Effect of Physiological Fluids Contamination on Selected Mechanical Properties of Acrylate Bone Cement
Abstract
1. Introduction
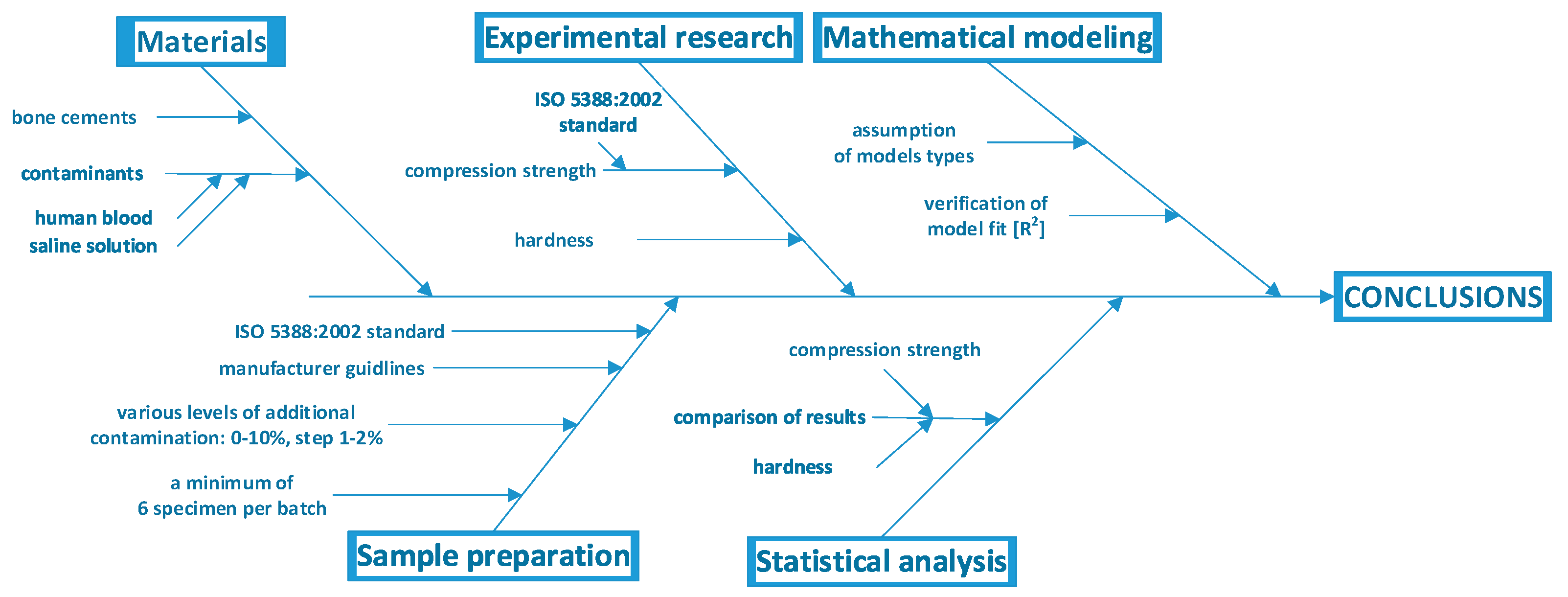
2. Materials and Methods
2.1. Substrate and Sample Preparation
2.2. Compressive Strength and Hardness Tests
2.3. Statistical Analysis and Mathematical Modelling
3. Results
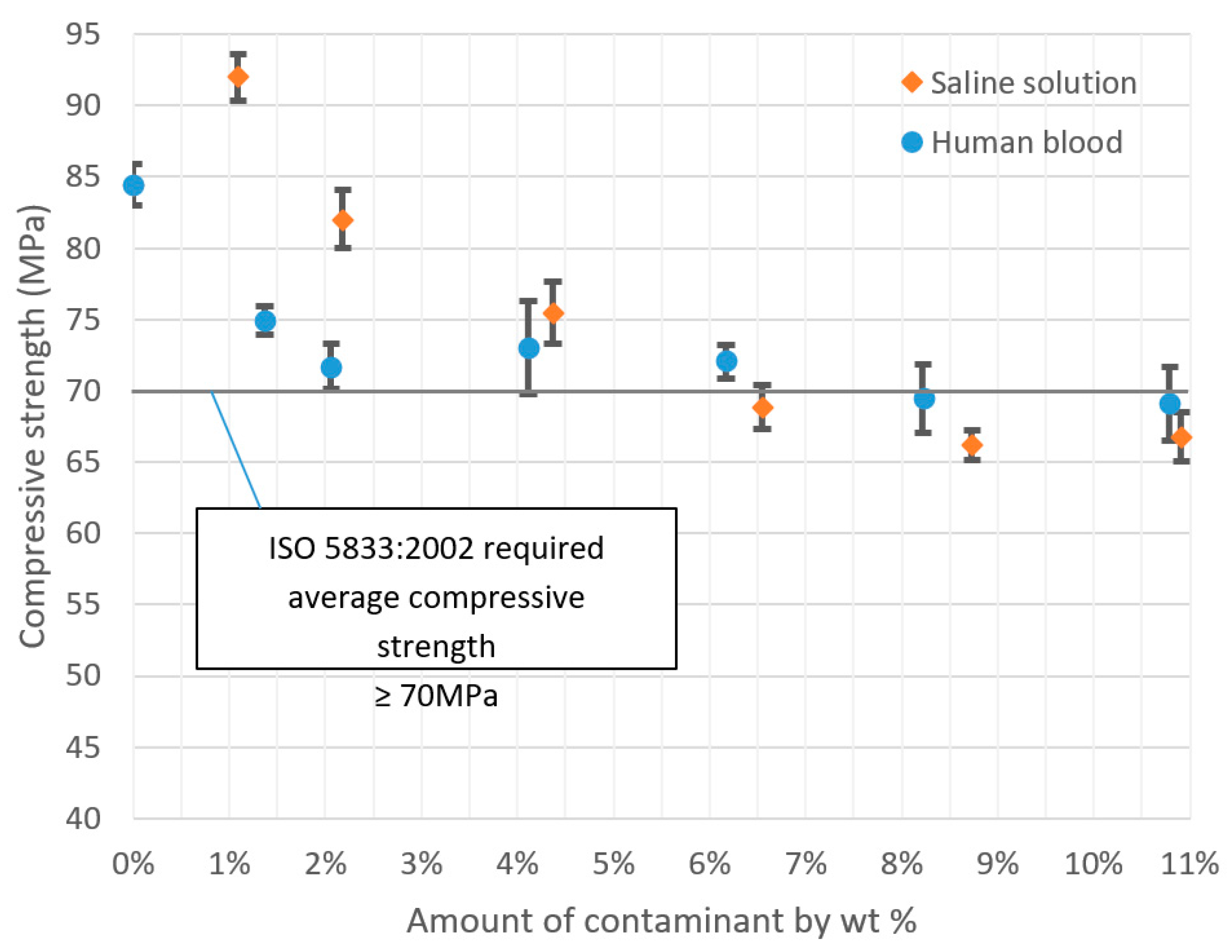
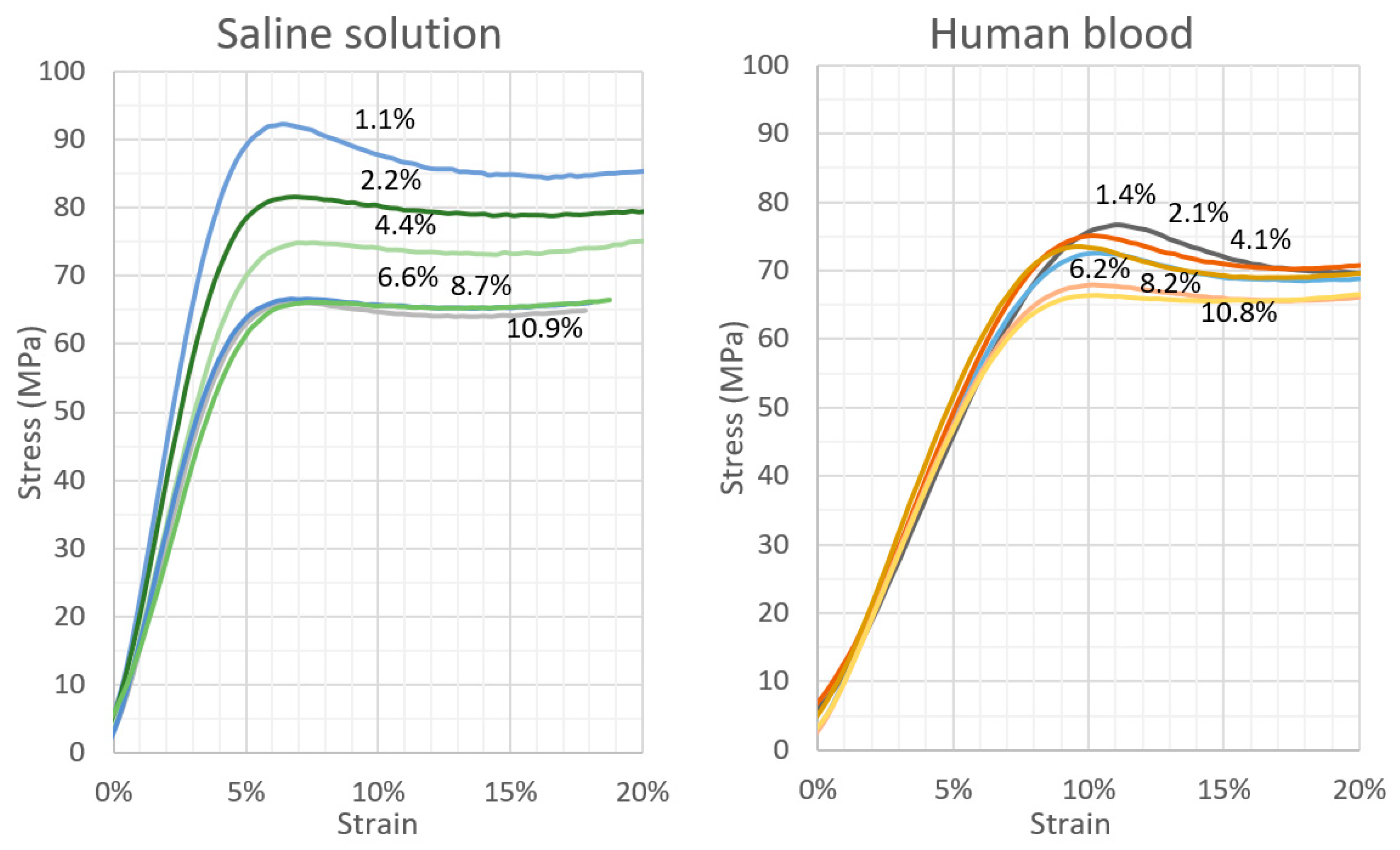
3.1. Compression Strength
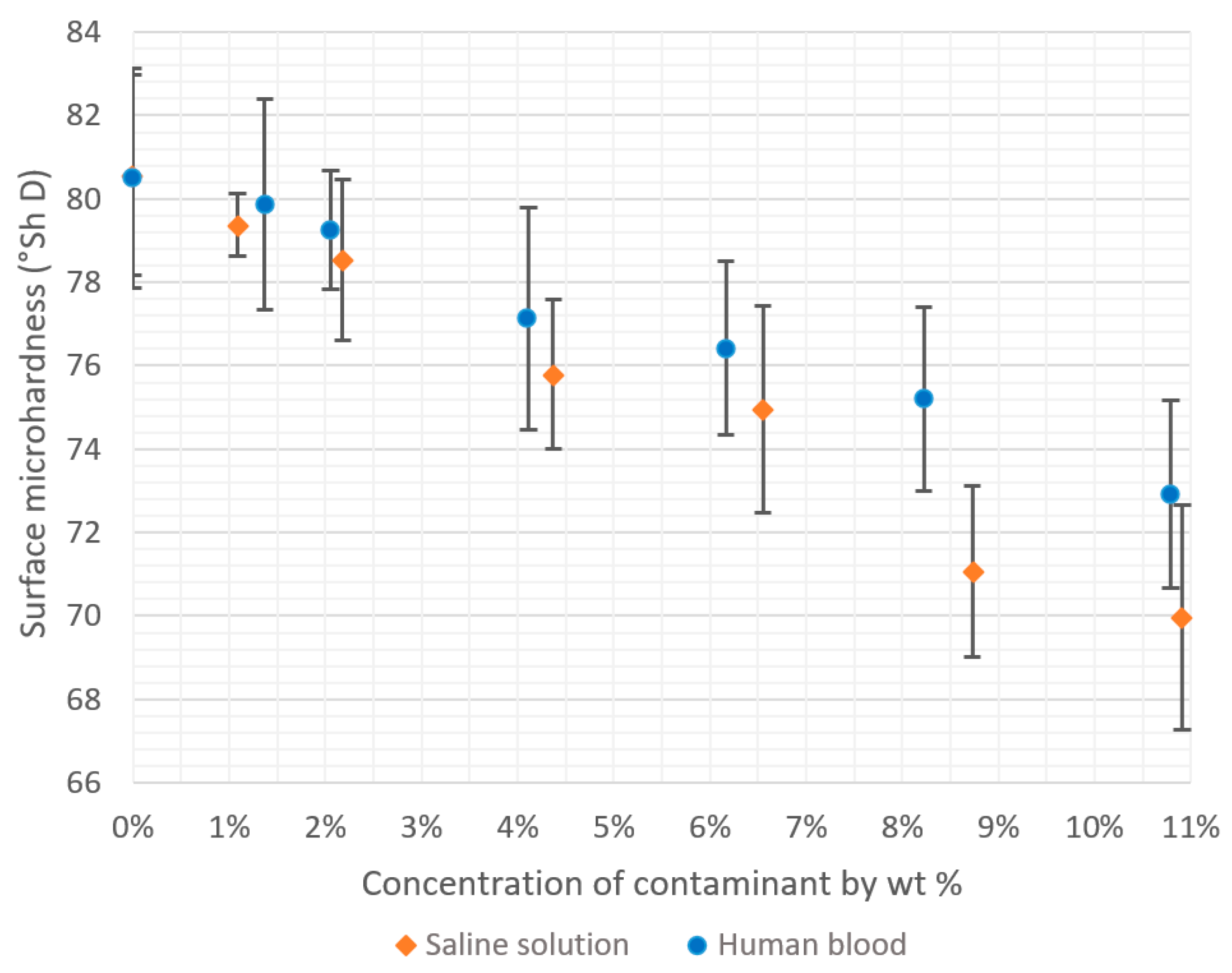
3.2. Hardness
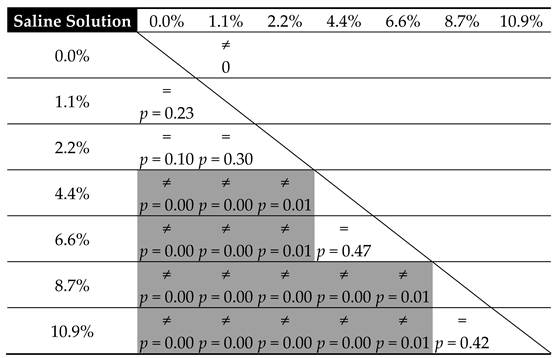
3.3. Statistical Analysis
3.4. Mathematical Modelling
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kang, I.-G.; Park, C.-I.; Lee, H.; Kim, H.-E.; Lee, S.-M. Hydroxyapatite Microspheres as an Additive to Enhance Radiopacity, Biocompatibility, and Osteoconductivity of Poly (methyl methacrylate) Bone Cement. Materials 2018, 11, 258. [Google Scholar] [CrossRef]
- Kim, S.B.; Kim, Y.J.; Yoon, T.L.; Park, S.A.; Cho, I.H.; Kim, E.J.; Kim, I.A.; Shin, J.-W. The characteristics of a hydroxyapatite–chitosan–PMMA bone cement. Biomaterials 2004, 25, 5715–5723. [Google Scholar] [CrossRef]
- Santos, J.G.F., Jr.; Pita, V.J.R.R.; Melo, P.A.; Nele, M.; Pinto, J.C. Effect of process variables on the preparation of artificial bone cements. Braz. J. Chem. Eng. 2013, 30, 865–876. [Google Scholar] [CrossRef]
- Jiang, H.-J.; Xu, J.; Qiu, Z.-Y.; Ma, X.-L.; Zhang, Z.-Q.; Tan, X.-X.; Cui, Y.; Cui, F.-Z. Mechanical Properties and Cytocompatibility Improvement of Vertebroplasty PMMA Bone Cements by Incorporating Mineralized Collagen. Materials 2015, 8, 2616–2634. [Google Scholar] [CrossRef]
- Liu, H.; Liu, B.; Gao, C.; Meng, B.; Yang, H.; Yu, H.; Yang, L. Injectable, biomechanically robust, biodegradable and osseointegrative bone cement for percutaneous kyphoplasty and vertebroplasty. Int. Orthop. 2018, 42, 125–132. [Google Scholar] [CrossRef]
- Magnússon, B.; Pétursson, P.; Edmunds, K.; Magnúsdóttir, G.; Halldórsson, G.; Jónsson, H., Jr.; Gargiulo, P. Improving planning and post-operative assessment for Total Hip Arthroplasty. Eur. J. Transl. Myol. 2015, 25, 101. [Google Scholar] [CrossRef]
