Atom Probe Tomography Analysis of TiCx Powders Synthesized by SHS in Al/Fe/Cu–Ti–C Systems
Abstract
1. Introduction
2. Experimental Methods
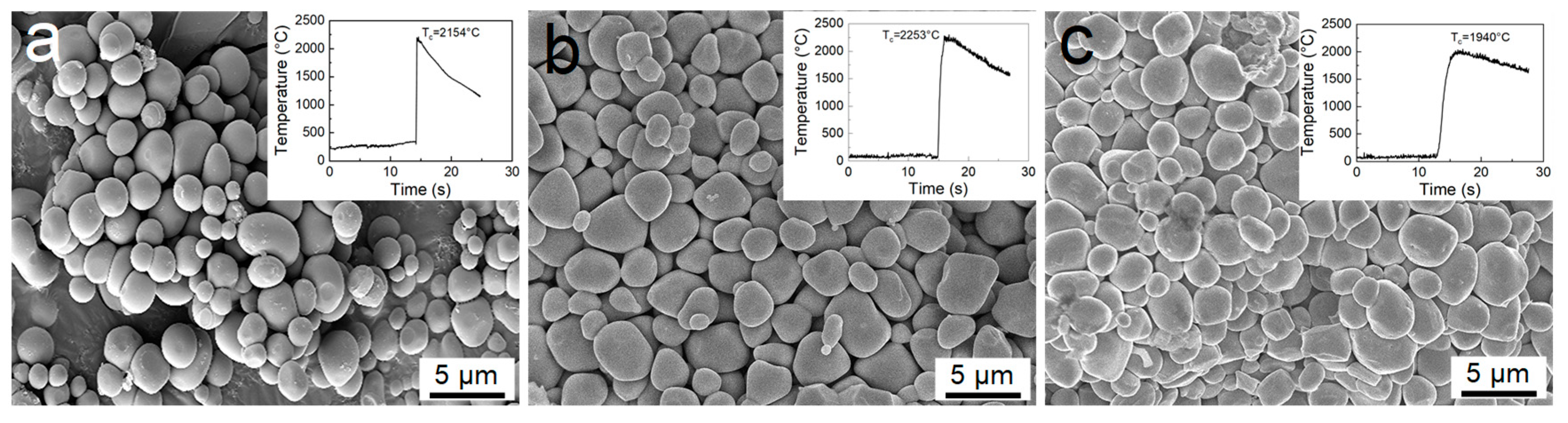
2.1. SHS Experiments and the Synthesized TiCx Powders
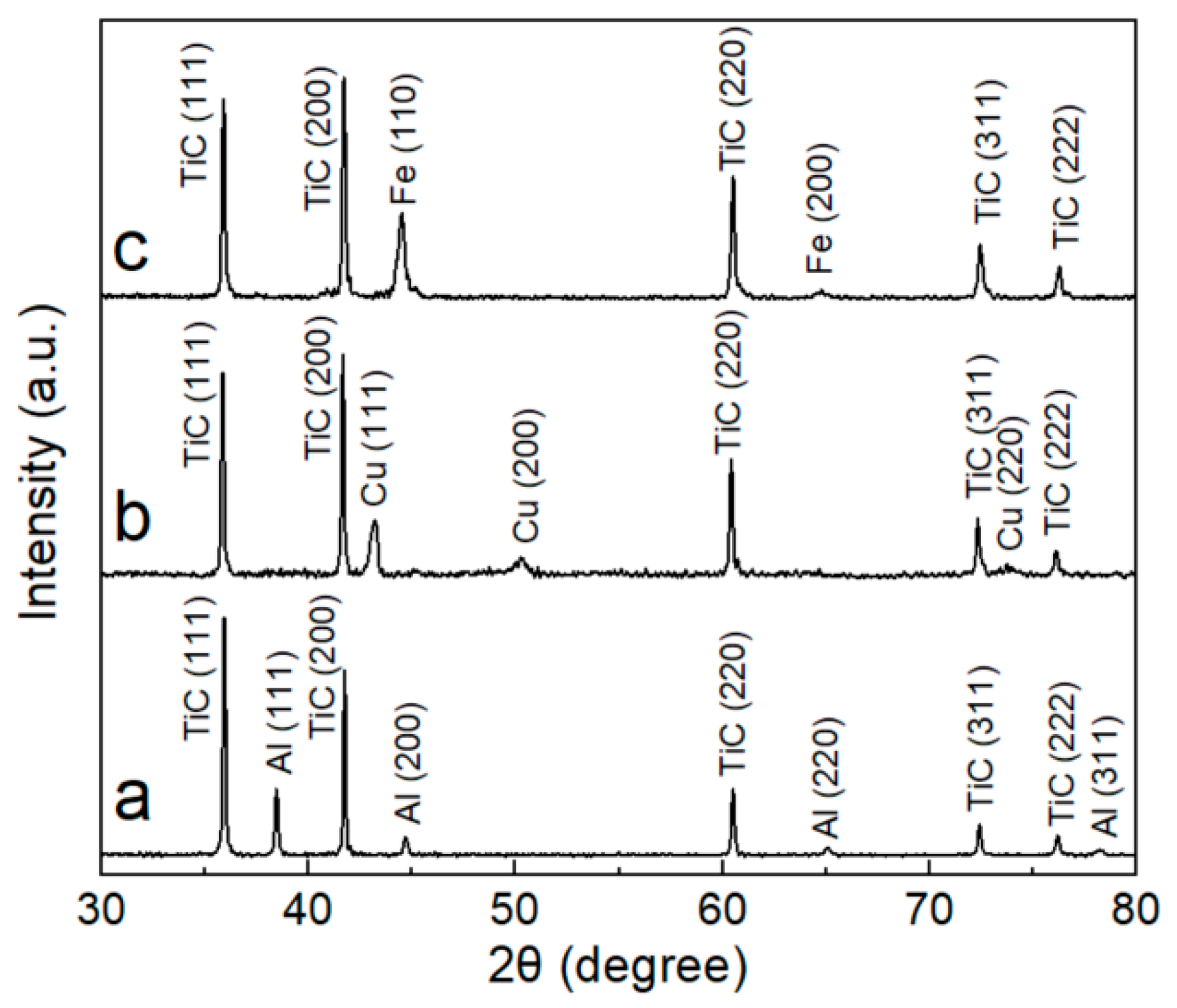
2.2. XRD Analysis
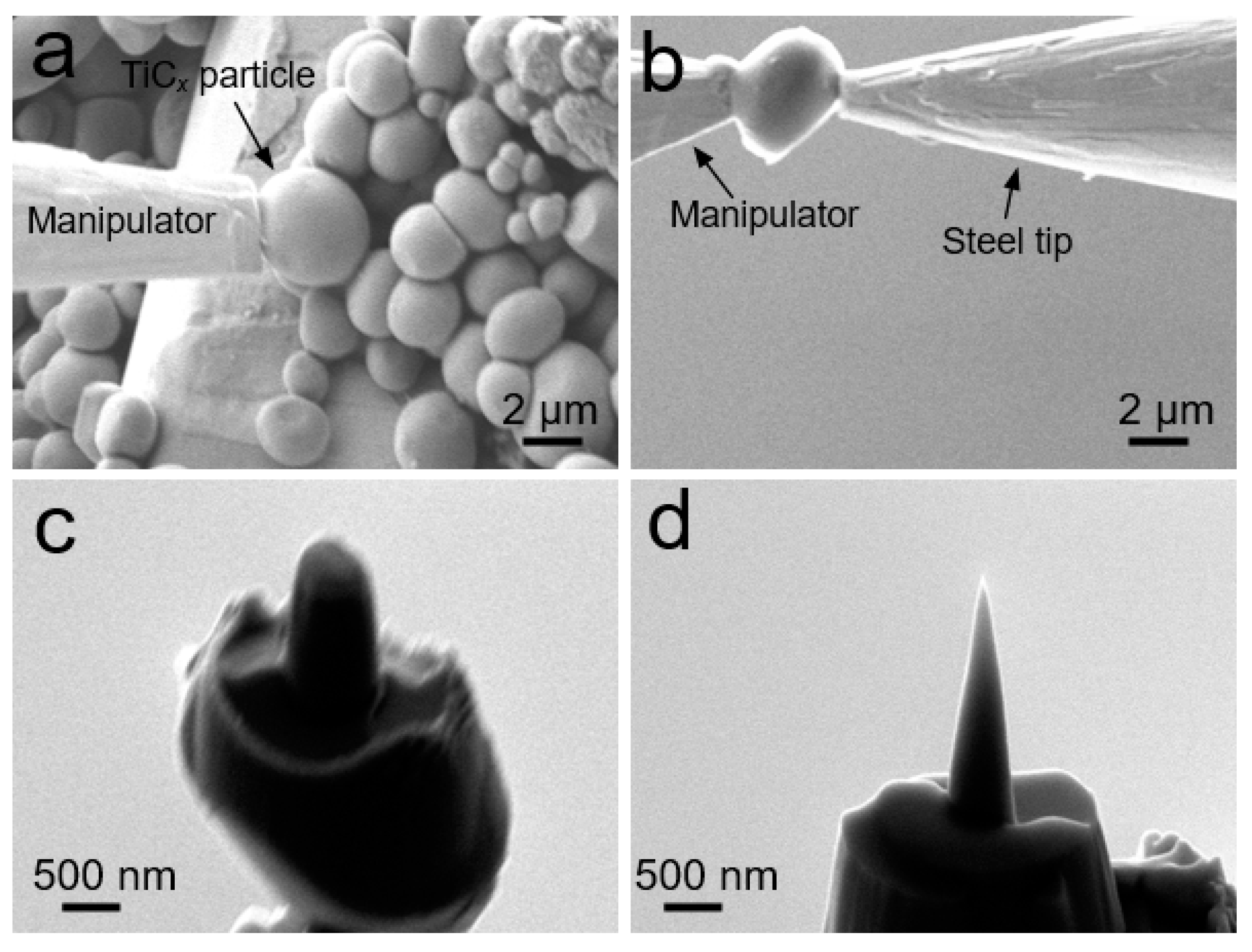
2.3. APT Specimen Preparation
2.4. APT Experiment and Data Analysis
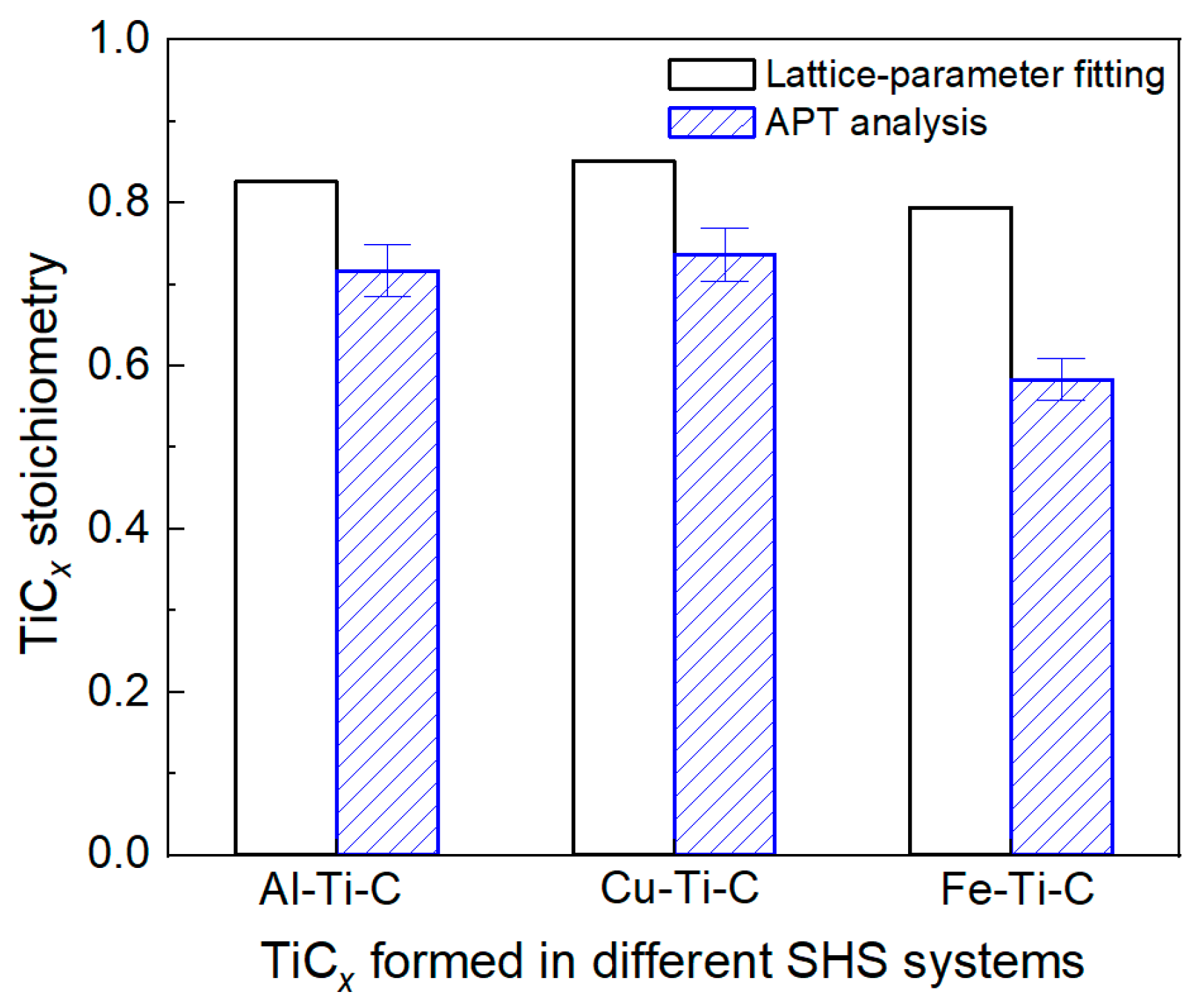
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Abkowitz, S.; Abkowitz, S.M.; Fisher, H.; Schwartz, P.J. CermeTi (R) discontinuously reinforced Ti-matrix composites: Manufacturing, properties, and applications. JOM 2004, 56, 37–41. [Google Scholar] [CrossRef]
- AlMangour, B.; Baek, M.S.; Grzesiak, D.; Lee, K.A. Strengthening of stainless steel by titanium carbide addition and grain refinement during selective laser melting. Mater. Sci. Eng. A 2018, 712, 812–818. [Google Scholar] [CrossRef]
- Boving, H.J.; Hintermann, H.E. Wear-resistant hard titanium carbide coatings for space applications. Tribol. Int. 1990, 23, 129–133. [Google Scholar] [CrossRef]
- Durlu, N. Titanium carbide based composites for high temperature applications. J. Eur. Ceram. Soc. 1999, 19, 2415–2419. [Google Scholar] [CrossRef]
- Parashivamurthy, K.I.; Kumar, R.K.; Seetharamu, S.; Chandrasekharaiah, M.N. Review on TiC reinforced steel composites. J. Mater. Sci. 2001, 36, 4519–4530. [Google Scholar] [CrossRef]
- Rajabi, A.; Ghazali, M.J.; Syarif, J.; Daud, A.R. Development and application of tool wear: A review of the characterization of TiC-based cermets with different binders. Chem. Eng. J. 2014, 255, 445–452. [Google Scholar] [CrossRef]
- Tjong, S.C.; Ma, Z.Y. Microstructural and mechanical characteristics of in situ metal matrix composites. Mater. Sci. Eng. R 2000, 29, 49–113. [Google Scholar] [CrossRef]
- Massalski, T.B.; Okamoto, H.; Subramanian, P.R.; Kacprzak, L. Binary Alloy Phase Diagrams, 2nd ed.; ASM International: Materials Park, OH, USA, 1990. [Google Scholar]
