Randomized Controlled Clinical Trial of Nanostructured Carbonated Hydroxyapatite for Alveolar Bone Repair
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis and Physicochemical Characterization of Nanostructured Carbonated Hydroxyapatite
2.2. Ethical Considerations
2.3. Selection of Research Participant
2.4. Pre-Surgical Procedures
2.5. Surgical Procedure
2.6. Surgical Reentry
2.7. Sample Obtention
2.8. Descriptive Histological Analysis
2.9. Histomorphometric Analysis
2.10. Statistical Analysis
3. Results
3.1. Analysis of the Tested Biomaterial
3.2. Clinical Analysis
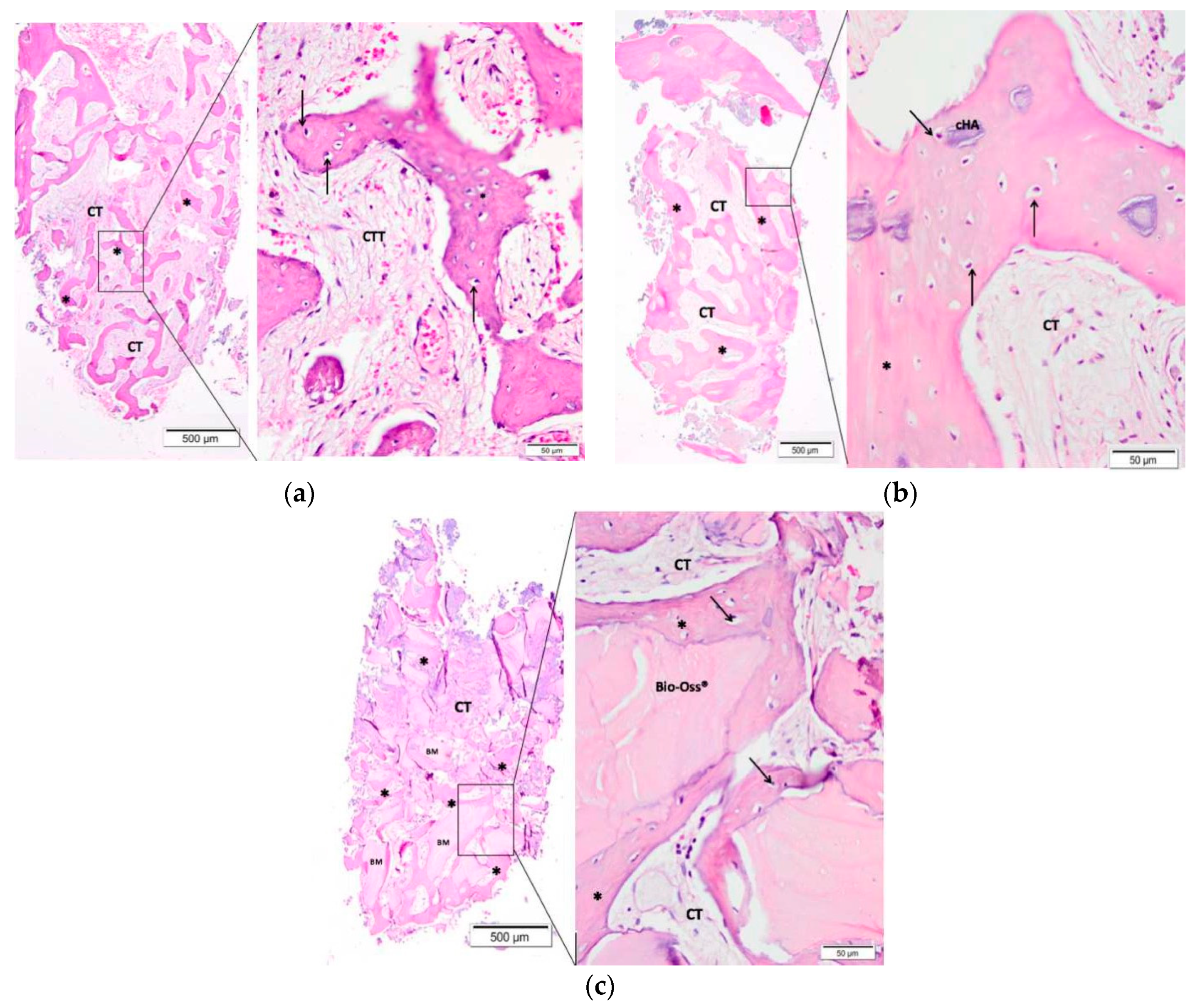
3.3. Descriptive Histological Evaluation
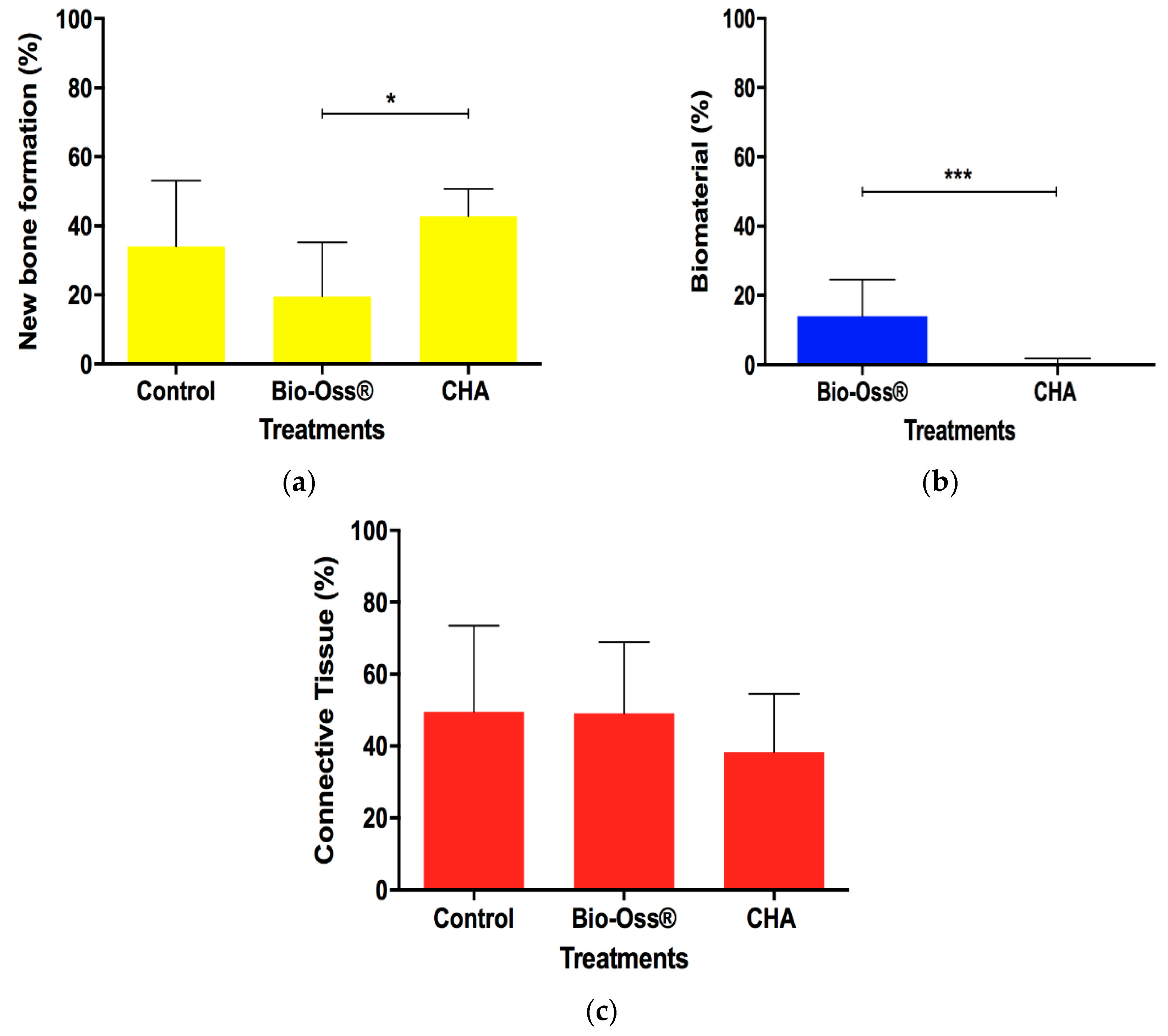
3.4. Histomorphometric Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mordenfeld, A.; Johansson, C.B.; Albrektsson, T.; Hallman, M. A randomized and controlled clinical trial of two different compositions of deproteinized bovine bone and autogenous bone used for lateral ridge augmentation. Clin. Oral Implants Res. 2014, 25, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Jambhekar, S.; Kernen, F.; Bidra, A.S. Clinical and histologic outcomes of socket grafting after flapless tooth extraction: A systematic review of randomized controlled clinical trials. J. Prosthet. Dent. 2015, 113, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Masaki, C.; Nakamoto, T.; Mukaibo, T.; Kondo, Y.; Hosokawa, R. Strategies for alveolar ridge reconstruction and preservation for implant therapy. J. Prosthodont. Res. 2015, 59, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Osorio, L.B.; Menezes, L.M.; Assaf, J.H.; Soares, A.V.; Veiga, M.L.; Stuani, M.B.S. Post-extraction evaluation of sockets with one plate loss—A microtomographic and histological study. Clin. Oral Implants Res. 2016, 27, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Vignoletti, F.; Matesanz, P.; Rodrigo, D.; Figuero, E.; Martin, C.; Sanz, M. Surgical protocols for ridge preservation after tooth extraction. A systematic review. Clin. Oral Implants Res. 2012, 23, 22–38. [Google Scholar] [CrossRef] [PubMed]
