Advantage of Alveolar Ridge Augmentation with Bioactive/Bioresorbable Screws Made of Composites of Unsintered Hydroxyapatite and Poly-L-lactide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Uncalcined and Unsintered Hydroxyapatite/Poly-L-lactide Composite Screws
2.2. Subjects
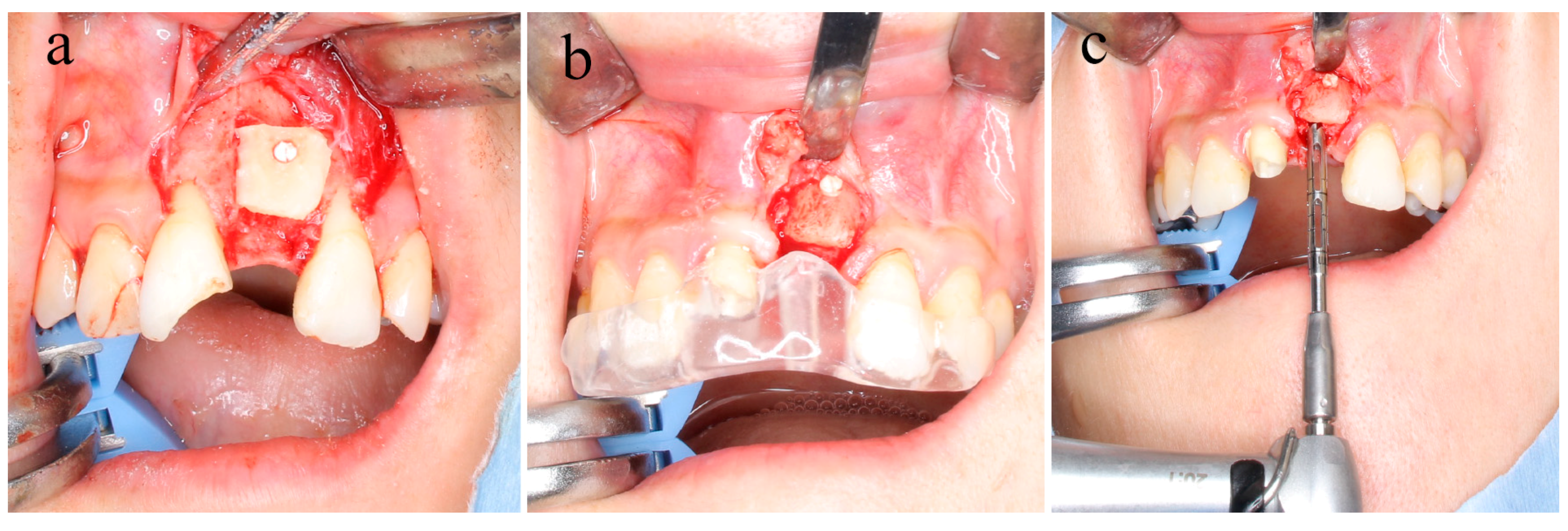
2.3. Surgical Bone Augmentation Procedure
2.4. Sample Collection
2.5. Preparation for Histological Evaluation
2.6. Immunohistochemistry
3. Results
3.1. Clinical Evaluation
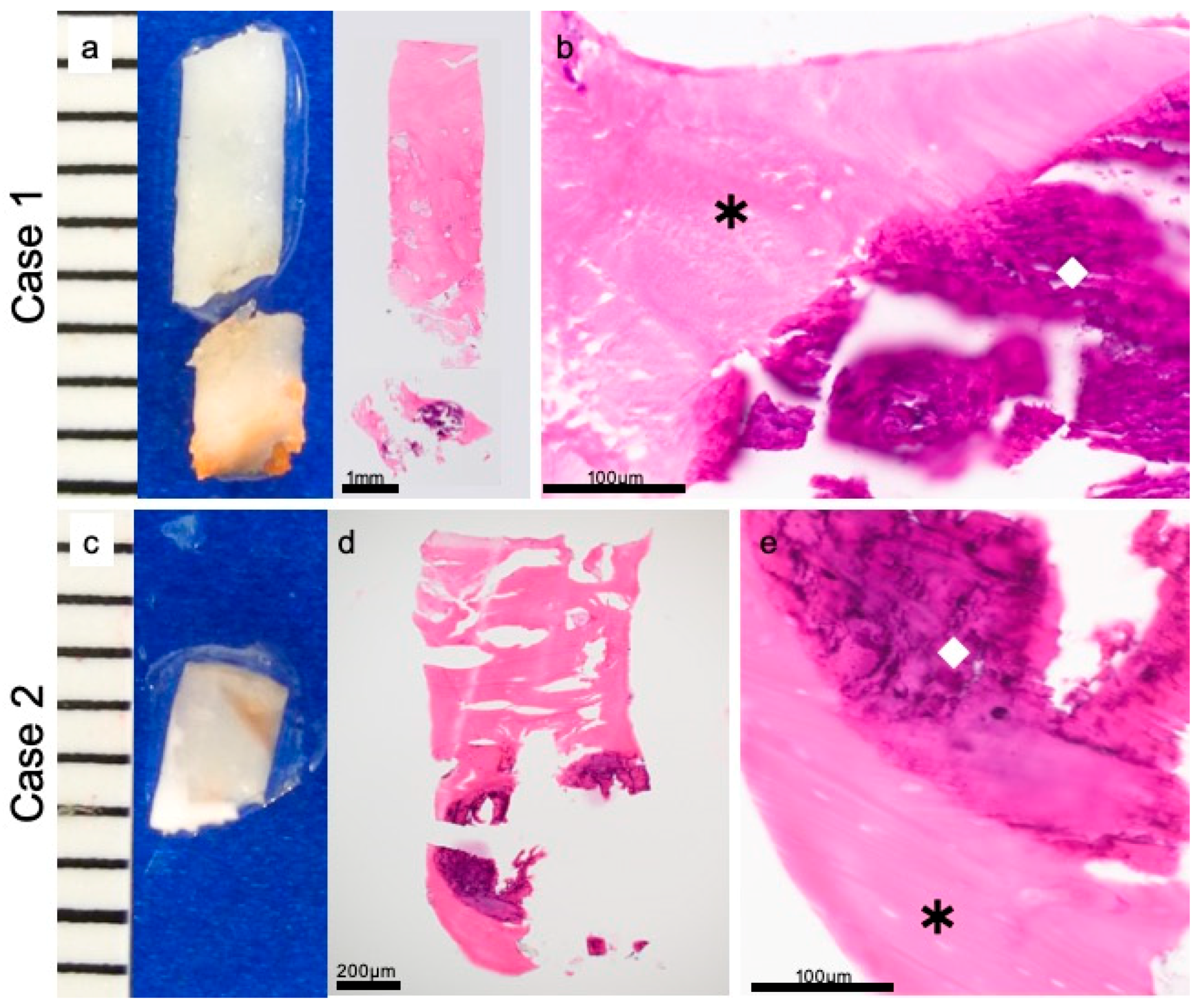
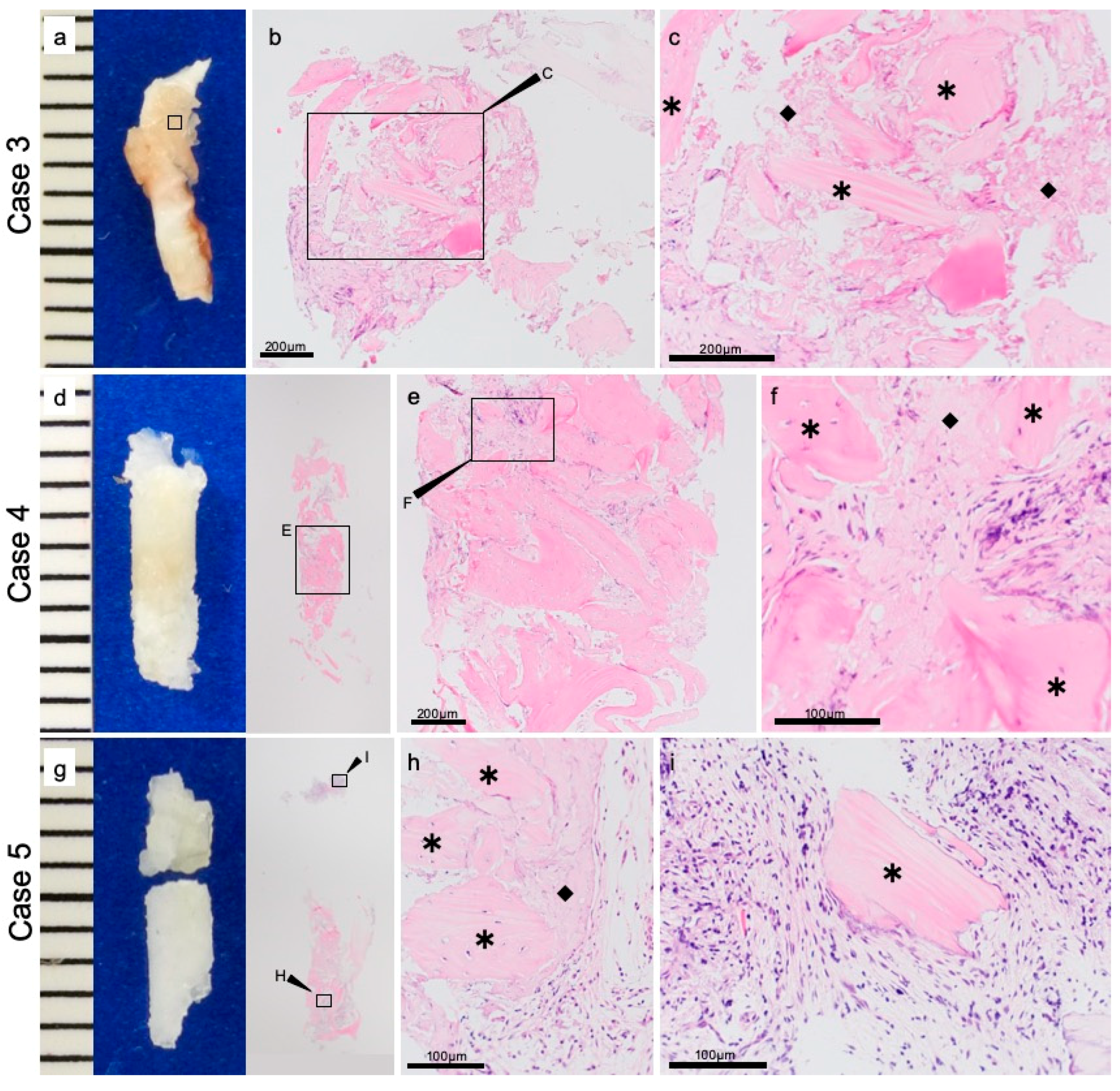
3.2. Histopathological Evaluations
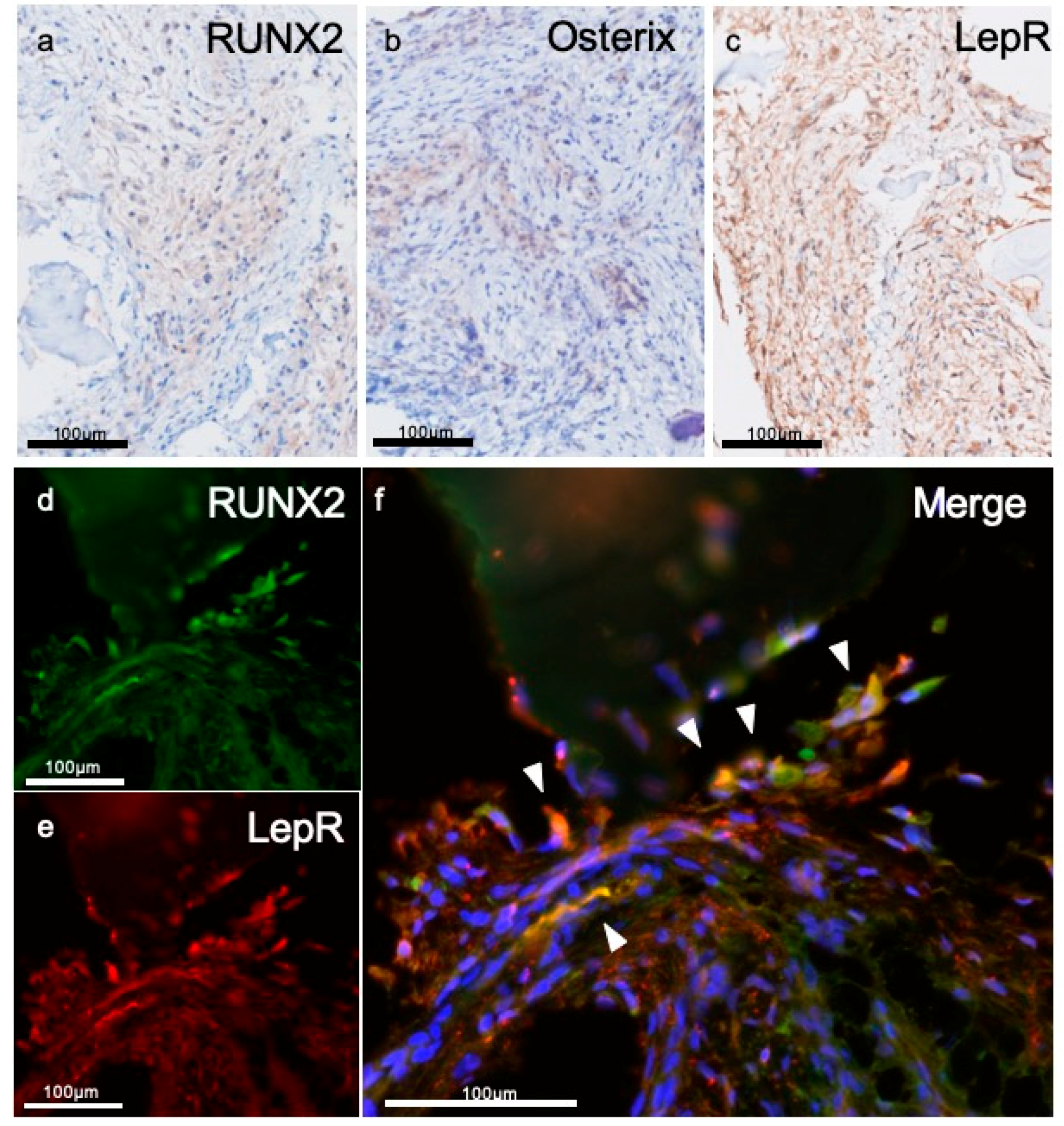
3.3. Immunohistochemical Evaluations
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Schmidt, B.L.; Perrott, D.H.; Mahan, D.; Kearns, G. The removal of plates and screws after le Fort I osteotomy. J. Oral Maxillofac. Surg. 1998, 56, 184–188. [Google Scholar] [CrossRef]
- Shikinami, Y.; Okuno, M. Bioresorbable devices made of forged composites of hydroxyapatite (HA) particles and poly-L-lactide (PLLA): Part I. Basic characteristics. Biomaterials 1999, 20, 859–877. [Google Scholar] [CrossRef]
