Microstructure and Pitting Corrosion of Austenite Stainless Steel after Crack Arrest
Abstract
1. Introduction
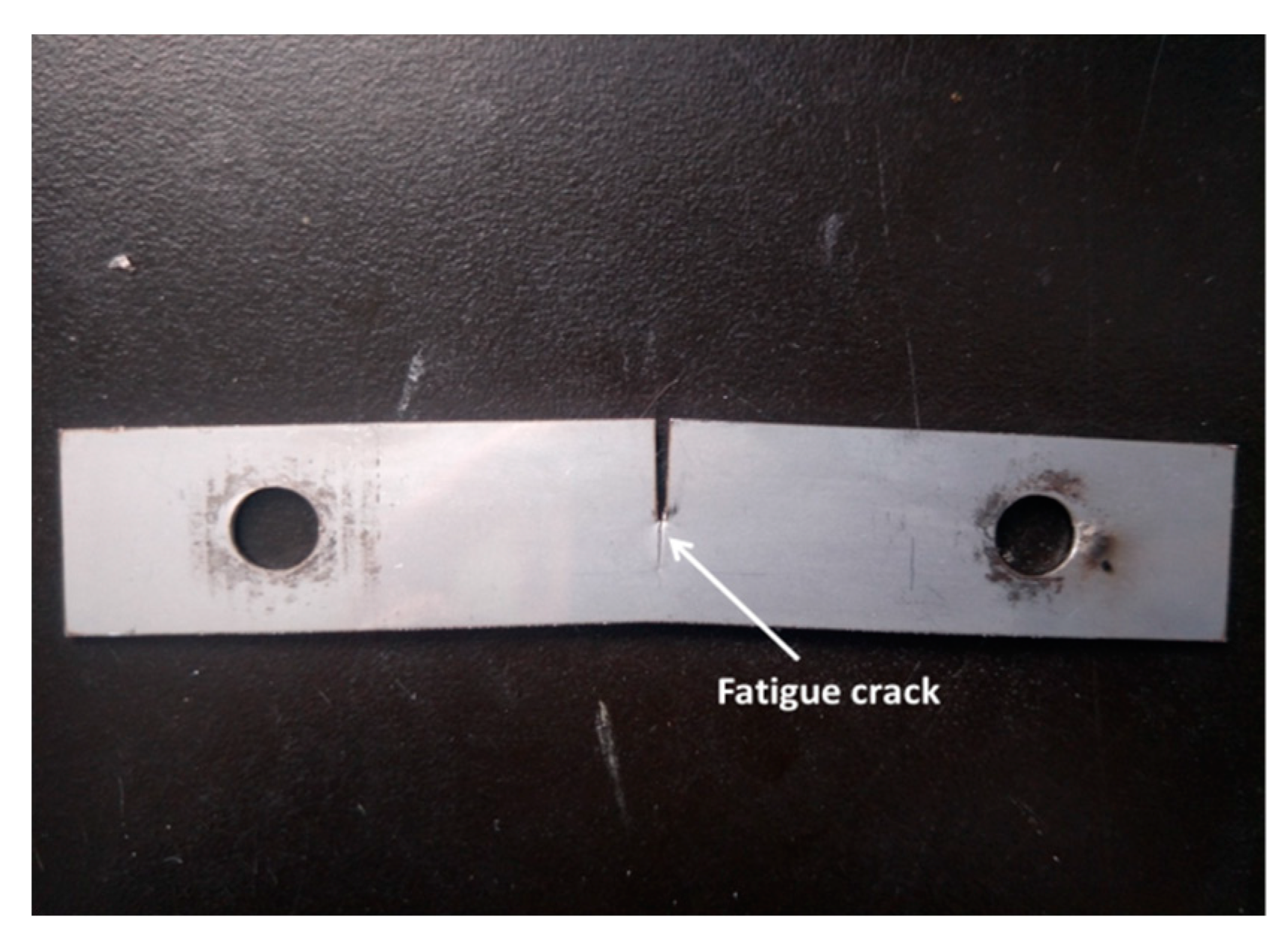
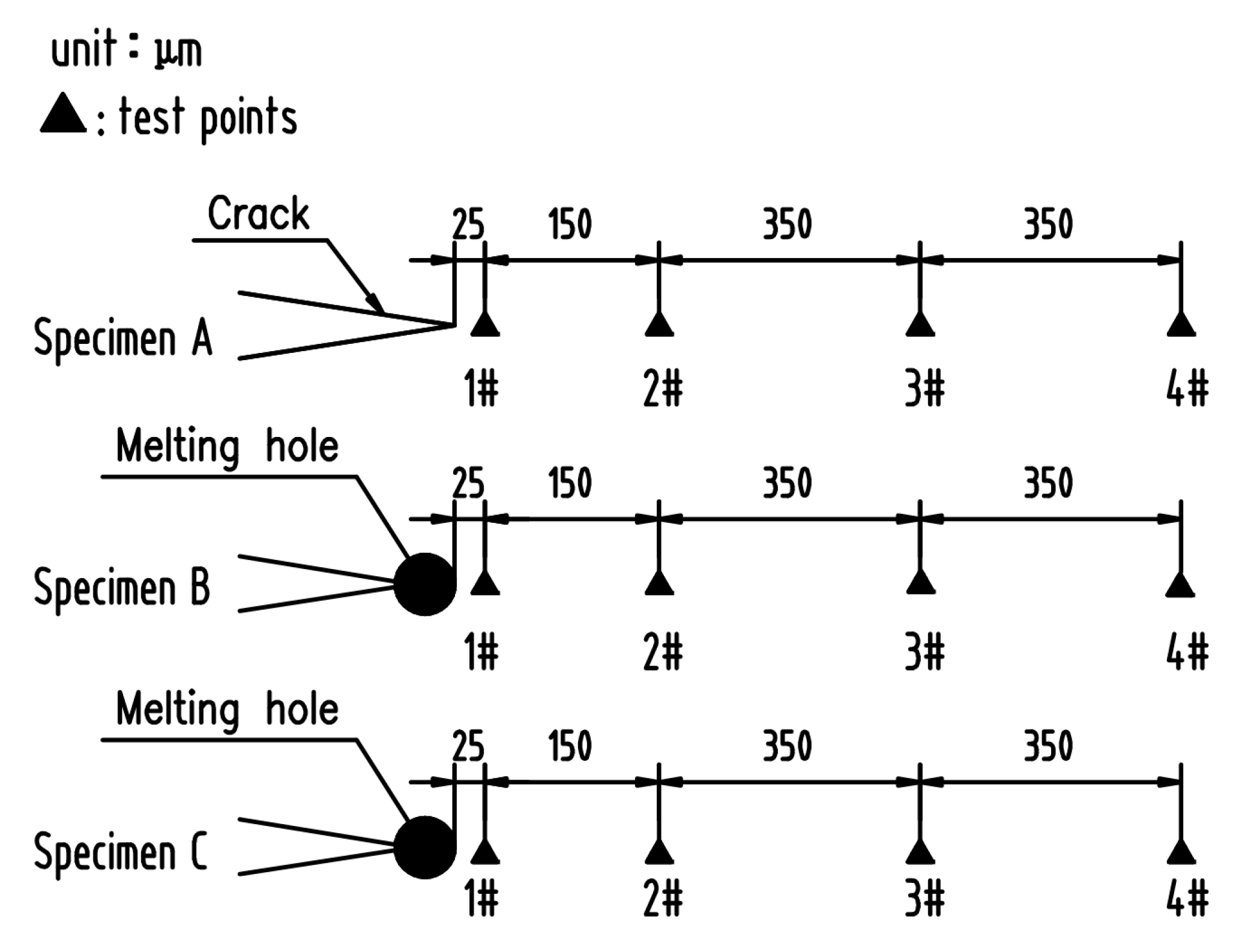
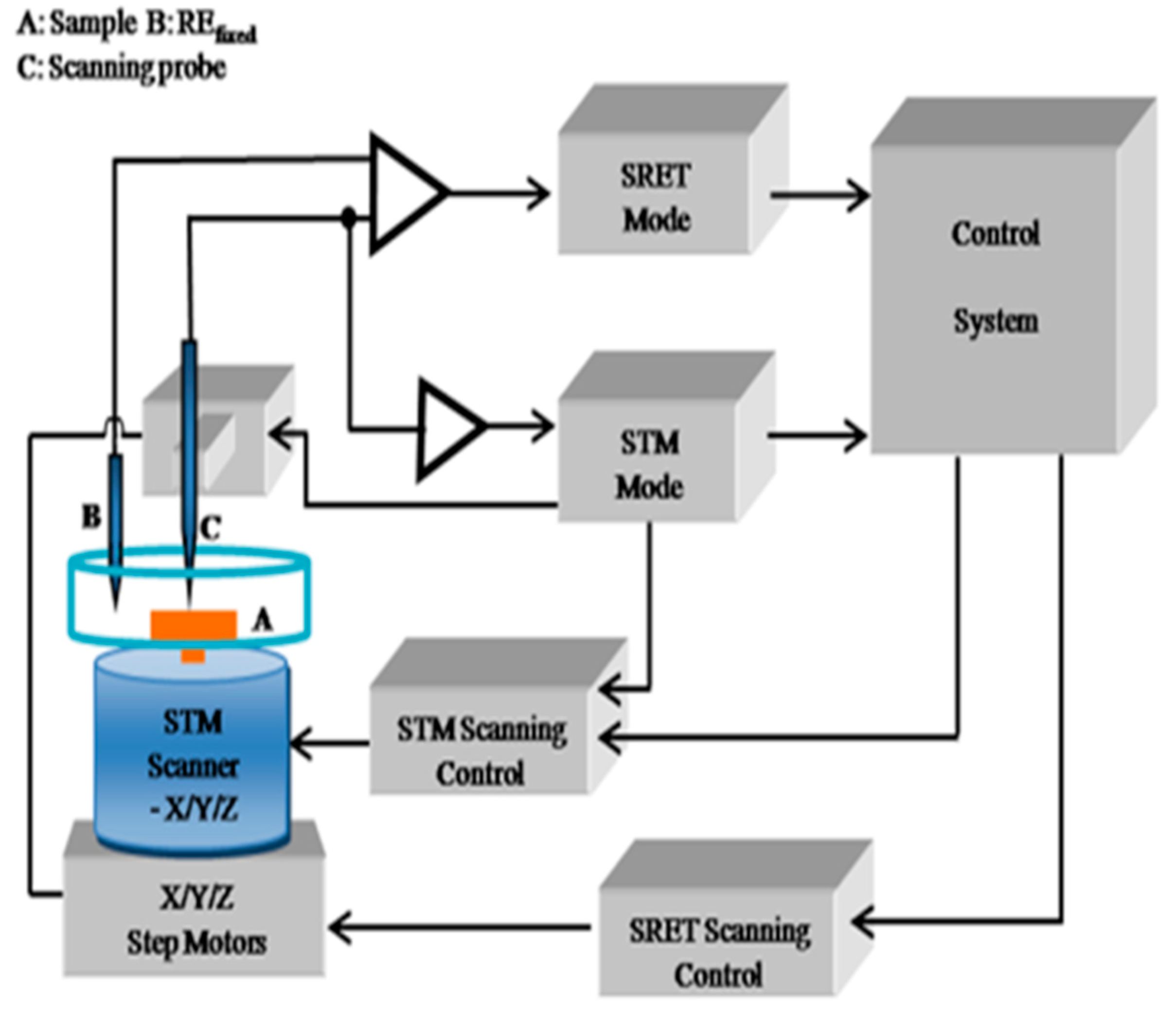
2. Materials and Methods
3. Results and Discussion
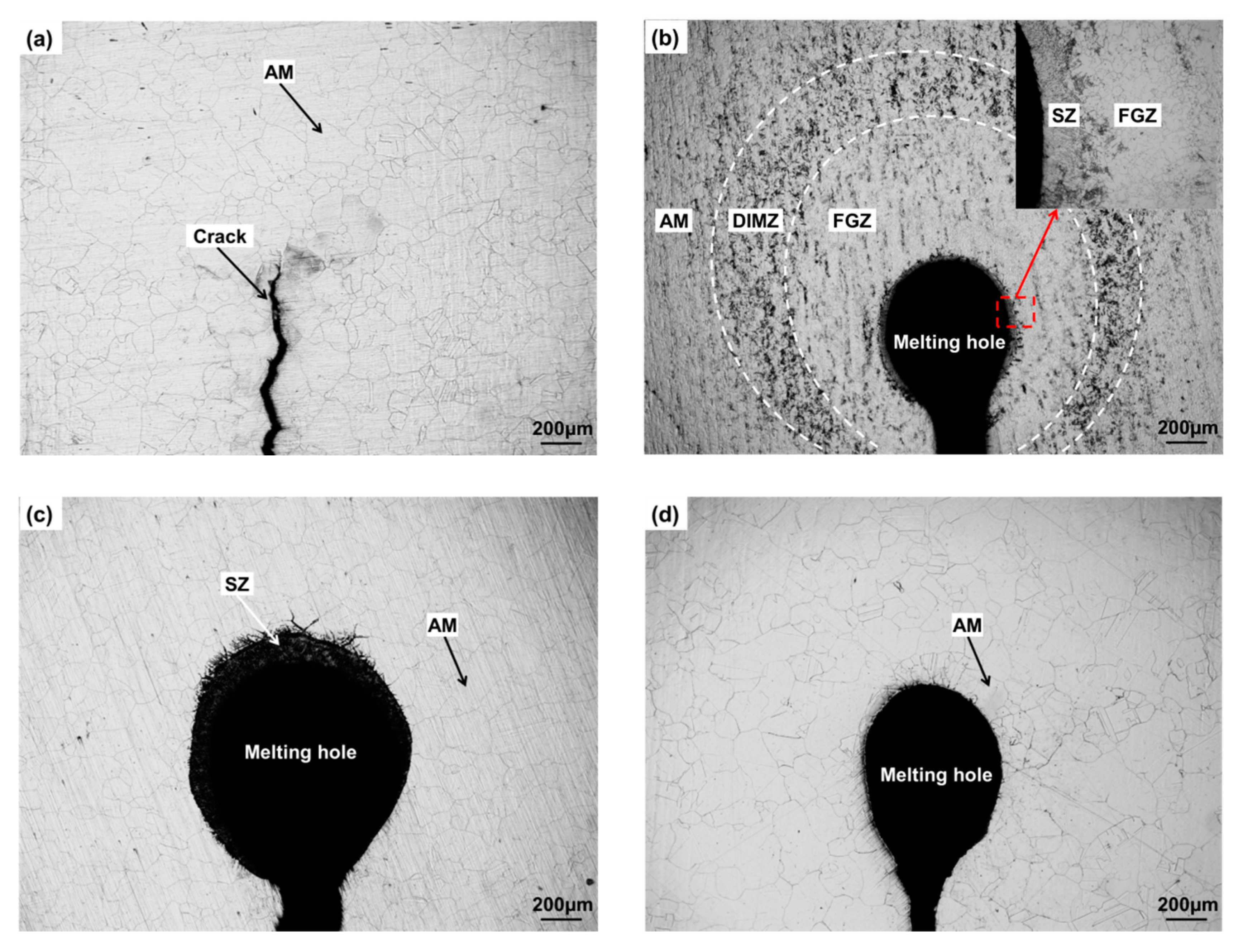
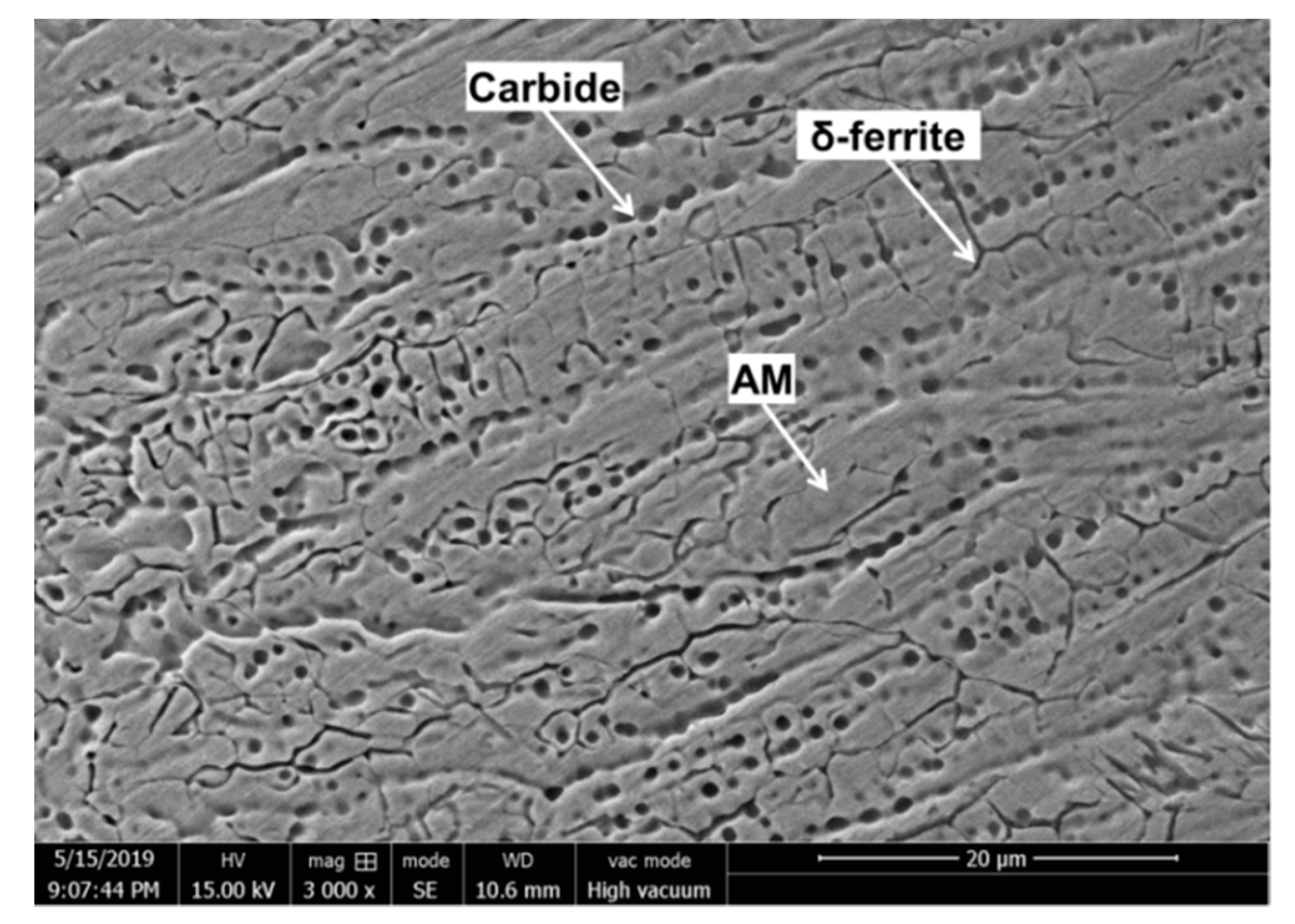
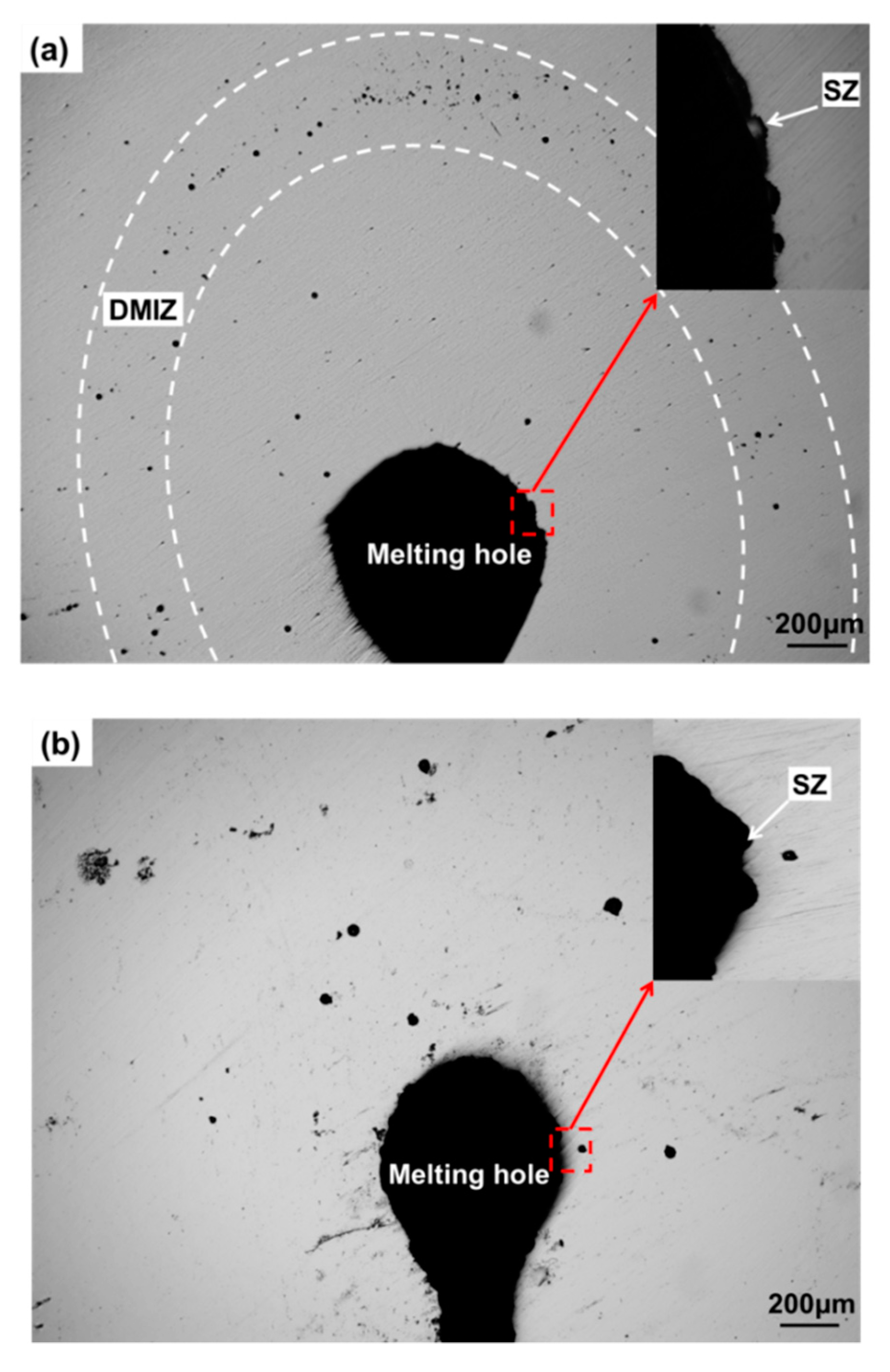
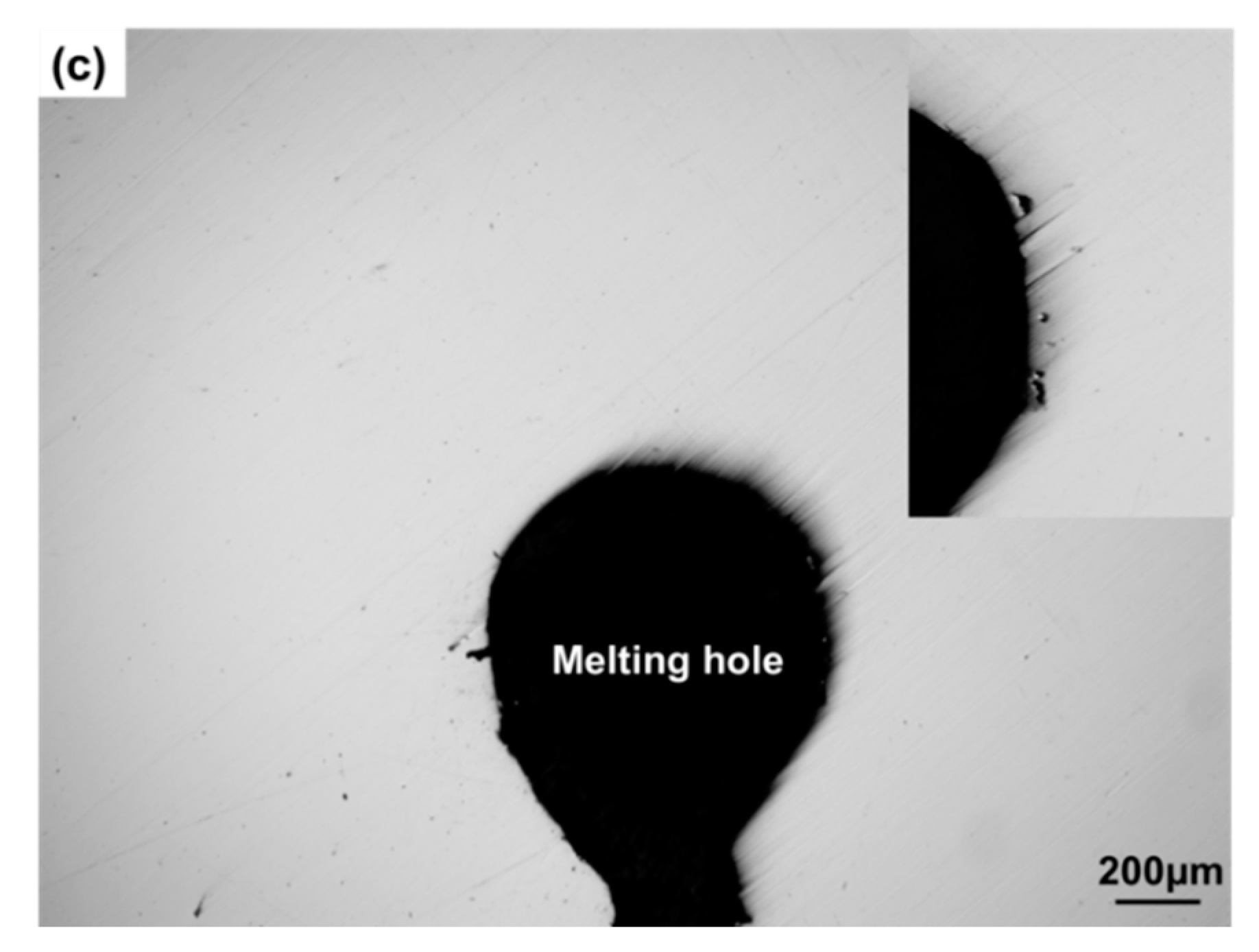
3.1. Analysis of Microstructure Morphology near Crack Tip or Melting Hole
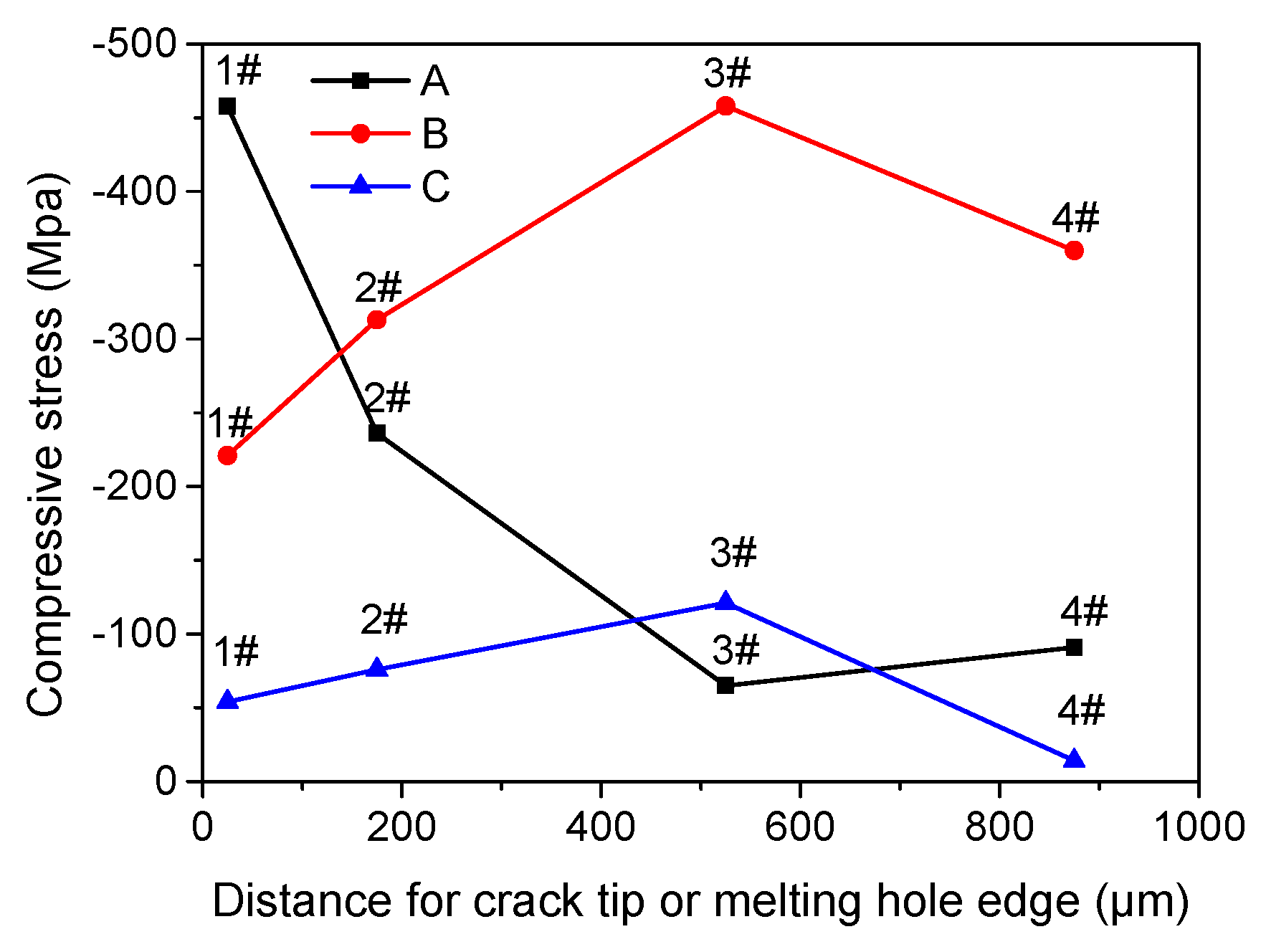
3.2. Residual Stress near Crack Tip or Melting Hole Using Nano-indentation
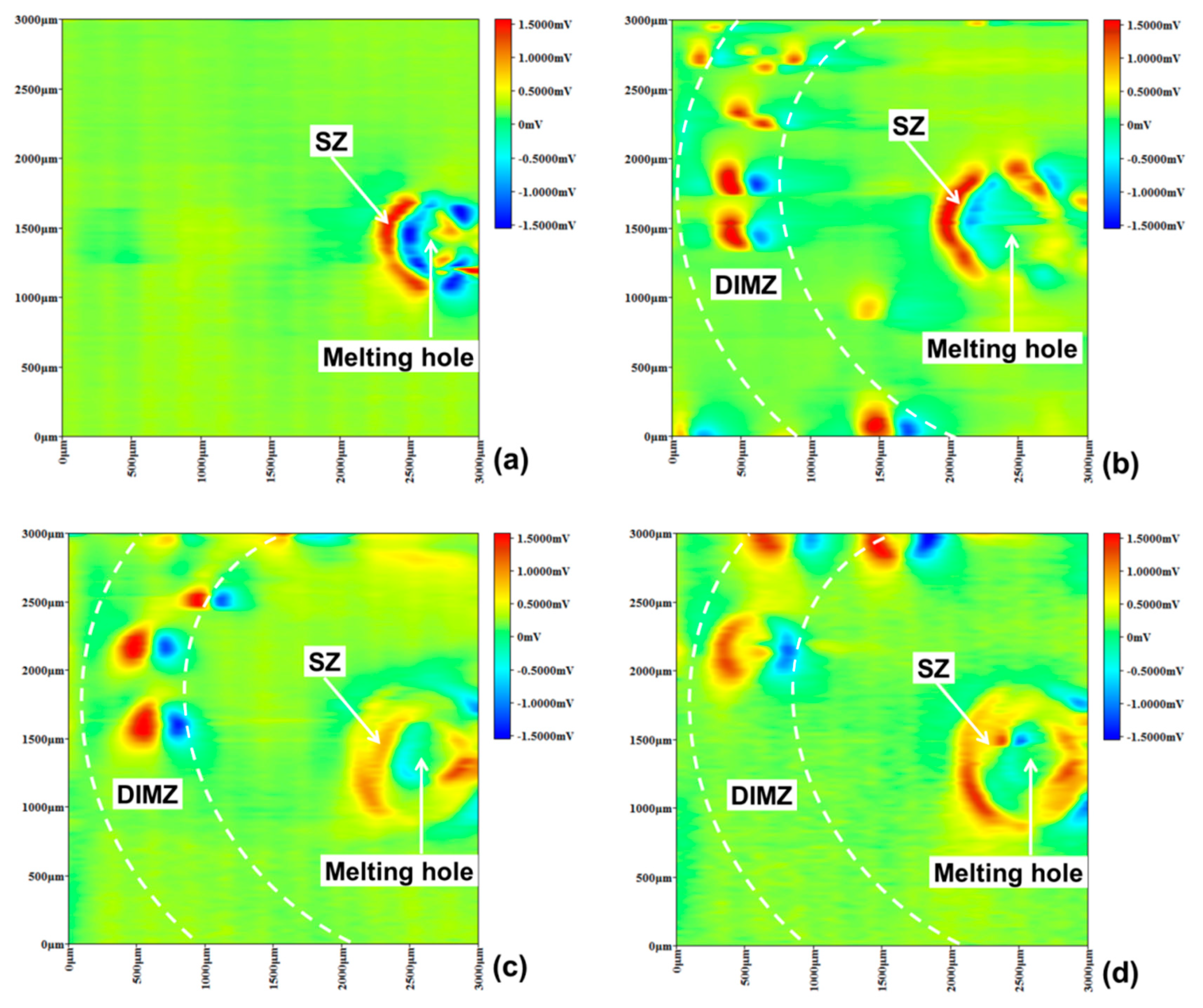
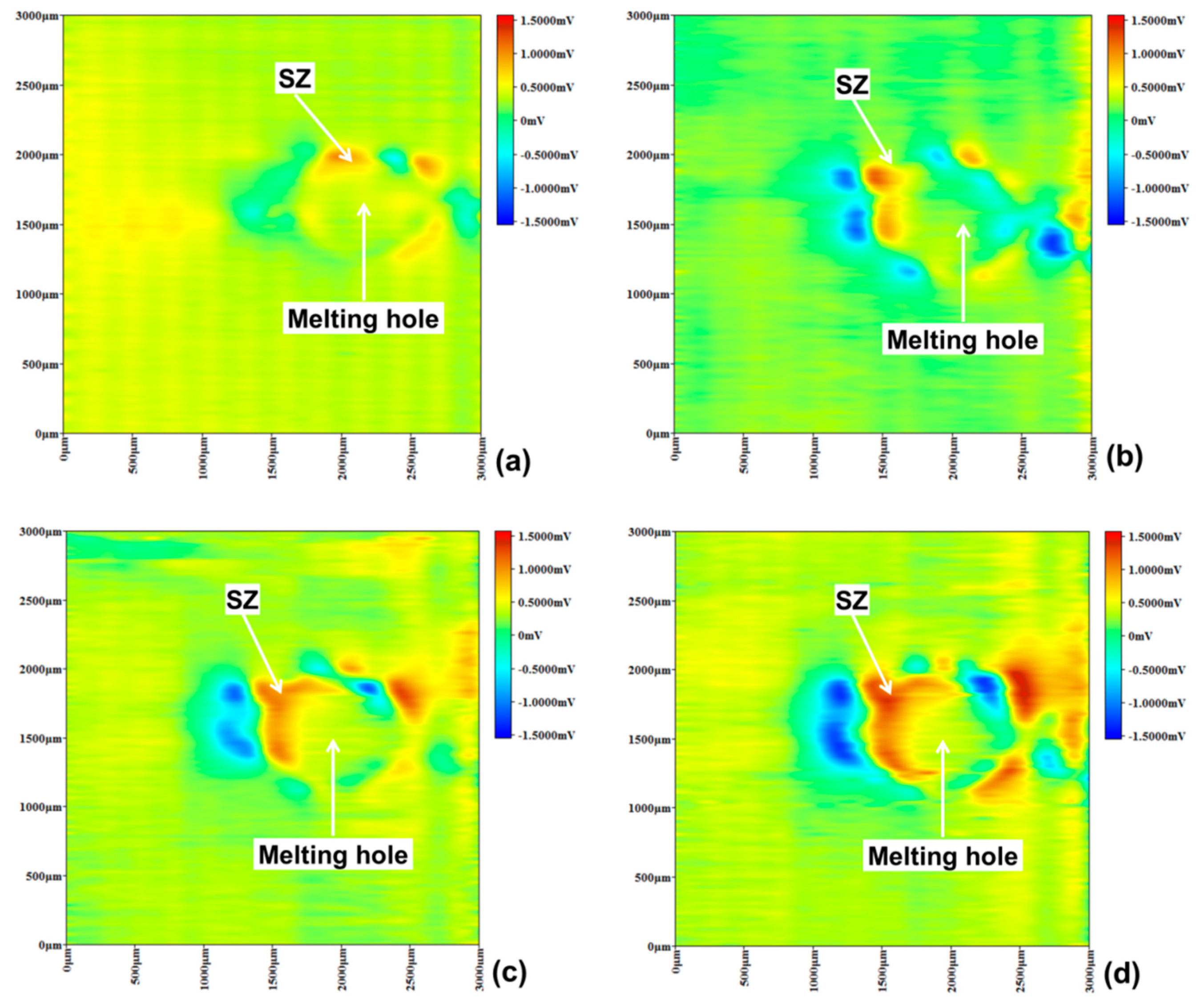
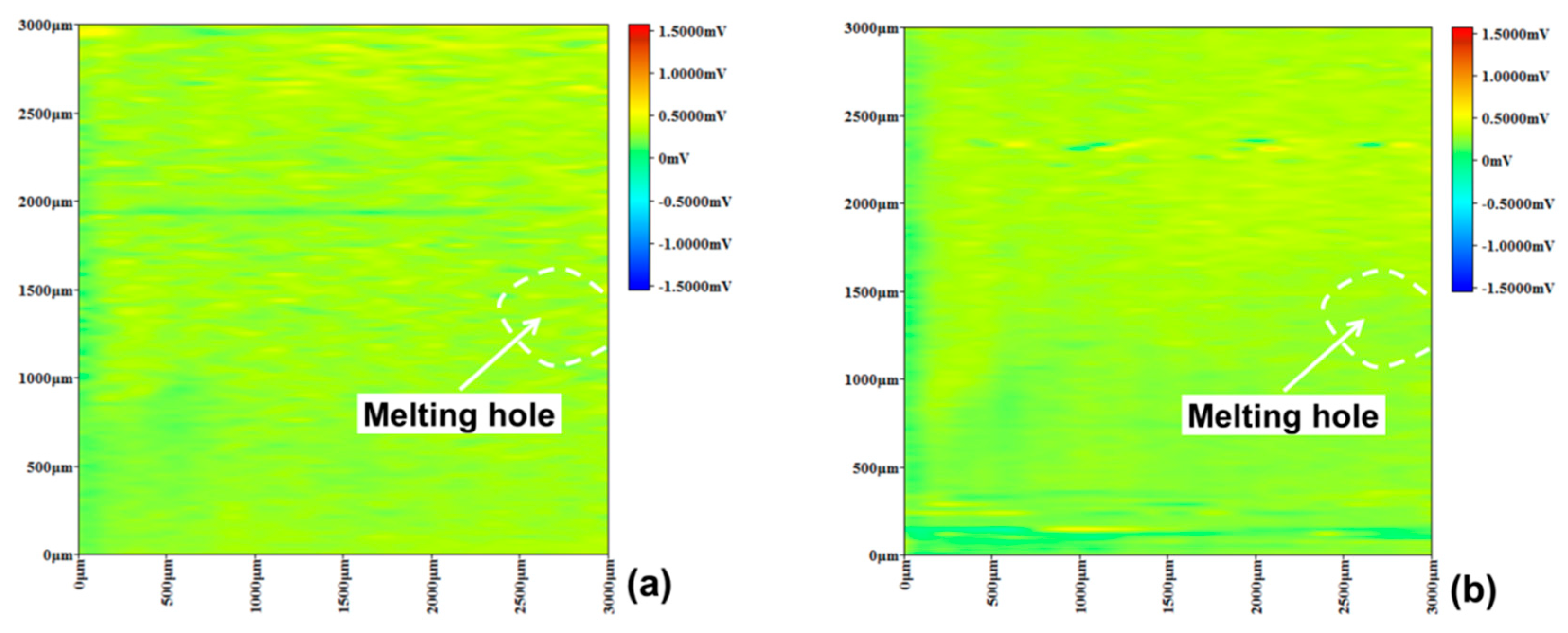
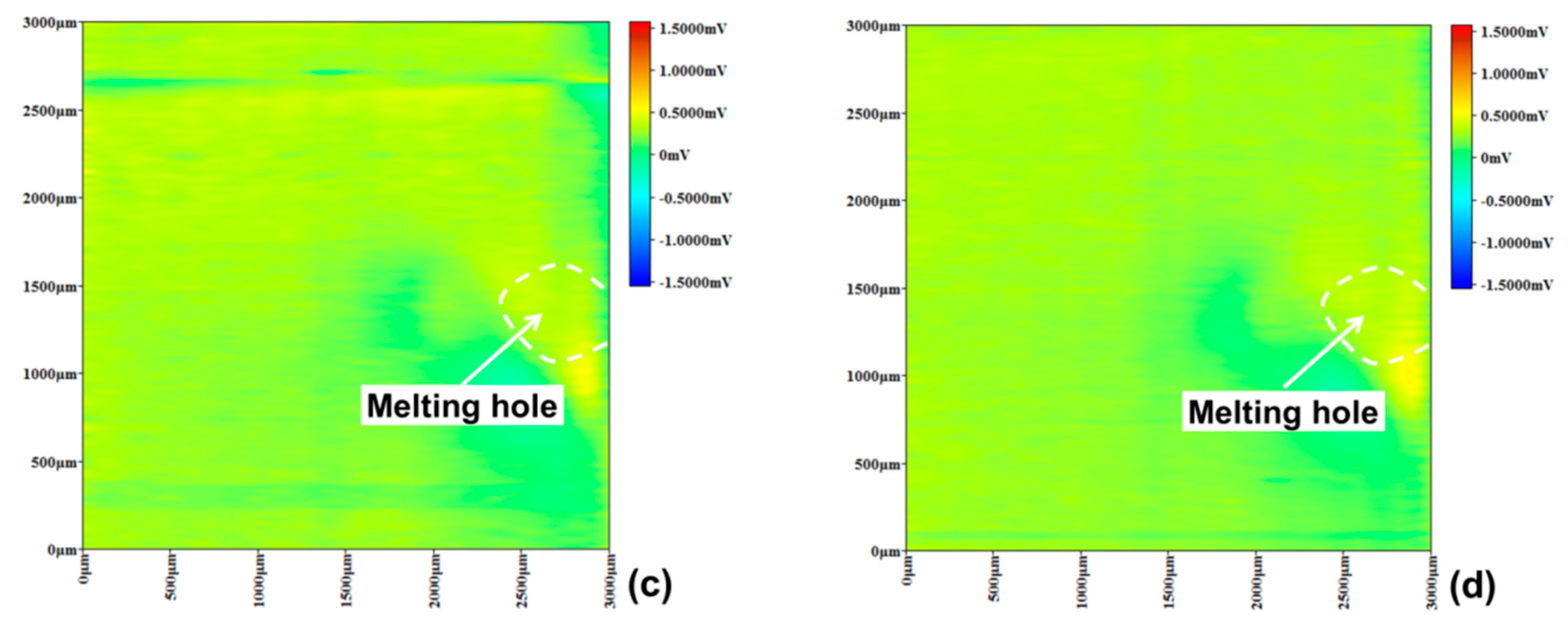
3.3. Pitting Corrosion near Melting Hole
3.4. Pitting Corrosion Morphology in 10% FeCl3 Solution
