 30, 257–264. [Google Scholar] [CrossRef]
- Bergman, A.; Ebel, D.; Liu, F.; Stone, J.; Wang, A.; Zeng, W.; Chen, L.; Dilzer, S.; Lasseter, K.; Herman, G.; et al. Absolute bioavailability of sitagliptin, an oral dipeptidyl peptidase-4 inhibitor, in healthy volunteers. Biopharm. Drug Dispos. 2007, 28, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Herman, G.A.; Stevens, C.; Van Dyck, K.; Bergman, A.; Yi, B.; De Smet, M.; Snyder, K.; Hilliard, D.; Tanen, M.; Tanaka, W.; et al. Pharmacokinetics and pharmacodynamics of sitagliptin, an inhibitor of dipeptidyl peptidase IV, in healthy subjects: Results from two randomized, double-blind, placebo-controlled studies with single oral doses. Clin. Pharmacol. Ther. 2005, 78, 675–688. [Google Scholar] [CrossRef] [PubMed]
- Al-Dhubiab, B.E.; Nair, A.B.; Kumria, R.; Attimarad, M.; Harsha, S. Formulation and evaluation of nano based drug delivery system for the buccal delivery of acyclovir. Colloids Surf. B Biointerfaces 2015, 136, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Al-Dhubiab, B.E.; Nair, A.B.; Kumria, R.; Attimarad, M.; Harsha, S. Development and evaluation of buccal films impregnated with selegiline-loaded nanospheres. Drug Deliv. 2016, 23, 2154–2162. [Google Scholar] [CrossRef] [PubMed]
- Netsomboon, K.; Bernkop-Schnürch, A. Mucoadhesive vs. mucopenetrating particulate drug delivery. Eur. J. Pharm. Biopharm. 2016, 98, 76–89. [Google Scholar] [CrossRef]
- Nagaraja, S.H.; Al-Dhubiab, B.E.; Tekade, R.K.; Venugopala, K.N.; Ghorpade, R.V.; Meravanige, G.; Alqadheeb, A. Novel preparation and effective delivery of mucoadeshive nanoparticles containing anti-diabetic drug. Indian J. Pharm. Educ. Res. 2019, 53, S43–S49. [Google Scholar] [CrossRef]
- Pallavi, S.; Patil, S.V.; Patil, S.S.; Shinde, S. Preparation and evaluation of mucoadhesive nanoparticles of rosuvastatin. Indian J. Pharm. Educ. Res. 2018, 80, 428–433. [Google Scholar] [CrossRef]
- Ways, M.; Mohammed, T.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan and its derivatives for application in mucoadhesive drug delivery systems. Polymers 2018, 10, 267. [Google Scholar] [CrossRef]
- Haq Asif, A.; Harsha, S.; Hodalur Puttaswamy, N.; Al-Dhubiab, B.E. An effective delivery system of sitagliptin using optimized mucoadhesive nanoparticles. Appl. Sci. 2018, 8, 861. [Google Scholar] [CrossRef]
- SreeHarsha, N.; Ramnarayanan, C.; Al-Dhubiab, B.E.; Nair, A.B.; Hiremath, J.G.; Venugopala, K.N.; Satish, R.T.; Attimarad, M.; Shariff, A. Mucoadhesive particles: A novel, prolonged-release nanocarrier of sitagliptin for the treatment of diabetics. BioMed. Res. 2019, 2019, 3950942. [Google Scholar] [CrossRef] [PubMed]
- Vauthier, C.; Bouchemal, K. Methods for the preparation and manufacture of polymeric nanoparticles. Pharm. Res. 2009, 26, 1025–1058. [Google Scholar] [CrossRef] [PubMed]
- Harsha, S.N.; Aldhubiab, B.E.; Nair, A.B.; Alhaider, I.A.; Attimarad, M.; Venugopala, K.N.; Srinivasan, S.; Gangadhar, N.; Asif, A.H. Nanoparticle formulation by Buchi B-90 nano spray dryer for oral mucoadhesion. Drug Des. Devel. Ther. 2015, 9, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Ahire, E.; Thakkar, S.; Darshanwad, M.; Misra, M. Parenteral nanosuspensions: A brief review from solubility enhancement to more novel and specific applications. Acta Pharm. Sin. 2018, 8, 733–755. [Google Scholar] [CrossRef] [PubMed]
