Influence of Nanostructuration on PbTe Alloys Synthesized by Arc-Melting
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
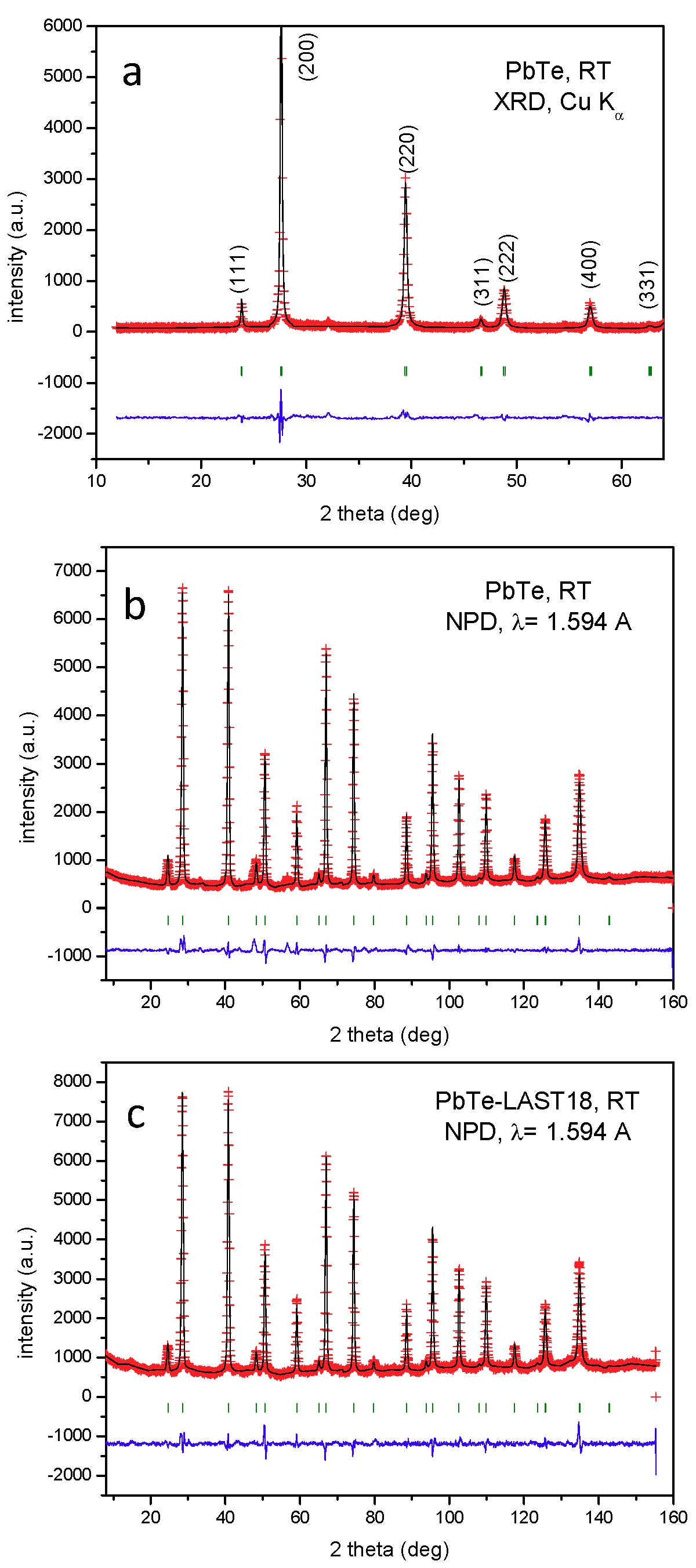
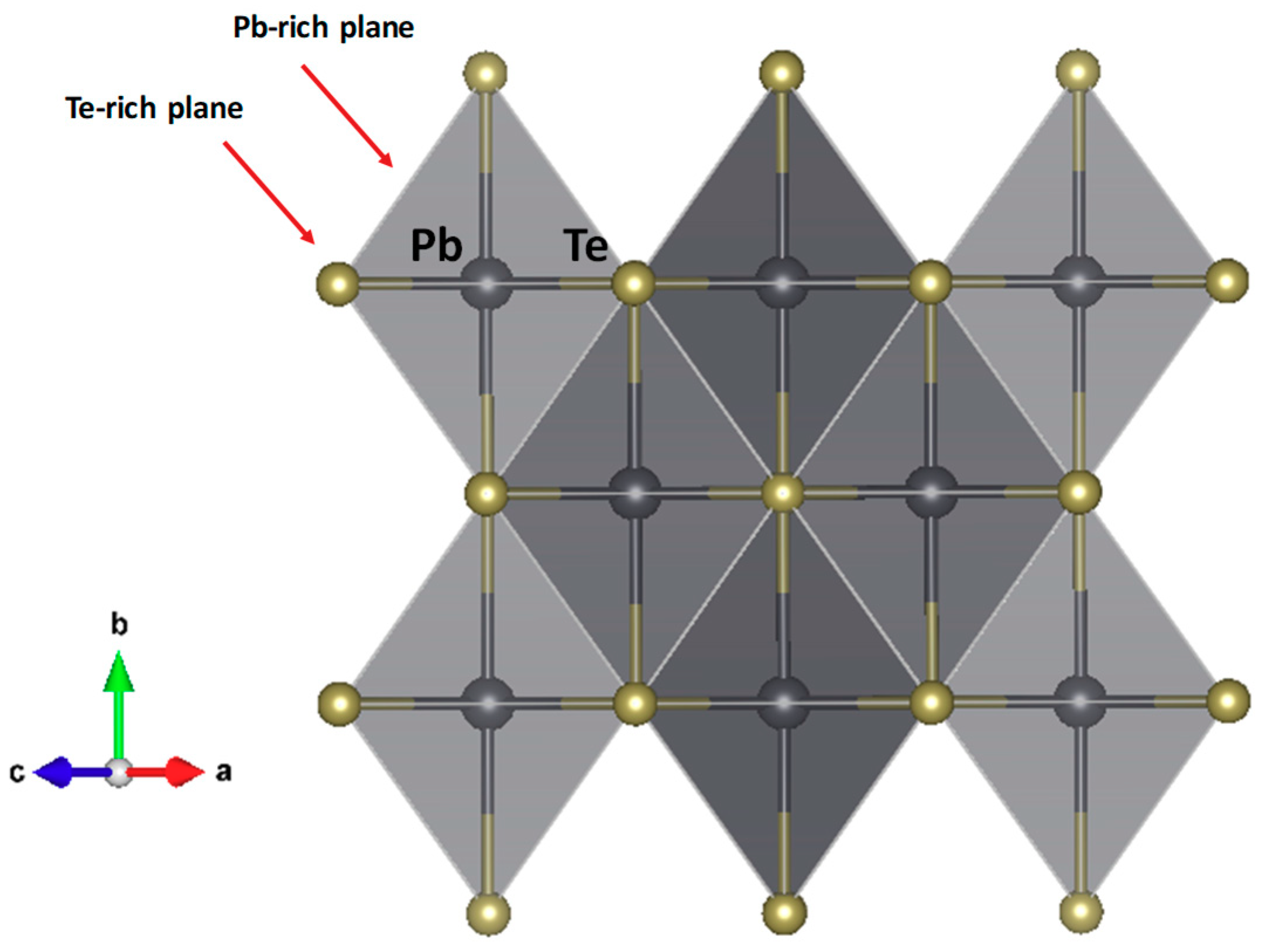
3.1. Crystal Structure
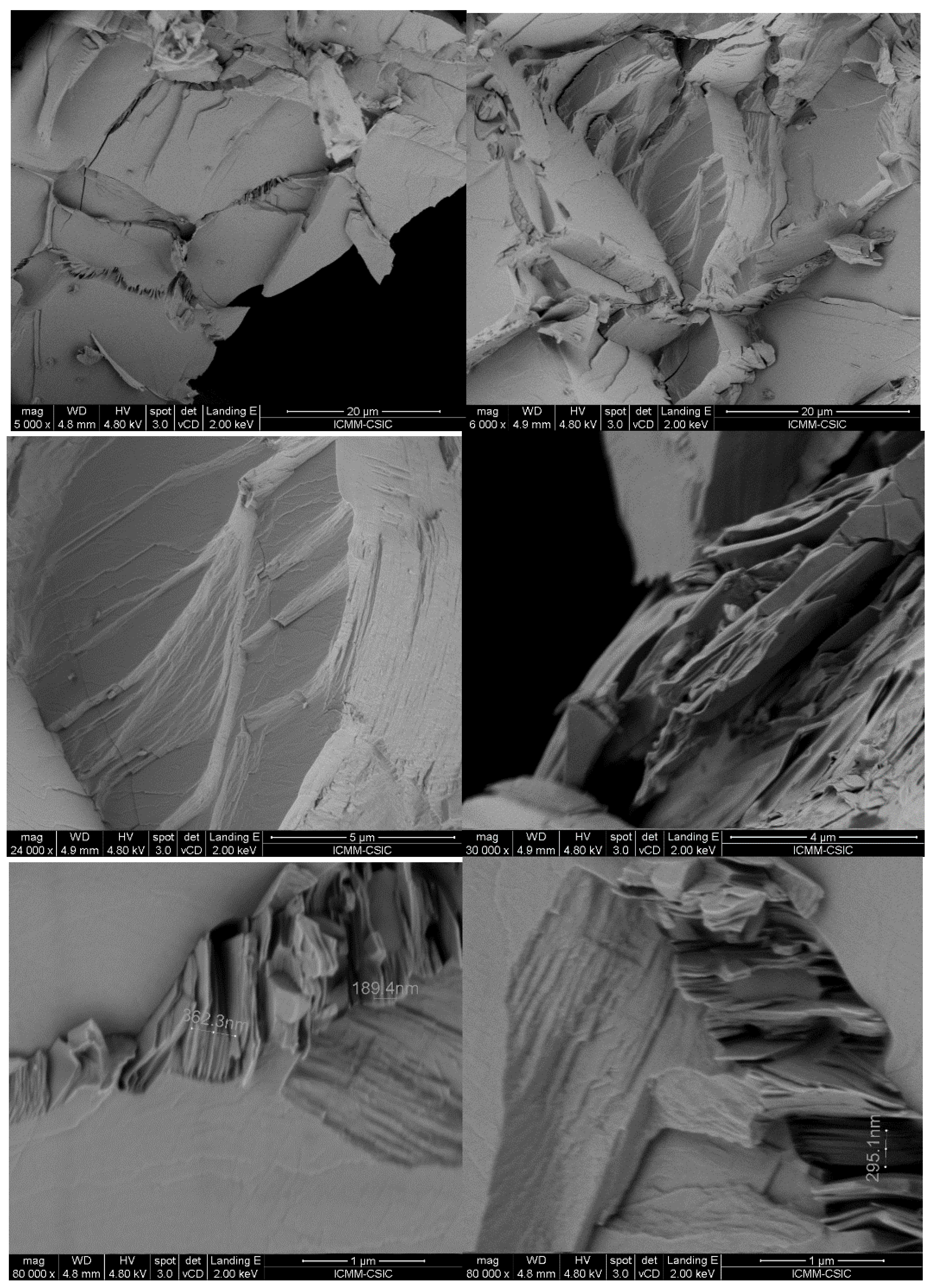
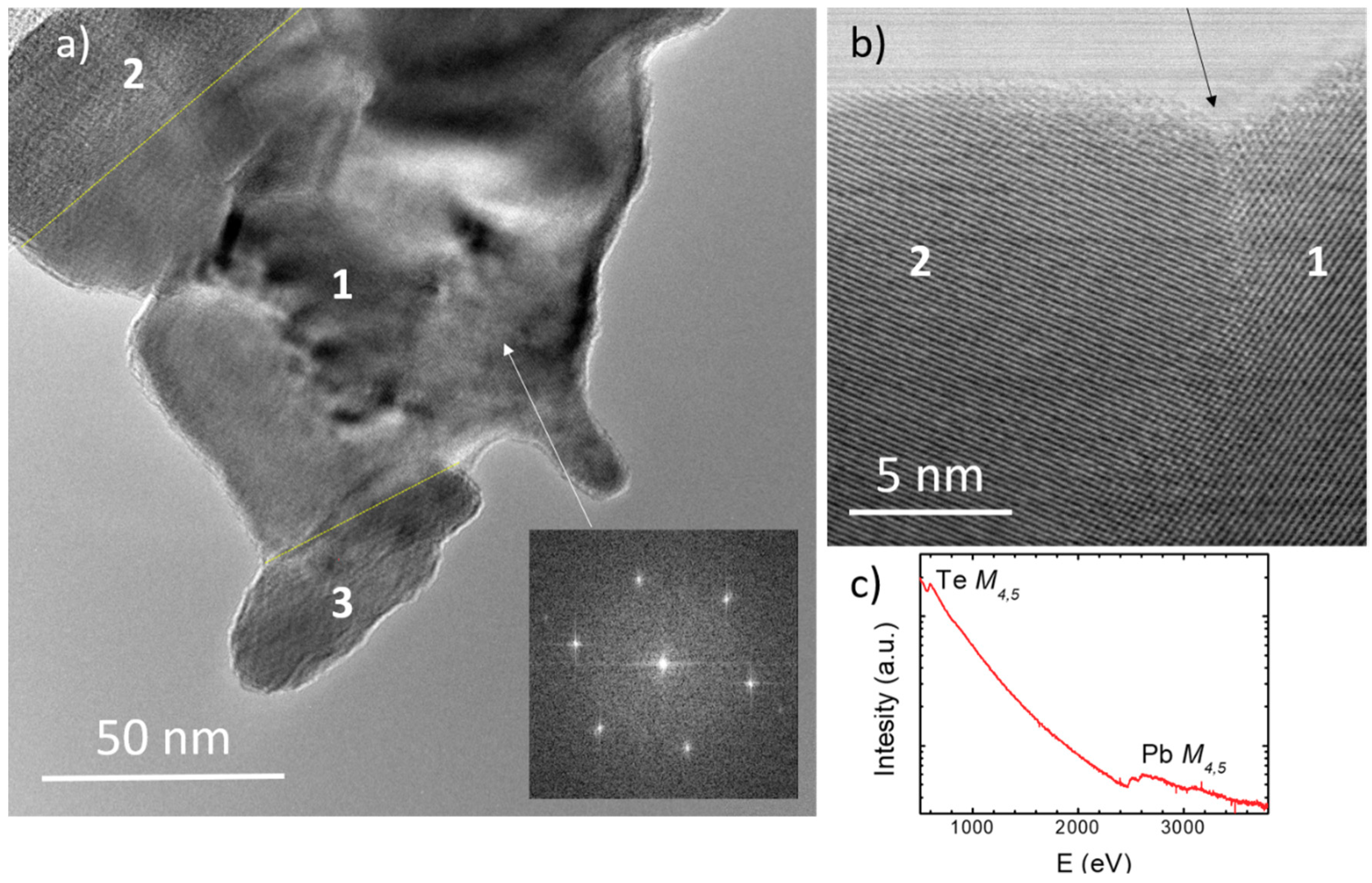
3.2. Nanostructuration
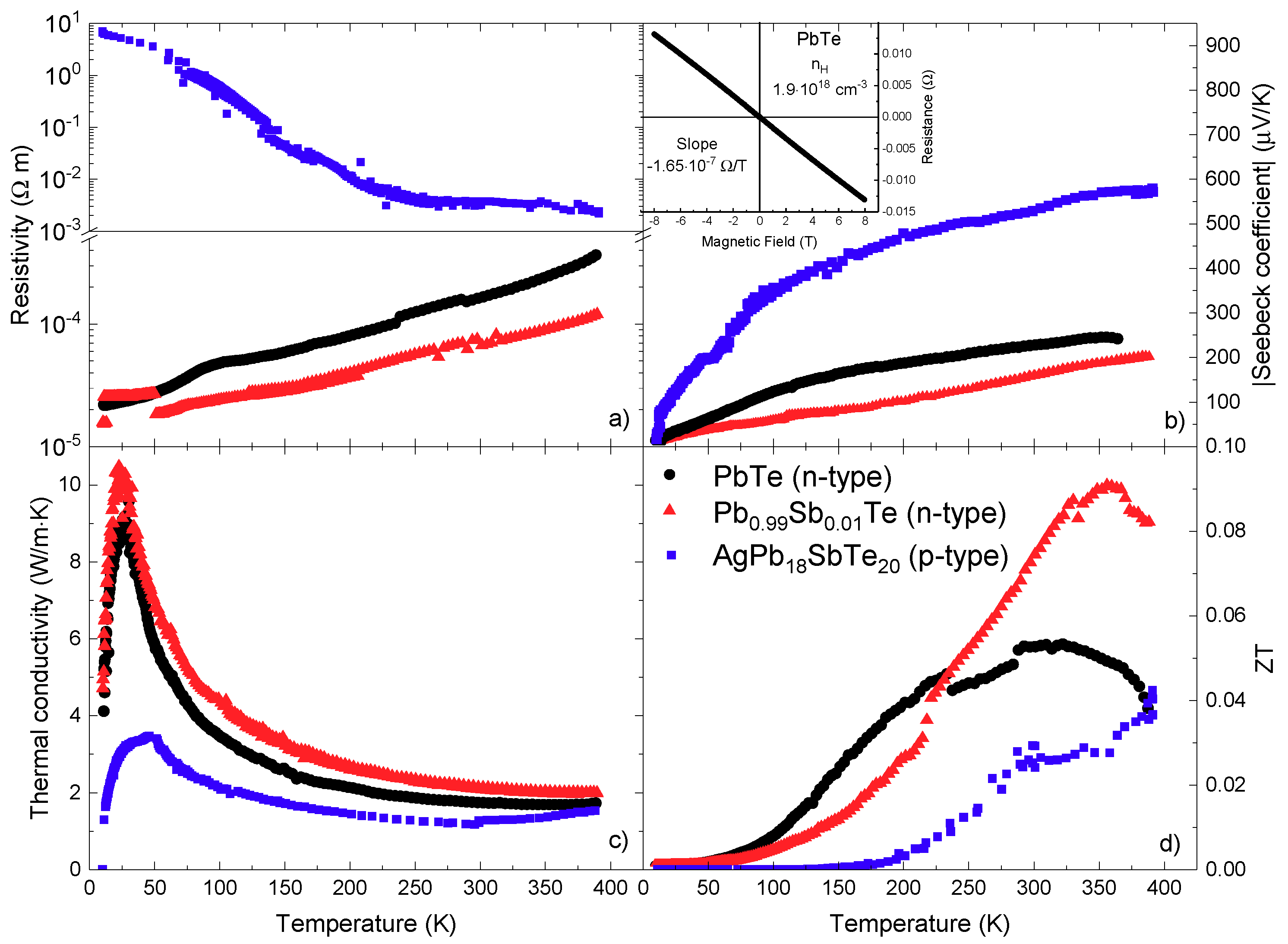
3.3. Transport Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Snyder, G.J.; Toberer, E.S. Complex thermoelectric materials. Nat. Mater. 2008, 7, 105–114. [Google Scholar] [CrossRef]
- Pei, Y.; Wang, H.; Snyder, G.J. Band engineering of thermoelectric materials. Adv. Mater. 2012, 24, 6125–6135. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Shi, X.; LaLonde, A.; Wang, H.; Chen, L.; Snyder, G.J. Convergence of electronic bands for high performance bulk thermoelectrics. Nature 2011, 473, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Biswas, K.; He, J.; Blum, I.D.; Wu, C.I.; Hogan, T.P.; Seidman, D.N.; Dravid, V.P.; Kanatzidis, M.G. High-performance bulk thermoelectrics with all-scale hierarchical architectures. Nature 2012, 489, 414–418. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Wang, D.; Wu, H.; Xiao, Y.; Zhang, Y.; He, D.; Feng, Y.; Hao, Y.-J.; Dong, J.-F.; Chetty, R.; et al. High thermoelectric performance in low-cost SnS 0.91 Se 0.09 crystals. Science 2019, 365, 1418–1424. [Google Scholar] [CrossRef]
- Chang, C.; Wu, M.; He, D.; Pei, Y.; Wu, C.-F.; Wu, X.; Yu, H.; Zhu, F.; Wang, K.; Chen, Y.; et al. 3D charge and 2D phonon transports leading to high out-of-plane ZT in n-type SnSe crystals. Science 2018, 360, 778–783. [Google Scholar] [CrossRef]
- Imasato, K.; Kang, S.D.; Snyder, G.J. Exceptional thermoelectric performance in Mg 3 Sb 0.6 Bi 1.4 for low-grade waste heat recovery. Energy Environ. Sci. 2019, 12, 965–971. [Google Scholar] [CrossRef]
- Imasato, K.; Kang, S.D.; Ohno, S.; Snyder, G.J. Band engineering in Mg3Sb2 by alloying with Mg3Bi2 for enhanced thermoelectric performance. Mater. Horizons 2018, 5, 59–64. [Google Scholar] [CrossRef]
- Kanatzidis, M.G. Nanostructured thermoelectrics: The new paradigm? Chem. Mater. 2010, 22, 648–659. [Google Scholar] [CrossRef]