- Calasans Maia, M.D.; Resende, R.; Fernandes, G.; Calasans Maia, J.; Alves, A.T.; Granjeiro, J.M. A randomized controlled clinical trial to evaluate a new xenograft for alveolar socket presevation. Clin. Oral Implants Res. 2013, 25, 1125–1130. [Google Scholar] [CrossRef]
- Liu, J.; Schmidlin, P.R.; Philipp, A.; Hild, N.; Tawse-Smith, A.; Duncan, W. Novel bone substitute material in alveolar bone healing following tooth extraction: An experimental study in sheep. Clin. Oral Implants Res. 2016, 27, 762–772. [Google Scholar] [CrossRef]
- Tan, W.L.; Wong, T.L.T.; Wong, M.C.M.; Lang, N.P. A systematic review of post-extractional alveolar hard and soft tissue dimensional changes in humans. Clin. Oral Implants Res. 2012, 23, 1–21. [Google Scholar] [CrossRef]
- Long, W.G., Jr.; Einhorn, T.A.; Koval, K.; McKee, M.; Smith, W.; Sanders, R.; Watson, T. Bone grafts and bone graft substitutes in orthopaedic trauma surgery: A critical analysis. J. Bone Jt. Surg. Am. 2007, 89, 649–658. [Google Scholar] [CrossRef]
- Wood, R.A.; Mealey, B.L. Histologic comparison of healing after tooth extraction with ridge preservation using mineralized versus demineralized freeze-dried bone allograft. J. Periodontol. 2012, 83, 329–336. [Google Scholar] [CrossRef]
- Chiara, G.; Letizia, F.; Lorenzo, F.; Edoardo, S.; Diego, S.; Stefano, S.; Eribeto, B.; Barbara, Z. Nanostructured biomaterials for tissue engineered bone tissue reconstruction. Int. J. Mol. Sci. 2012, 13, 737–757. [Google Scholar] [CrossRef]
- Chaves, D.; Nunes, L.S.S.; Oliveira, R.V.; Holgado, L.A.; Filho, H.N.; Matsumoto, M.A.; Ribeiro, D.A. Bovine hydroxyapatite (Bio-Oss®) induces osteocalcin, RANK-L and osteoprotegerin expression in sinus lift of rabbits. J. Cranio Maxillofac. Surg. 2012, 40, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Calasans Maia, M.D.; Sartoretto, S.C.; Mourão, C.F.A.B.; Uzeda, M.J.; Alves, A.T.N.N.; Granjeiro, J.M. Maxillary Sinus Augmentation with a New Xenograft: A Randomized Controlled Clinical Trial. Clin. Implant Dent. Relat. Res. 2015, 17, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Oortgiesen, D.A.; Plachokova, A.S.; Geenen, C.; Meijer, G.J.; Walboomers, X.F.; Van den Beucken, J.J.; Jansen, J.A. A three-dimensional cell culture model to study the mechano-biological behavior in periodontal ligament regeneration. J. Clin. Periodontol. 