- Pétursson, Þ.; Edmunds, K.J.; Gíslason, M.K.; Magnússon, B.; Magnúsdóttir, G.; Halldórsson, G.; Jónsson, H.; Gargiulo, P. Bone Mineral Density and Fracture Risk Assessment to Optimize Prosthesis Selection in Total Hip Replacement. Comput. Math. Methods Med. 2015, 2015, 1–7. [Google Scholar] [CrossRef]
- Rodriguez, L.; Chari, J.; Aghyarian, S.; Gindri, I.; Kosmopoulos, V.; Rodrigues, D. Preparation and Characterization of Injectable Brushite Filled-Poly (Methyl Methacrylate) Bone Cement. Materials 2014, 7, 6779–6795. [Google Scholar] [CrossRef]
- Sun, X.; Wu, Z.; He, D.; Shen, K.; Liu, X.; Li, H.; Jin, W. Bioactive injectable polymethylmethacrylate/silicate bioceramic hybrid cements for percutaneous vertebroplasty and kyphoplasty. J. Mech. Behav. Biomed. Mater. 2019, 96, 125–135. [Google Scholar] [CrossRef]
- Zhu, J.; Jiang, G.; Qiu, Z.; Lu, J.; Shen, F.; Cui, F.-Z. Modification of Poly (methyl methacrylate) Bone Cement for Vertebroplasty. J. Biomater. Tissue Eng. 2018, 8, 607–616. [Google Scholar] [CrossRef]
- Lee, C. The Importance of Establishing the Best Bone-Cement Interface. In The Well-Cemented Total Hip Arthroplasty; Springer: Berlin/Heidelberg, Germany, 2005; pp. 119–124. ISBN 978-3-540-24197-3. [Google Scholar]
- Khellafi, H.; Bouziane, M.M.; Djebli, A.; Mankour, A.; Bendouba, M.; Bachir Bouiadjra, B.A.; Ould Chikh, E.B. Investigation of Mechanical Behaviour of the Bone Cement (PMMA) under Combined Shear and Compression Loading. J. Biomim. Biomater. Biomed. Eng. 2019, 41, 37–48. [Google Scholar] [CrossRef]
- Pahlevanzadeh, F.; Bakhsheshi-Rad, H.R.; Ismail, A.F.; Aziz, M.; Chen, X.B. Development of PMMA-Mon-CNT bone cement with superior mechanical properties and favorable biological properties for use in bone-defect treatment. Mater. Lett. 2019, 240, 9–12. [Google Scholar] [CrossRef]
- Saha, S.; Pal, S. Mechanical properties of bone cement: A review. J. Biomed. Mater. Res. 1984, 18, 435–462. [Google Scholar] [CrossRef]
- Wiegand, M.J.; Faraci, K.L.; Reed, B.E.; Hasenwinkel, J.M. Enhancing mechanical properties of an injectable two-solution acrylic bone cement using a difunctional crosslinker: Enhancing mechanical properties of an injectable bone cement. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 783–790. [Google Scholar] [CrossRef]
- Zhao, K.; Pi, B.; Zhao, L.; Tian, S.; Ge, J.; Yang, H.; Sha, W.; Wang, L. Influence of N-acetyl cysteine (NAC) and 2-methylene-1,3-dioxepane (MDO) on the properties of polymethyl methacrylate (PMMA) bone cement. RSC Adv. 2019, 9, 11833–11841. [Google Scholar] [CrossRef]