- Hasegawa, S.; Ishii, S.; Tamura, J.; Furukawa, T.; Neo, M.; Matsusue, Y.; Shikinami, Y.; Okuno, M.; Nakamura, T. A 5–7 year in vivo study of high-strength hydroxyapatite/poly(L-lactide) composite rods for the internal fixation of bone fractures. Biomaterials 2006, 27, 1327–1332. [Google Scholar] [CrossRef] [PubMed]
- Shikinami, Y.; Okuno, M. Bioresorbable devices made of forged composites of hydroxyapatite (HA) particles and poly-L-lactide (PLLA). Part II: Practical properties of miniscrews and miniplates. Biomaterials 2001, 22, 3197–3211. [Google Scholar] [CrossRef]
- Sukegawa, S.; Kanno, T.; Manabe, Y.; Matsumoto, K.; Sukegawa-Takahashi, Y.; Masui, M.; Furuki, Y. Biomechanical Loading Evaluation of Unsintered hydroxyapatite/poly-L-lactide Plate System in bilateral Sagittal Split ramus Osteotomy. Materials 2017, 10, 764. [Google Scholar] [CrossRef] [PubMed]
- Sukegawa, S.; Kanno, T.; Katase, N.; Shibata, A.; Takahashi, Y.; Furuki, Y. Clinical evaluation of an unsintered hydroxyapatite/poly-L-lactide osteoconductive composite device for the internal fixation of maxillofacial fractures. J. Craniofac. Surg. 2016, 27, 1391–1397. [Google Scholar] [CrossRef] [PubMed]
- Sukegawa, S.; Kawai, H.; Nakano, K.; Kanno, T.; Takabatake, K.; Nagatsuka, H.; Furuki, Y. Feasible advantage of bioactive/bioresorbable devices made of forged composites of hydroxyapatite particles and poly-L-lactide