2012, 39, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Dias, R.R.; Sehn, F.P.; Santos, T.S.; Silva, E.R.; Chaushu, G.; Xavier, S.P. Corticocancellous fresh-frozen allograft bone blocks for augmenting atrophied posterior mandibles in humans. Clin. Oral Implants Res. 2016, 27, 39–46. [Google Scholar] [CrossRef]
- Maretaningtias, M.; Matsuura, A.; Hirata, I.; Kubo, T.; Kato, K.; Akagawa, K. New development of carbonate apatite-chitosan scaffold based on lyophilization technique for bone tissue engineering. Dent. Mater. J. 2013, 32, 317–325. [Google Scholar]
- Calasans Maia, M.D.; Calasans Maia, J.; Santos, S.; Mavropoulos, E.; Farina, M.; Lima, I.; Lopes, R.T.; Rossi, A.; Granjeiro, J.M. Short-term in vivo evaluation of zinc-containing calcium phosphate using a normalized procedure. Mater. Sci. Eng. C 2014, 41, 309–319. [Google Scholar] [CrossRef]
- Saijo, H.; Chung, U.I.; Igawa, K.; Mori, Y.; Chikazu, D. Clinical application of artificial bone in the maxillofacial region. J. Artif. Organs 2008, 11, 171–176. [Google Scholar] [CrossRef]
- LeGeros, R.Z. Calcium phosphates in oral biology. Monogr. Oral Sci. 1991, 15, 1–201. [Google Scholar]
- Hasegawa, M.; Doi, Y.; Uchida, A. Cell-mediated bioresorption of sintered carbonate apatitein rabbits. J. Bone Jt. Surg. Am. 2003, 85, 142–147. [Google Scholar] [CrossRef]
- Calasans Maia, M.D.; Melo, B.R.; Resende, R.F.B.; Louro, R.S.; Sartoretto, S.C.; Granjeiro, J.M.; Alves, G.G. Cytocompatibility and biocompatibility of nanostructured carbonated hydroxyapatite spheres for bone repair. J. Appl. Oral Sci. 2015, 23, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Valiense, H.; Barreto, M.; Resende, R.; Alves, A.T.; Rossi, A.M.; Mavropoulos, E.; Granjeiro, J.M.; Calasans Maia, M.D. In vitro and in vivo evaluation of strontium-containing nanostructured carbonated hydroxyapatite/sodium alginate for sinus lift in rabbits. J. Biomed. Mater. Res. B 2016, 104, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Martinez, V.; Calasans-Maia, M.D.; Granjeiro, J.M.; Rossi, A.M.; Santana, R.B. Microspheres of alginate encapsulated minocycline-loaded nanocrystalline carbonated hydroxyapatite: Therapeutic potential and effects on bone regeneration. Int. J. Nanomed. 2019, 14, 4559–4571. [Google Scholar]