- Biswas, K.; He, J.; Zhang, Q.; Wang, G.; Uher, C.; Dravid, V.P.; Kanatzidis, M.G. Strained endotaxial nanostructures with high thermoelectric figure of merit. Nat. Chem. 2011, 3, 160–166. [Google Scholar] [CrossRef]
- Vaqueiro, P.; Powell, A.V. Recent developments in nanostructured materials for high-performance thermoelectrics. J. Mater. Chem. 2010, 20, 9577. [Google Scholar] [CrossRef]
- Zhao, L.D.; Dravid, V.P.; Kanatzidis, M.G. The panoscopic approach to high performance thermoelectrics. Energy Environ. Sci. 2014, 7, 251–268. [Google Scholar] [CrossRef]
- Sootsman, J.R.; Pcionek, R.J.; Kong, H.; Uher, C.; Kanatzidis, M.G. Strong Reduction of Thermal Conductivity in Nanostructured PbTe Prepared by Matrix Encapsulation. Chem. Mater. 2006, 18, 4993–4995. [Google Scholar] [CrossRef]
- Witting, I.T.; Chasapis, T.C.; Ricci, F.; Peters, M.; Heinz, N.A.; Hautier, G.; Snyder, G.J. The Thermoelectric Properties of Bismuth Telluride. Adv. Electron. Mater. 2019, 5, 1800904. [Google Scholar] [CrossRef]
- Dong, J.; Sun, F.; Tang, H.; Pei, J.; Zhuang, H.; Hu, H.; Zhang, B.; Pan, Y.; Li, J. Medium-temperature thermoelectric GeTe: vacancy suppression and band structure engineering leading to high performance. Energy Environ. Sci. 2019, 12, 1396–1403. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Lin, S.; Chen, Z.; Pei, Y. Realizing the High Thermoelectric Performance of GeTe by Sb-Doping and Se-Alloying. Chem. Mater. 2017, 29, 605–611. [Google Scholar] [CrossRef]
- Hong, M.; Zou, J.; Chen, Z. Thermoelectric GeTe with Diverse Degrees of Freedom Having Secured Superhigh Performance. Adv. Mater. 2019, 31, 1807071. [Google Scholar] [CrossRef]
- Perumal, S.; Samanta, M.; Ghosh, T.; Shenoy, U.S.; Bohra, A.K.; Bhattacharya, S.; Singh, A.; Waghmare, U.V.; Biswas, K. Realization of High Thermoelectric Figure of Merit in GeTe by Complementary Co-doping of Bi and In. Joule 2019, 3, 2565–2580. [Google Scholar] [CrossRef]
- Srinivasan, B.; Gellé, A.; Halet, J.-F.; Boussard-Pledel, C.; Bureau, B. Detrimental Effects of Doping Al and Ba on the Thermoelectric Performance of GeTe. Materials (Basel). 2018, 11, 2237. [Google Scholar] [CrossRef]
