Broadening the Photoluminescence Excitation Spectral Bandwidth of YVO4:Eu3+ Nanoparticles via a Novel Core-Shell and Hybridization Approach
Abstract
:1. Introduction
2. Experimental
2.1. Materials
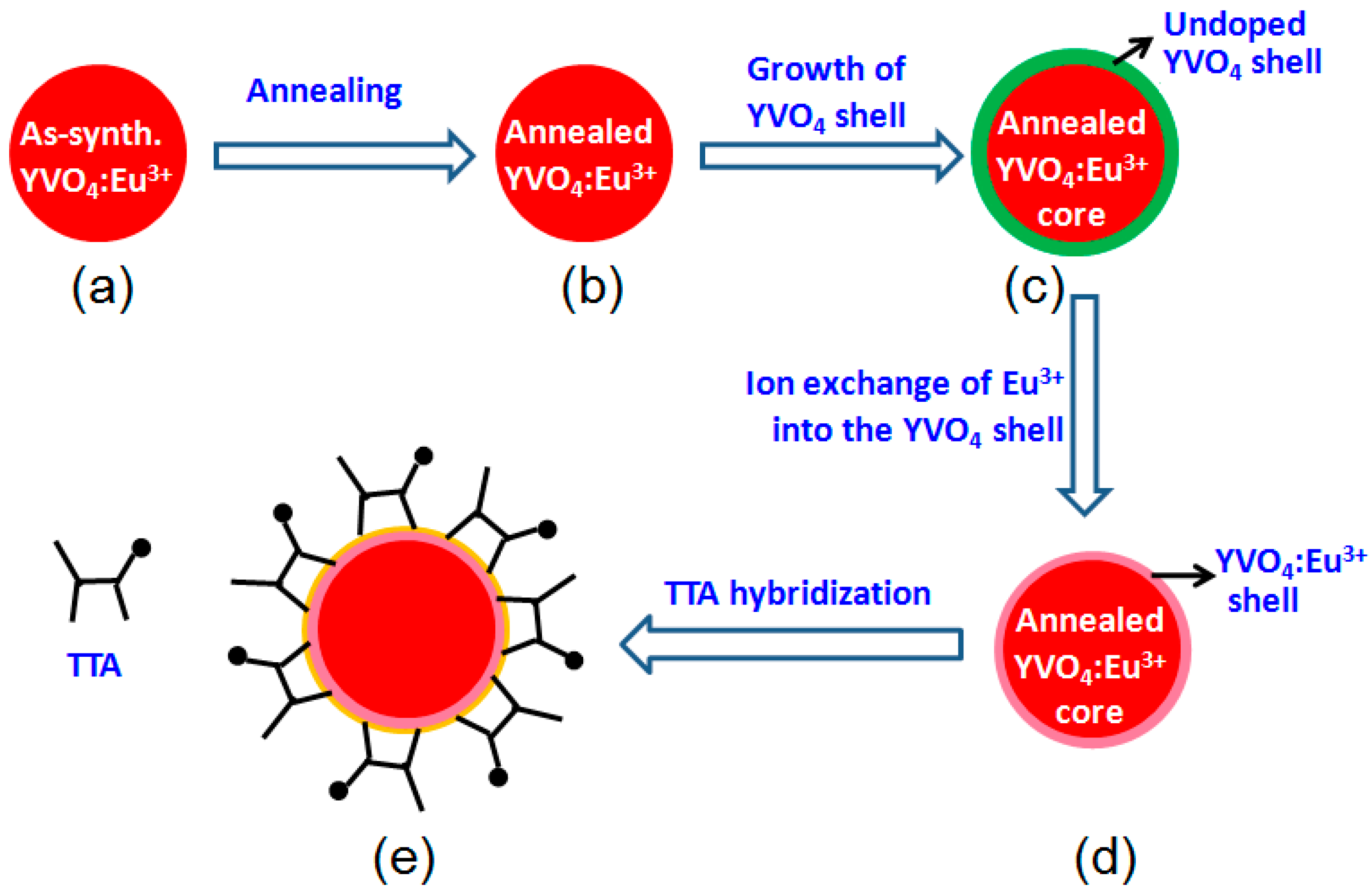
2.2. Synthesis and Annealing of YVO4:Eu3+ Nanoparticles
2.3. Synthesis of YVO4:Eu3+@YVO4 Core-Shell Nanoparticles
2.4. Synthesis of YVO4:Eu3+@ YVO4:Eu3+ Nanoparticles
2.5. Synthesis of YVO4:Eu3+@YVO4:Eu3+–TTA Nanoparticles
2.6. Characterization
3. Results and Discussion
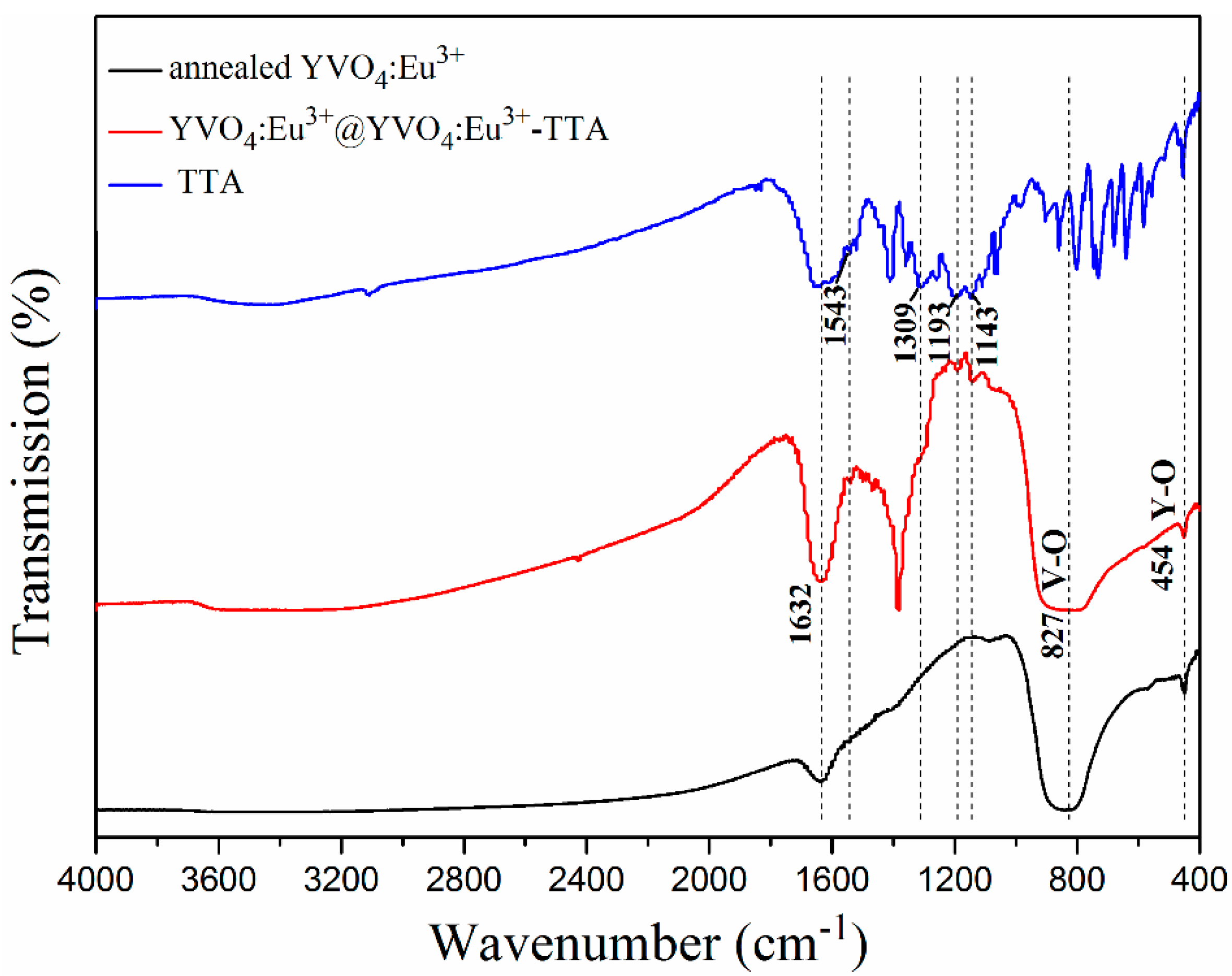
3.1. Microstructural Characteristics
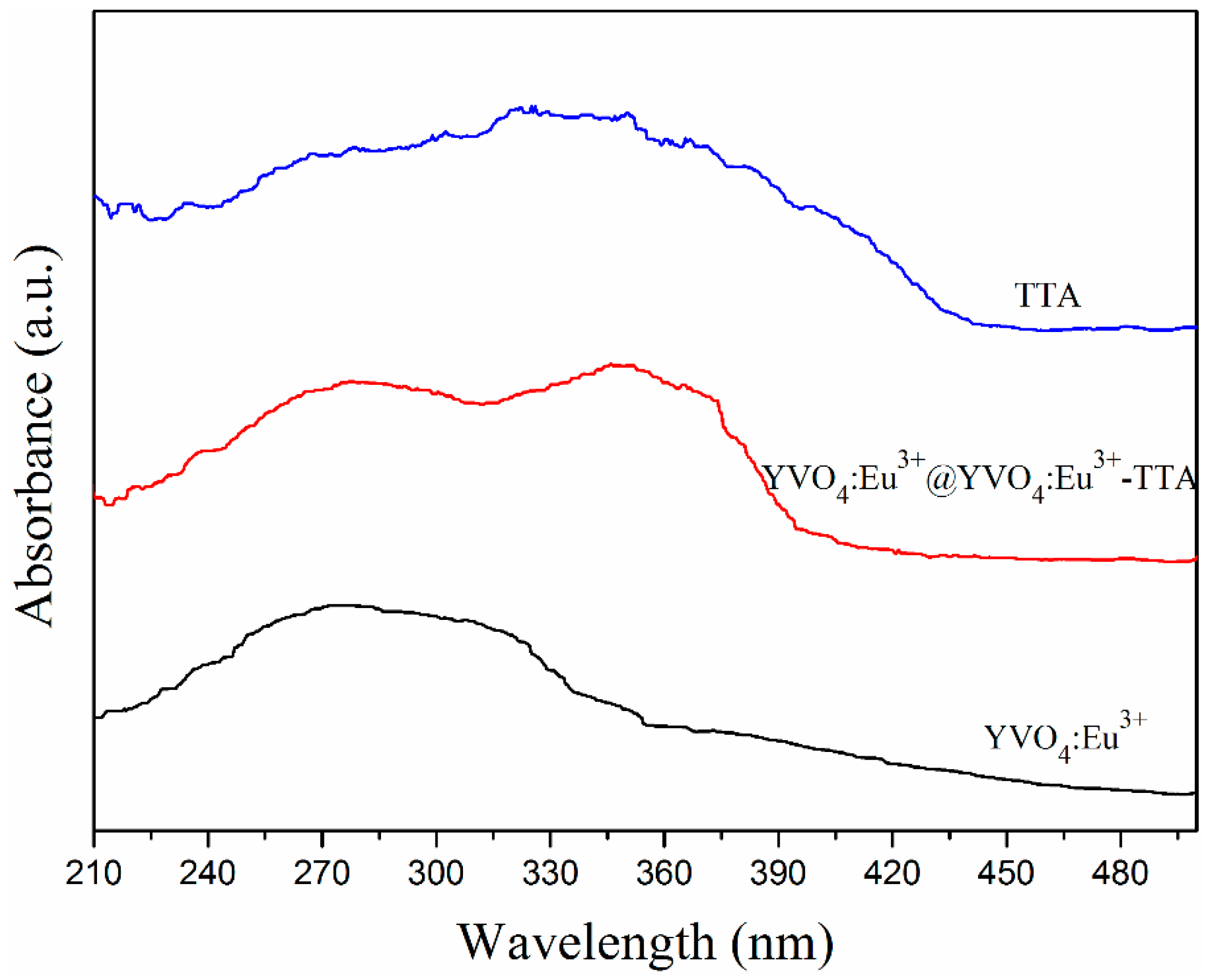
3.2. UV–Vis Absorption Spectra
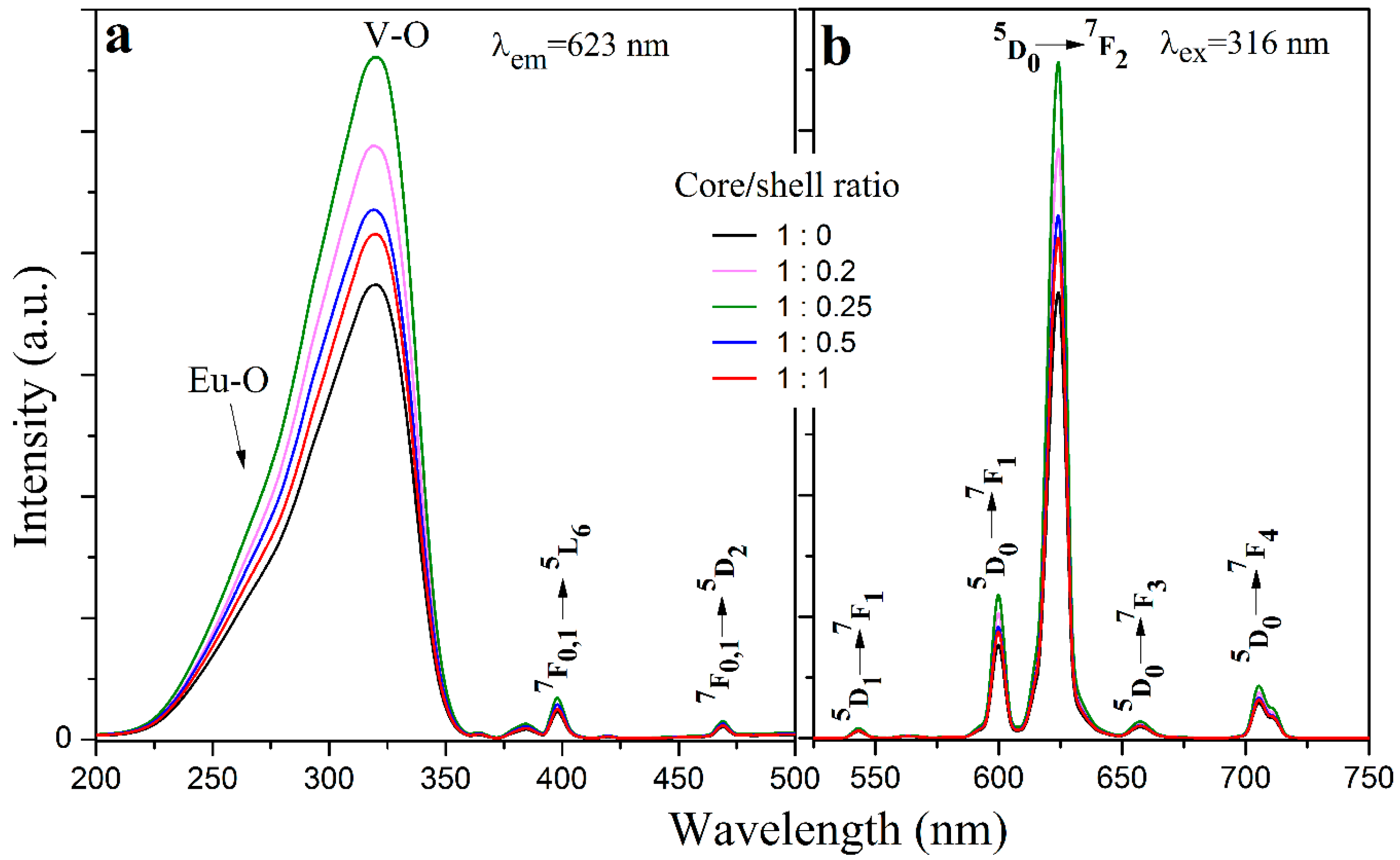
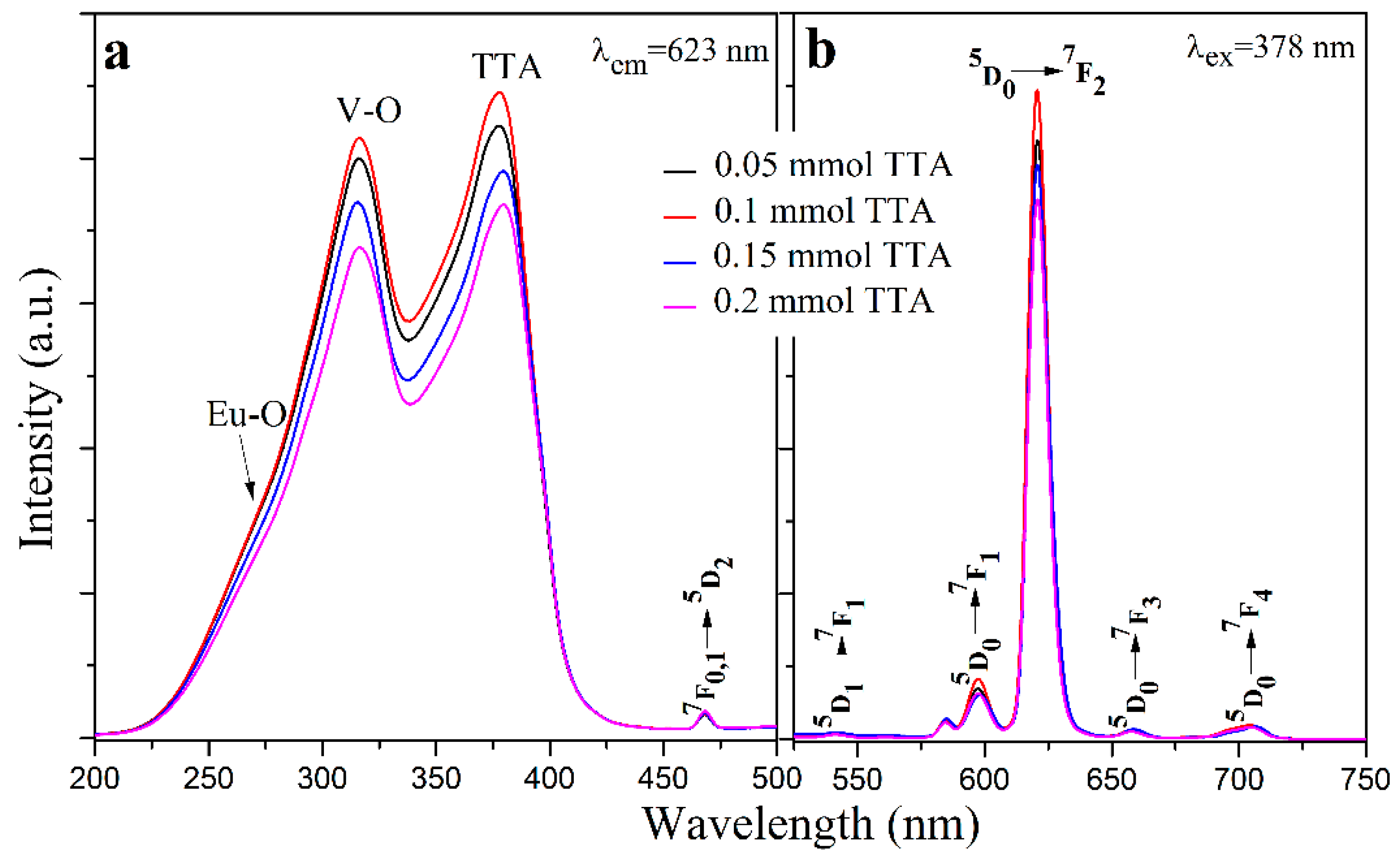
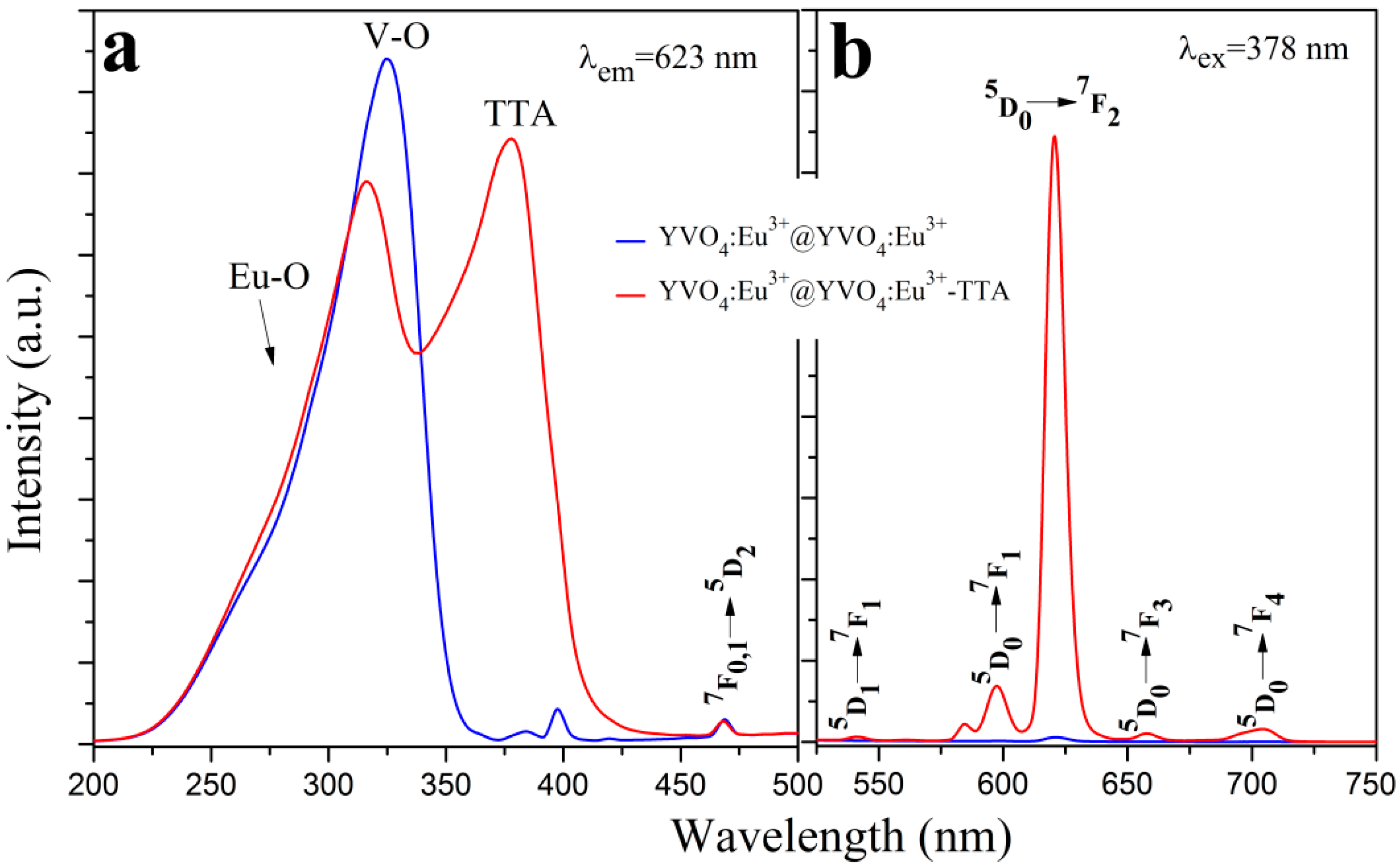
3.3. Photoluminescence Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Feugnet, G.; Bussac, C.; Larat, C.; Schwarz, M.; Pocholle, J.P. High-efficiency TEM(00) Nd:YVO(4) laser longitudinally pumped by a high-power array. Opt. Lett. 1995, 20, 157–159. [Google Scholar] [CrossRef]
- Croitoru, G.; Pavel, N. Passive Q-switching by Cr4+:YAG saturable absorber of buried depressed-cladding waveguides obtained in Nd-doped media by femtosecond laser beam writing. Materials 2018, 11, 1689. [Google Scholar]
