Europium-Doped Tellurite Glasses: The Eu2+ Emission in Tellurite, Adjusting Eu2+ and Eu3+ Emissions toward White Light Emission
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
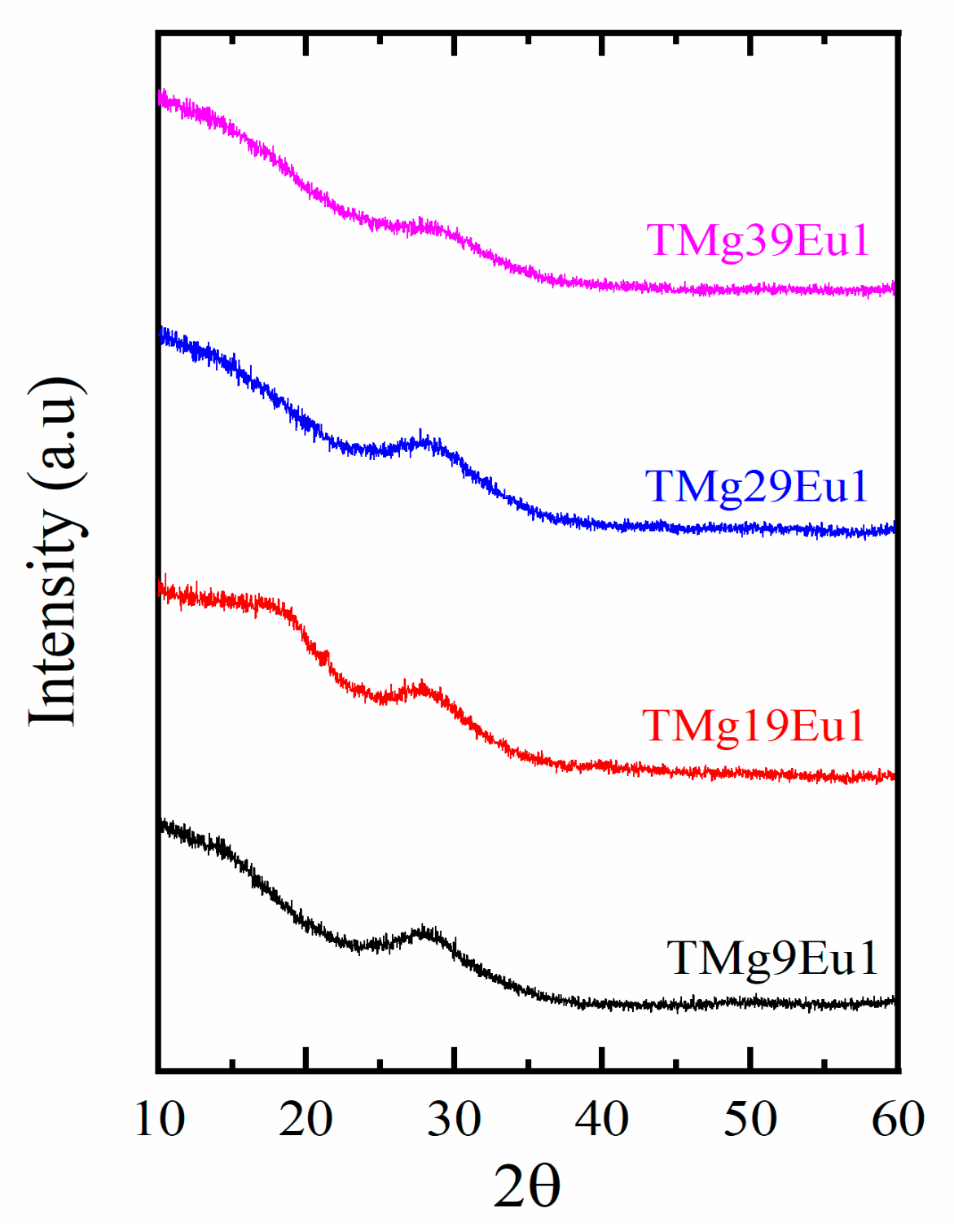
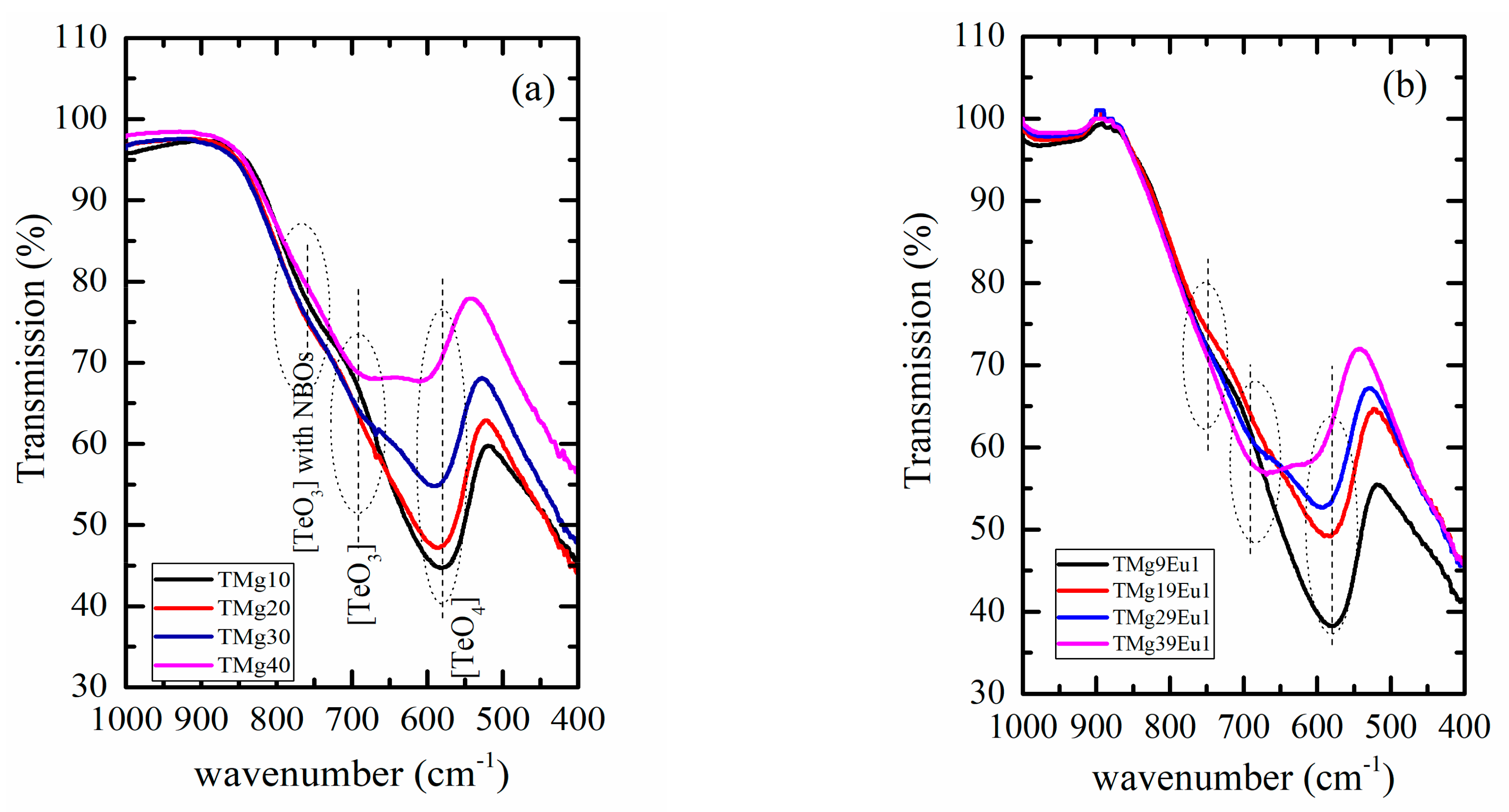
3.1. Structural and Thermal Investigation
3.2. Photoluminescence Spectroscopy
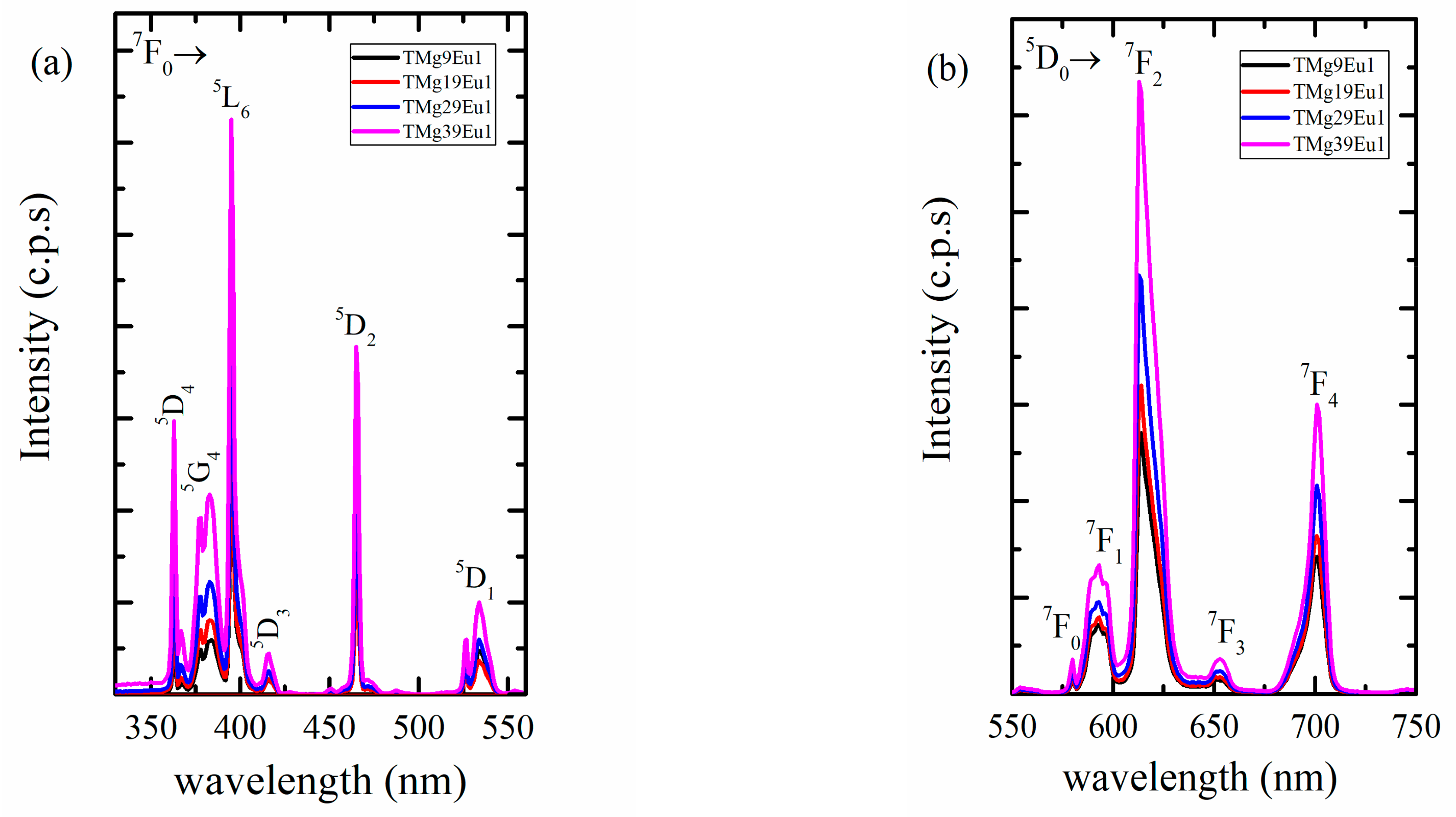
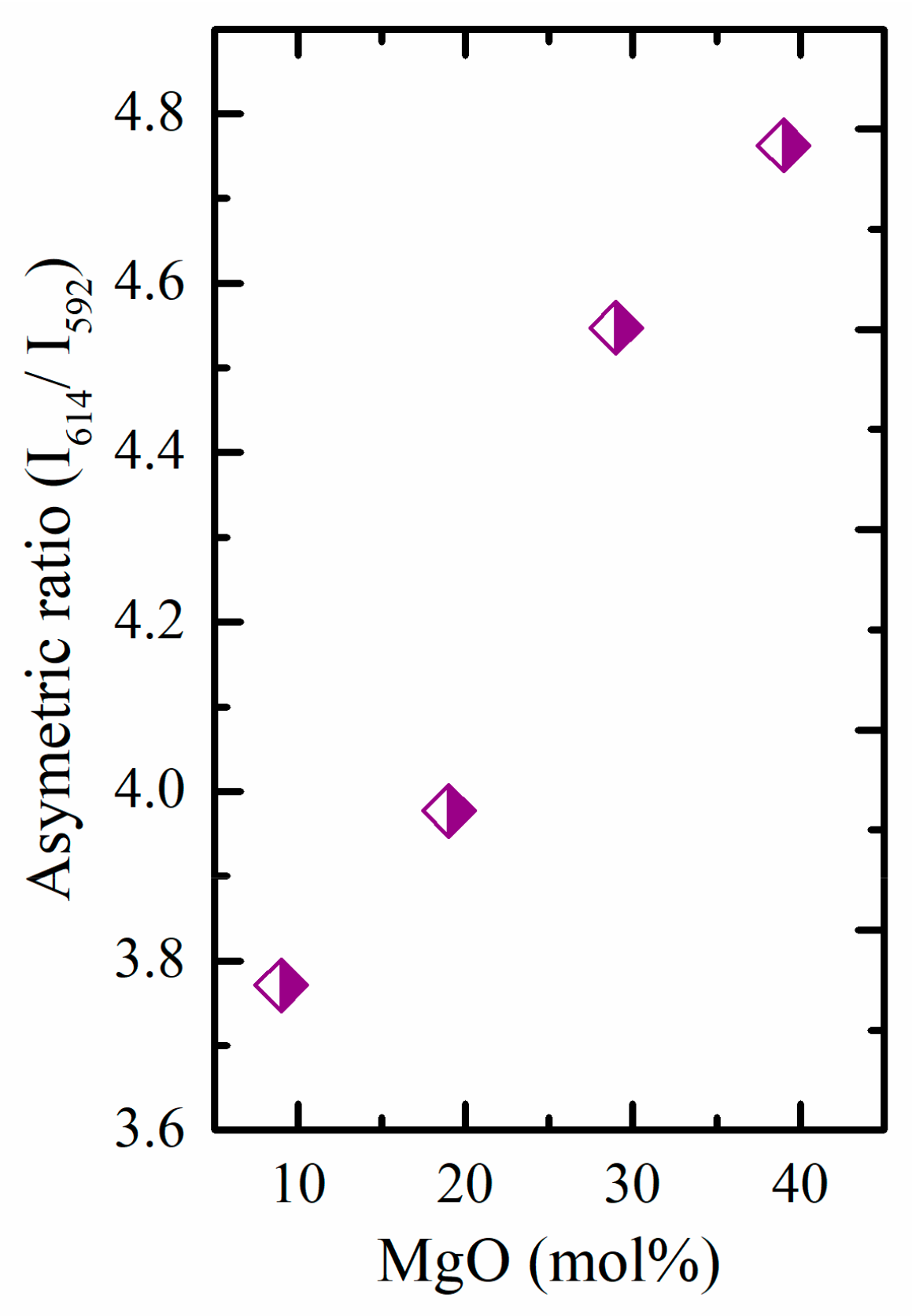
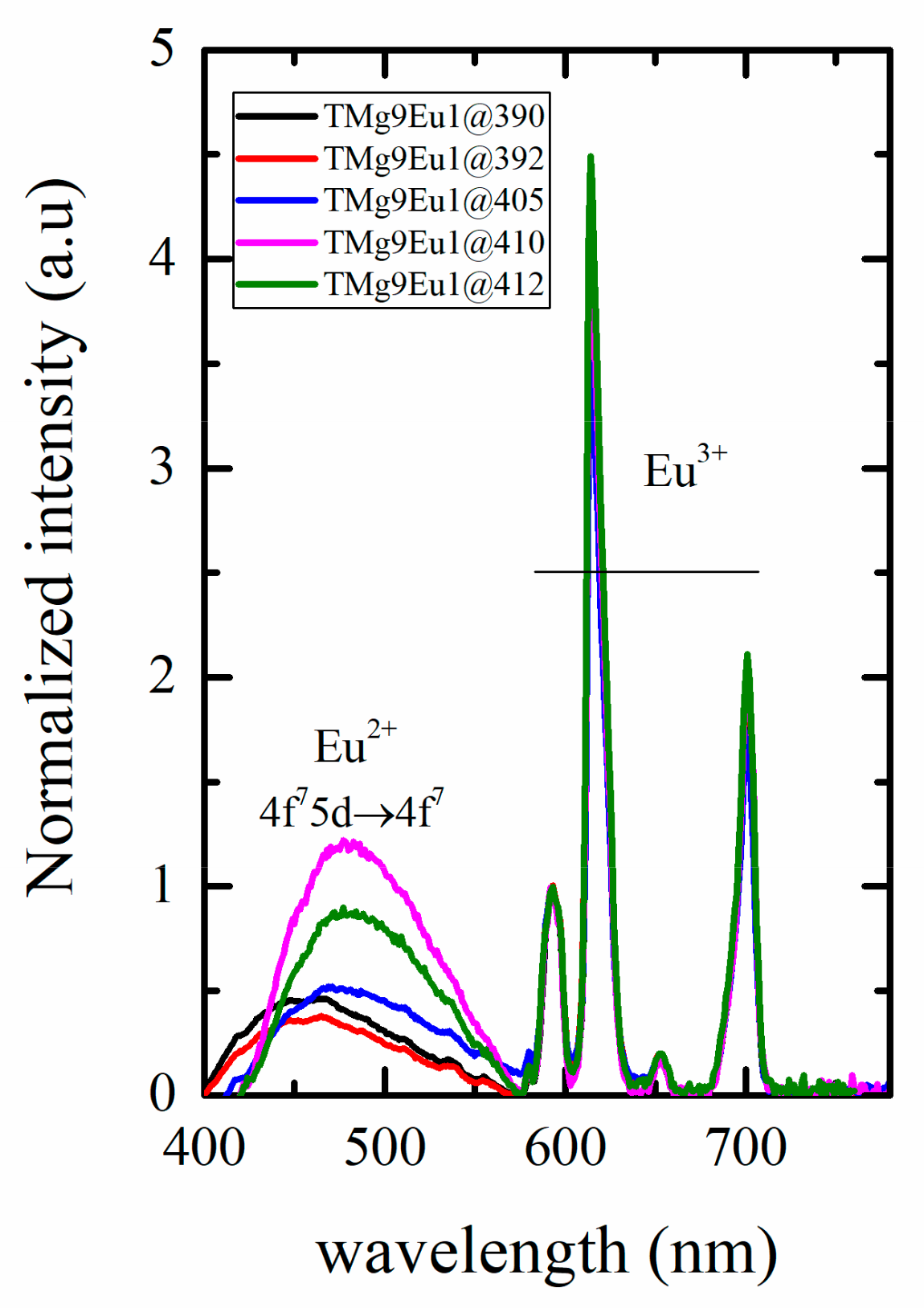
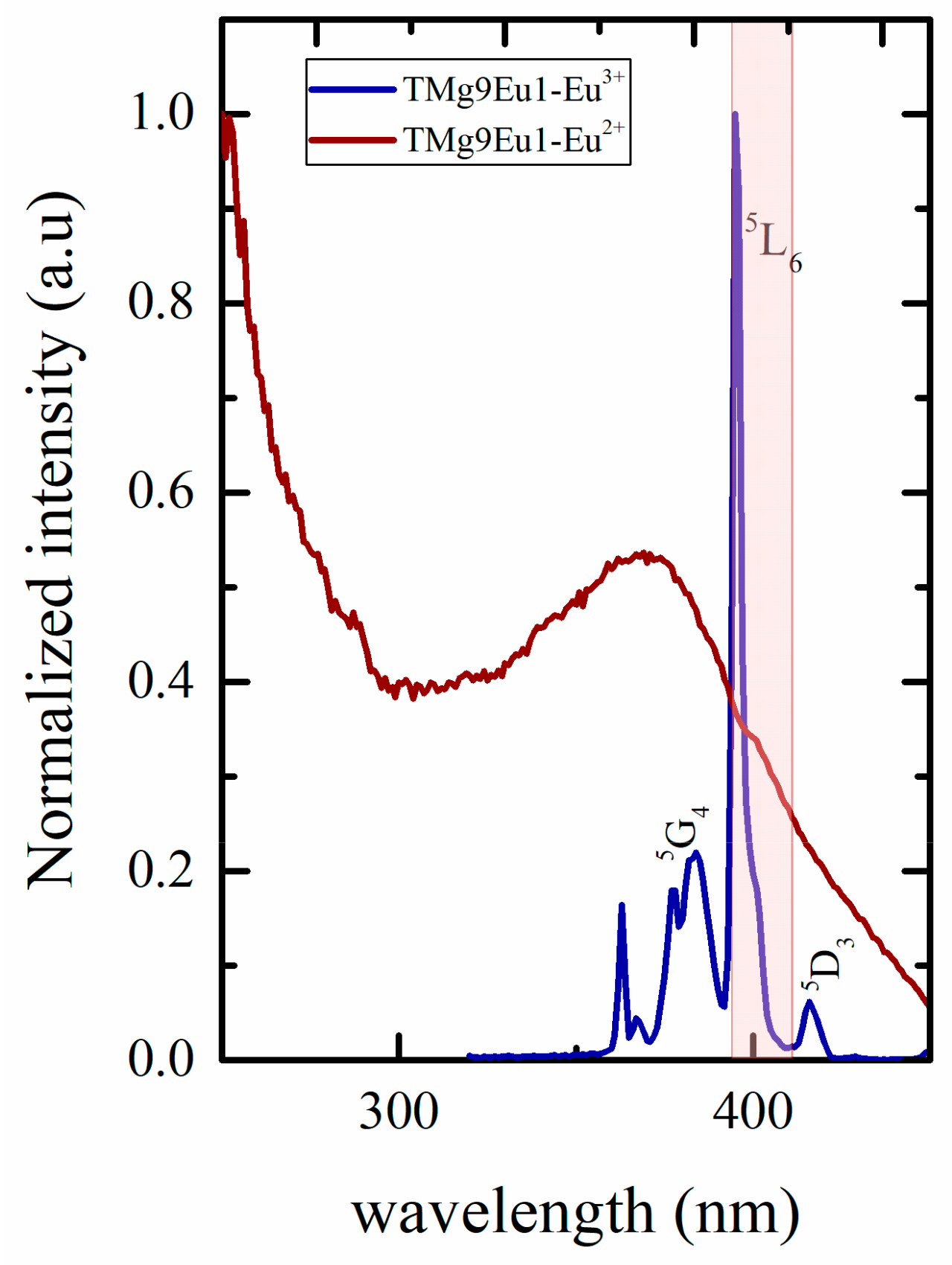
3.2.1. Photoluminescence of Eu3+
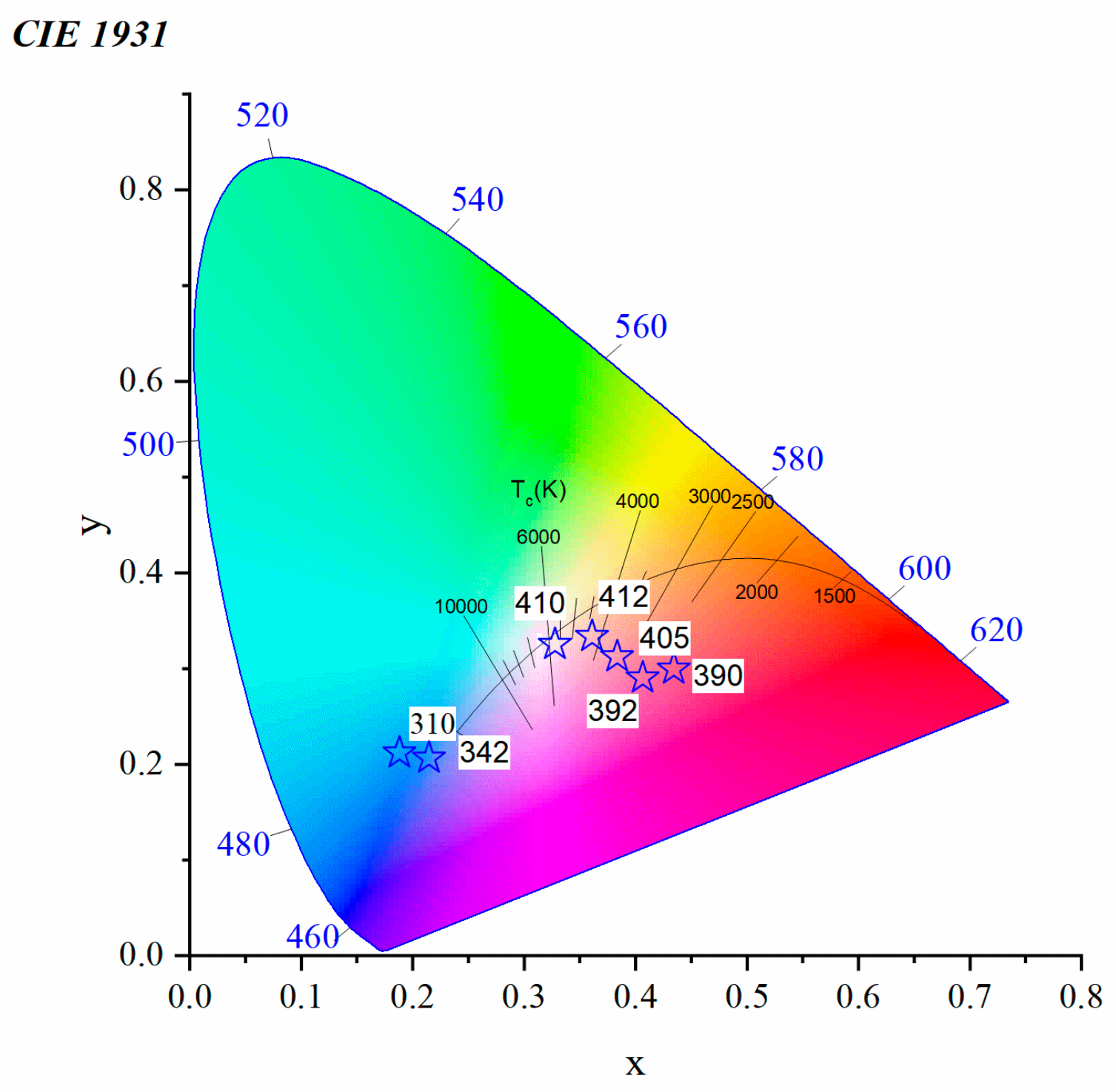
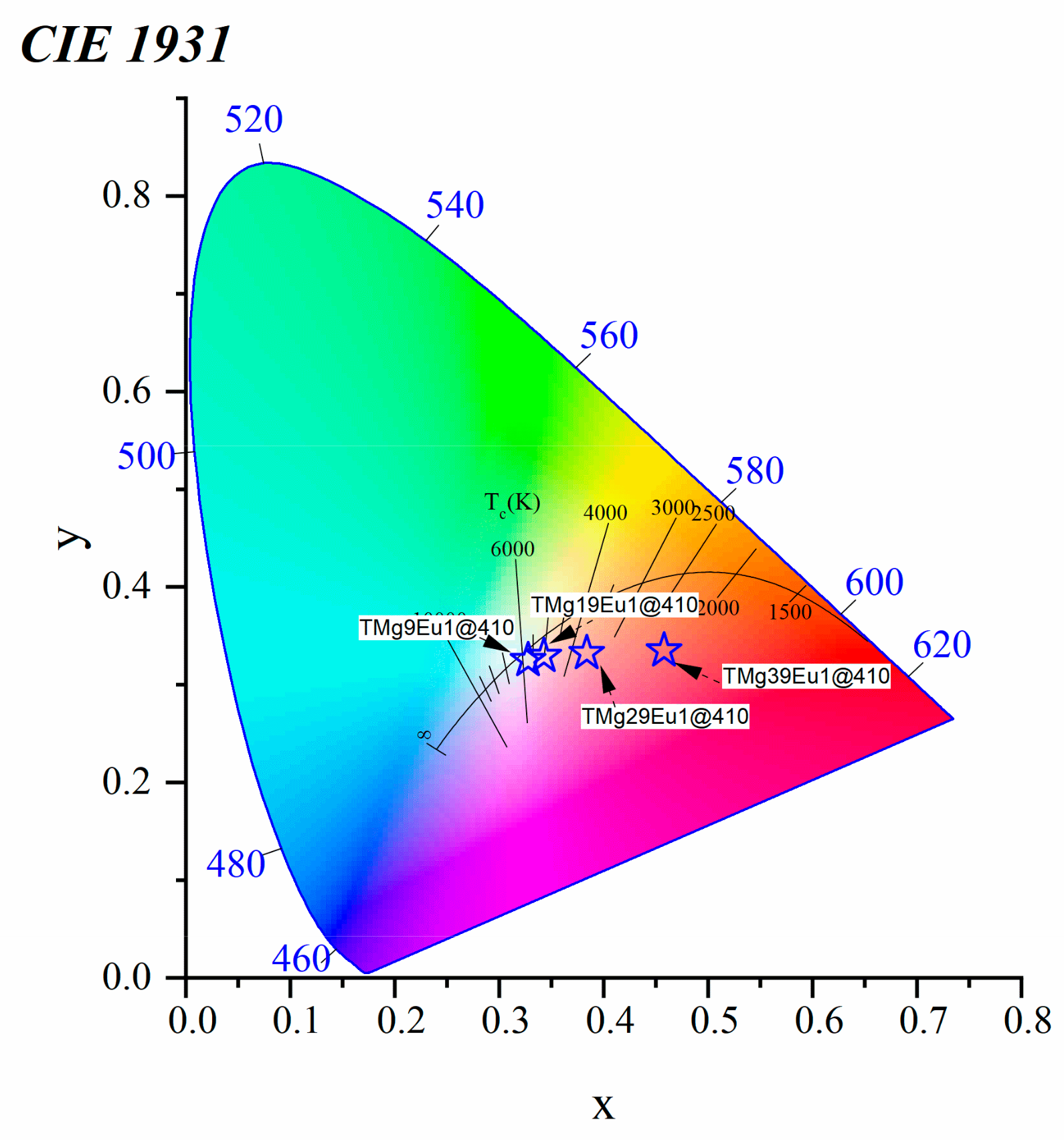
3.2.2. Toward White Light Emission
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rolli, R.; Montagna, M.; Chaussedent, S.; Monteil, A.; Tikhomirov, V.; Ferrari, M. Erbium-doped tellurite glasses with high quantum efficiency and broadband stimulated emission cross section at 1.5 μm. Opt. Mater. 2003, 21, 743–748. [Google Scholar] [CrossRef] [Green Version]
- Hirashima, H.; Ide, M.; Yoshida, T. Memory switching of V2O5TeO2 glasses. J. Non-Cryst. Solids 1986, 86, 327–335. [Google Scholar] [CrossRef]
- Chen, F.; Xu, T.; Dai, S.; Nie, Q.; Shen, X.; Zhang, J.; Wang, X. Linear and non-linear characteristics of tellurite glasses within TeO2–Bi2O3–TiO2 ternary system. Opt. Mater. 2010, 32, 868–872. [Google Scholar] [CrossRef]
- Kaur, A.; Khanna, A.; Pesquera, C.; González, F.; Sathe, V. Preparation and characterization of lead and zinc tellurite glasses. J. Non-Cryst. Solids 2010, 356, 864–872. [Google Scholar] [CrossRef]
- Mahato, K.; Rai, S.; Rai, A. Optical studies of Eu3 doped oxyfluoroborate glass. Spectrochim Acta A 2004, 60, 979–985. [Google Scholar] [CrossRef]