- May, A.F.; Fleurial, J.-P.; Snyder, G.J. Thermoelectric performance of lanthanum telluride produced via mechanical alloying. Phys. Rev. B 2008, 78, 125205. [Google Scholar] [CrossRef]
- May, A.F.; Singh, D.J.; Snyder, G.J. Influence of band structure on the large thermoelectric performance of lanthanum telluride. Phys. Rev. B 2009, 79, 153101. [Google Scholar] [CrossRef]
- Gomez, S.J.; Cheikh, D.; Vo, T.; Von Allmen, P.; Lee, K.; Wood, M.; Snyder, G.J.; Dunn, B.S.; Fleurial, J.-P.; Bux, S.K. Synthesis and Characterization of Vacancy-Doped Neodymium Telluride for Thermoelectric Applications. Chem. Mater. 2019. [Google Scholar] [CrossRef]
- Cheikh, D.; Hogan, B.E.; Vo, T.; Von Allmen, P.; Lee, K.; Smiadak, D.M.; Zevalkink, A.; Dunn, B.S.; Fleurial, J.-P.; Bux, S.K. Praseodymium Telluride: A High-Temperature, High-ZT Thermoelectric Material. Joule 2018, 2, 698–709. [Google Scholar] [CrossRef]
- Ma, J.M.; Clarke, S.M.; Zeier, W.G.; Vo, T.; Von Allmen, P.; Jeffrey Snyder, G.; Kaner, R.B.; Fleurial, J.-P.; Bux, S.K. Mechanochemical synthesis and high temperature thermoelectric properties of calcium-doped lanthanum telluride La3−xCaxTe4. J. Mater. Chem. C 2015, 3, 10459–10466. [Google Scholar] [CrossRef]
- Zhao, L.D.; Wu, H.J.; Hao, S.Q.; Wu, C.I.; Zhou, X.Y.; Biswas, K.; He, J.Q.; Hogan, T.P.; Uher, C.; Wolverton, C.; et al. All-scale hierarchical thermoelectrics: MgTe in PbTe facilitates valence band convergence and suppresses bipolar thermal transport for high performance. Energy Environ. Sci. 2013, 6, 3346–3355. [Google Scholar] [CrossRef]
- Pei, Y.; Wang, H.; Gibbs, Z.M.; LaLonde, A.D.; Snyder, J.G. Thermopower enhancement in Pb1-xMnxTe alloys and its effect on thermoelectric efficiency. NPG Asia Mater. 2012, 4, 1–6. [Google Scholar] [CrossRef]
- Lalonde, A.D.; Pei, Y.; Wang, H.; Jeffrey Snyder, G. Lead telluride alloy thermoelectrics. Mater. Today 2011, 14, 526–532. [Google Scholar] [CrossRef]
- Chen, Z.; Jian, Z.; Li, W.; Chang, Y.; Ge, B.; Hanus, R.; Yang, J.; Chen, Y.; Huang, M.; Snyder, G.J.; et al. Lattice Dislocations Enhancing Thermoelectric PbTe in Addition to Band Convergence. Adv. Mater. 2017, 29, 1606768. [Google Scholar] [CrossRef]