- Arnold, J.C.; Venditti, N.P. Effects of environment on the creep properties of a poly (ethylmethacrylate) based bone cement. J. Mater. Sci. Mater. Med. 2001, 12, 707–717. [Google Scholar] [CrossRef]
- Arnold, J.C.; Venditti, N.P. Prediction of the long-term creep behaviour of hydroxyapatite-filled polyethylmethacrylate bone cements. J. Mater. Sci. Mater. Med. 2007, 18, 1849–1858. [Google Scholar] [CrossRef]
- Balin, A. Materiałowo Uwarunkowane Procesy Adaptacyjne i Trwałość Cementów Stosowanych w Chirurgii Kostnej; Wydawnictwo Politechniki Śląskiej: Gliwice, Poland, 2004. [Google Scholar]
- Santos, J.G.F., Jr.; Pita, V.J.R.R.; Melo, P.A.; Nele, M.; Pinto, J.C. Production of bone cement composites: Effect of fillers, co-monomer and particles properties. Braz. J. Chem. Eng. 2011, 28, 229–241. [Google Scholar] [CrossRef]
- Tham, W.L.; Chow, W.S.; Mohd Ishak, Z.A. Simulated body fluid and water absorption effects on poly(methyl methacrylate)/hydroxyapatite denture base composites. Express Polym. Lett. 2010, 4, 517–528. [Google Scholar] [CrossRef]
- Karpiński, S. Maksymiuk Seasoning Polymethyl Methacrylate (PMMA) Bone Cements with Incorrect Mix Ratio. Materials 2019, 12, 3073. [Google Scholar] [CrossRef]
- Jawhar, A.; Stetzelberger, V.; Kollowa, K.; Obertacke, U. Tourniquet application does not affect the periprosthetic bone cement penetration in total knee arthroplasty. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 2071–2081. [Google Scholar] [CrossRef]
- Miller, A.J.; Stimac, J.D.; Smith, L.S.; Feher, A.W.; Yakkanti, M.R.; Malkani, A.L. Results of Cemented vs Cementless Primary Total Knee Arthroplasty Using the Same Implant Design. J. Arthroplast. 2018, 33, 1089–1093. [Google Scholar] [CrossRef]
- Ayre, W.N.; Denyer, S.P.; Evans, S.L. Ageing and moisture uptake in polymethyl methacrylate (PMMA) bone cements. J. Mech. Behav. Biomed. Mater. 2014, 32, 76–88. [Google Scholar] [CrossRef]
- Karpinski, R.; Szabelski, J.; Maksymiuk, J. Analysis of the properties of bone cement with respect to its manufacturing and typical service lifetime conditions. MATEC Web Conf. 2018, 244, 01004. [Google Scholar] [CrossRef]
