Corrosion Behavior of an AISI/SAE Steel Cut by Electropulsing
Abstract
1. Introduction
2. Materials and Methods
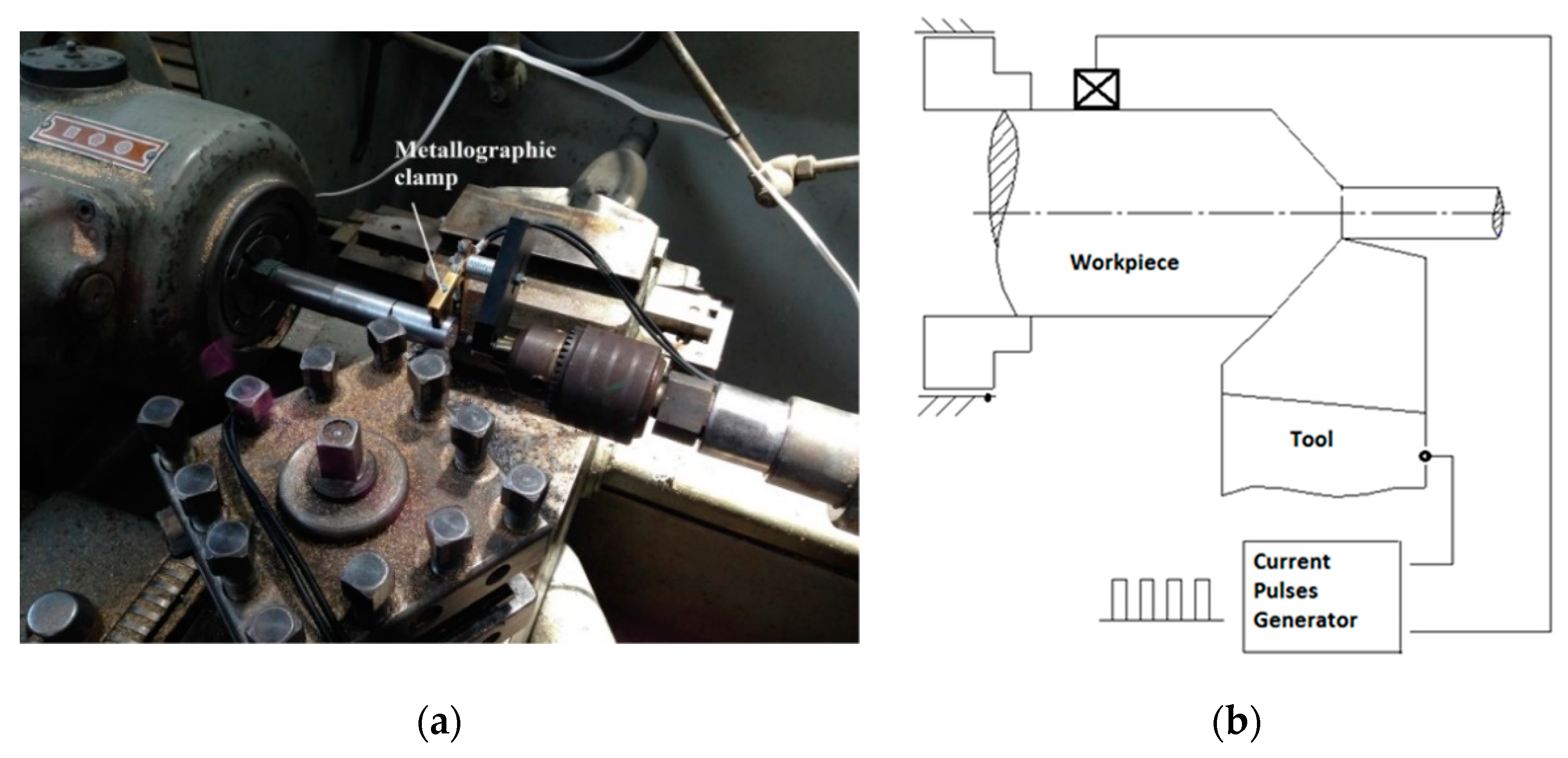
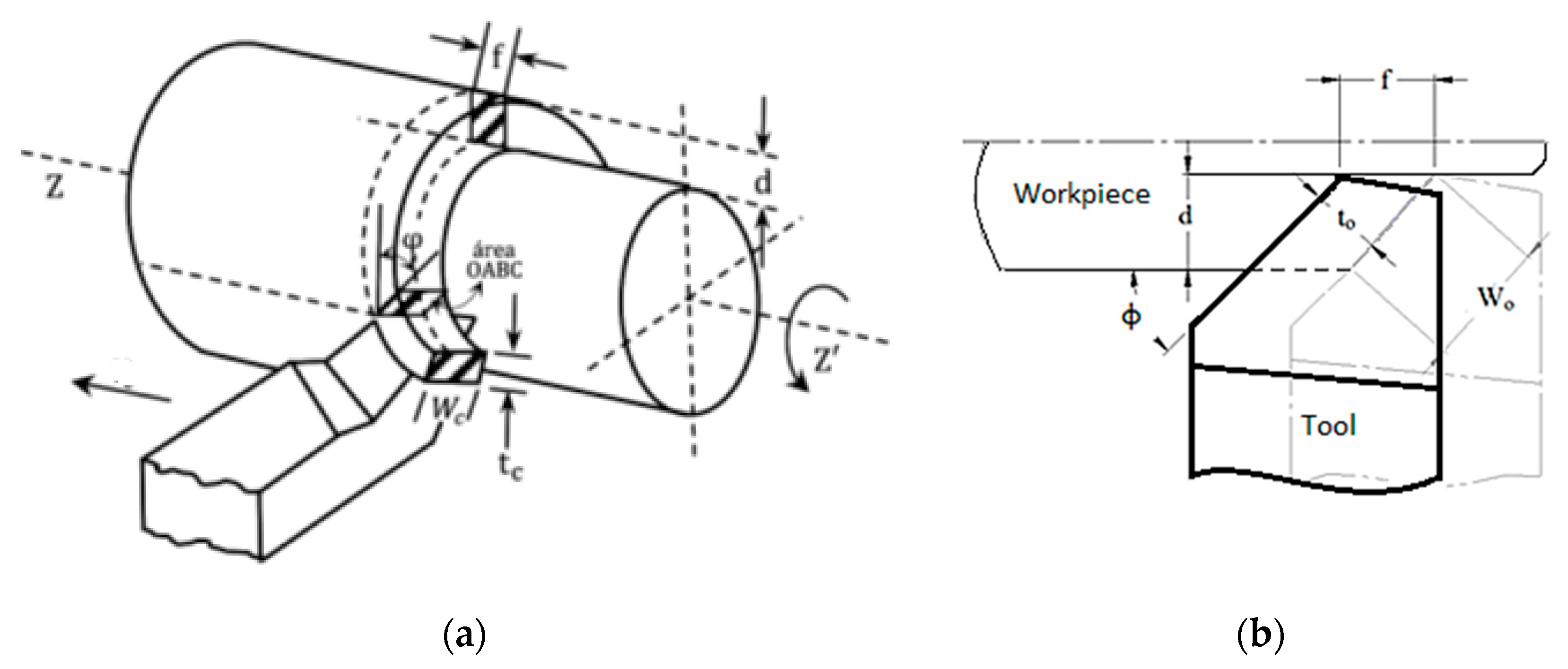
2.1. Test Specimens and Tools
2.2. Gravimetric Analysis and Salt Spray Test
