1. Introduction
Due to the harmfulness of Pb to the human body and environment, Pb-free solders received an increasing attention in research and application in packaging in recent years [
1,
2]. At present, Sn-Ag-Cu series solders are the most widely used Pb-free solders for their favorable performances [
3]. However, other solders are being developed due to a high Ag cost and lower drop reliability from Sn-Ag-Cu solders [
4]. Sn-Bi solder is one of them due to its low melting point (139 °C) and good mechanical properties [
5]. However, large amounts of Bi in Sn-58Bi eutectic increases the brittleness of the solder alloy because of the brittleness nature of Bi phases [
6]. In Sn-Bi solder, Bi atoms can also exist as a solid solution in the β-Sn phase, which acts as a solid solution strengthening. Therefore, Sn-Bi solders with lower Bi content, especially Sn-Bi solid solution solders provide potential application to replace Sn-Ag-Cu series solders due to their similar melting point [
7,
8]. Wang et al. [
9] investigated the interfacial behavior in Sn-Bi solid solution solder on Cu and found that Sn-Bi solder with low Bi content presented lower growth of intermetallic compound (IMC) thickness and higher joint strength compared with pure Sn solder. Ye et al. [
10] studied the mechanical properties of Sn-Bi solder with different Bi content and found that the hardness of Sn-Bi solder with a Bi content of about 10% was the highest among all studied Sn-Bi solder alloys. Lai et al. [
11,
12] compared Sn-10Bi solder with Sn-Ag-Cu solder, and found that Sn-10Bi solder presented higher creep resistance. Therefore, Sn-10Bi solder provides a possible replacement for Sn-Ag-Cu solder.
It is well known that intermetallic compounds (IMCs) are formed by the reaction of solder with substrate to realize the interconnecting between them during soldering. The IMCs between Sn-based solder and Cu are mainly Cu
6Sn
5 and Cu
3Sn, which will gradually grow and thicken with the service time. Due to the embrittlement of IMCs, solder joints are prone to brittle fracture due to excessive growth of IMCs during service, which deteriorates the reliability of solder joints [
13]. Therefore, various methods were used to inhibit the excessive growth of IMCs. At present, doping trace elements is a relatively common and practical method. The effects of doping various elements in solder alloy on the interfacial reaction and reliability of solder joints were studied [
14]. It has been found that the addition of trace Zn was very effective in controlling the interfacial reaction with Cu. The formation of Cu
3Sn IMC, the consumption of Cu, and the formation of interfacial voids were significantly reduced by the addition of Zn [
15,
16,
17]. El-Daly et al. [
18,
19] explored the effects of Zn addition on microstructure, thermal behavior, and tensile creep properties of Sn-1.0Ag-0.3Cu solder alloy, and Zn addition could improve the tensile strength, ductility and creep resistance. Kang et al. [
15] observed that a small amount of Zn added into Sn-Ag-Cu solder can reduce supercooling during solidification, thereby inhibiting the formation of large Ag
3Sn plates in solder. Mokhtari et al. [
20] doped a trace amount of Zn into Sn-58Bi solder and found that the addition of 0.7 wt% Zn significantly inhibited the thickness growth of IMC in Sn-58Bi solder joint under reflow and thermal aging. Adding a small amount of Zn to Sn-0.7Cu solder was studied by Wang et al. and found that it can significantly reduce the formation of Cu
6Sn
5, and when the amount of doped Zn exceeded 0.8 wt%, the IMC would become Cu
5Zn
8 [
21]. Wang et al. also found that the addition of 0.2 wt% Zn to pure Sn solder significantly suppressed the thickness growth of IMCs [
22].
In this paper, we used Sn-10Bi solder as the solder matrix, and tried to incorporate trace amounts of Zn into it. The interfacial reaction and atomic migration mechanism of solder/Cu joints during thermal aging were then studied.