- Pei, Y.; Tan, G.; Feng, D.; Zheng, L.; Tan, Q.; Xie, X.; Gong, S.; Chen, Y.; Li, J.-F.; He, J.; et al. Integrating Band Structure Engineering with All-Scale Hierarchical Structuring for High Thermoelectric Performance in PbTe System. Adv. Energy Mater. 2017, 7, 1601450. [Google Scholar] [CrossRef]
- Hsu, K.F.; Loo, S.; Guo, F.; Chen, W.; Dyck, J.S.; Uher, C.; Hogan, T.; Polychroniadis, E.K.; Kanatzidis, M.G. Cubic AgPbmSbTe2+m: Bulk Thermoelectric Materials with High Figure of Merit. Science 2004, 303, 818–821. [Google Scholar] [CrossRef]
- Luo, Z.-Z.; Cai, S.; Hao, S.; Bailey, T.P.; Su, X.; Spanopoulos, I.; Hadar, I.; Tan, G.; Luo, Y.; Xu, J.; et al. High Figure of Merit in Gallium-Doped Nanostructured n-Type PbTe-xGeTe with Midgap States. J. Am. Chem. Soc. 2019, 141, 16169–16177. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, J.-F.; Nan, C.-W.; Zhou, M.; Liu, W.; Zhang, B.-P.; Kita, T. High-performance Ag0.8Pb18+xSbTe20 thermoelectric bulk materials fabricated by mechanical alloying and spark plasma sintering. Appl. Phys. Lett. 2006, 88, 092104. [Google Scholar] [CrossRef]
- Kosuga, A.; Uno, M.; Kurosaki, K.; Yamanaka, S. Thermoelectric properties of stoichiometric Ag1−xPb18SbTe20 (x = 0, 0.1, 0.2). J. Alloys Compd. 2005, 391, 288–291. [Google Scholar] [CrossRef]
- Ahmad, S.; Mahanti, S.D.; Hoang, K.; Kanatzidis, M.G. Ab initio studies of the electronic structure of defects in PbTe. Phys. Rev. B 2006, 74, 155205. [Google Scholar] [CrossRef]
- Pei, Y.; Gibbs, Z.M.; Gloskovskii, A.; Balke, B.; Zeier, W.G.; Snyder, G.J. Optimum carrier concentration in n-type PbTe thermoelectrics. Adv. Energy Mater. 2014, 4, 1–12. [Google Scholar] [CrossRef]
- Harman, T.C.; Taylor, P.J.; Walsh, M.P.; LaForge, B.E. Quantum Dot Superlattice Thermoelectric Materials and Devices. Science 2002, 297, 2229–2232. [Google Scholar] [CrossRef]
- Venkatasubramanian, R.; Siivola, E.; Colpitts, T.; O’Quinn, B. Thin-film thermoelectric devices with high room-temperature figures of merit. Nature 2001, 413, 597–602. [Google Scholar] [CrossRef]