2.3. Polarization Curves (PC) and Linear Polarization Resistance (LPR)
- NEQ is the equivalent number;
- fi is the fraction of the alloy element;
- ni is the element’s valence;
- ai is the atomic mass.
2.4. Determination of Corrosion Areas on Cross Sections
2.5. Micrographic Determination of the Grain Shape Factor and Microhardness
3. Results
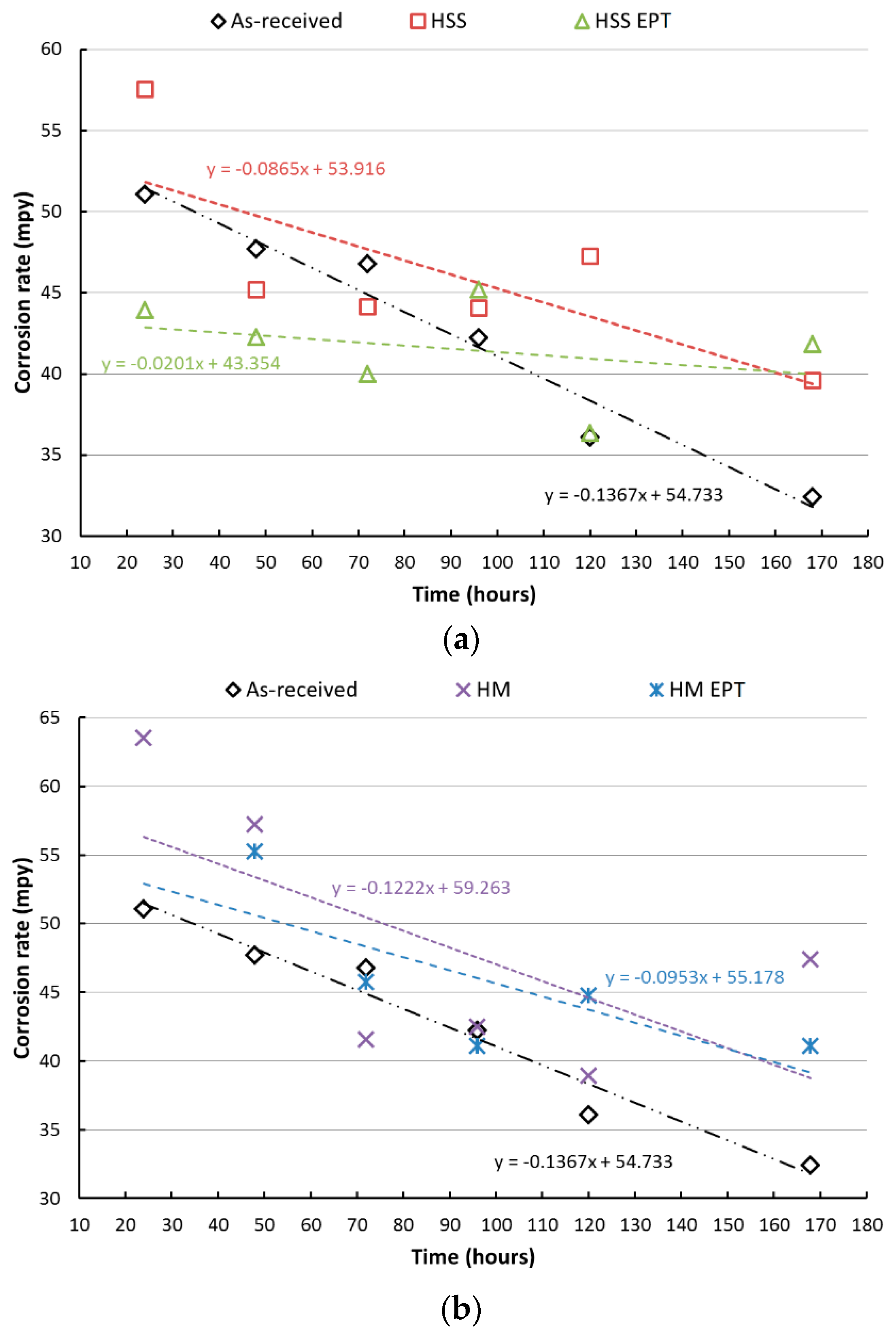
3.1. Gravimetric Analysis and Salt Spray Test
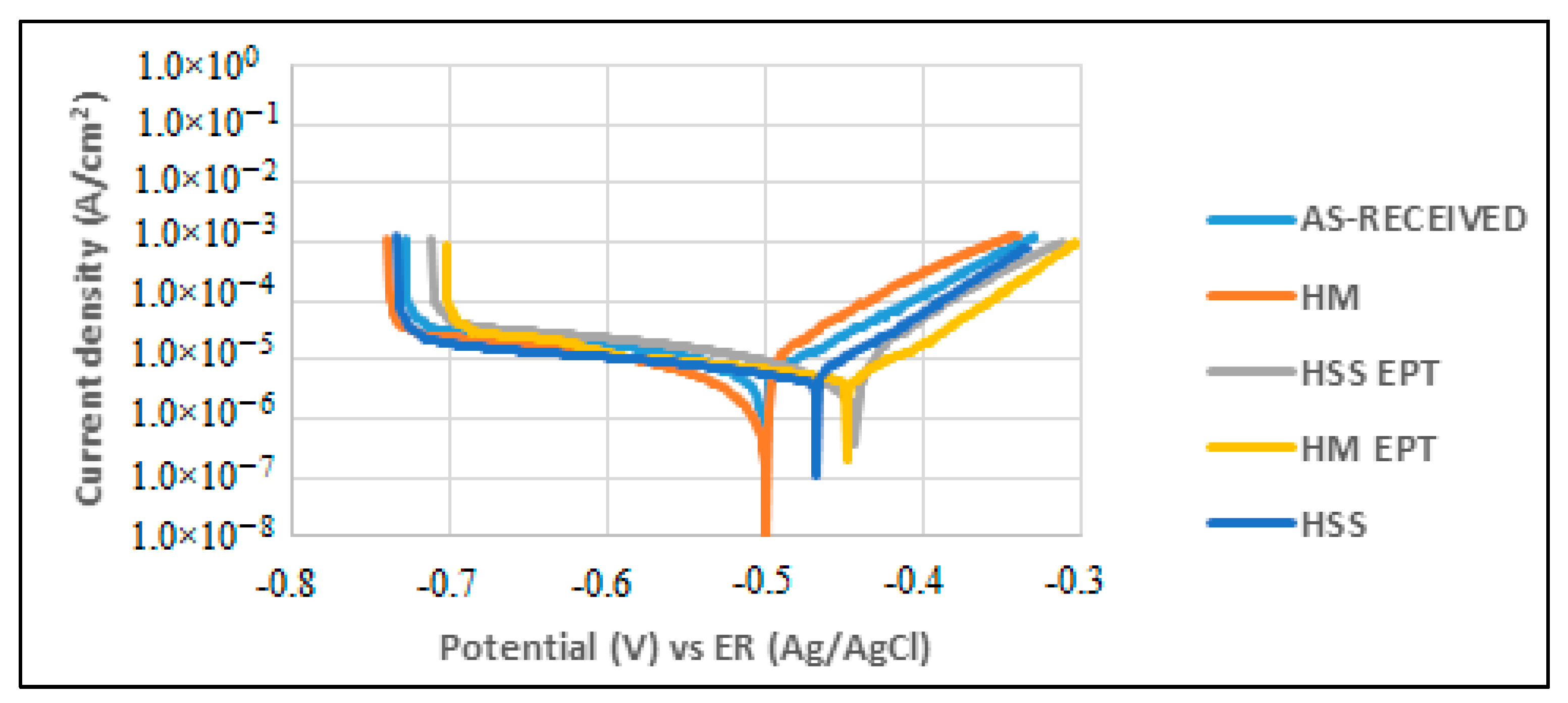
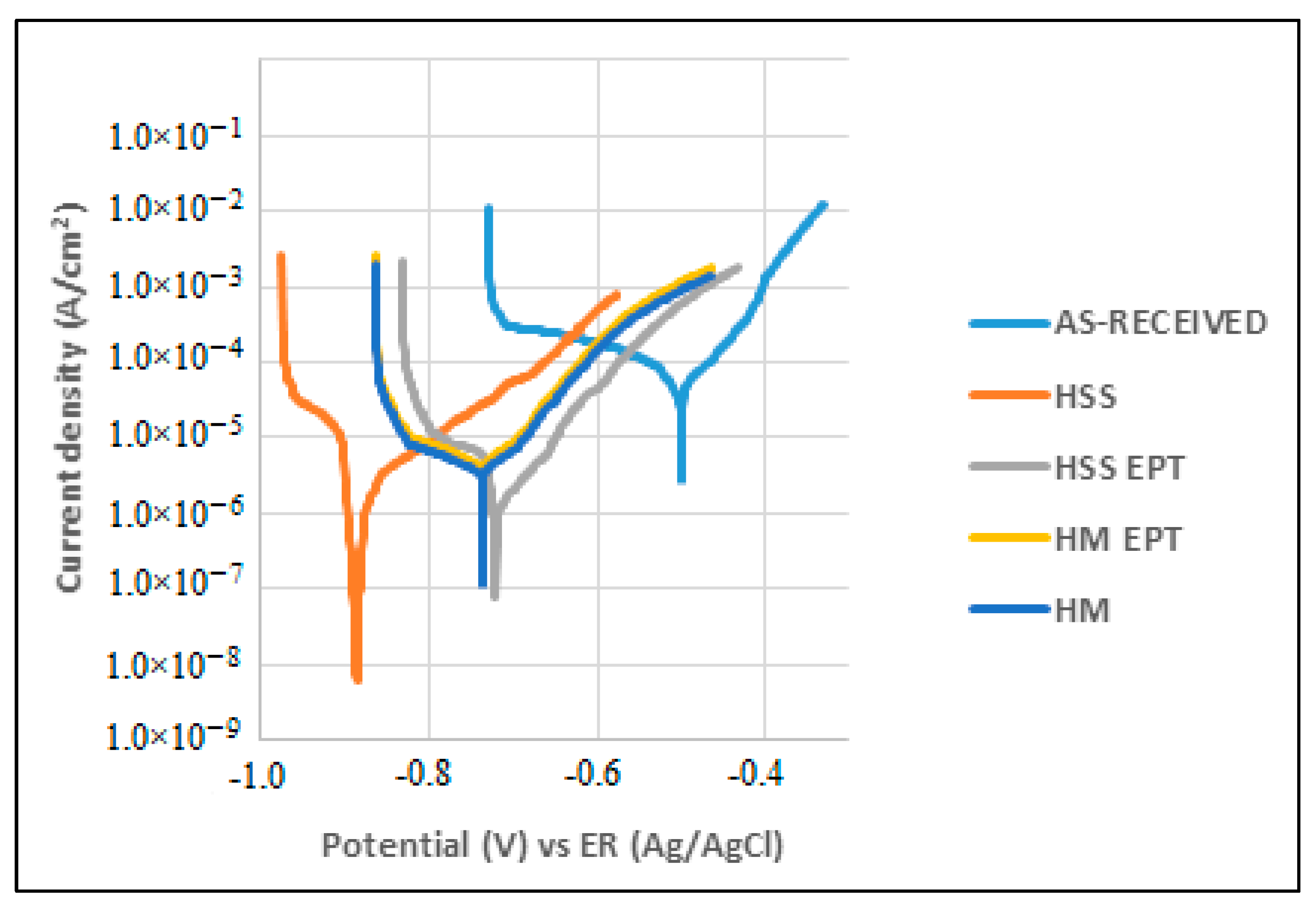