- Guemmaz, M.; Mosser, A.; Boudoukha, L.; Grob, J.J.; Raiser, D.; Sens, J.C. Ion beam synthesis of non-stoichiometric titanium carbide: Composition structure and nanoindentation studies. Nucl. Instrum. Methods Phys. Res. Sect. B 1996, 111, 263–270. [Google Scholar] [CrossRef]
- Lipatnikov, V.N.; Rempel, A.A.; Gusev, A.I. Atomic ordering and hardness of nonstoichiometric titanium carbide. Int. J. Refract. Met. H. 1997, 15, 61–64. [Google Scholar] [CrossRef]
- Fernandes, J.C.; Anjinho, C.; Amaral, P.M.; Rosa, L.ı.G.; Rodr´ıguez, J.; Mart´ınez, D.; Oliveira, F.A.C.; Shohoji, N. Characterisation of solar-synthesised TiCx (x = 0.50, 0.625, 0.75, 0.85, 0.90 and 1.0) by X-ray diffraction, density and Vickers microhardness. Mater. Chem. Phys. 2002, 77, 711–718. [Google Scholar] [CrossRef]
- Hugosson, H.W.; Korzhavyi, P.; Jansson, U.; Johansson, B.; Eriksson, O. Phase stabilities and structural relaxations in substoichiometric TiC1−x. Phys. Rev. B 2001, 63, 165116. [Google Scholar] [CrossRef]
- Lin, Q.; Shen, P.; Yang, L.; Jin, S.; Jiang, Q. Wetting of TiC by molten Al at 1123–1323K. Acta Mater. 2011, 59, 1898–1911. [Google Scholar] [CrossRef]
- Dudiy, S.V.; Lundqvist, B.I. Wetting of TiC and TiN by metals. Phys. Rev. B 2004, 69, 125421(1)–125421(16). [Google Scholar] [CrossRef]
- Frumin, N.; Frage, N.; Polak, M.; Dariel, M.P. Wettability and phase formation in the TiCx/Al system. Scr. Mater. 1997, 37, 1263–1267. [Google Scholar] [CrossRef]
- Hollander, H.E.J. Electrical conductivity and thermoelectric effect in single-crystal TiC. J. Appl. Phys. 1961, 32, 996–997. [Google Scholar] [CrossRef]
- Kumar, N.; Natarajan, G.; Dumpala, R.; Pandian, R.; Bahuguna, A.; Srivastava, S.K.; Ravindran, T.R.; Rajagopalan, S.; Dash, S.; Tyagi, A.K.; et al. Microstructure and phase composition dependent tribological properties of TiC/a-C nanocomposite thin films. Surf. Coat. Technol. 2014, 258, 557–565. [Google Scholar] [CrossRef]