- Lewis, G. Properties of acrylic bone cement: State of the art review. J. Biomed. Mater. Res. 1997, 38, 155–182. [Google Scholar] [CrossRef]
- Matuszewski, Ł.; Olchowik, G.; Mazurkiewicz, T.; Kowalczyk, B.; Zdrojewska, A.; Matuszewska, A.; Ciszewski, A.; Gospodarek, M.; Morawik, I. Biomechanical parameters of the BP-enriched bone cement. Eur. J. Orthop. Surg. Traumatol. 2014, 24, 435–441. [Google Scholar] [CrossRef][Green Version]
- Postler, A.; Lützner, C.; Beyer, F.; Tille, E.; Lützner, J. Analysis of Total Knee Arthroplasty revision causes. BMC Musculoskelet. Disord. 2018, 19, 55. [Google Scholar] [CrossRef]
- Ajit Singh, V.; Chun Haw, B.; Haseeb, A.; Shuan Ju Teh, C. Hand-mixed vancomycin versus commercial tobramycin cement revisited: A study on mechanical and antibacterial properties. J. Orthop. Surg. 2019, 27, 230949901983961. [Google Scholar] [CrossRef]
- Balin, A. Cementy w Chirurgii Kostnej; Wydawnictwo Politechniki Śląskiej: Gliwice, Poland, 2016; ISBN 978-83-7880-351-5. [Google Scholar]
- Sayeed, Z.; Padela, M.T.; El-Othmani, M.M.; Saleh, K.J. Acrylic bone cements for joint replacement. In Biomedical Composites; Elsevier: Amsterdam, The Netherlands, 2017; pp. 199–214. ISBN 978-0-08-100752-5. [Google Scholar]
- Slane, J.; Vivanco, J.; Rose, W.; Ploeg, H.-L.; Squire, M. Mechanical, material, and antimicrobial properties of acrylic bone cement impregnated with silver nanoparticles. Mater. Sci. Eng. C 2015, 48, 188–196. [Google Scholar] [CrossRef]
- Tan, J.H.; Koh, B.T.; Ramruttun, A.K.; Wang, W. Compression and flexural strength of bone cement mixed with blood. J. Orthop. Surg. 2016, 24, 240–244. [Google Scholar] [CrossRef]
- Slane, J.; Vivanco, J.; Meyer, J.; Ploeg, H.-L.; Squire, M. Modification of acrylic bone cement with mesoporous silica nanoparticles: Effects on mechanical, fatigue and absorption properties. J. Mech. Behav. Biomed. Mater. 2014, 29, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Khandaker, M.; Vaughan, M.; Morris, T.; White, J.; Meng, Z. Effect of additive particles on mechanical, thermal, and cell functioning properties of poly(methyl methacrylate) cement. Int. J. Nanomed. 2014, 9, 2699. [Google Scholar] [CrossRef] [PubMed]
- Webster, T.J. The influence of nano MgO and BaSO4 particle size additives on properties of PMMA bone cement. Int. J. Nanomed. 2008, 3, 125. [Google Scholar] [CrossRef]