3.2. Potentiostatic Polarization Curves (PC) and Linear Polarization Resistance (LPR)
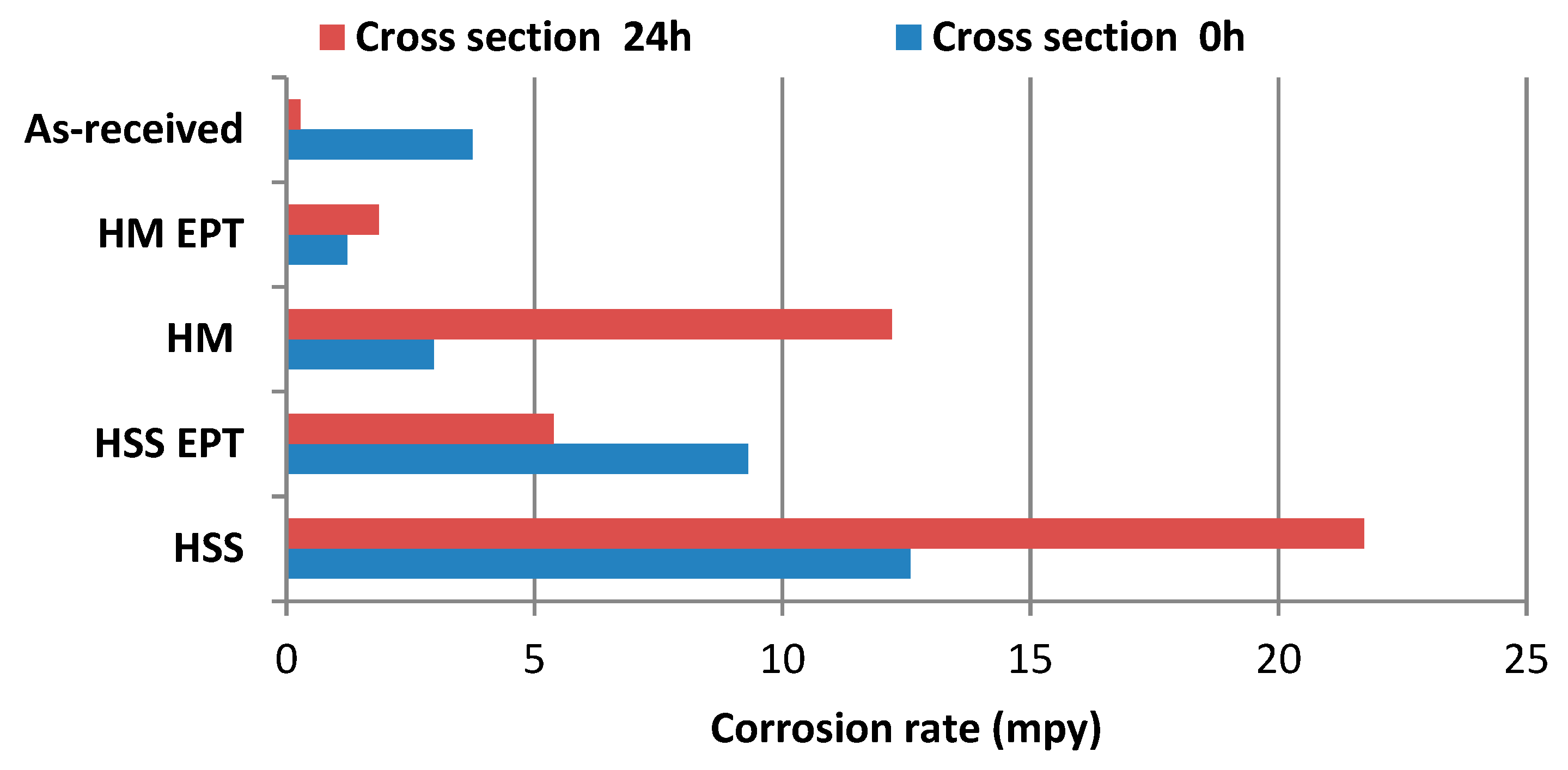
3.3. Corrosion Evolution in Circular Crowns from Cross Sections Subjected to Salt Spray Chamber
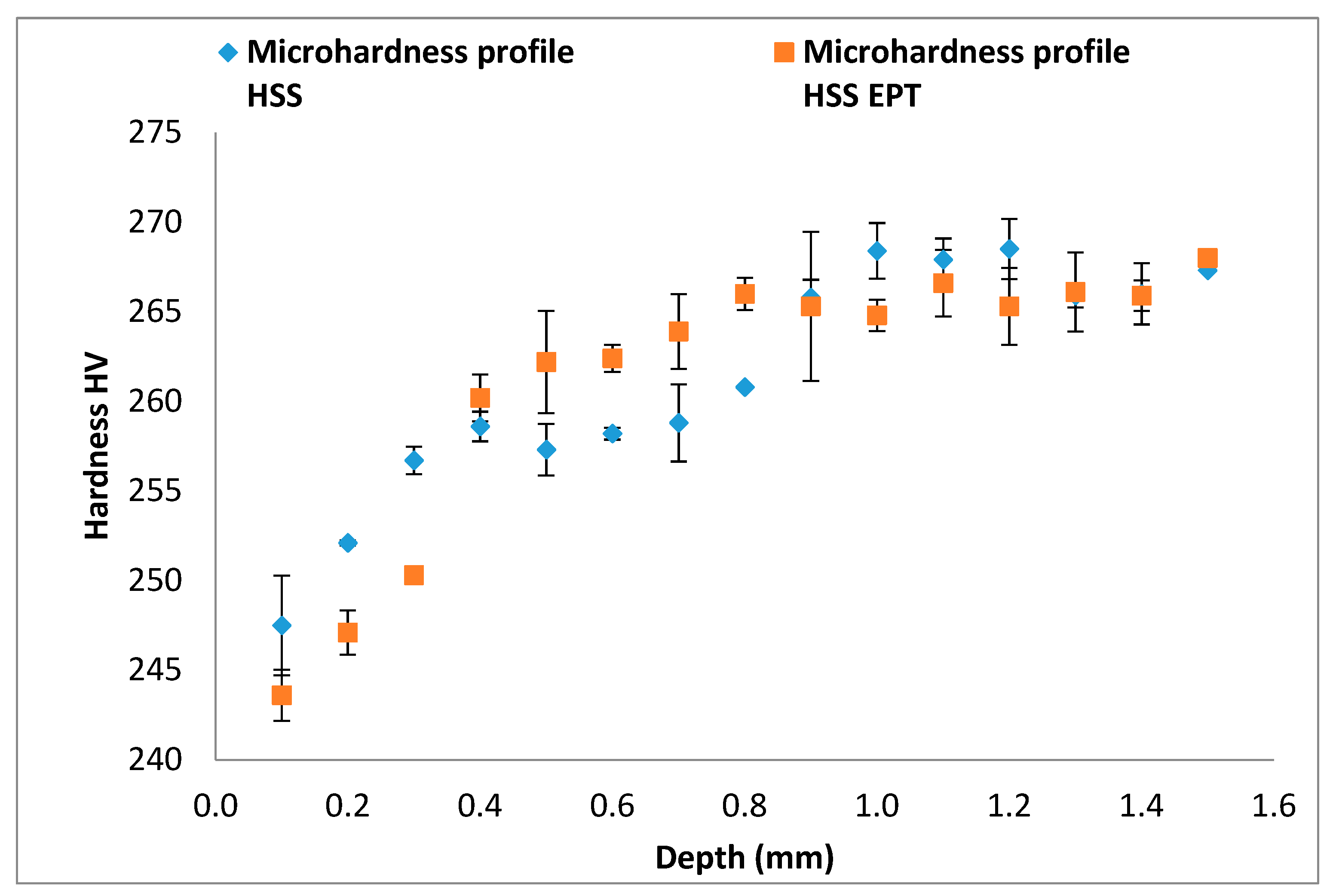
3.4. Micrographic Determination of the Grain Shape Factor h and Microhardness
4. Conclusions
- A study of the effect of turning assisted with high-density electropulsing, on the general corrosion behavior of an AISI/SAE 1045 steel, has been successfully carried out.