- Yang, Y.F.; Jin, S.B.; Jiang, Q.C. Effect of reactant C/Ti ratio on the stoichiometry, morphology of TiCx and mechanical properties of TiCx–Ni composite. Crystengcomm 2013, 15, 852–855. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.; Wang, J.; Jiang, Q. Lattice parameter and stoichiometry of TiCx produced in alloyed Ti–C systems by self-propagating high-temperature synthesis. J. Am. Chem. Soc. 2008, 91, 3813–3816. [Google Scholar]
- Yang, Y.F.; Wang, H.Y.; Zhang, J.; Zhao, R.Y.; Liang, Y.H.; Jiang, Q.C. Lattice parameter and stoichiometry of TiCx produced in the Ti–C and Ni–Ti–C systems by self-propagating high-temperature synthesis. J. Am. Chem. Soc. 2008, 91, 2736–2739. [Google Scholar]
- Chen, Y.S.; Haley, D.; Gerstl, S.S.A.; London, A.J.; Sweeney, F.; Wepf, R.A.; Rainforth, W.M.; Bagot, P.A.J.; Moody, M.P. Direct observation of individual hydrogen atoms at trapping sites in a ferritic steel. Science 2017, 355, 1196–1199. [Google Scholar] [CrossRef]
- Danoix, F.; Bemont, E.; Maugis, P.; Blavette, D. Atom probe tomography I. Early stages of precipitation of NbC and NbN in ferritic steels. Adv. Eng. Mater. 2006, 8, 1202–1205. [Google Scholar] [CrossRef]
- Kolli, R.P.; Seidman, D.N. Co-precipitated and collocated carbides and Cu-rich precipitates in a Fe–Cu steel characterized by atom-probe tomography. Microsc. Microanal. 2014, 20, 1727–1739. [Google Scholar] [CrossRef] [PubMed]
- Seol, J.B.; Raabe, D.; Choi, P.; Park, H.S.; Kwak, J.H.; Park, C.G. Direct evidence for the formation of ordered carbides in a ferrite-based low-density Fe–Mn–Al–C alloy studied by transmission electron microscopy and atom probe tomography. Scr. Mater. 2013, 68, 348–353. [Google Scholar] [CrossRef]
- Takahashi, J.; Kawakami, K.; Kobayashi, Y.; Tarui, T. The first direct observation of hydrogen trapping sites in TiC precipitation-hardening steel through atom probe tomography. Scr. Mater. 2010, 63, 261–264. [Google Scholar] [CrossRef]
- Takahashi, J.; Kawakami, K.; Tarui, T. Direct observation of hydrogen-trapping sites in vanadium carbide precipitation steel by atom probe tomography. Scr. Mater. 2012, 67, 213–216. [Google Scholar] [CrossRef]