- Balin, A. Preliminary results of surgical cement modification with glassy carbon. Physiotherapy 2008, 16, 63–69. [Google Scholar] [CrossRef]
- Palma, P.; Marques, J.; Falacho, R.; Correia, E.; Vinagre, A.; Santos, J.; Ramos, J. Six-Month Color Stability Assessment of Two Calcium Silicate-Based Cements Used in Regenerative Endodontic Procedures. J. Funct. Biomater. 2019, 10, 14. [Google Scholar] [CrossRef]
- Brooks, I.; Lin, P.; Palumbo, G.; Hibbard, G.D.; Erb, U. Analysis of hardness–tensile strength relationships for electroformed nanocrystalline materials. Mater. Sci. Eng. A 2008, 491, 412–419. [Google Scholar] [CrossRef]
- Pavlina, E.J.; Van Tyne, C.J. Correlation of Yield Strength and Tensile Strength with Hardness for Steels. J. Mater. Eng. Perform. 2008, 17, 888–893. [Google Scholar] [CrossRef]
- Tiryakioğlu, M.; Robinson, J.S.; Salazar-Guapuriche, M.A.; Zhao, Y.Y.; Eason, P.D. Hardness–strength relationships in the aluminum alloy 7010. Mater. Sci. Eng. A 2015, 631, 196–200. [Google Scholar] [CrossRef]
- Zhang, P.; Li, S.X.; Zhang, Z.F. General relationship between strength and hardness. Mater. Sci. Eng. A 2011, 529, 62–73. [Google Scholar] [CrossRef]
- Ferraris, S.; Miola, M.; Bistolfi, A.; Fucale, G.; Crova, M.; Massé, A.; Verné, E. In Vitro Comparison between Commercially and Manually Mixed Antibiotic-Loaded Bone Cements. J. Appl. Biomater. Biomech. 2010, 8, 166–174. [Google Scholar] [CrossRef]
- Wang, T.; Pelletier, M.H.; Bertollo, N.; Crosky, A.; Walsh, W.R. Cement-Implant Interface Contamination: Possible Reason of Inferior Clinical Outcomes for Rough Surface Cemented Stems. Open Orthop. J. 2013, 7, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.; Pruitt, L.; Ries, M.; Gundiah, N. Fracture and fatigue properties of acrylic bone cement. J. Arthroplast. 2000, 15, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.P.; Harris, W.H. In vitro and in vivo studies of pressurization of femoral cement in total hip arthroplasty. J. Arthroplast. 1993, 8, 585–591. [Google Scholar] [CrossRef]