- Chander, N.; Khan, A.F.; Chandrasekhar, P.S.; Thouti, E.; Swami, S.K.; Dutta, V.; Komarala, V.K. Reduced ultraviolet light induced degradation and enhanced light harvesting using YVO4:Eu3+ down-shifting nano-phosphor layer in organometal halide perovskite solar cells. Appl. Phys. Lett. 2014, 105, 033904. [Google Scholar] [CrossRef]
- Shokouhimehr, M.; Rafiaei, S.M. Combustion synthesized YVO4:Eu3+ phosphors: Effect of fuels on nanostructure and luminescence properties. Ceram. Int. 2017, 43, 11469–11473. [Google Scholar] [CrossRef]
- Huang, C.K.; Chen, Y.C.; Hung, W.B.; Chen, T.M.; Sun, K.W.; Chang, W.-L. Enhanced light harvesting of Si solar cells via luminescent down-shifting using YVO4:Bi3+,Eu3+ nanophosphors. Prog. Photovolt. Res. Appl. 2013, 21, 1507–1513. [Google Scholar] [CrossRef]
- Xu, W.; Song, H.; Yan, D.; Zhu, H.; Wang, Y.; Xu, S.; Bai, X.; Dong, B.; Liu, Y. YVO4:Eu3+,Bi3+ UV to visible conversion nano-films used for organic photovoltaic solar cells. J. Mater. Chem. 2011, 21, 12331–12336. [Google Scholar] [CrossRef]
- Peng, Y.; Liu, J.; Zhang, K.; Luo, H.; Li, J.; Xu, B.; Han, L.; Li, X.; Yu, X. Near-infrared luminescent and antireflective in SiO2/YVO4:Yb3+ bilayer films for c-Si solar cells. Appl. Phys. Lett. 2011, 99, 121110. [Google Scholar] [CrossRef]