4. Discussion
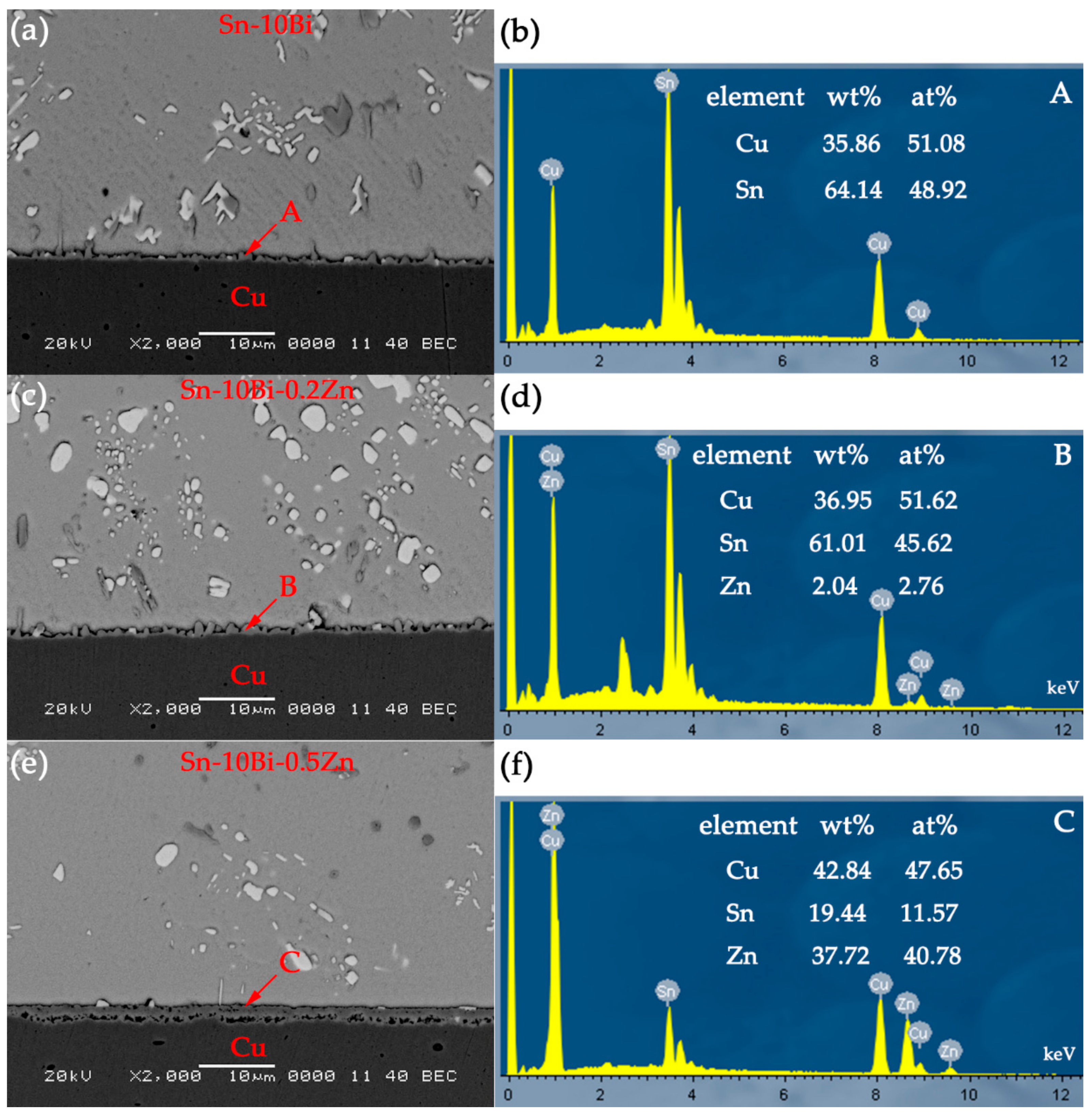
The compositions of interfacial IMCs in Sn-10Bi/Cu joints are Cu
6Sn
5 and Cu
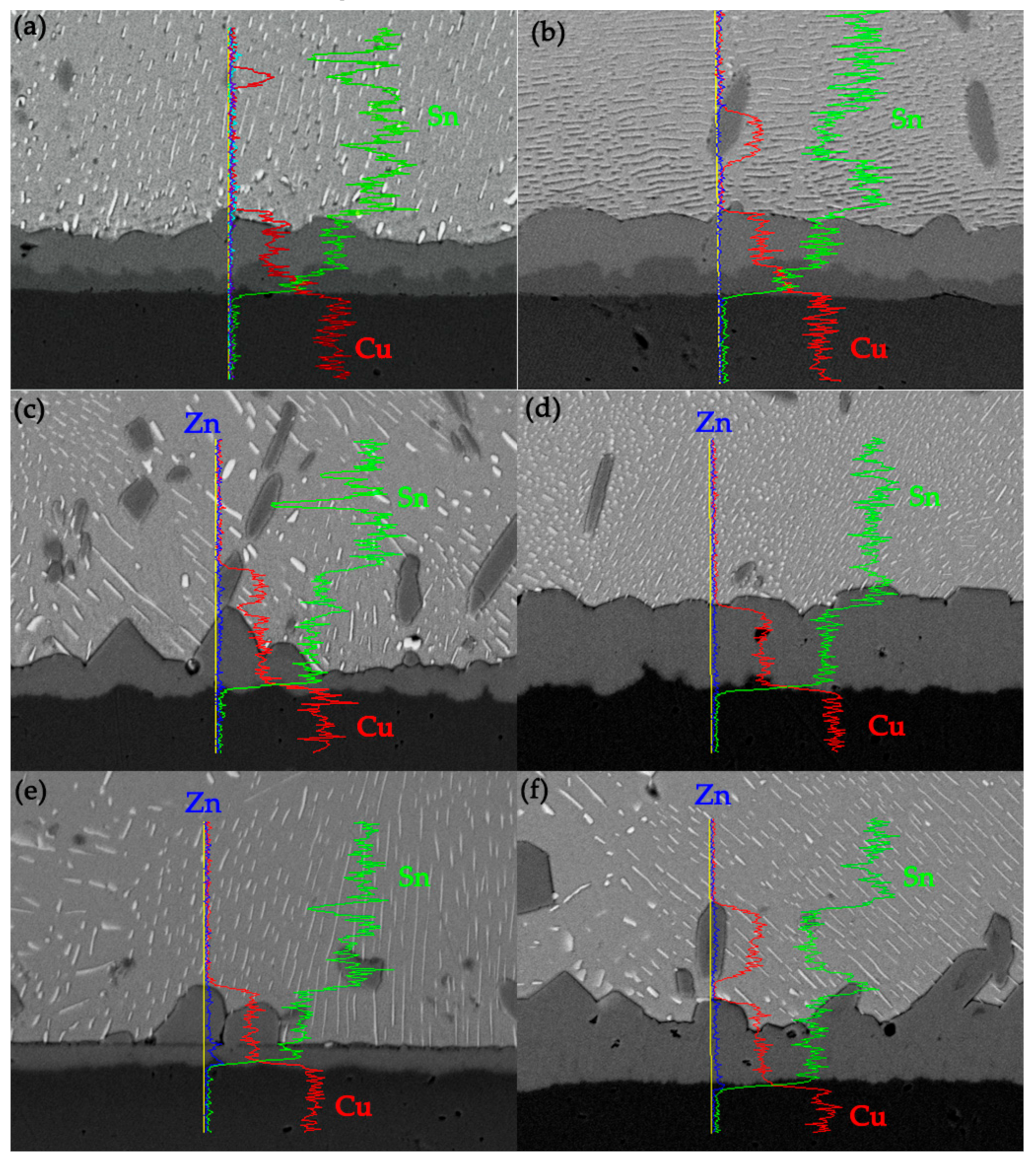
3Sn during isothermal aging. It can be seen from the line scan at the interface in
Figure 5a,b that Cu atoms are mainly presented in Cu substrate and IMC layer, and Sn atoms are mainly presented inside the solder matrix and IMC layer. Bi atoms are distributed in the solder matrix and do not enter the IMC layer and Cu substrate. With Zn doped into solder, the elemental composition at the interface changed. The atomic percentage at point 2 in
Table 1 is 60.11% for Cu atoms, 36.62% for Sn atoms, and 3.27% for Zn atoms. Therefore, Zn atoms in the Sn-10Bi-0.2Zn solder diffused into IMC and replaced some Sn atoms to form Cu
6(Sn, Zn)
5 [
23]. Since these Zn atoms in IMC hindered the diffusion of Cu atoms into solder and Sn atoms towards substrate, they inhibited the formation and growth of the Cu
6(Sn, Zn)
5 IMC layer. By performing a line scan at the interface of Sn-10Bi-0.2Zn/Cu solder joints aged at 150 °C for 40 days and 170 °C for 30 days, the content of Zn atoms in the IMC range obviously increased, which indicates that Zn atoms in Sn-10Bi-0.2Zn solder were dissolved into IMC, as shown in
Figure 5c,d. As reported in a previous paper [
24], Zn atoms were easily segregated at the interface between Cu substrate and IMC layer to form CuZn solid solution with Cu atoms, but it was not easily to be observed at the interface in
Figure 5c.
With more Zn addition, Zn atoms in Sn-10Bi-0.5Zn solder continuously diffused from the solder matrix to the interface and combined with Cu atoms to form Cu
5Zn
8 IMCs. Since the Gibbs free energy (∆
G) of Cu
5Zn
8 phase (−12.34 kJ/mol) was much lower than that of Cu
6Sn
5 phase (−7.42 kJ/mol) [
25], Zn atoms were more reactive than Sn atoms with Cu atoms, and accordingly the formation of Cu
5Zn
8 was superior to Cu
6Sn