- Kamboj, S.; Bala, S.; Nair, A.B. Solid lipid nanoparticles: An effective lipid based technology for poorly water soluble drugs. Int. J. Pharm. Sci. Rev. Res. 2010, 5, 78–90. [Google Scholar]
- Rizvi, S.A.; Saleh, A.M. Applications of nanoparticle systems in drug delivery technology. Saudi Pharm. J. 2018, 26, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.B.; Al-Dhubiab, B.E.; Shah, J.; Attimarad, M.; Harsha, S. Poly (lactic acid-co-glycolic acid) nanospheres improved the oral delivery of candesartan cilexetil. Indian J. Pharm. Educ. Res. 2017, 51, 571–579. [Google Scholar] [CrossRef]
- Harsha, S. Dual drug delivery system for targeting H. pylori in the stomach: Preparation and in vitro characterization of amoxicillin-loaded Carbopol® nanospheres. Int. J. Nanomed. 2012, 7, 4787. [Google Scholar] [CrossRef]
- Kumria, R.; Nair, A.B.; Al-Dhubiab, B.E. Loratidine buccal films for allergic rhinitis: Development and evaluation. Drug Dev. Ind. Pharm. 2014, 40, 625–631. [Google Scholar] [CrossRef]
- Sosnik, A.; das Neves, J.; Sarmento, B. Mucoadhesive polymers in the design of nano-drug delivery systems for administration by non-parenteral routes: A review. Prog. Polym. Sci. 2014, 39, 2030–2075. [Google Scholar] [CrossRef]
- Li, C.L.; Martini, L.G.; Ford, J.L.; Roberts, M. The use of hypromellose in oral drug delivery. J. Pharm. Pharmacol. 2005, 57, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Sekaran, C.B.; Rani, A.P. Development and validation of spectrophotometric method for the determination of DPP-4 inhibitor, sitagliptin, in its pharmaceutical preparations. Eclética Química 2010, 35, 45–53. [Google Scholar] [CrossRef]
- Morsy, M.A.; Nair, A.B. Prevention of rat liver fibrosis by selective targeting of hepatic stellate cells using hesperidin carriers. Int. J. Pharm. 2018, 552, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Jug, M.; Hafner, A.; Lovrić, J.; Kregar, M.L.; Pepić, I.; Vanić, Ž.; Cetina-Čižmek, B.; Filipović-Grčić, J. An overview of in vitro dissolution/release methods for novel mucosal drug delivery systems. J. Pharm. Biomed. Anal. 2018, 147, 350–366. [Google Scholar] [CrossRef] [PubMed]
- Makovicky, P.; Tumova, E.; Volek, Z.; Makovicky, P.; Vodicka, P. Histological aspects of the small intestine under variable feed restriction: The effects of short and intense restriction on a growing rabbit model. Exp. Ther. Med. 2014, 8, 1623–1627. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Nair, A.B.; Al-Dhubiab, B.E. Preparation and evaluation of niosome gel containing acyclovir for enhanced dermal deposition. J. Liposome Res. 2017, 27, 283–292. [Google Scholar] [CrossRef]
- Crucho, C.I.; Barros, M.T. Polymeric nanoparticles: A study on the preparation variables and characterization methods. Mater. Sci. Eng. C 2017, 80, 771–784. [Google Scholar] [CrossRef]
- Maas, S.G.; Schaldach, G.; Littringer, E.M.; Mescher, A.; Griesser, U.J.; Braun, D.E.; Walzel, P.E.; Urbanetz, N.A. The impact of spray drying outlet temperature on the particle morphology of mannitol. Powder Technol. 2011, 213, 27–35. [Google Scholar] [CrossRef]