- Lebre, F.; Sridharan, R.; Sawkins, M.J.; Kelly, D.J.; O’Brien, F.J.; Lavelle, E.C. The shape and size of hydroxyapatite particles dictate inflammatory responses following implantation. Sci. Rep. 2017, 7, 2922. [Google Scholar] [CrossRef] [PubMed]
- Mourão, C.F.A.B.; Lourenço, E.S.; Nascimento, J.R.B.; Machado, R.C.M.; Leite, P.E.C.; Rossi, A.; Granjeiro, J.M.; Alves, G.G.; Maia, M.D.C. Does the Association of Blood-derived Growth Factors to Nanostructured Carbonated Hydroxyapatite Contributes to the Maxillary Sinus Floor Elevation? A Randomized Clinical Trial. Clin. Oral Investig. 2019, 23, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Hauser, F.; Gaydarov, N.; Badoud, I.; Vazquez, L.; Bernard, J.P.; Ammann, P. Clinical and histological evaluation of postextraction platelet-rich fibrin socket filling: A prospective randomized controlled study. Implant Dent. 2013, 22, 295–303. [Google Scholar] [CrossRef]
- Mitri, F.; Alves, G.; Fernandes, G.; Knig, B.; Rossi, A.J.; Granjeiro, J.M. Cytocompatibility of porous biphasic calcium phosphate granules with human mesenchymal cells by a multiparametric assay. J. Artif. Organs 2012, 36, 535–542. [Google Scholar] [CrossRef]
- Lomelino, R.O.; Castro-Silva, I.I.; Linhares, A.B.; Alves, G.G.; Santos, S.R.; Gameiro, V.S. The association of human primary bone cells with biphasic calcium phosphate (bTCP/HA 70:30) granules increases bone repair. J. Mater. Sci. Mater. Med. 2012, 23, 781–788. [Google Scholar] [CrossRef]
- Ribeiro, C.C.; Barrias, C.C.; Barbosa, M.A. Calcium phosphate-alginate microspheres as enzyme delivery matrices. Biomaterials 2004, 25, 4363–4373. [Google Scholar] [CrossRef]