5.Therefore, in
Figure 1e, Cu atoms preferentially reacted with Zn atoms to form Cu
5Zn
8 at a distance from the Cu substrate, and then reacted with Sn atoms to form Cu
6Sn
5 between Cu
5Zn
8 and Cu substrate. Since the reaction was insufficient within a shorter soldering time, the generated Cu
6Sn
5 could not fill the gap between Cu
5Zn
8 and Cu substrate. A significant gap existed between Cu
5Zn
8 and Cu substrate. After aging with a shorter time, two IMC layers were also observed at the interface. It was found by EDX analysis that the compound near the solder matrix was Cu
5Zn
8, and the compound near Cu substrate was Cu
6 (Sn, Zn)
5, as shown at point 3 and 4. Moreover, the thickness of Cu
6(Sn, Zn)
5 layer increased with aging temperature and time, which indicates that during aging, Sn and Zn atoms were continuously migrated towards the interface and reacted with Cu atoms to form Cu
6(Sn, Zn)
5 IMCs. Liu et al. [
26] also reported the evolution of the metastable Cu
5Zn
8 phase at the interface of Sn-3.7Ag-0.9Zn/Cu joints. After 1 min of soldering, the metastable Cn
5Zn
8 phase was formed. Then, part of the Cu
5Zn
8 layer was transformed into the stable Cu
6Sn
5 phase near Cu plate. With aging time increasing, according to EDX analysis, the compound at point 5 is Cu
6(Sn, Zn)
5, and the thin layered compound at point 6 is Cu
5Zn
8. Therefore, due to the instability of Cu
5Zn
8 at high temperature [
27], Cu
5Zn
8 gradually decomposed into Zn atoms and Cu atoms during aging. Cu atoms combined with Sn atoms to form a more stable Cu
6Sn
5, while Zn atoms entered the continuously generated Cu
6Sn
5 to form Cu
6(Sn, Zn)
5 [
26]. Therefore, the interface in 0.5Zn contained solder joints after aging at 130 and 150 °C was composed of Cu
6(Sn, Zn)
5, Cu
5Zn
8, and Cu
6(Sn, Zn)
5. After aging at 170 °C, the Cu
5Zn
8 IMC layer was completely decomposed within a short aging time, and the interface was mainly composed of Cu
6(Sn, Zn)
5.