in alveolar bone augmentation: A preliminary study. Int. J. Med. Sci. 2019, 16, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Sukegawa, S.; Kanno, T.; Kawai, H.; Shibata, A.; Takahashi, Y.; Nagatsuka, H.; Furuki, Y. Long-term bioresorption of bone fixation devices made from composites of unsintered hydroxyapatite particles and poly-L-lactide. J. Hard Tissue Biol. 2015, 24, 219–224. [Google Scholar] [CrossRef]
- Middleton, J.C.; Tipton, A.J. Synthetic biodegradable polymers as orthopedic devices. Biomaterials 2000, 21, 2335–2346. [Google Scholar] [CrossRef]
- Matsusue, Y.; Nakamura, T.; Iida, H.; Shimizu, K. A long-term clinical study on drawn poly-L-lactide implants in orthopaedic surgery. J. Long-Term Eff. Med. Implant. 1997, 7, 119–137. [Google Scholar]
- Kallela, P.L.; Suuronen, R.; Ranta, P.; Iizuka, T.; Lindqvist, C.; Lindqvist, C. Osteotomy site healing following mandibular sagittal split osteotomy and rigid fixation with polylactide biodegradable screws. Int. J. Oral Maxillofac. Surg. 1999, 28, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Bergsma, J.E.; de Bruijn, W.C.; Rozema, F.R.; Bos, R.R.; Boering, G. Late degradation tissue response to poly(L-lactide) bone plates and screws. Biomaterials 1995, 16, 25–31. [Google Scholar] [CrossRef]