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gallo, F.; Satapathy, S.; Ravi-Chandar, K. Melting and cavity growth in the vicinity of crack tips subjected to short-duration current pulses. IEEE Trans. Magn. 2009, 45, 584–586. [Google Scholar] [CrossRef]
- Gallo, F.; Satapathy, S.; Ravi-Chandar, K. Melting and crack growth in electrical conductors subjected to short-duration current pulses. Int. J. Fract. 2001, 167, 183–193. [Google Scholar] [CrossRef]
- Yu, T.; Deng, D.W.; Wang, G.; Zhang, H.C. Crack healing in SUS304 stainless steel by electropulsing. J. Clean. Prod. 2016, 113, 989–994. [Google Scholar] [CrossRef]
- Chang, F.Q.; Zhang, H.H.; Yuan, H.H. Electrothermic effect around the crack tip in the thin conductive plate. J. Mech. Strength 2003, 25, 544–547. [Google Scholar]
- Fu, Y.M.; Tian, Z.G.; Zheng, L.J. Thermal stress field when crack arrest in an axial symmetry metal die using electromagnetic heating. Appl. Math. Mech. 2006, 27, 371–376. [Google Scholar] [CrossRef]
- Hasebe, N.; Bucher, C.; Heuer, R. Heat conduction and thermal stress induced by an electric current in an infinite thin plate containing an elliptical hole with an edge crack. Int. J. Solids Struct. 2010, 47, 138–147. [Google Scholar] [CrossRef][Green Version]
- Liu, Q.Q.; Thomas, J.C. Joule heating behaviors around through crack emanating from circular hole under electric load. Eng. Frac. Mech. 2014, 123, 2–20. [Google Scholar] [CrossRef]
- Deng, D.W.; Liu, Q.Q.; Li, X.N. Effects of pulsed current on microstructure and properties of crack tip in remanufacturing blank. Rare Metal. Mater. Eng. 2017, 8, 2208–2213. [Google Scholar]
- Lin, H.Q.; Zhao, Y.G.; Gao, Z.M. Effects of pulse current stimulation on the thermal fatigue crack propagation behavior of CHWD steel. Mater. Sci. Eng. A 2008, 478, 93–100. [Google Scholar] [CrossRef]