- The study demonstrates that the electropulsing improves the corrosion behavior of AISI/SAE 1045 steel after the turning process, especially with the use of Hard Metal HM cutting tool (1.23 mpy at 0 h and 1.86 mpy at 24 h). Meanwhile, for the HSS tool, similar behaviors were obtained, with values of 9.31 mpy at 0 h and 5.31 mpy at 24 h.
- The grain shape factor h at the edges of specimens turned with HM-EPT were slightly higher than those of their counterparts turned without EPT, consequently reducing the number of grain edges or limits, which diminishes susceptibility to intergranular corrosion.
- Additionally, the assistance with electropulsing has shown that it reduces surface microhardness of turned workpieces (a reduction of 12.3 HV in the specimens turned with the HM cutting tool, and 6.4 HV in specimens turned with the HSS cutting tool). The lowest microhardness values were found for the nearest point to edges. From a depth of 1.5 mm and on, values of microhardness become very similar to conventional turning with HM tools. This finding sheds light on the effect through the depth of the energy added through electropulsing.
- Finally, since AISI/SAE 1045 steel has widespread use in the industry, the reported improvements on corrosion resistance points out the convenience of future works on turning processes with HM tools as well as electropulsing assistance.
Author Contributions
Funding
Conflicts of Interest
References
- Shojaeipour, S. Sustainable manufacturing process planning. Int. J. Adv. Manuf. Technol. 2015, 78, 1347–1360. [Google Scholar] [CrossRef]
- Vinodh, S.; Ben Ruben, R.; Asokan, P. Life cycle assessment integrated value stream mapping framework to ensure sustainable manufacturing: A case study. Clean Technol. Environ. Policy 2016, 18, 279–295. [Google Scholar] [CrossRef]
- Dornfeld, D.A. Moving Towards Green and Sustainable Manufacturing. Int. J. Precis. Eng. Manuf. Green Technol. 2014, 1, 63–66. [Google Scholar] [CrossRef]
- Le Bourhis, F.; Kerbrat, O.; Hascoet, J.Y.; Mognol, P. Sustainable manufacturing: Evaluation and modeling of environmental impacts in additive manufacturing. Int. J. Adv. Manuf. Technol. 2013, 69, 1927–1939. [Google Scholar] [CrossRef]
- Chandrasekaran, K.; Marimuthu, P.; Raja, K. Machining Performance of TiCN/Al2O3 Multilayer and B-TiC Nano Multilayer Coated Inserts on Martensitic Stainless Steel in CNC Turning. In Emerging Trends in Science, Engineering and Technology, Lecture Notes in Mechanical Engineering; Sathiyamoorthy, S., Elizabeth Caroline, B., Gnana Jayanthi, J., Eds.; Springer India: Delhi, India, 2012; pp. 261–271. [Google Scholar]
- Khamel, S.; Ouelaa, N.; Bouacha, K. Analysis and prediction of tool wear, surface roughness and cutting forces in hard turning with CBN tool. J. Mech. Sci. Technol. 2012, 26, 3605–3616. [Google Scholar] [CrossRef]
- Zou, B.; Chen, M.; Li, S. Study on finish-turning of NiCr20TiAl nickel-based alloy using Al2O3/TiN-coated carbide tools. Int. J. Adv. Manuf. Technol. 2011, 53, 81. [Google Scholar] [CrossRef]
- Sohrabpoor, H.; Parsa, S.; Teimouri, R. Investigation of lubricant condition and machining parameters while turning of AISI 4340. Int. J. Adv. Manuf. Technol. 2015, 76, 2099–2116. [Google Scholar] [CrossRef]
- Hadad, M.; Hadi, M. An investigation on surface grinding of hardened stainless steel S34700 and aluminum alloy AA6061 using minimum quantity of lubrication (MQL) technique. Int. J. Adv. Manuf. Technol. 2013, 68, 2145–2158. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, L.; Yang, Y.; He, N.; Zhao, W. Machining distortion minimization for the manufacturing of aeronautical structure. Int. J. Adv. Manuf. Technol. 2014, 73, 1765–1773. [Google Scholar] [CrossRef]
- Kohlhoff, T.; Solter, J.; Brinksmeier, E. Influence of the Turning Process on the Distortion of Disks for Gear Manufacture. Prod. Eng. 2011, 5, 613–620. [Google Scholar] [CrossRef]
- Sánchez, A.J.; González, H.A.; Montilla, C.A.; Kallewaard, V. Effect of electroplastic cutting on the manufacturing process and surface properties. J. Mater. Process. Technol. 2015, 222, 327–334. [Google Scholar] [CrossRef]
- Sánchez, A.J.; González, H.A.; Montilla, C.A.; Kallewaard, V. Turning process assisted in situ by short time current pulses. In Proceedings of the Manufacturing Engineering Society International Conference MESIC, Barcelona, Spain, 22–24 July 2015. [Google Scholar]