- Kumar, G.M.; Bhaktha, B.S.; Rao, D.N. Self-quenching of spontaneous emission in Sm3 doped lead-borate glass. Opt. Mater. 2006, 28, 1266–1270. [Google Scholar] [CrossRef]
- Man, S.; Pun, E.; Chung, P. Tellurite glasses for 1.3 μm optical amplifiers. Opt. Commun. 1999, 168, 369–373. [Google Scholar] [CrossRef]
- Driesen, K.; Lenaerts, P.; Binnemans, K.; Görller-Walrand, C. Influence of heat treatment on the intensities of f–f transitions in lanthanide-doped sol–gel glasses. Phys. Chem. Chem. Phys. 2002, 4, 552–555. [Google Scholar] [CrossRef]
- Othman, H.; Arzumanyan, G.; Möncke, D. The influence of different alkaline earth oxides on the structural and optical properties of undoped, Ce-doped, Sm-doped, and Sm/Ce co-doped lithium alumino-phosphate glasses. Opt. Mater. 2016, 62, 689–696. [Google Scholar] [CrossRef]
- Othman, H.A.; Elkholy, H.S.; Hager, I.Z. Spectroscopic investigation of samarium-doped lead oxyflouroborate glasses using photo and cathode luminescence. Int. J. Appl. Glass Sci. 2016, 8, 313–321. [Google Scholar] [CrossRef]
- Xia, Z.; Zhang, Y.; Molokeev, M.S.; Atuchin, V.V. Structural and Luminescence Properties of Yellow-Emitting NaScSi2O6:Eu2 Phosphors: Eu2 Site Preference Analysis and Generation of Red Emission by Codoping Mn2 for White-Light-Emitting Diode Applications. J. Phys. Chem. C 2013, 117, 20847–20854. [Google Scholar] [CrossRef]
- Reisfeld, R.; Velapoldi, R.A.; Boehm, L.; Ish-Shalom, M. Transition probabilities of europium in phosphate glasses. J. Phys. Chem. 1971, 75, 3980–3983. [Google Scholar] [CrossRef]