- Lin, C.J.; Li, Y.; Lon, B. Developments of scanning electrochemical probes and their applications in studying of localized corrosions. Electrochem 2009, 2, 122–128. [Google Scholar]
- Shao, M.H.; Fu, Y.; Hu, R.G. A study on pitting corrosion of aluminum alloy 2024-T3 by scanning microreference electrode technique. Mater. Sci. Eng. A 2003, 344, 323–327. [Google Scholar] [CrossRef]
- Du, R.G.; Yan, L.I.; Liu, Y. Study on localized corrosion behavior of reinforcing steel by scanning microelectrode technique. J. Build. Mater. 2008, 11, 33–37. [Google Scholar]
- Xu, H.; Liu, Y.; Chen, W. Corrosion behavior of reinforcing steel in simulated concrete pore solutions: A scanning micro-reference electrode study. Electrochim. Acta 2009, 54, 4067–4072. [Google Scholar] [CrossRef]
- Ye, C.Q.; Hu, R.G.; Li, Y. Probing the vertical profiles of potential in a thin layer of solution closed to electrode surface during localized corrosion of stainless steel. Corros. Sci. 2012, 61, 242–245. [Google Scholar] [CrossRef]
- Hammar, O.; Svensson, U. Solidification and Casting of Metals; The Metals Society: London, UK, 1979. [Google Scholar]
- Schino, A.D.; Mecozzi, M.G.; Barteri, M. Slidification mode and residual ferrite in low-Ni austenitic stainless steels. J. Mater. Sci. 2000, 35, 375–380. [Google Scholar] [CrossRef]
- Tamura, I. Deformation-induced martensitic transformation and transformation-induced plasticity in steels. Metal. Sci. 2015, 16, 245–253. [Google Scholar] [CrossRef]
- Suresh, S.; Giannakopoulos, A.E. A new method for estimating residual stresses by instrumented sharp indentation. Acta Mater. 1998, 46, 5755–5767. [Google Scholar] [CrossRef]