A line scan of interface of Sn-10Bi-0.5Zn/Cu joints aged at 150 °C for 40 days showed that there were two peaks in the content of Zn atoms, as shown in
Figure 5e: One at the linear compounds in IMC and the other at the interface of IMC and Cu. For solder joint aged at 170 °C, as shown in
Figure 5f, the Cu
5Zn
8 compounds disappeared, and the content of Zn atoms in Cu
5Zn
8 compounds that diffused into solder became remarkable, which indicates that Cu
5Zn
8 continuously decomposed with the increase of aging time. The instability of Cu
5Zn
8 also affected the IMC between Cu
5Zn
8 and solder. It can be inferred that accompanying with the decomposition of Cu
5Zn
8, Cu
6Sn
5 layer between Cu
5Zn
8 and solder partly detached from IMC layer according to the special IMC morphology in joints aged at 130 and 150 °C for longer time. In
Figure 5c,e,f, the peaks of Zn atoms content appear at the interface between Cu and IMC, indicating that Zn atoms diffused into Cu substrate to form Cu
5Zn
8 after continuous aging. Moreover, the presence of Zn atoms in Cu
6(Sn, Zn)
5 prevented the reaction between Cu substrate and Cu
6(Sn, Zn)
5 to form Cn
3Sn between them.
Since the growth of IMC at solder/Cu during solid aging is volume diffusion-controlled, it is well known that it follows the classical diffusion equation:
where
X represents the IMC thickness,
X0 represents the initial IMC thickness,
D is the diffusivity, and
t is the aging time. The slope of the fitted line,
, represents the growth rate of IMC.
Figure 6a,b are results on the thickness of IMCs in Sn-10Bi/Cu and Sn-10Bi-0.2Zn/Cu solder joints at different aging temperatures and aging times. The linear fit confirms that the thickness growth of IMCs in Sn-10Bi/Cu and Sn-10Bi-0.2Zn/Cu solder joints is proportional to
t0.5.
Figure 6c is the thickness of IMCs in Sn-10Bi-0.5Zn/Cu solder joints after aging at different temperatures. It is obvious that the thickness growth of IMC in Sn-10Bi-0.5Zn/Cu solder joints did not follow the classical diffusion formula because of the formation and dissolution of Cu
5Zn
8 layer and the spalling effect of Cu
6(Sn, Zn)
5 IMC layer.
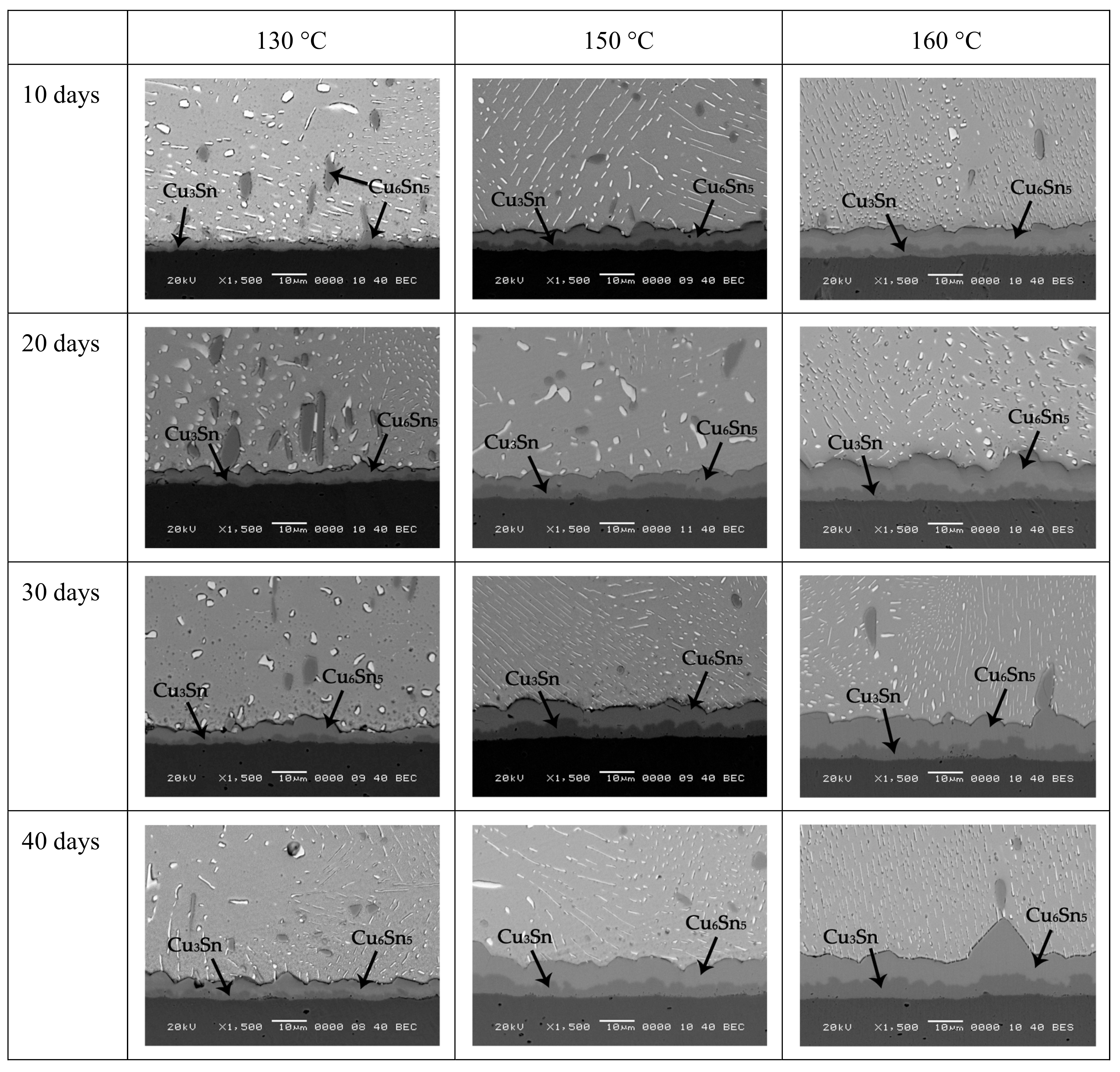
According to the data in