- Li, X.; Anton, N.; Arpagaus, C.; Belleteix, F.; Vandamme, T.F. Nanoparticles by spray drying using innovative new technology: The Büchi Nano Spray Dryer B-90. J. Control. Release 2010, 147, 304–310. [Google Scholar] [CrossRef]
- Harsha, S.; Al-Dhubiab, B.E.; Nair, A.B.; Al-Khars, M.; Al-Hassan, M.; Rajan, R.; Attimarad, M.; Venugopala, K.N.; Asif, A.H. Novel drying technology of microsphere and its evaluation for targeted drug delivery for lungs. Dry. Technol. 2015, 33, 502–512. [Google Scholar] [CrossRef]
- Heng, D.; Lee, S.H.; Ng, W.K.; Tan, R.B. The nano spray dryer B-90. Expert Opin. Drug Deliv. 2011, 8, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.; Nair, A.B.; Jacob, S.; Patel, R.K.; Shah, H.; Shehata, T.M.; Morsy, M.A. Nanoemulsion based vehicle for effective ocular delivery of moxifloxacin using experimental design and pharmacokinetic study in rabbits. Pharmaceutics 2019, 11, 230. [Google Scholar] [CrossRef] [PubMed]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable controlled-release polymers and polymeric nanoparticles: Mechanisms of controlling drug release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [PubMed]
- Hanafy, N.A.; Leporatti, S.; El-Kemary, M.A. Mucoadhesive hydrogel nanoparticles as smart biomedical drug delivery system. Appl. Sci. 2019, 9, 825. [Google Scholar] [CrossRef]
- Schneider, F.; Koziolek, M.; Weitschies, W. In Vitro and in vivo test methods for the evaluation of gastroretentive dosage forms. Pharmaceutics 2019, 11, 416. [Google Scholar] [CrossRef]





| Source | Degree of Freedom | Sum of Squares | Mean Square | F Ratio |
|---|---|---|---|---|
| Model | 6 | 0.9103593 | 0.151727 | 20.7187 |
| Error | 13 | 0.0952013 | 0.007323 | Prob > F |
| Total | 19 | 1.0055606 | 0.159050 | <0.0001 * |
| Lack of Fit | 8 | 0.04569042 | 0.005711 | 0.5768 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nair, A.B.; Sreeharsha, N.; Al-Dhubiab, B.E.; Hiremath, J.G.; Shinu, P.; Attimarad, M.; Venugopala, K.N.; Mutahar, M. HPMC- and PLGA-Based Nanoparticles for the Mucoadhesive Delivery of Sitagliptin: Optimization and In Vivo Evaluation in Rats. Materials 2019, 12, 4239. https://doi.org/10.3390/ma12244239
Nair AB, Sreeharsha N, Al-Dhubiab BE, Hiremath JG, Shinu P, Attimarad M, Venugopala KN, Mutahar M. HPMC- and PLGA-Based Nanoparticles for the Mucoadhesive Delivery of Sitagliptin: Optimization and In Vivo Evaluation in Rats. Materials. 2019; 12(24):4239. https://doi.org/10.3390/ma12244239
Chicago/Turabian StyleNair, Anroop B., Nagaraja Sreeharsha, Bandar E. Al-Dhubiab, Jagadeesh G. Hiremath, Pottathil Shinu, Mahesh Attimarad, Katharigatta N. Venugopala, and Mohamed Mutahar. 2019. "HPMC- and PLGA-Based Nanoparticles for the Mucoadhesive Delivery of Sitagliptin: Optimization and In Vivo Evaluation in Rats" Materials 12, no. 24: 4239. https://doi.org/10.3390/ma12244239
APA StyleNair, A. B., Sreeharsha, N., Al-Dhubiab, B. E., Hiremath, J. G., Shinu, P., Attimarad, M., Venugopala, K. N., & Mutahar, M. (2019). HPMC- and PLGA-Based Nanoparticles for the Mucoadhesive Delivery of Sitagliptin: Optimization and In Vivo Evaluation in Rats. Materials, 12(24), 4239. https://doi.org/10.3390/ma12244239