- Wang, W.; Poudel, B.; Wang, D.; Ren, Z.F. Synthesis of PbTe nanoboxes using a solvothermal technique. Adv. Mater. 2005, 17, 2110–2114. [Google Scholar] [CrossRef]
- Szczech, J.R.; Higgins, J.M.; Jin, S. Enhancement of the thermoelectric properties in nanoscale and nanostructured materials. J. Mater. Chem. 2011, 21, 4037–4055. [Google Scholar] [CrossRef]
- Dow, H.S.; Oh, M.W.; Kim, B.S.; Park, S.D.; Min, B.K.; Lee, H.W.; Wee, D.M. Effect of Ag or Sb addition on the thermoelectric properties of PbTe. J. Appl. Phys. 2010, 108, 113709. [Google Scholar] [CrossRef]
- Wang, S.; Yang, Z.; Sun, Y.; Xiao, Y.; Zhao, L. Synergistically optimizing charge and phonon transport properties in n-type PbTe via introducing ternary compound AgSb(Se, Te)2. J. Alloys Compd. 2020, 815, 152463. [Google Scholar] [CrossRef]
- Quarez, E.; Hsu, K.-F.; Pcionek, R.; Frangis, N.; Polychroniadis, E.K.; Kanatzidis, M.G. Nanostructuring, Compositional Fluctuations, and Atomic Ordering in the Thermoelectric Materials AgPbmSbTe2+m. The Myth of Solid Solutions. J. Am. Chem. Soc. 2005, 127, 9177–9190. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cai, K.F.; Du, Y.; Wang, H.F.; Shen, S.Z.; Li, X.L.; Wang, Y.Y.; Zhou, C.W. Preparation and thermoelectric properties of AgPb18SbTe20−xSex (x = 1, 2, 4) materials. Curr. Appl. Phys. 2012, 12, 188–192. [Google Scholar] [CrossRef]
- Han, M.-K.; Hoang, K.; Kong, H.; Pcionek, R.; Uher, C.; Paraskevopoulos, K.M.; Mahanti, S.D.; Kanatzidis, M.G. Substitution of Bi for Sb and its Role in the Thermoelectric Properties and Nanostructuring in Ag1−xPb18MTe20 (M = Bi, Sb) (x = 0, 0.14, 0.3). Chem. Mater. 2008, 20, 3512–3520. [Google Scholar] [CrossRef]
- Wu, J.; Yang, J.-Y.; Zhang, J.-S.; Li, G.; Peng, J.-Y.; Xiao, Y.; Fu, L.-W.; Liu, Q.-Z. Thermoelectric Properties of Sn-Substituted AgPbmSbTem+2 via the Route of Mechanical Alloying and Plasma-Activated Sintering. J. Electron. Mater. 2012, 41, 1100–1104. [Google Scholar] [CrossRef]
- Gharsallah, M.; Serrano-Sánchez, F.; Bermúdez, J.; Nemes, N.M.; Martínez, J.L.; Elhalouani, F.; Alonso, J.A. Nanostructured Bi2Te3 Prepared by a Straightforward Arc-Melting Method. Nanoscale Res. Lett. 2016, 11, 4–10. [Google Scholar] [CrossRef]