Figure 6a, the interfacial IMCs in Sn-10Bi/Cu solder joints continues to grow during isothermal aging, and its growth rate is accelerated with aging temperature increasing. The growth rate of the interfacial IMCs in Sn-10Bi/Cu solder joints is 0.915 μm/day
1/2, 1.370 μm/day
1/2, and 1.605 μm/day
1/2 under 130, 150, and 160 °C, respectively. Therefore, an obvious difference on them with the effect of aging temperature does not exist. However, the addition of 0.2Zn obviously affects the growth rate of interfacial IMC layer. It can be seen from
Figure 6b that the growth rate of IMCs at the interface of Sn-10Bi-0.2Zn/Cu under aging temperature of 130 and 150 °C is evidently reduced, and the influence of aging temperature on the growth rate is accelerated. Comparing
Figure 6a with
Figure 6b, it is found that the thickness of IMCs in Sn-10Bi joints grew from 3.2 to 6.3 μm at 130 °C, while that in Sn-10Bi-0.2Zn solder joints grew from 1.9 to 2.8 μm. The growth rate of IMCs of Sn-10Bi-0.2Zn solder joints is much lower than that of Sn-10Bi joints, indicating that the addition of 0.2Zn greatly inhibited the reaction of Sn atoms in the solder with Cu at 130 °C. However, the inhibition effect is deteriorated at higher aging temperature. For example, the IMCs in Sn-10Bi solder joints aged at 150 °C grew from 6. 2 to 11 μm, while the IMCs of Sn-10Bi-0.2Zn grew from 2.9 to 6.3 μm. Accordingly, the IMCs growth rate of Sn-10Bi and Sn-10Bi-0.2Zn solder joints is 1.370 and 1.252 μm/day
1/2, respectively. Overall, it is clearly seen that the diffusion rate increased as the aging temperature increased.
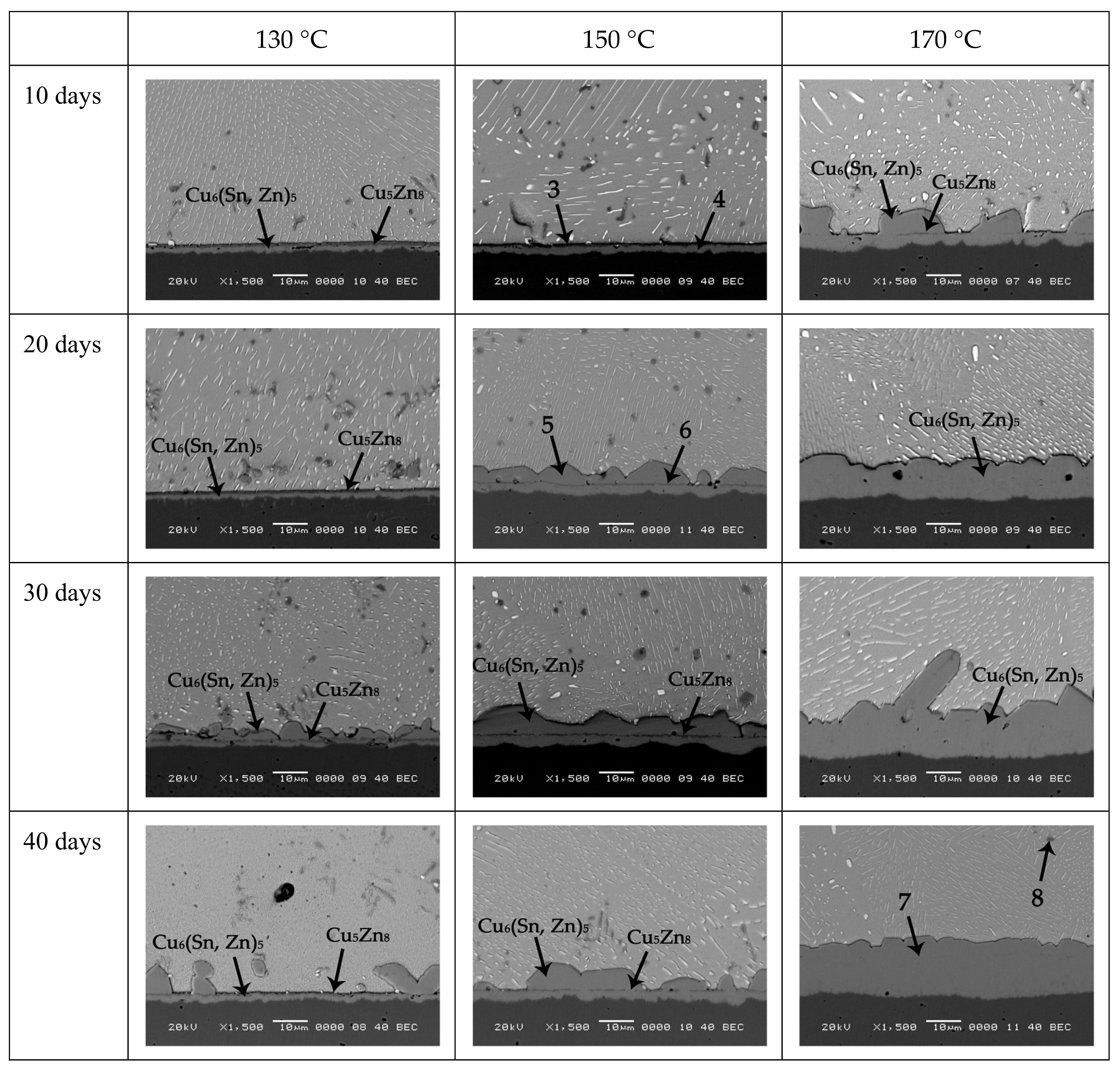
It is obvious that the growth of IMCs in Sn-10Bi-0.5Zn did not follow the classical diffusion formula. In
Figure 6c, during aging at 130 °C for 10 or 20 days, the overall growth of IMC was very slow due to the simultaneous reaction of Cu atoms with Zn and Sn atoms. With aging period prolonging to 30 days, the IMC grew rapidly because the reaction between Sn and Cu atoms became the dominant reaction. After 30 days aging, Cu
6(Sn, Zn)
5 was produced between Cu
5Zn
8 and solder matrix, which induced an increase on IMC thickness. With aging days of 40 days, the spalling effect of Cu