- Capobianco, J.A.; Proulx, P.P.; Bettinelli, M.; Negrisolo, F. Absorption and emission spectroscopy ofEu3 in metaphosphate glasses. Phys. Rev. B 1990, 42, 5936–5944. [Google Scholar] [CrossRef] [PubMed]
- Farias, A.M.; Sandrini, M.; Viana, J.R.M.; Baesso, M.L.; Bento, A.C.; Rohling, J.H.; Guyot, Y.; Ligny, D.D.; Nunes, L.A.O.; Gandra, F.G.; et al. Emission tunability and local environment in europium-doped OH−-free calcium aluminosilicate glasses for artificial lighting applications. Mater. Chem. Phys. 2015, 156, 214–219. [Google Scholar] [CrossRef]
- Prasad, V.R.; Babu, S.; Ratnakaram, Y.C. Luminescence performance of Eu3 doped lead free zinc phosphate glasses for red emission. In AIP Conference Proceedings, International Conference on Condensed Matter and Applied Physics (ICC 2015); American Institute of Physics (AIP Publishing): Melville, NY, USA, 2016; Volume 1728, p. 020394. [Google Scholar] [CrossRef]
- Deopa, N.; Kaur, S.; Prasad, A.; Joshi, B.; Rao, A. Spectral studies of Eu3 doped lithium lead alumino borate glasses for visible photonic applications. Opt. Laser Technol. 2018, 108, 434–440. [Google Scholar] [CrossRef]
- Divina, R.; Suthanthirakumar, P.; Naseer, K.A.; Marimuthu, K. Luminescence studies on Eu3 ions doped telluroborate glasses for photonic applications. Dae Solid State Phys. Symp. 2018 2019. [Google Scholar] [CrossRef]
- Elkholy, H.; Othman, H.; Hager, I.; Ibrahim, M.; de Ligny, D. Thermal and optical properties of binary magnesium tellurite glasses and their link to the glass structure. J. Alloys Compd 2019. (under review). [Google Scholar]
- Luo, Y.-R. Comprehensive Handbook of Chemical Bond Energies; CRC Press: Boca Raton, FL, USA; p. 2007. [CrossRef]