- Huang, X.Y.; Wang, J.X.; Yu, D.C.; Ye, S.; Zhang, Q.Y.; Sun, X.W. Spectral conversion for solar cell efficiency enhancement using YVO4: Bi3+, Ln3+ (Ln = Dy, Er, Ho, Eu, Sm, and Yb) phosphors. J. Appl. Phys. 2011, 109, 113526. [Google Scholar] [CrossRef]
- Ho, W.-J.; You, B.-J.; Liu, J.-J.; Bai, W.-B.; Syu, H.-J.; Lin, C.-F. Photovoltaic performance enhancement of silicon solar cells based on combined ratios of three species of europium-doped phosphors. Materials 2018, 11, 845. [Google Scholar] [CrossRef]
- Ho, W.-J.; Deng, Y.-J.; Liu, J.-J.; Feng, S.-K.; Lin, J.-C. Photovoltaic performance characterization of textured silicon solar cells using luminescent down-shifting Eu-doped phosphor particles of various dimensions. Materials 2017, 10, 21. [Google Scholar] [CrossRef]
- Riwotzki, K.; Haase, M. Colloidal YVO4:Eu and YP0.95V0.05O4:Eu nanoparticles: Luminescence and energy transfer processes. J. Phys. Chem. B 2001, 105, 12709–12713. [Google Scholar] [CrossRef]
- Liao, Y.; Chen, N.; Du, G. Strong luminescence enhancement of YVO4:Eu3+,Ba2+ phosphors prepared by a solvothermal method. J. Alloys Compd. 2013, 561, 214–219. [Google Scholar] [CrossRef]