- Maté Sanchez de Val, J.E.; Calvo-Guirado, J.L.; Gómez-Moreno, G.; Pérez-Albacete Martínez, C.; Mazón, P.; De Aza, P.N. Influence of hydroxyapatite granule size, porosity, and crystallinity on tissue reaction in vivo. Part A: Synthesis, characterization of the materials, and SEM analysis. Clin. Oral Implants Res. 2016, 11, 1331–1338. [Google Scholar] [CrossRef]
- Maté Sanchez de Val, J.E.; Calvo-Guirado, J.L.; Gómez-Moreno, G.; Pérez-Albacete Martínez, C.; Mazón, P.; De Aza, P.N. Influence of hydroxyapatite granule size, porosity, and crystallinity on tissue reaction in vivo. Part B: A comparative study with biphasic synthetic biomaterials. Clin. Oral Implants Res. 2018, 11, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Nancollas, G.H. Calcium Orthophosphates: Crystallization and Dissolution. Chem. Rev. 2008, 11, 4628–4669. [Google Scholar] [CrossRef] [PubMed]
- LeGeros, R. Calcium Phosphate-Based Osteoinductive Materials. Chem. Rev. 2008, 11, 4742–4753. [Google Scholar] [CrossRef] [PubMed]
- Handoll, H.H.; Watts, A.C. Bone grafts and bone substitutes for treating distal radial fractures in adults. Cochrane Database Syst. Rev. 2008, 2, CD006836. [Google Scholar] [CrossRef]
- Habibovic, P.; Juhl, M.V.; Clyens, S.; Martinetti, R.; Dolcini, L.; Theilgaard, N.; Van Blitterswijk, C.A. Comparison of two carbonated apatite ceramics in vivo. Acta Biomater. 2010, 6, 2219–2226. [Google Scholar] [CrossRef]
- Landia, E.G.; Celottia, G.; Logroscinob, A.; Tampieria, A. Carbonated hydroxyapatite as bone substitute. J. Eur. Ceram. Soc. 2003, 23, 2931–2937. [Google Scholar] [CrossRef]
- Shu, C.; Wang, Y.; Lv, H.; Peng, Z.; Yao, K. Synthesis of carbonated hydroxyapatite nanofibers by mechanochemical methods. Ceram. Int. 2005, 31, 135–138. [Google Scholar] [CrossRef]
- Mavropoulos, E.; Hausen, M.; Costa, A.M.; Albuquerque, S.R.; Alves, G.G.; Granjeiro, J.M.; Rossi, A.M. Biocompatibility of carbonated hydroxyapatite nanoparticles with different crystallinities. Key Eng. Mater. 2012, 1463, 331–336. [Google Scholar] [CrossRef]
- Sartoretto, S.C.; Alves, A.T.N.N.; Resende, R.F.B.; Rossi, A.M.; Calasans Maia, M.D.; Granjeiro, J.M. Histological evaluation of biocompatibility and biodegradation of nanostructured carbonateapatites. Implant News 2013, 6, 138–143. [Google Scholar]