- Toji, Y.; Miyamoto, G.; Raabe, D. Carbon partitioning during quenching and partitioning heat treatment accompanied by carbide precipitation. Acta Mater. 2015, 86, 137–147. [Google Scholar] [CrossRef]
- Yao, M.J.; Dey, P.; Seol, J.B.; Choi, P.; Herbig, M.; Marceau, R.K.W.; Hickel, T.; Neugebauer, J.; Raabe, D. Combined atom probe tomography and density functional theory investigation of the Al off-stoichiometry of κ-carbides in an austenitic Fe–Mn–Al–C low density steel. Acta Mater. 2016, 106, 229–238. [Google Scholar] [CrossRef]
- Weidow, J. Atom probe tomography analysis of WC powder. Ultramicroscopy 2013, 132, 295–299. [Google Scholar] [CrossRef]
- Weidow, J.; Andren, H.O. Grain and phase boundary segregation in WC–Co with small V, Cr or Mn additions. Acta Mater. 2010, 58, 3888–3894. [Google Scholar] [CrossRef]
- Weidow, J.; Andren, H.O. Grain and phase boundary segregation in WC–Co with TiC, ZrC, NbC or TaC additions. Int. J. Refract. Met. Hard Mater. 2011, 29, 38–43. [Google Scholar] [CrossRef]
- Weidow, J.; Andren, H.O. APT analysis of WC-Co based cemented carbides. Ultramicroscopy 2011, 111, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Weidow, J.; Johansson, S.; Andren, H.O.; Wahnstrom, G. Transition Metal Solubilities in WC in Cemented Carbide Materials. J. Am. Ceram. Soc. 2011, 94, 605–610. [Google Scholar] [CrossRef]
- Yousfi, M.A.; Norgren, S.; Andren, H.O.; Falk, L.K.L. Chromium segregation at phase boundaries in Cr–doped WC–Co cemented carbides. Mater. Charact. 2018, 144, 48–56. [Google Scholar] [CrossRef]
- Jin, S.; Shen, P.; Zhou, D.; Jiang, Q. Self-propagating high-temperature synthesis of nano-TiCx particles with different shapes by using carbon nano-tube as C source. Nanoscale Res. Lett. 2011, 6, 515. [Google Scholar] [CrossRef] [PubMed]
- Angseryd, J.; Liu, F.; Andren, H.O.; Gerstl, S.S.; Thuvander, M. Quantitative APT analysis of Ti(C,N). Ultramicroscopy 2011, 111, 609–614. [Google Scholar] [CrossRef]
- Thuvander, M.; Weidow, J.; Angseryd, J.; Falk, L.K.; Liu, F.; Sonestedt, M.; Stiller, K.; Andren, H.O. Quantitative atom probe analysis of carbides. Ultramicroscopy 2011, 111, 604–608. [Google Scholar] [CrossRef]