- Langes Handbook of Chemistry, 12th ed.; Edited by John A. Dean. McGraw-Hill: New York, NY 1978; Volume 10020. J. Pharm. Sci. 1979, 68, 805–806. [CrossRef]
- Babu, S.S.; Jang, K.; Cho, E.J.; Lee, H.; Jayasankar, C.K. Thermal, structural and optical properties of Eu3 -doped zinc-tellurite glasses. J. Phys. D Appl. Phys. 2007, 40, 5767–5774. [Google Scholar] [CrossRef]
- Rada, S.; Culea, M.; Culea, E. Structure of TeO2·B2O3 glasses inferred from infrared spectroscopy and DFT calculations. J. Non-Cryst. Solids 2008, 354, 5491–5495. [Google Scholar] [CrossRef]
- Noguera, O.; Merle-Méjean, T.; Mirgorodsky, A.; Smirnov, M.; Thomas, P.; Champarnaud-Mesjard, J.-C. Vibrational and structural properties of glass and crystalline phases of TeO2. J. Non-Cryst. Solids 2003, 330, 50–60. [Google Scholar] [CrossRef]
- Rada, S.; Dan, V.; Rada, M.; Culea, E. Gadolinium-environment in borate–tellurate glass ceramics studied by FTIR and EPR spectroscopy. J. Non-Cryst. Solids 2010, 356, 474–479. [Google Scholar] [CrossRef]
- Gowda, V.V.; Reddy, C.N.; Radha, K.; Anavekar, R.; Etourneau, J.; Rao, K. Structural investigations of sodium diborate glasses containing PbO, Bi2O3 and TeO2: Elastic property measurements and spectroscopic studies. J. Non-Cryst. Solids 2007, 353, 1150–1163. [Google Scholar] [CrossRef]
- Jha, A.; Shen, S.; Naftaly, M. Structural origin of spectral broadening of 1.5-μm emission inEr3 -doped tellurite glasses. Phys. Rev. B 2000, 62, 6215–6227. [Google Scholar] [CrossRef]
- Rada, S.; Culea, M.; Culea, E. Toward Modeling Phosphate Tellurate Glasses: The Devitrification and Addition of Gadolinium Ions Behavior. J. Phys. Chem. A 2008, 112, 11251–11255. [Google Scholar] [CrossRef]