- Furukawa, T.; Matsusue, Y.; Yasunaga, T.; Nakagawa, Y.; Okada, Y.; Shikinami, Y.; Okuno, M.; Nakamura, T. Histomorphometric study on high-strength hydroxyapatite/poly(L-lactide) composite rods for internal fixation of bone fractures. J. Biomed. Mater. Res. 2000, 50, 410–419. [Google Scholar] [CrossRef]
- Dong, Q.N.; Kanno, T.; Bai, Y.; Sha, J.; Hideshima, K. Bone Regeneration Potential of Uncalcined and Unsintered Hydroxyapatite/Poly L-lactide Bioactive/Osteoconductive Sheet Used for Maxillofacial Reconstructive Surgery: An In Vivo Study. Materials 2019, 11, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tanner, K.E.; Gurav, N.; Di Silvio, L. In Vitro osteoblastic response to 30 vol% hydroxyapatite-polyethylene composite. J. Biomed. Mater. Res. A 2007, 81, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Karsenty, G. The complexities of skeletal biology. Nature 2003, 423, 316–318. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Regulation of bone development and extracellular matrix protein genes by RUNX2. Cell Tissue Res. 2010, 339, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; Zhou, X.; Kunkel, G.; Zhang, Z.; Deng, J.M.; Behringer, R.R.; de Crombrugghe, B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 2002, 108, 17–29. [Google Scholar] [CrossRef]