- Storms, E.K. The Refractory Carbides; Academic Press: New York, NY, USA, 1967. [Google Scholar]
- Marques, L.S.A.; Fernandes, A.C.; Vaz, F.; Ramos, M.M.D. Influence of oxygen addition on the structural and elastic properties of tic thin films. Plasma Process Polym. 2007, 4, 195–199. [Google Scholar] [CrossRef][Green Version]
- Sundgren, J.E.; Johansson, B.O.; Hentzell, H.T.G.; Karlsson, S.E. Mechanisms of reactive sputtering of titanium nitride and titanium carbide III: Influence of substrate bias on composition and structure. Thin Solid Films 1983, 105, 385–393. [Google Scholar] [CrossRef]
- Shikama, T.; Shinno, H.; Fukutomi, M.; Fujitsuka, M.; Kitajima, M.; Okada, M. Properties of TixC1−x films coated on molybdenum by magnetron sputtering. Thin Solid Films 1983, 101, 233–242. [Google Scholar] [CrossRef]
- Jiang, C.C.; Goto, T.; Hirai, T. Preparation of titanium carbide plates by chemical vapour deposition. J. Mater. Sci. 1990, 25, 1086–1093. [Google Scholar] [CrossRef]
- Nickl, J.J.; Reiche, M.; Vesped, R. Chemical vapor deposition in the titanium-carbon system. In Proceedings of the 3rd International Conference on CVD, Salt Lake City, UT, USA, 24–27 April 1972. [Google Scholar]
- Cadoff, I.; Nielsen, J.P.; Miller, E. Properties of Arc-Melted vs. Powder Metallurgy Titanium Carbide; Plansee Proceedings: Pergamon, Turkey; Oxford, UK, 1955. [Google Scholar]
- JCPDS powder diffraction file, card no. 32-1383.
- Ramaekers, P.P.J.; Metselaar, R. Composition, Defect Structure and Bonding in Titanium Carbide, in SPECIAL CERAMICS 8; The Institute of Ceramics: Shelton, CT, USA; Stoke-on-Trent, UK, 1986. [Google Scholar]
- Rudy, E.; Harmon, D.P.; Brukl, C.E. Ternary Phase Equilibria in Transition Metal-Boron-Carbon-Silicon Systems. Part I. Related Binary Systems. Volume II. Ti-C and Zr-C system; Aerojet-General Corp.: Sacramento, CA, USA, 1965. [Google Scholar]
- Ehrlich, P. Über die binären systeme des titans mit den elementen stickstoff, kohlenstoff, bor und beryllium. Z. Anorg. Chem. 1949, 259, 1–41. [Google Scholar] [CrossRef]