6(Sn, Zn)
5 was faster than its formation, which induced a decrease on IMC thickness. It should be noted that the decomposition of Cu
5Zn
8 always occurred, which affected the formation of Cu
6Sn
5 and Cu
3Sn between Cu
5Zn
8 and solder. At an aging temperature of 150 °C, the reaction was accelerated due to the increase on atomic diffusion. The reaction between Sn and Cu atoms became the dominant reaction during 10 to 30 days, and the IMC continued to grow during this period. The thickness of IMC also decreased after 30 days due to the spalling effect of Cu
6(Sn, Zn)
5. When the temperature increased to 170 ° C, the reaction was furtherly accelerated. Combined with
Figure 5, it is found that the formation and decomposition of Cu
5Zn
8 completed within 10 days aging. As shown in
Figure 5, during aging from 10 days to 40 days, Zn atoms diffused into the solder and Cu matrix, while Sn atoms continuously reacted with Cu atoms to induce the growth of IMCs.
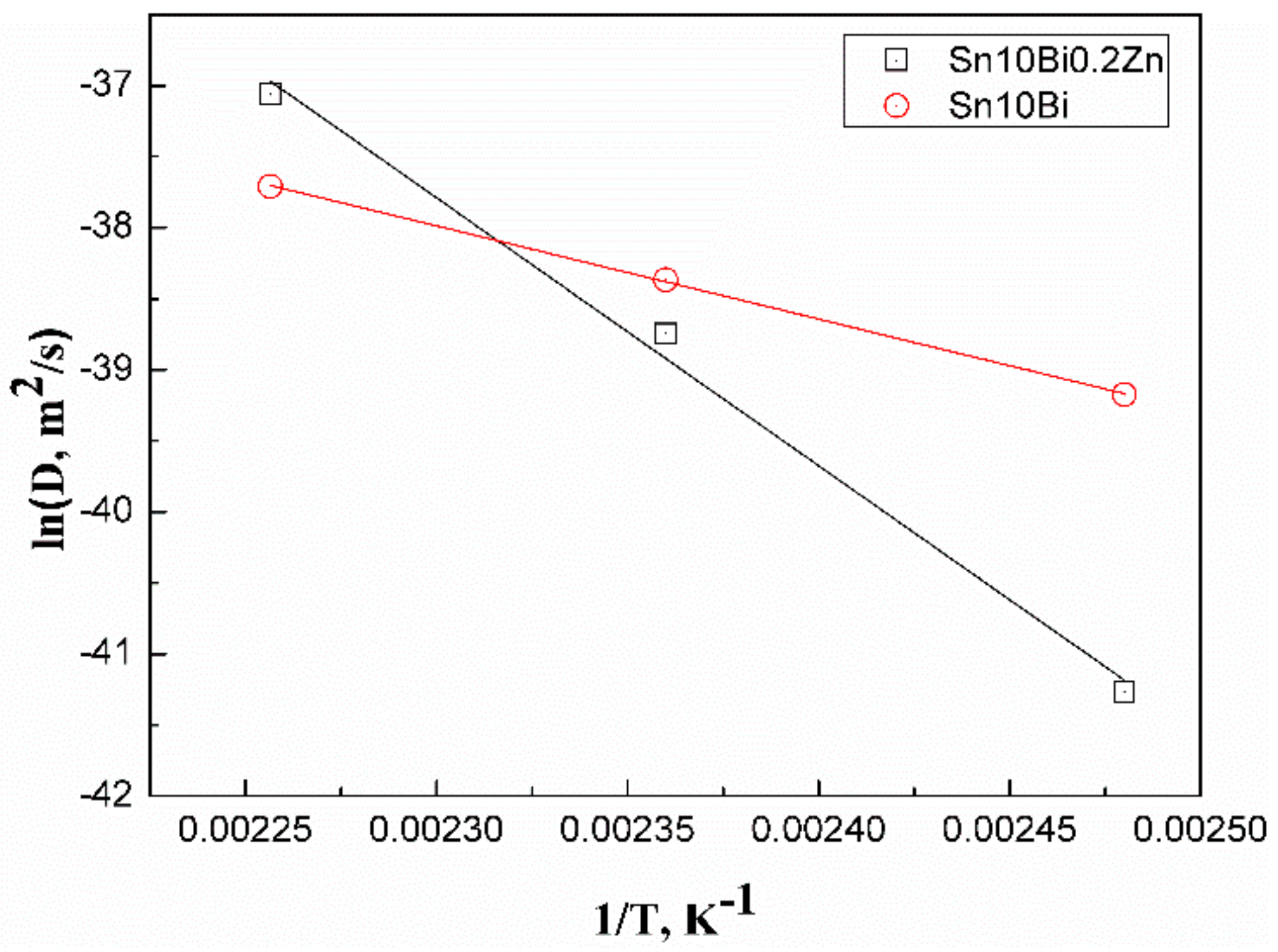
In order to explore the reason why the growth of IMC is slowed after the addition of 0.2Zn, the activation energy
Q of the solder is calculated. In Fick’s first law, the diffusion coefficient
D can be expressed by:
where
D0 is the diffusion constant,
R is the general gas constant,
T is the absolute temperature, and
Q is the activation energy. This formula can be rewritten as:
Therefore, in the curve with 1/
T as the
x-axis and
as the
y-axis, the slope of the curve indicates the activation energy
Q. The greater the activation energy is, the smaller the diffusion coefficient is, and the slower the IMC growth is. The diffusion coefficient
D and aging temperature
T of the growth of IMCs in Sn-10Bi/Cu and Sn-10Bi-0.2Zn/Cu solder joints are listed in
Table 2.
The Arrhenius plots are drawn from the relationship between aging temperature and diffusion coefficient with the results shown in
Figure 7. Accordingly, the activation energy
Q can be calculated. The activation energy of IMCs growth of Sn-10Bi/Cu is 54.61 kJ/mol, and that of Sn-10Bi-0.2Zn/Cu is 157.19 kJ/mol. The activation energy of IMCs growth of Sn-10Bi-0.2Zn/Cu is much higher than that of Sn-10Bi/Cu. Therefore, the energy required for the interfacial reaction of the Sn-10Bi-0.2Zn/Cu joint is much higher than that of Sn-10Bi/Cu joint, and the formation rate of IMC is also slowed down.