- Takeshi, S.; Ogato, H.; Isobe, T.; Wawayama, T.; Niikura, S. Effects of Citrate Additive on Transparency and Photostability Properties of YVO4:Bi3+,Eu3+ Nanophosphor. J. Electrochem. Soc. 2010, 157, J74–J80. [Google Scholar] [CrossRef]
- Chen, D.; Yu, Y.; Huang, P.; Lin, H.; Shan, Z.; Zeng, L.; Yang, A.; Wang, Y. Color-tunable luminescence for Bi3+/Ln3+:YVO4 (Ln = Eu, Sm, Dy, Ho) nanophosphors excitable by near-ultraviolet light. Phys. Chem. Chem. Phys. 2010, 12, 7775–7778. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, Y.; Du, G.; Chen, N.; Zhang, A. Broadband UV excited LaF3:Eu3+ based inorganic–organic mixed hybrid nanoparticles for strong luminescence. J. Nanosci. Nanotechnol. 2018, 18, 1876–1881. [Google Scholar] [CrossRef] [PubMed]
- Carlos, L.D.; Ferreira, R.A.S.; de Zea Bermudez, V.; Ribeiro, S.J.L. Lanthanide-containing light-emitting organic–inorganic hybrids: A Bet on the Future. Adv. Mater. 2009, 21, 509–534. [Google Scholar] [CrossRef]
- He, Y.; Chen, N.; Du, G. Synthesis of LaOF:Eu3+ Nanoparticles with strong luminescence enhanced by organic ligands. J. Am. Ceram. Soc. 2014, 97, 1931–1936. [Google Scholar] [CrossRef]
- Tang, L.; Gui, W.; Ding, K.; Chen, N.; Du, G. Ion exchanged YVO4:Eu3+ nanoparticles and their strong luminescence enhanced by energy transfer of thenoyltrifluoroacetone ligands. J. Alloys Compd. 2014, 590, 277–282. [Google Scholar] [CrossRef]