- Mizoguchi, T.; Pinho, S.; Ahmed, J.; Kunisaki, Y.; Hanoun, M.; Mendelson, A.; Ono, N.; Kronenberg, H.M.; Frenette, P.S. Osterix marks distinct waves of primitive and definitive stromal progenitors during bone marrow development. Dev. Cell 2014, 29, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Arai, A.; Udagawa, N.; Hiraga, T.; Lijuan, Z.; Ito, S.; Komori, T.; Moriishi, T.; Matsuo, K.; Shimoda, K.; et al. Osteogenic factor Runx2 marks a subset of leptin receptor-positive cells that sit Atop the bone marrow stromal cell hierarchy. Sci. Rep. 2017, 7, 4928. [Google Scholar] [CrossRef] [PubMed]
| Patient Number | Sex (Male/Female) | Age (Years) | Screw Length (mm) | Number of Screws | Presence or Absence of u-HA/PLLA Screws in the Specimen | Period from the Screw Placement to Evaluation (Day) |
|---|---|---|---|---|---|---|
| 1 | Female | 44 | 8.0 | 2 | Presence | 246 |
| 2 | Female | 57 | 8.0 | 2 | Presence | 209 |
| 3 | Female | 59 | 8.0 | 2 | Absence | 219 |
| 4 | Female | 55 | 12.0 | 3 | Presence | 203 |
| 5 | Male | 55 | 12.0 | 1 | Absence | 223 |
| 6 | Female | 33 | 12.0 | 1 | Presence | 209 |
| 7 | Male | 50 | 8.0 | 2 | Presence | 226 |