Furtherly, the intrinsic atomic migration and interaction during aging were investigated.
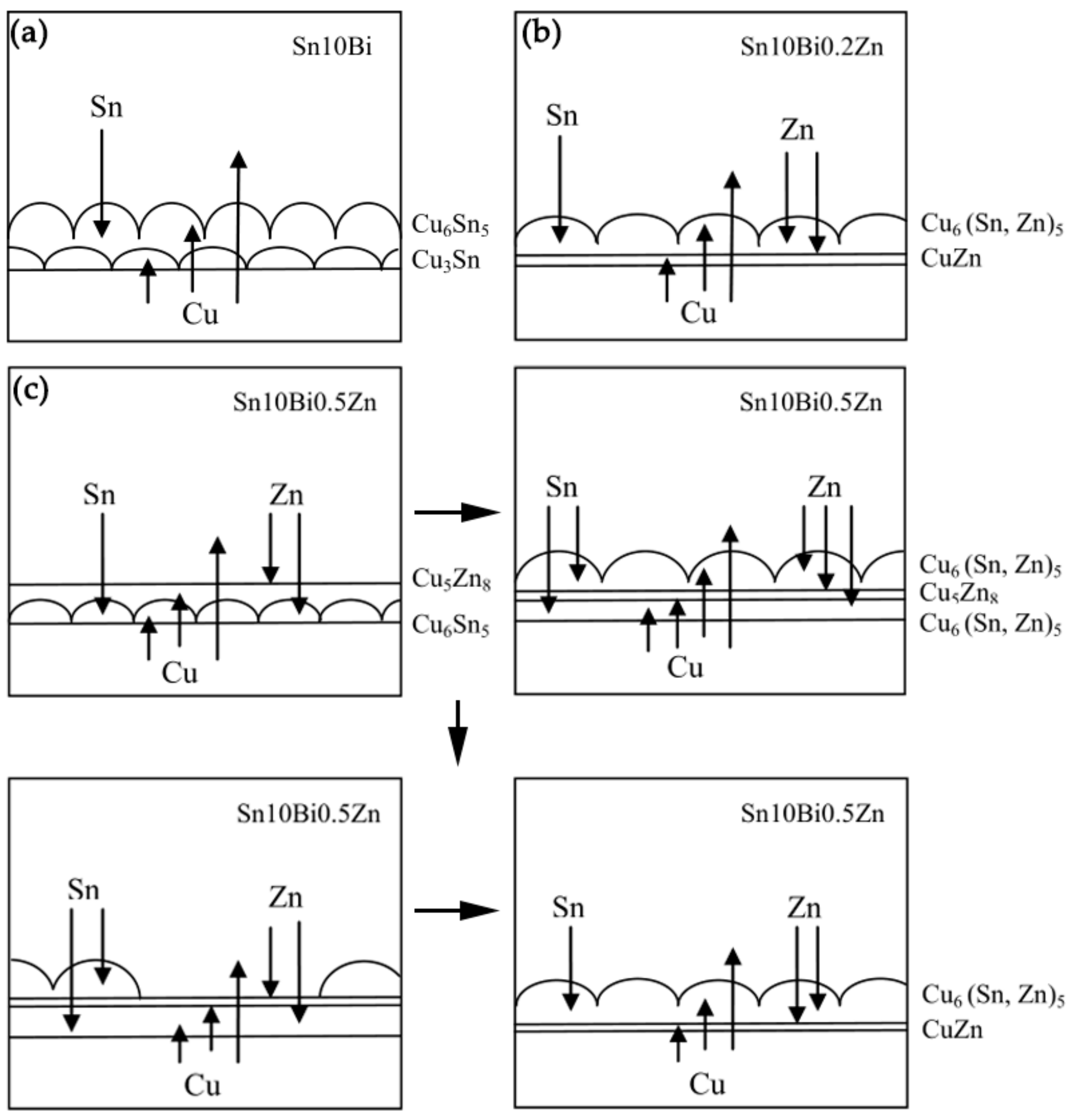
Figure 8 shows the atomic diffusion and reaction at the interface during isothermal aging. After the temperature increased, the atoms became active and diffused to each other. In Sn-10Bi/Cu solder joints shown in
Figure 8a, Bi atoms were mainly dissolved in β-Sn phase, and did not enroll in the reaction. Sn and Cu atoms were the main diffusing atoms. During aging, Sn atoms continuously diffused from solder to Cu substrate and Cu atoms diffused from substrate to the solder side. After reaction, Cu
6Sn
5 was continuously formed at the interface between solder and Cu substrate, as shown in Equation (4). Therefore, the Cu
6Sn
5 IMC layer formed during soldering continuously grew with aging.
Moreover, Cu
3Sn was also formed at the interface between Cu
6Sn
5 and Cu substrate due to the reaction of the diffused Cu atoms with Cu
6Sn
5, as shown in the Equation (5).
It can be seen that Cu6Sn5 was formed at the interface between solder and IMC and Cu3Sn was formed at the interface between Cu substrate and IMC. This indicates that the growth of Cu6Sn5 was mainly controlled by the diffusion of Sn atoms, while the growth of Cu3Sn was mainly controlled by the diffusion of Cu atoms. The formation of Cu6Sn5 and Cu3Sn led to a continuous increase on IMC thickness at the interface.
After the addition of 0.2% Zn, the change on the interfacial reaction occurred. Zn atoms in the solder did not react with Sn atoms, but continuously diffused towards the interface and Cu
6Sn
5 IMCs. As shown in the EDX of point 9, Zn atoms diffused into the Cu
6Sn
5 crystal structure and replaced some Sn atoms to form Cu
6(Sn, Zn)
5. At the same time, Zn atoms in IMCs hindered the inter diffusion of Sn atoms and Cu atoms, thus suppressing the formation of Cu
6Sn
5. Since the activation energy of Cu
5Zn
8 is much smaller than that of Cu
3Sn, there was no Cu