- Gbureck, U.; Grübel, S.; Thull, R.; Barralet, J.E. Modified PMMA cements for a hydrolysis resistant metal–polymer interface in orthopaedic applications. Acta Biomater. 2005, 1, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Spierings, P.T.J. Testing and Performance of Bone Cements. In The Well-Cemented Total Hip Arthroplasty; Springer: Berlin/Heidelberg, Germany, 2005; pp. 67–78. ISBN 978-3-540-24197-3. [Google Scholar]
- De Wijn, J.R. Porous Polymethylmethacrylate Cement; Dissertation University of Nijmegen: Nijmegen, The Netherlands, 1982. [Google Scholar]
- Bergmann, G.; Deuretzbacher, G.; Heller, M.; Graichen, F.; Rohlmann, A.; Strauss, J.; Duda, G.N. Hip contact forces and gait patterns from routine activities. J. Biomech. 2001, 34, 859–871. [Google Scholar] [CrossRef]
- Kutzner, I.; Heinlein, B.; Graichen, F.; Bender, A.; Rohlmann, A.; Halder, A.; Beier, A.; Bergmann, G. Loading of the knee joint during activities of daily living measured in vivo in five subjects. J. Biomech. 2010, 43, 2164–2173. [Google Scholar] [CrossRef]
- Krause, W.; Mathis, R.S. Fatigue properties of acrylic bone cements: Review of the literature. J. Biomed. Mater. Res. 1988, 22, 37–53. [Google Scholar] [CrossRef]
- Kolmas, J.; Groszyk, E.; Kwiatkowska-Różycka, D. Substituted Hydroxyapatites with Antibacterial Properties. BioMed Res. Int. 2014, 2014, 1–15. [Google Scholar] [CrossRef]
- Kolmas, J.; Krukowski, S.; Laskus, A.; Jurkitewicz, M. Synthetic hydroxyapatite in pharmaceutical applications. Ceram. Int. 2016, 42, 2472–2487. [Google Scholar] [CrossRef]
- Pajor, K.; Pajchel, L.; Kolmas, J. Hydroxyapatite and Fluorapatite in Conservative Dentistry and Oral Implantology—A Review. Materials 2019, 12, 2683. [Google Scholar] [CrossRef]
- Alves, N.M.; Gómez Ribelles, J.L.; Mano, J.F. Enthalpy relaxation studies in polymethyl methacrylate networks with different crosslinking degrees. Polymer 2005, 46, 491–504. [Google Scholar] [CrossRef]
- Sasaki, T.; Uchida, T.; Sakurai, K. Effect of crosslink on the characteristic length of glass transition of network polymers. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 1958–1966. [Google Scholar] [CrossRef]
- Yang, J.-M.; Lu, C.-S.; Hsu, Y.-G.; Shih, C.-H. Mechanical properties of acrylic bone cement containing PMMA-SiO2 hybrid sol-gel material. J. Biomed. Mater. Res. 1997, 38, 143–154. [Google Scholar] [CrossRef]
- Deb, S.; Braden, M.; Bonfield, W. Effect of crosslinking agents on poly (ethylmethacrylate) bone cements. J. Mater. Sci. Mater. Med. 1997, 8, 829–833. [Google Scholar] [CrossRef]