- Mialon, G.; Gohin, M.; Gacoin, T.; Boilot, J.-P. High temperature strategy for oxide nanoparticle synthesis. ACS Nano 2008, 2, 2505–2512. [Google Scholar] [CrossRef]
- Lin, Q.; Xu, Y.; Fu, E.; Baber, S.; Bao, Z.; Yu, L.; Deng, S.; Kundu, J.; Hollingsworth, J.; Bauer, E.; et al. Polymer-assisted chemical solution approach to YVO4:Eu nanoparticle networks. J. Mater. Chem. 2012, 22, 5835–5839. [Google Scholar] [CrossRef]
- Filho, P.C.D.; Gacoin, T.; Boilot, J.-P.; Walton, R.I.; Serra, O.A. Synthesis and luminescent properties of REVO4−REPO4 (RE = Y, Eu, Gd, Er, Tm, or Yb) heteronanoparticles: A promising class of phosphors for excitation from NIR to VUV. J. Phys. Chem. C 2015, 119, 24062–24074. [Google Scholar] [CrossRef]
- Zhang, F.; Che, R.; Li, X.; Yao, C.; Yang, J.; Shen, D.; Hu, P.; Li, W.; Zhao, D. Direct imaging the upconversion nanoparticle core/shell structure at the subnanometer level: Shell thickness dependence in upconverting optical properties. Nano Lett. 2012, 12, 2852–2858. [Google Scholar] [CrossRef] [PubMed]
- Yi, G.-S.; Chow, G.-M. Water-soluble NaYF4:Yb,Er(Tm)/NaYF4/polymer core/shell/shell nanoparticles with significant enhancement of upconversion fluorescence. Chem. Mater. 2007, 19, 341–343. [Google Scholar] [CrossRef]