- Waber, J.T.; Chiotti, P.; Miner, W.N. International Symposium on Compounds of Interest in Nuclear Reactor Technology; Metallurgical Society of the AIMMPE: Boulder, CO, USA, 1961. [Google Scholar]
- Bittner, H.; Goretzki, H. Magnetische untersuchungen der carbide TiC, ZrC, HfC, VC, NbC und TaC. Mon. Chem. Verwandte Teile Wiss. 1962, 93, 1000–1004. [Google Scholar] [CrossRef]
- Norton, J.T.; Lewis, R.K. Properties of Non-Stoichiometric Metallic Carbides Final Report; Advanced Metals Research Corporation: Somerville, MA, USA, April 1964. [Google Scholar]
- Rassaerts, H.; Benesovsky, F.; Nowotny, H. Investigation of the Ti– and Hf–Cr–C systems. Planseeber. Pulvermetall. 1966, 14, 23–28. [Google Scholar]
- Riaz, S.; Flower, H.M. Changes inγ–TiC stoichiometry during heat treatment of TiC reinforced Ti composites. Mater. Sci. Technol. 2013, 15, 1341–1348. [Google Scholar] [CrossRef]
- Gringoz, A.; Glandut, N.; Valette, S. Electrochemical hydrogen storage in TiC0.6, not in TiC0.9. Electrochem. Commun. 2009, 11, 2044–2047. [Google Scholar] [CrossRef]
- Miracle, D.B.; Lipsitt, H.A. Mechanical properties of fine-grained substoichiomebic titanium carbide. J. Am. Ceram. Soc. 1983, 66, 592–597. [Google Scholar] [CrossRef]
- Guemmaz, M.; Moraitis, G.; Mosser, A.; Khan, M.A.; Parlebasb, J.C. Experimental and theoretical study of the electronic structure of ion-beam-synthesized substoichiometric titanium carbides. J. Electron. Spectrosc. 1997, 83, 173–179. [Google Scholar] [CrossRef]
- Nguyen, J.; Glandut, N.; Jaoul, C.; Lefort, P. Hydrogen insertion in substoichiometric titanium carbide. Int. J. Hydrog. Energy 2015, 40, 8562–8570. [Google Scholar] [CrossRef]
- Vasanthakumar, K.; Bakshi, S.R. Effect of C/Ti ratio on densification, microstructure and mechanical properties of TiCx prepared by reactive spark plasma sintering. Ceram. Int. 2018, 44, 484–494. [Google Scholar] [CrossRef]
- Wolfe, D.E.; Singh, J. Titanium carbide coatings deposited by reactive ion beam-assisted, electron beam-physical vapor deposition. Surf. Coat. Technol. 2000, 124, 142–153. [Google Scholar] [CrossRef]