- Li, C.H.; Jiang, S.; Zhang, K. Pulse current-assisted hot-forming of light metal alloy. Int. J. Adv. Manuf. Technol. 2012, 63, 931. [Google Scholar] [CrossRef]
- Renaldi, K.; Kellens, W.; Dewulf1, J.; Duflou, R. Exergy efficiency definitions for manufacturing processes. In Proceedings of the 18th CIRP International 329 Conference on Life Cycle Engineering, Technische Universität Braunschweig, Braunschweig, Germany, 2–4 May 2011. [Google Scholar]
- Li, W.; Zein, A.; Kara, S.; Herrmann, C.H. An Investigation into Fixed Energy Consumption of Machine Tools. In Proceedings of the 18th CIRP International 268 Conference on Life Cycle Engineering, Technische Universität Braunschweig, Braunschweig, Germany, 2–4 May 2011. [Google Scholar]
- Jawahir, I.S.; Jayal, A.D. Product and Process Innovation for Modeling of Sustainable Machining Processes. Institute for Sustainable Manufacturing. In Advances in Sustainable Manufacturing; Springer: Berlin, Germany, 2011; pp. 301–307. [Google Scholar]
- Neugebauer, R.; Wertheim, R.; Harzbecker, C. Energy and Resources Efficiency in the Metal Cutting Industry. In Advances in Sustainable Manufacturing; Springer: Berlin, Germany, 2011; pp. 249–259. [Google Scholar]
- Ye, Z.; Zhang, Z.; Jin, X.; Xiao, M.; Su, J. Study of hybrid additive manufacturing based on pulse laser wire depositing and milling. Int. J. Adv. Manuf. Technol. 2016, 88, 2237. [Google Scholar] [CrossRef]
- Kapil, S.; Legesse, F.; Kulkarni, P.; Joshi, P.; Desai, A.; Karunakaran, K.P. Hybrid-Layered Manufacturing Using Tungsten Inert Gas Cladding. Prog. Addit. Manuf. 2016, 1, 79. [Google Scholar] [CrossRef]
- Salandro, W.A.; Jones, J.J.; Bunget, C.; Mears, L.; Roth, J.T. Electrically Assisted Forming: Modeling and Control; Springer: Berlin, Germany, 2015. [Google Scholar]
- Fottovati, B.; Namdari, N.; Dehghandikolaei, A. On coating techniques for surface protection: A review. J. Manuf. Mater. Process. 2019, 3, 28. [Google Scholar] [CrossRef]
- Trinstancho, J.L.; Sánchez, M.; Sandoval, R.; Orozco, V.M.; Almeraya, F.; Chacón, J.G.; Gonzalez, J.G.; Martínez, A. Electrochemical impedance spectroscopy investigation of alloy inconel 718 in molten salts at high temperature. Int. J. Electrochem. Sci. 2011, 6, 419–431. [Google Scholar]
- Zhang, B.; Wang, H.; Zhang, S.; Song, G.; Kure-Chu, S.; Wang, X.; Kuang, J.; Tang, G. Effect of electropulsing-ultrasonic surface treatment on the surface properties and the corrosion behavior of 45 steel. J. Mater. Res. 2016, 31, 2114. [Google Scholar] [CrossRef]
- Ye, X.; Yang, Y.; Tang, G. Microhardness and corrosion behavior of surface gradient oxide coating on the titanium alloy strips under high energy electro-pulsing treatment. Surf. Coat. Technol. 2014, 258, 467–484. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.; Han, E.; Ke, W. Effects of Surface Machining by a Lathe on Microstructure of Near Surface Layer and Corrosion Behavior of SA182 Grade 304 Stainless Steel in Simulated Primary Water. Corros. Sci. Technol. 2019, 18, 1–7. [Google Scholar]
- Ashby, M.; Johanson, K. Materials and Design: The Art and Science of Material Selection in Product Design; Butterworth-Heinemann: Oxford, UK, 2008. [Google Scholar]
- Compañía General de Aceros. SAE 1020 y SAE 1045. Aceros de Ingeniería Al Carbono. 2007. Catálogo en Línea Disponible en. Available online: http://repository.unilibre.edu.co/bitstream/handle/10901/7826/VasquezTorresEdwinLibardo2013Anexos (accessed on 13 August 2019).
- Groover, M.P. Fundamentals of Modern Manufacturing: Materials Procesess and Systems; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2015. [Google Scholar]
- Standard Practice for Preparing, Cleaning, and Evaluation Corrosion Test Specimens; ASTM G1; ASTM International: West Conshohocken, PA, USA, 2003.
- Standard Practice for Operating Salt Spray (Fog) Apparatus; ASTM B117; ASTM International: West Conshohocken, PA, USA, 2018.
- Standard Test Method for Conducting Potentiodynamic Polarization Resistance Measurements; ASTM G 59–97; ASTM International: West Conshohocken, PA, USA, 1997.
- DrafSight 2018 (Dassault Systemes), T.M. Available online: https://www.draftsight.com/ (accessed on 1 August 2019).
- Standard Test Methods for Determining Average Grain Size; ASTM E112-13; ASTM International: West Conshohocken, PA, USA, 2013.
- Minitab 18®. Available online: https://www.minitab.com/es-mx/downloads/ (accessed on 1 August 2019).