- Deb, S.; Vazquez, B.; Bonfield, W. Effect of crosslinking agents on acrylic bone cements based on poly (methylmethacrylate). J. Biomed. Mater. Res. 1997, 37, 465–473. [Google Scholar] [CrossRef]
- Topoleski, L.D.; Ducheyne, P.; Cuckler, J.M. A fractographic analysis of in vivo poly (methyl methacrylate) bone cement failure mechanisms. J. Biomed. Mater. Res. 1990, 24, 135–154. [Google Scholar] [CrossRef]
- Bialoblocka-Juszczyk, E.; Baleani, M.; Cristofolini, L.; Viceconti, M. Fracture properties of an acrylic bone cement. Acta Bioeng. Biomech. 2008, 10, 21. [Google Scholar]
- Hughes, K.F.; Ries, M.D.; Pruitt, L.A. Structural degradation of acrylic bone cements due toin vivo and simulated aging. J. Biomed. Mater. Res. 2003, 65A, 126–135. [Google Scholar] [CrossRef]
- Oonishi, H.; Akiyama, H.; Takemoto, M.; Kawai, T.; Yamamoto, K.; Yamamuro, T.; Oonishi, H.; Nakamura, T. The long-term in vivo behavior of polymethyl methacrylate bone cement in total hip arthroplasty. Acta Orthop. 2011, 82, 553–558. [Google Scholar] [CrossRef]
- Nottrott, M.; Mølster, A.O.; Gjerdet, N.R. Time dependent mechanical properties of bone cement. Anin vitro study over one year. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 83B, 416–421. [Google Scholar] [CrossRef]

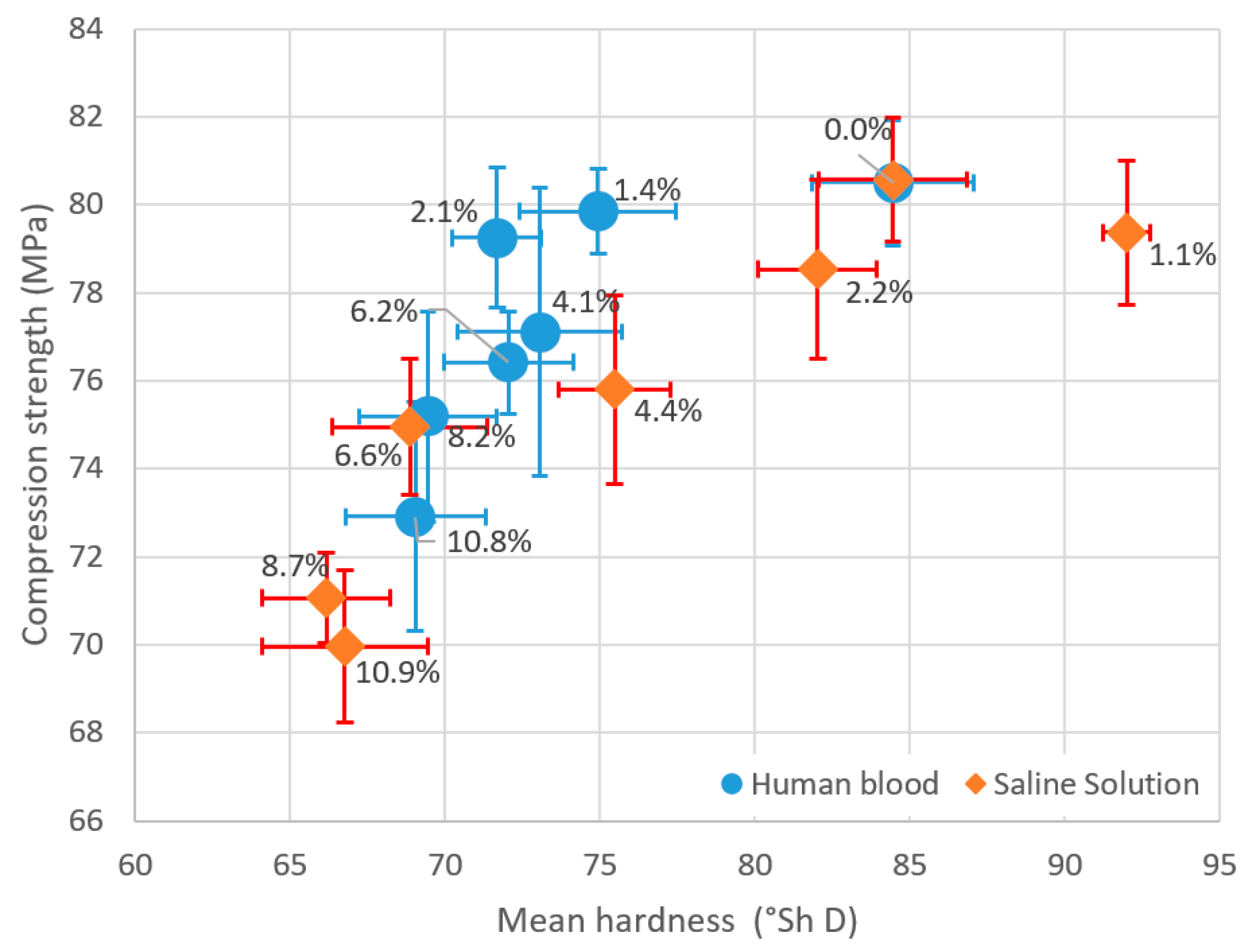
| Contamination Level | Mean Compression Strength (MPa) | SD (MPa) | CV |
|---|---|---|---|
| Human blood | |||
| 0.0% | 84.45 | 1.42 | 1.7% |
| 1.4% | 74.94 | 0.97 | 1.3% |
| 2.1% | 71.68 | 1.59 | 2.2% |
| 4.1% | 73.06 | 3.28 | 4.5% |
| 6.2% | 72.04 | 1.16 | 1.6% |
| 8.2% | 69.45 | 2.40 | 3.5% |
| 10.8% | 69.05 | 2.60 | 3.8% |
| Contamination Level | Mean Compression Strength (MPa) | SD (MPa) | CV |
|---|---|---|---|
| Saline solution | |||
| 0.0% | 84.45 | 1.42 | 1.7% |
| 1.1% | 92.00 | 1.63 | 1.8% |
| 2.2% | 82.02 | 2.04 | 2.5% |
| 4.4% | 75.48 | 2.14 | 2.8% |
| 6.6% | 68.86 | 1.55 | 2.3% |
| 8.7% | 66.17 | 1.03 | 1.6% |
| 10.9% | 66.77 | 1.72 | 2.6% |
| Contamination Level | Mean Hardness | SD (°Sh D) | CV |
|---|---|---|---|
| (°Sh D) | |||
| Human blood | |||
| 0.0% | 80.50 | 2.63 | 3.3% |
| 1.4% | 79.86 | 2.53 | 3.2% |
| 2.1% | 79.25 | 1.43 | 1.8% |
| 4.1% | 77.11 | 2.66 | 3.5% |