- Velasco, D.S.; de Moura, A.P.; Medina, A.N.; Baesso, M.L.; Rubira, A.F.; Cremona, M.; Bento, A.C. Preparation, characterization, and spectroscopic properties of PC/PMMA doped blends: Study of the effect of rare-earth doping on luminescence, quenching rate, and lifetime enhancement. J. Phys. Chem. B 2010, 114, 5657–5660. [Google Scholar] [CrossRef]
- Meng, Q.G.; Boutinaud, P.; Franville, A.-C.; Zhang, H.J.; Mahiou, R. Preparation and characterization of luminescent cubic MCM-48 impregnated with an Eu3+ β-diketonate complex. Microporous Mesoporous Mater. 2003, 65, 127–136. [Google Scholar] [CrossRef]
- Guan, J.B.; Chen, B.; Sun, Y.Y.; Liang, H.; Zhang, Q.J. Effects of synergetic ligands on the thermal and radiative properties of Eu(TTA)3nL-doped poly(methyl methacrylate). J. Non-Cryst. Solids 2005, 351, 849–855. [Google Scholar] [CrossRef]
- Hirano, S.; Yogo, T.; Kikuta, K.; Sakamoto, W.; Koganei, H. Synthesis of Nd:YVO4 Thin Films by a Sol-Gel Method. J. Am. Ceram. Soc. 1996, 79, 3041–3044. [Google Scholar] [CrossRef]
- Wu, X.C.; Tao, Y.R.; Song, C.Y.; Mao, C.J.; Dong, L.; Zhu, J.J. Morphological control and luminescent properties of YVO4:Eu nanoparticles. J. Phys. Chem. B 2006, 110, 15791–15796. [Google Scholar] [CrossRef]