- Rada, S.; Culea, E.; Culea, M. Gadolinium doping of vanadate-tellurate glasses and glass ceramics. J. Mater. Sci. 2008, 43, 6480–6485. [Google Scholar] [CrossRef]
- Azianty, S.; Yahya, A. Enhancement of elastic properties by WO3 partial replacement of TeO2 in ternary (80−x)TeO2–20PbO–xWO3 glass system. J. Non-Cryst. Solids 2013, 378, 234–240. [Google Scholar] [CrossRef]
- Mohamed, E.; Ahmad, F.; Aly, K. Effect of lithium addition on thermal and optical properties of zinc–tellurite glass. J. Alloys Compd. 2012, 538, 230–236. [Google Scholar] [CrossRef]
- Rakpanich, S.; Wantana, N.; Ruangtaweep, Y.; Kaewkhao, J. Eu3+ doped borosilicate glass for solid-state luminescence material. JMMM 2017, 27, 35–38. [Google Scholar] [CrossRef]
- Kumar, M.V.; Jamalaiah, B.; Gopal, K.R.; Reddy, R. Novel Eu3 -doped lead telluroborate glasses for red laser source applications. J. Solid State Chem. 2011, 184, 2145–2149. [Google Scholar] [CrossRef]
- Ivankov, A.; Seekamp, J.; Bauhofer, W. Optical properties of Eu3 -doped zinc borate glasses. J. Lumin. 2006, 121, 123–131. [Google Scholar] [CrossRef]
- Kaur, S.; Jayasimhadri, M.; Rao, A. A novel red emitting Eu3 doped calcium aluminozincate phosphor for applications in w-LEDs. J. Alloys Compd. 2017, 697, 367–373. [Google Scholar] [CrossRef]
- Herrmann, A.; Fibikar, S.; Ehrt, D. Time-resolved fluorescence measurements on Eu3 - and Eu2 -doped glasses. J. Non-Cryst. Solids 2009, 355, 2093–2101. [Google Scholar] [CrossRef]
- Polisadova, E. Effects of matrix composition and Eu3 concentration on luminescence properties of phosphate glass. Funct. Mater. 2013, 20, 290–294. [Google Scholar] [CrossRef] [Green Version]