- Taiwaide, R.; Shukla, R.; Vashishtha, H.; Ingle, A.; Dayal, R. Effect of Grain Size on Degree of Sensitization of ChromeManganese Stainless Steel. ISIJ Int. 2013, 53, 2206–2212. [Google Scholar] [CrossRef]
- Jinghui, L.; Fuguo, L.; Xinkai, M.; Jiang, L.; Shan, L. Effect of grain boundary characteristic on intergranular corrosion and mechanical properties of severely sheared Al-Zn-Mg-Cu alloy. Mater. Sci. Eng. A 2018, 732, 53–62. [Google Scholar]
- Yu, S.; He, Y.; Li, S.; Wang, L. Effect of grain size on susceptibility to intergranular corrosion for austenitic stainless steel. J. Chin. Soc. Corros. Protect. 2013, 33, 70–74. [Google Scholar]
- Totten, G. Steel Heat Treatment. Metallurgy and Technologies; Taylor & Francis Group: Boca, FL, USA, 2006. [Google Scholar]



| Element | C | Mn | Si | S | P | Fe |
|---|---|---|---|---|---|---|
| % | 0.45 | 0.70 | 0.25 | 0.007 | 0.008 | Bal. |
| Tool | Spindle Speed (RPM) | Feed (mm/rev) | Depth of Cut (mm) | Rake Angle γ (°) | Principal Cutting Edge Angle (°) |
|---|---|---|---|---|---|
| HSS | 716 | 0.138 | 1 | 14 | 40 |
| HM | 914 | 0.174 | 1 | 3 | 30 |
| Test Condition | Uncut Chip Thickness to (mm) | Uncut Chip Width Wo (mm) | Chip Thickness Tc (mm) | Chip Width Wc (mm) | Chip Ratio rc | Shear Angle ϕ (°) | Shear Plane Area Ac (mm2) |
|---|---|---|---|---|---|---|---|
| HSS | 0.09 | 1.56 | 0.336 ± 0.037 | 2.116 ± 0.286 | 0.267 | 88.5 | 0.179 |
| HSS-EPT | 0.192 ± 0.012 | 1.924 ± 0.129 | 0.465 | 77.1 | 0.184 | ||
| HM | 0.09 | 2.00 | 0.556 ± 0.009 | 1.563 ± 0.0265 | 0.156 | 84.1 | 0.135 |
| HM-EPT | 0.543 ± 0.011 | 1.521 ± 0.255 | 0.161 | 83.8 | 0.135 |
| Tool | Test Condition | Frequency (Hz) | Pulse Width (µs) | IRMS (A) | Shear Plane Area Ac (mm2) | RMS Current Density Js (A/mm2) |
|---|---|---|---|---|---|---|
| HSS | EPT | 300 | 200 | 26.93 | 0.184 | 146.36 |
| HM | EPT | 300 | 200 | 26.93 | 0.135 | 199.48 |
| Test Condition | 0 h | 24 h | ||||
|---|---|---|---|---|---|---|
| Cathodic Slope βc (V) | Anodic Slopes βa (V) | Tafel Constant β (V) | Cathodic Slope βc (V) | Anodic Slopes βa (V) | Tafel Constant β (V) | |
| HSS | 55.87 | 252.12 | 19.86 | 46.58 | 121.42 | 14.62 |
| HM EPT | 68.67 | 57.18 | 13.55 | 118.29 | 65.34 | 18.28 |
| HSS EPT | 144.55 | 39.38 | 13.44 | 58.27 | 66.51 | 13.49 |
| HM | 245.98 | 58.5 | 20.52 | 361.83 | 68.75 | 25.09 |
| As-received | 318.84 | 69.13 | 24.67 | 38.73 | 70.59 | 10.86 |
| Test Condition | 0 h | 24 h | ||||
|---|---|---|---|---|---|---|
| Corrosion Current Icorr (μA) | Polarization Resistance LPR (Ω) | Corrosion Rate Vcorr (mpy) | Corrosion Current Icorr (μA) | Polarization Resistance LPR (Ω) | Corrosion Rate Vcorr (mpy) | |
| HSS | 97.68 | 203.3 | 12.58 | 168.79 | 86.61 | 21.73 |
| HSS EPT | 69.64 | 192.97 | 9.31 | 40.35 | 714.89 | 5.39 |
| HM | 25.59 | 801.83 | 2.97 | 105.3 | 238.22 | 12.21 |
| HM EPT | 9.04 | 1500.0 | 1.23 | 13.70 | 1330.0 | 1.86 |
| As-received | 29.15 | 846.2 | 3.75 | 2.2 | 4930 | 283.94 |
| Oxidized Areas (µm2) | ||||
|---|---|---|---|---|
| Time (min) | HSS | HSS-EPT | HM | HM-EPT |
| 15 | 241,594.8 | 23,150.5 | 90,355.2 | 25,293.3 |
| 30 | 416,886.0 | 114,623.6 | 379,033.5 | 104,153.2 |
| 45 | 674,922.8 | 426,903.3 | 575,725.0 | 242,215.0 |
| 60 | 742,718.2 | 592,543.5 | 639,871.2 | 346,970.7 |
| Test Condition | Grain Shape Factor h | ANOVA p-Value |
|---|---|---|
| HSS | 1.0230 ± 0.1239 | 0.498 |
| HSS-EPT | 1.0192 ± 0.1319 | |
| HM | 1.0980 ± 0.0849 | 0.007 |
| HM-EPT | 1.3727 ± 0.1558 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montilla, C.A.; González, H.A.; Kallewaard, V.; Tristancho, J.L. Corrosion Behavior of an AISI/SAE Steel Cut by Electropulsing. Materials 2019, 12, 3782. https://doi.org/10.3390/ma12223782
Montilla CA, González HA, Kallewaard V, Tristancho JL. Corrosion Behavior of an AISI/SAE Steel Cut by Electropulsing. Materials. 2019; 12(22):3782. https://doi.org/10.3390/ma12223782
Chicago/Turabian StyleMontilla, Carlos Alberto, Hernán Alberto González, Valentina Kallewaard, and José Luis Tristancho. 2019. "Corrosion Behavior of an AISI/SAE Steel Cut by Electropulsing" Materials 12, no. 22: 3782. https://doi.org/10.3390/ma12223782
APA StyleMontilla, C. A., González, H. A., Kallewaard, V., & Tristancho, J. L. (2019). Corrosion Behavior of an AISI/SAE Steel Cut by Electropulsing. Materials, 12(22), 3782. https://doi.org/10.3390/ma12223782