| 8 | Female | 58 | 8.0 | 2 | Absence | 212 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sukegawa, S.; Kawai, H.; Nakano, K.; Takabatake, K.; Kanno, T.; Nagatsuka, H.; Furuki, Y. Advantage of Alveolar Ridge Augmentation with Bioactive/Bioresorbable Screws Made of Composites of Unsintered Hydroxyapatite and Poly-L-lactide. Materials 2019, 12, 3681. https://doi.org/10.3390/ma12223681
Sukegawa S, Kawai H, Nakano K, Takabatake K, Kanno T, Nagatsuka H, Furuki Y. Advantage of Alveolar Ridge Augmentation with Bioactive/Bioresorbable Screws Made of Composites of Unsintered Hydroxyapatite and Poly-L-lactide. Materials. 2019; 12(22):3681. https://doi.org/10.3390/ma12223681
Chicago/Turabian StyleSukegawa, Shintaro, Hotaka Kawai, Keisuke Nakano, Kiyofumi Takabatake, Takahiro Kanno, Hitoshi Nagatsuka, and Yoshihiko Furuki. 2019. "Advantage of Alveolar Ridge Augmentation with Bioactive/Bioresorbable Screws Made of Composites of Unsintered Hydroxyapatite and Poly-L-lactide" Materials 12, no. 22: 3681. https://doi.org/10.3390/ma12223681
APA StyleSukegawa, S., Kawai, H., Nakano, K., Takabatake, K., Kanno, T., Nagatsuka, H., & Furuki, Y. (2019). Advantage of Alveolar Ridge Augmentation with Bioactive/Bioresorbable Screws Made of Composites of Unsintered Hydroxyapatite and Poly-L-lactide. Materials, 12(22), 3681. https://doi.org/10.3390/ma12223681