- Gharsallah, M.; Serrano-Sanchez, F.; Nemes, N.M.; Martinez, J.L.; Alonso, J.A. Influence of Doping and Nanostructuration on n-Type Bi2(Te0.8Se0.2)3 Alloys Synthesized by Arc Melting. Nanoscale Res. Lett. 2017, 12, 47. [Google Scholar] [CrossRef] [PubMed]
- Gharsallah, M.; Serrano-Sánchez, F.; Nemes, N.M.; Mompeán, F.J.; Martínez, J.L.; Fernández-Díaz, M.T.; Elhalouani, F.; Alonso, J.A. Giant Seebeck effect in Ge-doped SnSe. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef]
- Iwanaga, S.; Toberer, E.S.; LaLonde, A.; Snyder, G.J. A high temperature apparatus for measurement of the Seebeck coefficient. Rev. Sci. Instrum. 2011, 82, 063905. [Google Scholar] [CrossRef]
- Korkosz, R.J.; Chasapis, T.C.; Lo, S.; Doak, J.W.; Kim, Y.J.; Wu, C.-I.; Hatzikraniotis, E.; Hogan, T.P.; Seidman, D.N.; Wolverton, C.; et al. High ZT in p-Type (PbTe)1–2x(PbSe)x(PbS)x Thermoelectric Materials. J. Am. Chem. Soc. 2014, 136, 3225–3237. [Google Scholar] [CrossRef]
- Chen, N.; Gascoin, F.; Snyder, G.J.; Müller, E.; Karpinski, G.; Stiewe, C. Macroscopic thermoelectric inhomogeneities in (AgSbTe2)x(PbTe)1−x. Appl. Phys. Lett. 2005, 87, 171903. [Google Scholar] [CrossRef]
- Srinivasan, B.; Gucci, F.; Boussard-Pledel, C.; Cheviré, F.; Reece, M.J.; Tricot, S.; Calvez, L.; Bureau, B. Enhancement in thermoelectric performance of n-type Pb-deficit Pb-Sb-Te alloys. J. Alloys Compd. 2017, 729, 198–202. [Google Scholar] [CrossRef]
- Heremans, J.P.; Jovovic, V.; Toberer, E.S.; Saramat, A.; Kurosaki, K.; Charoenphakdee, A.; Yamanaka, S.; Snyder, G.J. Enhancement of Thermoelectric Efficiency in PbTe by Distortion of the Electronic Density of States. Science 2008, 321, 554–557. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Lalonde, A.; Iwanaga, S.; Snyder, G.J. High thermoelectric figure of merit in heavy hole dominated PbTe. Energy Environ. Sci. 2011, 4, 2085–2089. [Google Scholar] [CrossRef]
- Parashchuk, T.; Dashevsky, Z.; Wojciechowski, K. Feasibility of a high stable PbTe:In semiconductor for thermoelectric energy applications. J. Appl. Phys. 2019, 125, 245103. [Google Scholar] [CrossRef]