- Lu, B.T.; Chen, Z.K.; Luo, J.L. Pitting and stress corrosion cracking behavior in welded austenitic stainless steel. Electrochim. Acta. 2005, 50, 1391–1403. [Google Scholar] [CrossRef]
- Dadfar, M.; Fathi, M.H.; Karimzadeh, F. Effect of TIG welding on corrosion behavior of 316L stainless steel. Mater. Lett. 2007, 61, 2343–2346. [Google Scholar] [CrossRef]
- Mohammed-Ali, H.B.; Street, S.R.; Attallah, M.M. Effect of microstructure on the morphology of atmospheric corrosion pits in 304L stainless steel. Corrosion 2018, 74, 1373–1384. [Google Scholar] [CrossRef]
- Mudali, U.K.; Shankar, P.; Ningshen, S. On the pitting corrosion resistance of nitrogen alloyed cold worked austenitic stainless steels. Corros. Sci. 2002, 44, 2183–2198. [Google Scholar] [CrossRef]
- Peguet, L.; Malki, B.; Baroux, B. Effect of austenite stability on the pitting corrosion resistance of cold worked stainless steels. Corros. Sci. 2009, 51, 493–498. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.F. Effects of cold deformation on electrochemical corrosion behaviors of 304 stainless steel. Anti-Corros. Methods Mater. 2017, 64, 252–262. [Google Scholar] [CrossRef]
- Xu, C.C.; Hu, G. Effect of deformation-induced martensite on the pit propagation behavior of 304 stainless steel. Anti-Corros. Methods Mater. 2004, 51, 381–388. [Google Scholar]


| C | Si | Mn | Cr | Ni | P | S | N | Fe |
|---|---|---|---|---|---|---|---|---|
| 0.03 | 0.4 | 2.0 | 17.5 | 7.8 | 0.045 | 0.03 | 0.1 | Bal |
| Specimen | 1# | 2# | 3# | 4# |
|---|---|---|---|---|
| A | −458 | −236 | −65 | −91 |
| B | −221 | −313 | −458 | −360 |
| C | −54 | −76 | −121 | −14 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Pan, G.; Jiang, Y.; Chen, S.; Zou, S.; Li, W.; Xu, C.; Zhang, J. Microstructure and Pitting Corrosion of Austenite Stainless Steel after Crack Arrest. Materials 2019, 12, 4025. https://doi.org/10.3390/ma12244025
Zhang Z, Pan G, Jiang Y, Chen S, Zou S, Li W, Xu C, Zhang J. Microstructure and Pitting Corrosion of Austenite Stainless Steel after Crack Arrest. Materials. 2019; 12(24):4025. https://doi.org/10.3390/ma12244025
Chicago/Turabian StyleZhang, Zhuwu, Guangguo Pan, Yan Jiang, Song Chen, Song Zou, Wei Li, Chengwei Xu, and Jingwei Zhang. 2019. "Microstructure and Pitting Corrosion of Austenite Stainless Steel after Crack Arrest" Materials 12, no. 24: 4025. https://doi.org/10.3390/ma12244025
APA StyleZhang, Z., Pan, G., Jiang, Y., Chen, S., Zou, S., Li, W., Xu, C., & Zhang, J. (2019). Microstructure and Pitting Corrosion of Austenite Stainless Steel after Crack Arrest. Materials, 12(24), 4025. https://doi.org/10.3390/ma12244025