- Holt, J.B.; Munir, Z.A. Combustion synthesis of titanium carbide: Theory and experiment. J. Mater. Sci. 1986, 21, 251–259. [Google Scholar] [CrossRef]
- Frage, N.; Froumin, N.; Dariel, M.P. Wetting of TiC by non-reactive liquid metals. Acta Mater. 2002, 50, 237–245. [Google Scholar] [CrossRef]
- Zarrinfar, N.; Shipway, P.H.; Kennedy, A.R.; Saidi, A. Carbide stoichiometry in TiCx and Cu–TiCx produced by self-propagating high-temperature synthesis. Scr. Mater. 2002, 46, 121–126. [Google Scholar] [CrossRef]
- Sarian, S. Diffusion of 44Ti in TiCx. J. Appl. Phys. 1969, 40, 3515–3520. [Google Scholar] [CrossRef]
- Guemmaz, M.; Mosser, A.; Ahujab, R.; Johansson, B. Elastic properties of sub-stoichiometric titanium carbides Comparison of FP-LMTO calculations and experimental results. Solid State Commun. 1999, 110, 299–303. [Google Scholar] [CrossRef]
- Moisy-Maurice, V.; Lorenzelli, N.; Novion, C.H.D.; Convert, P. High temperature neutron diffraction study of the order-disorder transition in TiC1−x. Acta Metall. 1982, 30, 1769–1779. [Google Scholar] [CrossRef]
- Dunand, A.; Flack, H.D.; Yvon, K. Bonding study of TiC and TiN. I. High-precision x-ray-diffraction determination of the valence-electron density distribution, Debye-Waller temperature factors, and atomic static displacements in TiC0.94 and TiN0.99. Phys Rev B Condens Matter 1985, 31, 2299–2315. [Google Scholar] [CrossRef]
- Ordan’yan, S.S.; Tabatadze, G.S.; Kozlovskii, L.V. Compaction of nonstoichiometric titanium carbide in sintering. Poroshk. Metall. 1979, 7, 43–47. [Google Scholar]
- Dridi, Z.; Bouhafs, B.; Ruterana, P.; Aourag, H. First-principles calculations of vacancy effects on structural and electronic properties of TiCx and TiNx. J. Phys-Condens. Mat. 2002, 14, 10237–10249. [Google Scholar] [CrossRef]
- Ahuja, R.; Eriksson, O.; Wills, J.M.; Johansson, B. Structural, elastic, and high-pressure properties of cubic TiC, TiN, and TiO. Phys. Rev. B: Condens. Matter. 1996, 53, 3072–3079. [Google Scholar] [CrossRef]