- You, H.; Nogami, M. Optical Properties and Local Structure of Eu3 Ions in Sol–Gel TiO2–SiO2 Glasses. J. Phys. Chem. B 2004, 108, 12003–12008. [Google Scholar] [CrossRef]
- Linganna, K.; Jayasankar, C. Optical properties of Eu3 ions in phosphate glasses. Spectrochim. Acta A 2012, 97, 788–797. [Google Scholar] [CrossRef] [PubMed]
- Venkatramu, V.; Babu, P.; Jayasankar, C. Fluorescence properties of Eu3 ions doped borate and fluoroborate glasses containing lithium, zinc and lead. Spectrochim. Acta A 2006, 63, 276–281. [Google Scholar] [CrossRef]
- Babu, S.S.; Babu, P.; Jayasankar, C.; Sievers, W.; Tröster, T.; Wortmann, G. Optical absorption and photoluminescence studies of Eu3 -doped phosphate and fluorophosphate glasses. J. Lumin. 2007, 126, 109–120. [Google Scholar] [CrossRef]
- Swapna, K.; Mahamuda, S.; Rao, A.S.; Sasikala, T.; Packiyaraj, P.; Moorthy, L.R.; Prakash, G.V. Luminescence characterization of Eu 3 doped Zinc Alumino Bismuth Borate glasses for visible red emission applications. J. Lumin. 2014, 156, 80–86. [Google Scholar] [CrossRef]
- Maheshvaran, K.; Marimuthu, K. Concentration dependent Eu3 doped boro-tellurite glasses—Structural and optical investigations. J. Lumin. 2012, 132, 2259–2267. [Google Scholar] [CrossRef]
- Pisarski, W.; Pisarska, J.; Mączka, M.; Ryba-Romanowski, W. Europium-doped lead fluoroborate glasses: Structural, thermal and optical investigations. J. Mol. Struct. 2006, 792, 207–211. [Google Scholar] [CrossRef]
- Marimuthu, K.; Karunakaran, R.; Babu, S.S.; Muralidharan, G.; Arumugam, S.; Jayasankar, C. Structural and spectroscopic investigations on Eu3 -doped alkali fluoroborate glasses. J. Solid State Sci. 2009, 11, 1297–1302. [Google Scholar] [CrossRef]