- Boskey, A. Bone mineral crystal size. Osteoporos. Int. 2003, 14, 16–21. [Google Scholar]
- Takeuchi, A.; Munar, M.L.; Wakae, H.; Maruta, M.; Matsuya, S.; Tsuru, K.; Ishikawa, K. Effect of temperature on crystallinity of carbonate apatite foam prepared from a-tricalcium phosphate by hydrother-mal treatment. Biomed. Mater. Eng. 2009, 19, 205–211. [Google Scholar] [PubMed]
- Calasans Maia, M.D.; Ascoli, F.O.; Novellino, A.T.N.A.; Rossi, A.M.; Granjeiro, J.M. Comparative histological evaluation of tibial bone repair in rabbits treated with xenografts. Acta Ortop. Bras. 2009, 17, 340–343. [Google Scholar]
- Mordenfeld, A.; Hallman, M.; Johansson, C.B.; Albrektsson, T. Histological and histomorphometrical analyses of biopsies harvested 11 years after maxillary sinus floor augmentation with deproteinized bovine and autogenous bone. Clin. Oral Implants Res. 2010, 21, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Kohal, R.J.; Gubik, S.; Strohl, C.; Stampf, S.; Bächle, M.; Hurrle, A.A.; Patzelt, S.B.M. Effect of two different healing times on the mineralization of newly formed bone using a bovine bone substitute in sinus floor augmentation: A randomized, controlled, clinical and histological investigation. J. Periodontol. 2015, 42, 1052–1059. [Google Scholar] [CrossRef] [PubMed]

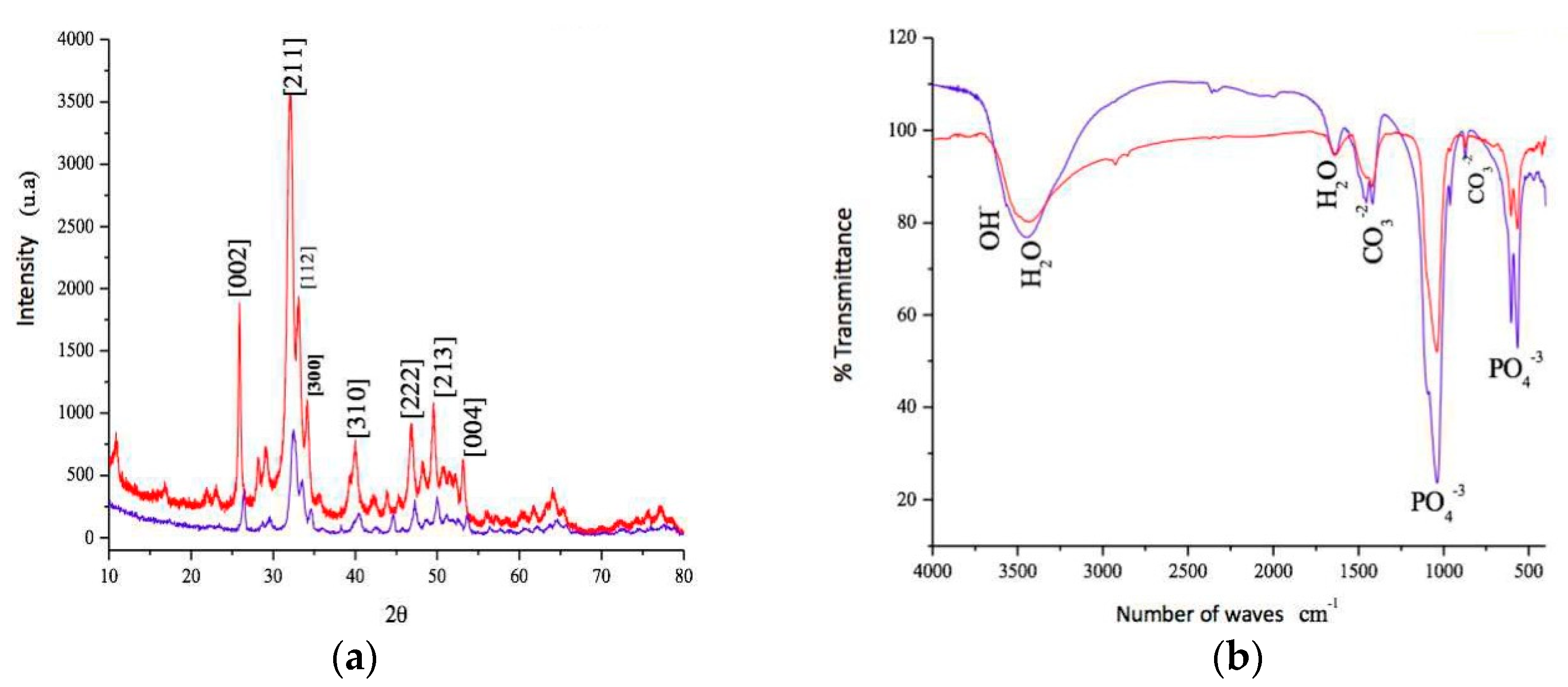
| Material | %Ca | %P | Ca/P | Surface Area (m2/g) | Porous Size (Å) | Porous Volume (cm3/g) |
|---|---|---|---|---|---|---|
| Bio-Oss® | 37, 50 | 17, 30 | 1, 68 | 80, 25 | 129, 90 | 0, 26 |
| CHA | 40, 13 | 18, 50 | 1, 53 | 93, 08 | 47, 31 | 0, 11 |
| Patient | Sex | Age | Tooth | Experimental Group |
|---|---|---|---|---|
| 1 | F | 40 | 25 ★ | 2 |
| 2 | M | 64 | 14 ★ | 1 |
| 3 | M | 34 | 36 ★ | 1 |
| 4 | F | 56 | 27 ★ | 3 |
| 5 | F | 31 | 24 ★ | 2 |
| 6 | M | 64 | 36 ★ | 1 |
| 7 | F | 35 | 26 ★ | 1 |
| 8 | F | 31 | 25 ★ | 2 |
| 9 | F | 43 | 15 * | 3 |
| 10 | F | 43 | 25 * | 3 |
| 11 | F | 42 | 11 ✚ | 2 |
| 12 | F | 42 | 12 ✚ | 2 |
| 13 | F | 56 | 36 ✚ | 1 |
| 14 | M | 44 | 12 ★ | 2 |
| 15 | M | 42 | 26 ★ | 3 |
| 16 | M | 59 | 16 ★ | 1 |
| 17 | M | 61 | 15 ★ | 1 |