- Wang, H.; Yu, M.; Lin, C.K.; Lin, J. Core–shell structured SiO2@YVO4:Dy3+/Sm3+ phosphor particles:Sol–gel preparation and characterization. J. Colloid Interface Sci. 2006, 300, 176–182. [Google Scholar] [CrossRef]
- Wang, Y.; Qin, W.P.; Zhang, J.S.; Cao, C.Y.; Lü, S.Z.; Ren, X.G. Photoluminescence of colloidal YVO4:Eu/SiO2 core/shell nanoparticles. Opt. Commun. 2009, 282, 1148–1153. [Google Scholar] [CrossRef]
- Hsu, C.; Powell, R.C. Energy transfer in europium doped yttrium vanadate crystals. J. Lumin. 1975, 10, 273–293. [Google Scholar] [CrossRef]
- Meyssamy, H.; Riwotzki, K.; Kornowski, A.; Naused, S.; Haase, M. Wet-chemical synthesis of doped colloidal nanomaterials: Particles and fibers of LaPO4:Eu, LaPO4:Ce, and LaPO4:Ce,Tb. J. Cryst. Growth 1999, 11, 840–844. [Google Scholar] [CrossRef]
- Jia, G.; Song, Y.H.; Yang, M.; Liu, K.; Zheng, Y.H.; You, H.P. Facile synthesis and luminescence properties of octahedral YVO4:Eu3+ microcrystals. J. Cryst. Growth 2009, 311, 4213–4218. [Google Scholar] [CrossRef]
- Liao, Y.; Zhen, Y.; Chen, N.; Du, G. Effect of Sr2+ doping on the luminescence properties of YVO4:Eu3+,Sr2+ particles prepared by a solvothermal method. J. Sol-Gel Sci. Technol. 2013, 65, 353–358. [Google Scholar] [CrossRef]