- Wang, X.-K.; Veremchuk, I.; Bobnar, M.; Zhao, J.-T.; Grin, Y. Solid solution Pb1−xEuxTe: constitution and thermoelectric behavior. Inorg. Chem. Front. 2016, 3, 1152–1159. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhao, L.-D. Charge and phonon transport in PbTe-based thermoelectric materials. npj Quantum Mater. 2018, 3, 55. [Google Scholar] [CrossRef]
- May, A.F.; Snyder, G.J. Introduction to Modeling Thermoelectric Transport at High Temperatures. In Materials, Preparation, and Characterization in Thermoelectrics; Rowe, D.M., Ed.; CRC Press: Boca Raton, FL, USA, 2012; pp. 1–18. ISBN 978-1-4398-7470-7. [Google Scholar]
- Tan, G.; Shi, F.; Hao, S.; Zhao, L.-D.; Chi, H.; Zhang, X.; Uher, C.; Wolverton, C.; Dravid, V.P.; Kanatzidis, M.G. Non-equilibrium processing leads to record high thermoelectric figure of merit in PbTe–SrTe. Nat. Commun. 2016, 7, 12167. [Google Scholar] [CrossRef]
- Song, Q.; Liu, T.-H.; Zhou, J.; Ding, Z.; Chen, G. Ab initio study of electron mean free paths and thermoelectric properties of lead telluride. Mater. Today Phys. 2017, 2, 69–77. [Google Scholar] [CrossRef]
- Srinivasan, B.; Fontaine, B.; Gucci, F.; Dorcet, V.; Saunders, T.G.; Yu, M.; Cheviré, F.; Boussard-Pledel, C.; Halet, J.-F.; Gautier, R.; et al. Effect of the Processing Route on the Thermoelectric Performance of Nanostructured CuPb18SbTe20. Inorg. Chem. 2018, 57, 12976–12986. [Google Scholar] [CrossRef]
- Qin, B.; Hu, X.; Zhang, Y.; Wu, H.; Pennycook, S.J.; Zhao, L. Comprehensive Investigation on the Thermoelectric Properties of p-Type PbTe-PbSe-PbS Alloys. Adv. Electron. Mater. 2019. [Google Scholar] [CrossRef]
- Nithiyanantham, U.; Ozaydin, M.F.; Tazebay, A.S.; Kundu, S. Low temperature formation of rectangular PbTe nanocrystals and their thermoelectric properties. New J. Chem. 2016, 40, 265–277. [Google Scholar] [CrossRef]

| PbTe | LAST-18 | |
|---|---|---|
| a(Å) | 6.4595(1) | 6.4590(1) |
| V(Å3) | 269.522(8) | 269.460(9) |
| Pb,Ag,Sb 4a(0,0,0) | ||
| focc Pb/Ag/Sb | 1.00/0.0/0.0 | 0.90/0.05/0.05 |
| B(Å2) | 1.80(3) | 1.75(4) |
| Te 4b (1/2,1/2,1/2) | ||
| focc Te | 0.985(6) | 0.96(1) |