- Duffy, J.A.; Harris, B.; Kamitsos, E.I.; Chryssikos, G.D.; Yiannopoulos, Y.D. Basicity Variation in Network Oxides: Distribution of Metal Ion Sites in Borate Glass Systems. J. Phys. Chem. B 1997, 101, 4188–4192. [Google Scholar] [CrossRef]
- Duffy, J.A. A review of optical basicity and its applications to oxidic systems. Geochim. Cosmochim. Acta 1993, 57, 3961–3970. [Google Scholar] [CrossRef]
- Dimitrov, V.; Sakka, S. Electronic oxide polarizability and optical basicity of simple oxides. I. J. Appl. Phys 1996, 79, 1736–1740. [Google Scholar] [CrossRef]
- Dimitrov, V.; Komatsu, T. Communication: Group optical basicity of tellurite glasses. Phys. Chem. Glasses-B 2011, 52, 138–141. [Google Scholar]
- Cicconi, M.R.; Veber, A.; Ligny, D.D.; Rocherullé, J.; Lebullenger, R.; Tessier, F. Chemical tunability of europium emission in phosphate glasses. J. Lumin. 2017, 183, 53–61. [Google Scholar] [CrossRef]
- Wang, C.; Peng, M.; Jiang, N.; Jiang, X.; Zhao, C.; Qiu, J. Tuning the Eu luminescence in glass materials synthesized in air by adjusting glass compositions. Mater. Lett. 2007, 61, 3608–3611. [Google Scholar] [CrossRef]


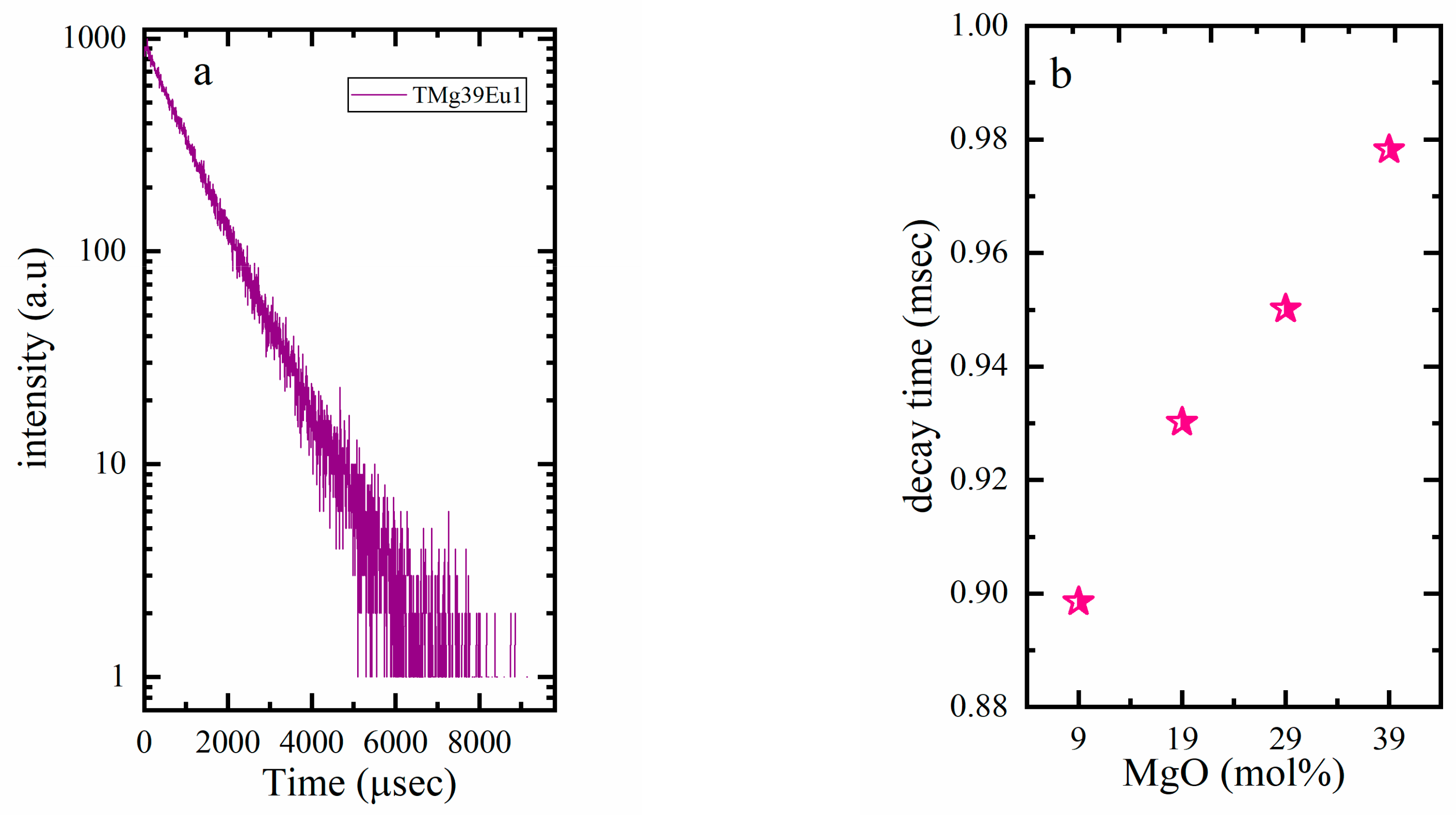


| Sample Key | Composition | Tg ± 1.5 °C | 1 Tg* ± 1.5 °C | ^th | Decay Rate (ms−1) |
|---|---|---|---|---|---|
| TMg9Eu1 | 90TeO2-9MgO-1Eu2O3 | 335 | 325 | 0.940 | 1.113 |
| TMg19Eu1 | 80TeO2-19MgO-1Eu2O3 | 360 | 354 | 0.925 | 1.075 |
| TMg29Eu1 | 70TeO2-29MgO-1Eu2O3 | 433 | 378 | 0.909 | 1.052 |
| TMg39Eu1 | 60TeO2-39MgO-1Eu2O3 | 452 | 450 | 0.890 | 1.022 |
| Sample Key | R (5D0→7F2/5D0→7F1) | Reference |