- Luwang, M.N.; Ningthoujam, R.S.; Srivastava, S.K.; Vatsa, R.K. Preparation of white light emitting YVO4: Ln3+ and silica-coated YVO4:Ln3+ (Ln3+ = Eu3+, Dy3+, Tm3+) nanoparticles by CTAB/n-butanol/hexane/water microemulsion route: Energy transfer and site symmetry studies. J. Mater. Chem. 2011, 21, 5326–5337. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Tang, L.; Chen, N.; Du, G. Broadening the Photoluminescence Excitation Spectral Bandwidth of YVO4:Eu3+ Nanoparticles via a Novel Core-Shell and Hybridization Approach. Materials 2019, 12, 3830. https://doi.org/10.3390/ma12233830
Huang J, Tang L, Chen N, Du G. Broadening the Photoluminescence Excitation Spectral Bandwidth of YVO4:Eu3+ Nanoparticles via a Novel Core-Shell and Hybridization Approach. Materials. 2019; 12(23):3830. https://doi.org/10.3390/ma12233830
Chicago/Turabian StyleHuang, Jianhua, Lu Tang, Nan Chen, and Guoping Du. 2019. "Broadening the Photoluminescence Excitation Spectral Bandwidth of YVO4:Eu3+ Nanoparticles via a Novel Core-Shell and Hybridization Approach" Materials 12, no. 23: 3830. https://doi.org/10.3390/ma12233830
APA StyleHuang, J., Tang, L., Chen, N., & Du, G. (2019). Broadening the Photoluminescence Excitation Spectral Bandwidth of YVO4:Eu3+ Nanoparticles via a Novel Core-Shell and Hybridization Approach. Materials, 12(23), 3830. https://doi.org/10.3390/ma12233830