3Sn production at the interface. With time increasing, the solubility of Zn atoms in Cu
6Sn
5 was saturated. Some Zn atoms diffused into Cu substrate to form the CuZn solid solution, and some were enriched at the interface between IMCs and Cu substrate. The enrichment of Zn atoms and the CuZn solid solution became diffusion barriers, hindering the diffusion of Cu atoms and Sn atoms and suppressing the formation of IMCs. Hence, the growth of IMC of Sn-10Bi-0.2Zn/Cu was much slower than that of Sn-10Bi/Cu, as shown in
Figure 8b.
It is possible that the content of Zn atoms in Sn-10Bi-0.2Zn/Cu solder joint was too small, and no obvious Cu
5Zn
8 compound was observed at the interface. However, the product at the interface changed when the content of Zn atoms increased to 0.5%. Firstly, Zn atoms diffused towards Cu substrate and reacted with Cu atoms to form Cu
5Zn
8, as shown in Equation (6).
Secondly, Cu atoms reacted with the diffused Sn atoms to form Cu
6Sn
5 between Cu
5Zn
8 and Cu substrate. Then, some Zn atoms diffused into the Cu
6Sn
5. As shown in the initial period of aging at 130 and 150 °C in
Figure 4, the top layer of the IMC had a flatter appearance. Thirdly, Cu atoms diffused into the solder to react with Sn atoms. Cu
6Sn
5 formed at the interface between solder and Cu
5Zn
8, and a small amount of Zn atoms were still diffused therein. At the same time, the decomposition of Cu
5Zn
8 began to occur due to its instability, as shown in Equation (7).
Part of the decomposed Cu atoms and Zn atoms diffused towards Cu
6Sn
5 between Cu
5Zn
8 and Cu substrate, while some of them diffused into the solder side. Simultaneously, Cu
6(Sn, Zn)
5 was also produced between the Cu
5Zn
8 layer and solder matrix. However, due to the poor adhered effect of Cu
6(Sn, Zn)
5 on Cu
5Zn
8, a part of Cu
6Sn
5 was easily separated from IMCs. After the decomposition of Cu
5Zn
8, part of Zn atoms diffused toward Cu substrate and were concentrated at the interface between IMCs and Cu substrate. Zn atoms that diffused into Cu substrate were mainly dissolved in the Cu atoms to form a small layer of CuZn solid solution. The interfacial evolution in Sn-10Bi-0.5Zn joints was then plotted in
Figure 8c.