| 18 | M | 66 | 25 ★ | 2 |
| 19 | M | 52 | 36 ★ | 2 |
| 20 | F | 42 | 36 ✚ | 3 |
| 21 | M | 52 | 26 ✚ | 2 |
| 22 | F | 56 | 46 ✚ | 1 |
| 23 | F | 54 | 13 ✚ | 3 |
| 24 | M | 43 | 16 ★ | 3 |
| 25 | F | 54 | 15 ★ | 1 |
| 26 | F | 30 | 27 ✚ | 1 |
| 27 | F | 54 | 15 ✚ | 3 |
| 28 | F | 54 | 23 ✚ | 3 |
| 29 | F | 54 | 14 ✚ | 2 |
| 30 | F | 54 | 25 ✚ | 3 |
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| - Age between 30 and 70 years - Good general health - Presence of a tooth for extraction - An adequate extraction site for the implant installation - A signed consent form | - Use of any medication that may alter or compromise the bone healing response - Smoker - Pregnancy or lactation - Contraindication of surgical treatment - Presenting with a known psychological disorder |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Resende, R.F.B.; Sartoretto, S.C.; Uzeda, M.J.; Alves, A.T.N.N.; Calasans-Maia, J.A.; Rossi, A.M.; Granjeiro, J.M.; Calasans-Maia, M.D. Randomized Controlled Clinical Trial of Nanostructured Carbonated Hydroxyapatite for Alveolar Bone Repair. Materials 2019, 12, 3645. https://doi.org/10.3390/ma12223645
Resende RFB, Sartoretto SC, Uzeda MJ, Alves ATNN, Calasans-Maia JA, Rossi AM, Granjeiro JM, Calasans-Maia MD. Randomized Controlled Clinical Trial of Nanostructured Carbonated Hydroxyapatite for Alveolar Bone Repair. Materials. 2019; 12(22):3645. https://doi.org/10.3390/ma12223645
Chicago/Turabian StyleResende, Rodrigo F. B., Suelen C. Sartoretto, Marcelo J. Uzeda, Adriana T. N. N. Alves, José A. Calasans-Maia, Alexandre M. Rossi, José Mauro Granjeiro, and Mônica D. Calasans-Maia. 2019. "Randomized Controlled Clinical Trial of Nanostructured Carbonated Hydroxyapatite for Alveolar Bone Repair" Materials 12, no. 22: 3645. https://doi.org/10.3390/ma12223645
APA StyleResende, R. F. B., Sartoretto, S. C., Uzeda, M. J., Alves, A. T. N. N., Calasans-Maia, J. A., Rossi, A. M., Granjeiro, J. M., & Calasans-Maia, M. D. (2019). Randomized Controlled Clinical Trial of Nanostructured Carbonated Hydroxyapatite for Alveolar Bone Repair. Materials, 12(22), 3645. https://doi.org/10.3390/ma12223645