- Grossman, J.C.; Mizel, A.; Cote, M.; Cohen, M.L.; Louie, S.G. Transition metals and their carbides and nitrides: Trends in electronic and structural properties. Phys. Rev. B 1999, 60, 6343–6347. [Google Scholar] [CrossRef]
- Jhi, S.; Ihm, J. Electronic structure and structural stability of TiCxN1−x alloys. Phys. Rev. B 1997, 56, 13826–13829. [Google Scholar] [CrossRef]
- Sha, W.; Chang, L.; Smith, G.D.W.; Cheng, L.; Mittemeijer, E.J. Some aspects of atom-probe analysis of Fe–C and Fe–N systems. Surf. Sci. 1992, 266, 416–423. [Google Scholar] [CrossRef]
- Kerans, R.J.; Mazdiyasni, K.S.; Ruh, R.; Lipsitt, H.A. Solubility of metals in substoichiometric TiC1−x. J. Am. Ceram. Soc. 1984, 67, 34–38. [Google Scholar] [CrossRef]
- Yu, R.; He, L.L.; Ye, H.Q. Effects of Si and Al on twin boundary energy of TiC. Acta Mater. 2003, 51, 2477–2484. [Google Scholar] [CrossRef]

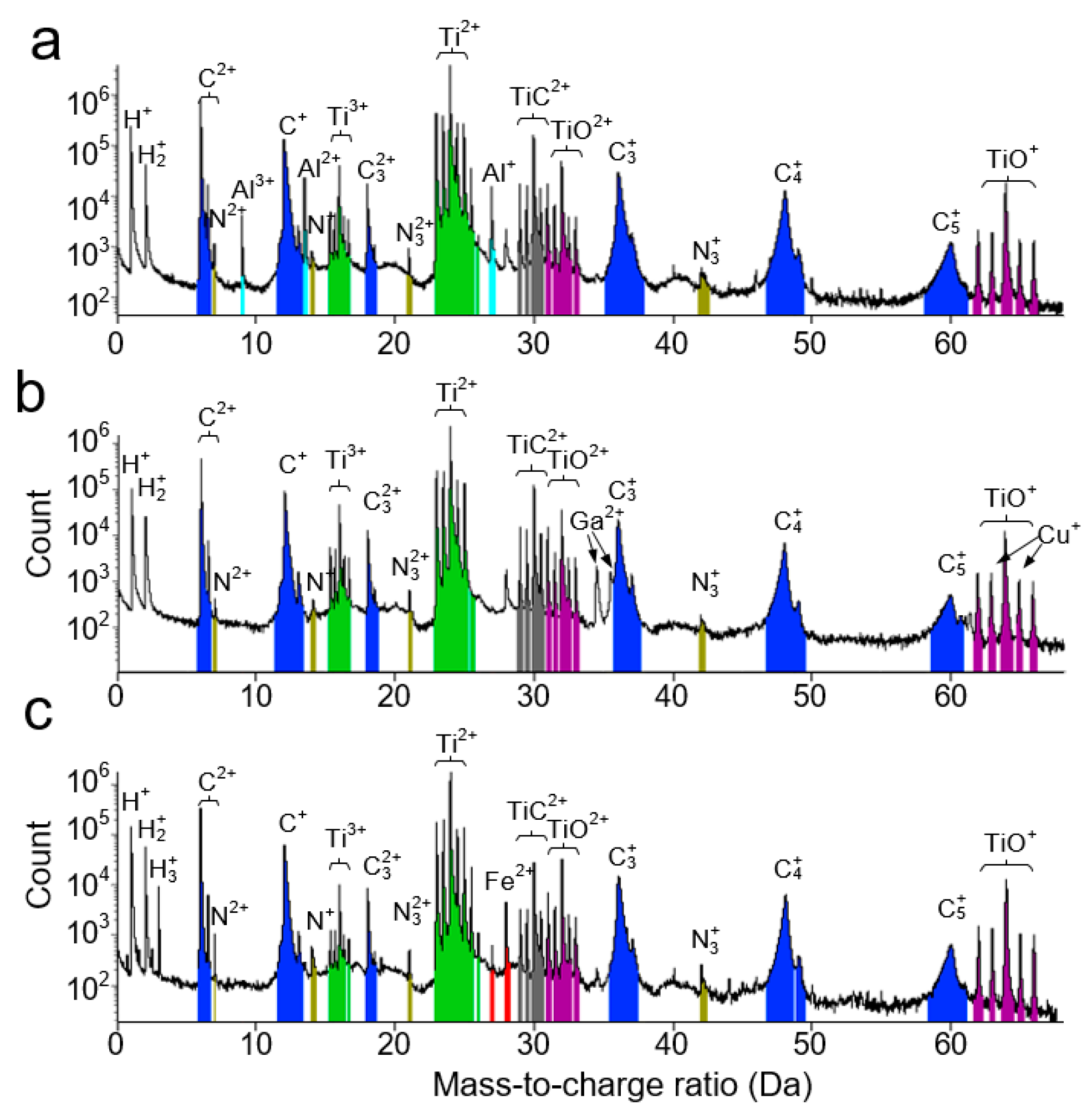
| Ti | C | O | N | Al | Cu | Fe | |
|---|---|---|---|---|---|---|---|
| TiCx in Al–Ti–C | 57 ± 1.0 | 39 ± 1.1 | 3.12 ± 0.06 | 0.073 ± 0.001 | 0.48 ± 0.01 | - | - |
| TiCx in Cu–Ti–C | 58 ± 1.0 | 41 ± 1.1 | 1.86 ± 0.01 | 0.052 ± 0.001 | - | 0.012 ± 0.001 | - |
| TiCx in Fe–Ti–C | 62 ± 1.0 | 35 ± 1.0 | 2.41 ± 0.04 | 0.185 ± 0.003 | - | 0.18 ± 0.01 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, S.; Su, H.; Sha, G. Atom Probe Tomography Analysis of TiCx Powders Synthesized by SHS in Al/Fe/Cu–Ti–C Systems. Materials 2019, 12, 4095. https://doi.org/10.3390/ma12244095
Jin S, Su H, Sha G. Atom Probe Tomography Analysis of TiCx Powders Synthesized by SHS in Al/Fe/Cu–Ti–C Systems. Materials. 2019; 12(24):4095. https://doi.org/10.3390/ma12244095
Chicago/Turabian StyleJin, Shenbao, Haokai Su, and Gang Sha. 2019. "Atom Probe Tomography Analysis of TiCx Powders Synthesized by SHS in Al/Fe/Cu–Ti–C Systems" Materials 12, no. 24: 4095. https://doi.org/10.3390/ma12244095
APA StyleJin, S., Su, H., & Sha, G. (2019). Atom Probe Tomography Analysis of TiCx Powders Synthesized by SHS in Al/Fe/Cu–Ti–C Systems. Materials, 12(24), 4095. https://doi.org/10.3390/ma12244095