|---|---|---|
| TMg9Eu1 | 3.77 | Current study |
| TMg19Eu1 | 3.98 | Current study |
| TMg29Eu1 | 4.55 | Current study |
| TMg39Eu1 | 4.76 | Current study |
| PKSAEu10 | 4.43 | [38] |
| PbFBEu10 | 3.92 | [39] |
| PKBFAEu10 | 4.69 | [40] |
| LiPbAlBEu3 | 2.024 | [16] |
| LiPbAlBEu7 | 2.468 | [16] |
| ZnAlBiBEu0.1 | 1.951 | [41] |
| ZnAlBiBEu2.5 | 2.78 | [41] |
| BTeMgKEu1 | 4.12 | [42] |
| BTeMgKEu2 | 4.24 | [42] |
| BTeMgKEu3 | 4.14 | [42] |
| PbFBAlWEu1 | 2.30 | [43] |
| TeZnNaLiNbEu1 | 3.73 | [44] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elkholy, H.; Othman, H.; Hager, I.; Ibrahim, M.; de Ligny, D. Europium-Doped Tellurite Glasses: The Eu2+ Emission in Tellurite, Adjusting Eu2+ and Eu3+ Emissions toward White Light Emission. Materials 2019, 12, 4140. https://doi.org/10.3390/ma12244140
Elkholy H, Othman H, Hager I, Ibrahim M, de Ligny D. Europium-Doped Tellurite Glasses: The Eu2+ Emission in Tellurite, Adjusting Eu2+ and Eu3+ Emissions toward White Light Emission. Materials. 2019; 12(24):4140. https://doi.org/10.3390/ma12244140
Chicago/Turabian StyleElkholy, Hagar, Hosam Othman, Ibrahim Hager, Medhat Ibrahim, and Dominique de Ligny. 2019. "Europium-Doped Tellurite Glasses: The Eu2+ Emission in Tellurite, Adjusting Eu2+ and Eu3+ Emissions toward White Light Emission" Materials 12, no. 24: 4140. https://doi.org/10.3390/ma12244140
APA StyleElkholy, H., Othman, H., Hager, I., Ibrahim, M., & de Ligny, D. (2019). Europium-Doped Tellurite Glasses: The Eu2+ Emission in Tellurite, Adjusting Eu2+ and Eu3+ Emissions toward White Light Emission. Materials, 12(24), 4140. https://doi.org/10.3390/ma12244140