| 6.2% | 76.42 | 2.09 | 2.7% |
| 8.2% | 75.19 | 2.21 | 2.9% |
| 10.8% | 72.91 | 2.25 | 3.1% |
| Contamination Level | Mean Hardness | SD (°Sh D) | CV |
|---|---|---|---|
| (°Sh D) | |||
| Saline solution | |||
| 0.0% | 80.57 | 2.40 | 3.0% |
| 1.1% | 79.37 | 0.75 | 0.9% |
| 2.2% | 78.53 | 1.92 | 2.4% |
| 4.4% | 75.80 | 1.80 | 2.4% |
| 6.6% | 74.95 | 2.49 | 3.3% |
| 8.7% | 71.07 | 2.05 | 2.9% |
| 10.9% | 69.97 | 2.68 | 3.8% |
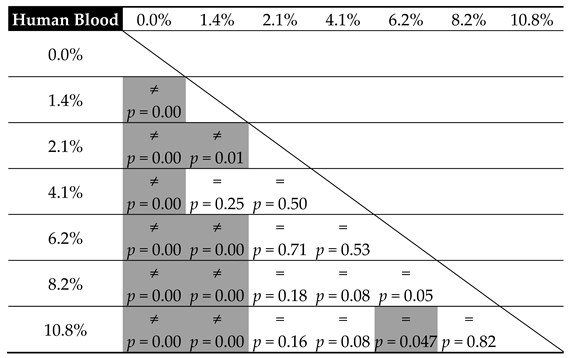
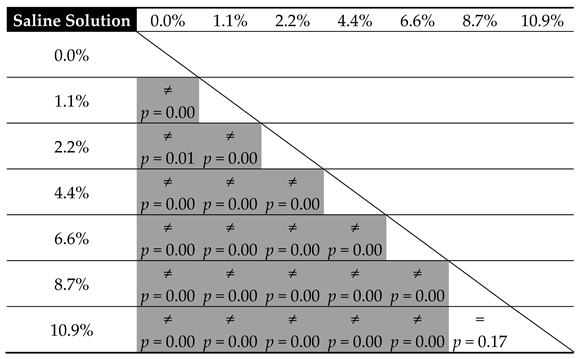
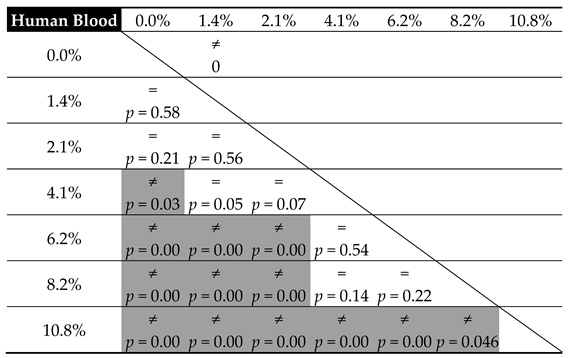
| Contaminant | Parameters of the Linear Model of Hardness Change (m × x + b) | ||
|---|---|---|---|
| m | b | R2 | |
| Human blood | −69.48 | 80.57 | 0.987 |
| Saline solution | −99.32 | 80.55 | 0.983 |
| Contaminant | Parameters of the Polynomial Model of Compression Strength Change (α1 × x3 + α2 × x2 + α3 × x + ε) | |||||
|---|---|---|---|---|---|---|
| Polynomial model degree | α1 | α2 | α3 | ε | R2 | |
| Human blood | 1st (linear) | - | - | −102.76 | 78.33 | 0.59 |
| 2nd (quadratic) | - | 1716 | −285.58 | 80.87 | 0.75 | |
| 3rd (cubic) | −56,651 | 10,954 | −659.22 | 83.20 | 0.88 | |
| Saline solution | 1st (linear) | - | - | −223.85 | 87.35 | 0.84 |
| 2nd (quadratic) | - | 1540 | −388.87 | 89.54 | 0.87 | |
| 3rd (cubic) | 61,137 | −8523 | 21.18 | 87.12 | 0.92 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karpiński, R.; Szabelski, J.; Maksymiuk, J. Effect of Physiological Fluids Contamination on Selected Mechanical Properties of Acrylate Bone Cement. Materials 2019, 12, 3963. https://doi.org/10.3390/ma12233963
Karpiński R, Szabelski J, Maksymiuk J. Effect of Physiological Fluids Contamination on Selected Mechanical Properties of Acrylate Bone Cement. Materials. 2019; 12(23):3963. https://doi.org/10.3390/ma12233963
Chicago/Turabian StyleKarpiński, Robert, Jakub Szabelski, and Jacek Maksymiuk. 2019. "Effect of Physiological Fluids Contamination on Selected Mechanical Properties of Acrylate Bone Cement" Materials 12, no. 23: 3963. https://doi.org/10.3390/ma12233963
APA StyleKarpiński, R., Szabelski, J., & Maksymiuk, J. (2019). Effect of Physiological Fluids Contamination on Selected Mechanical Properties of Acrylate Bone Cement. Materials, 12(23), 3963. https://doi.org/10.3390/ma12233963