| B(Å2) | 1.19(4) | 1.13(5) |
| Agreement factors | ||
| Rp (%) | 3.07 | 3.66 |
| Rwp (%) | 4.67 | 4.95 |
| RBragg (%) | 2.61 | 2.06 |
| χ2 | 8.22 | 3.04 |
| Distances (Å) | ||
| Pb-Te (x6) | 3.230(1) | 3.229(1) |
| Thermal Conductivity (W/m−1K−1) | Seebeck Coefficient (μV/K) | Resistivity (Ω m) | Synthesis Method | Synthesis Time | Density | Reference | |
|---|---|---|---|---|---|---|---|
| PbTeM (This Work) (n = 1.9 × 1018) | 1.6 | −215 | Arc-melting + cold pressing | ~1 h | ~96% | - | |
| PbTe | 2.25 (κlattice) | - | - | Quartz tubes (Liquid matrix encapsulation) | Several hours | - | [13] |
| 4.25 | 30 | Quartz tubes + SPS | ~60 h | - | [62] | ||
| 3.8 | 60 | Graphite coated quartz tubes | ~62 h | - | [10] | ||
| PbTe (n = 4.3 × 1018) | 2.1 | −170 | Graphite coated quartz tubes + Hot pressing | 8 h + 3 days of annealing | >98% | [35] | |
| PbTe (n = 9.4 × 1019) | 5.5 | −40 | >98% | ||||
| PbTe Nanocrystals | 1.85 | 150 | Low temperature route (353 K) in a micellar medium + SPS | ~3.5 h | ~ 98.5% | [63] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gainza, J.; Serrano-Sánchez, F.; Biskup, N.; Nemes, N.M.; Martínez, J.L.; Fernández-Díaz, M.T.; Alonso, J.A. Influence of Nanostructuration on PbTe Alloys Synthesized by Arc-Melting. Materials 2019, 12, 3783. https://doi.org/10.3390/ma12223783
Gainza J, Serrano-Sánchez F, Biskup N, Nemes NM, Martínez JL, Fernández-Díaz MT, Alonso JA. Influence of Nanostructuration on PbTe Alloys Synthesized by Arc-Melting. Materials. 2019; 12(22):3783. https://doi.org/10.3390/ma12223783
Chicago/Turabian StyleGainza, Javier, Federico Serrano-Sánchez, Neven Biskup, Norbert Marcel Nemes, José Luis Martínez, María Teresa Fernández-Díaz, and José Antonio Alonso. 2019. "Influence of Nanostructuration on PbTe Alloys Synthesized by Arc-Melting" Materials 12, no. 22: 3783. https://doi.org/10.3390/ma12223783
APA StyleGainza, J., Serrano-Sánchez, F., Biskup, N., Nemes, N. M., Martínez, J. L., Fernández-Díaz, M. T., & Alonso, J. A. (2019). Influence of Nanostructuration on PbTe Alloys Synthesized by Arc-Melting. Materials, 12(22), 3783. https://doi.org/10.3390/ma12223783







