New Approach to Preparation of Heat-Resistant “Lola-M” Fiber
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Monomers and Solvents
2.1.2. Synthesis of PANI
2.1.3. PANI Solutions
2.2. Selection of a Coagulant
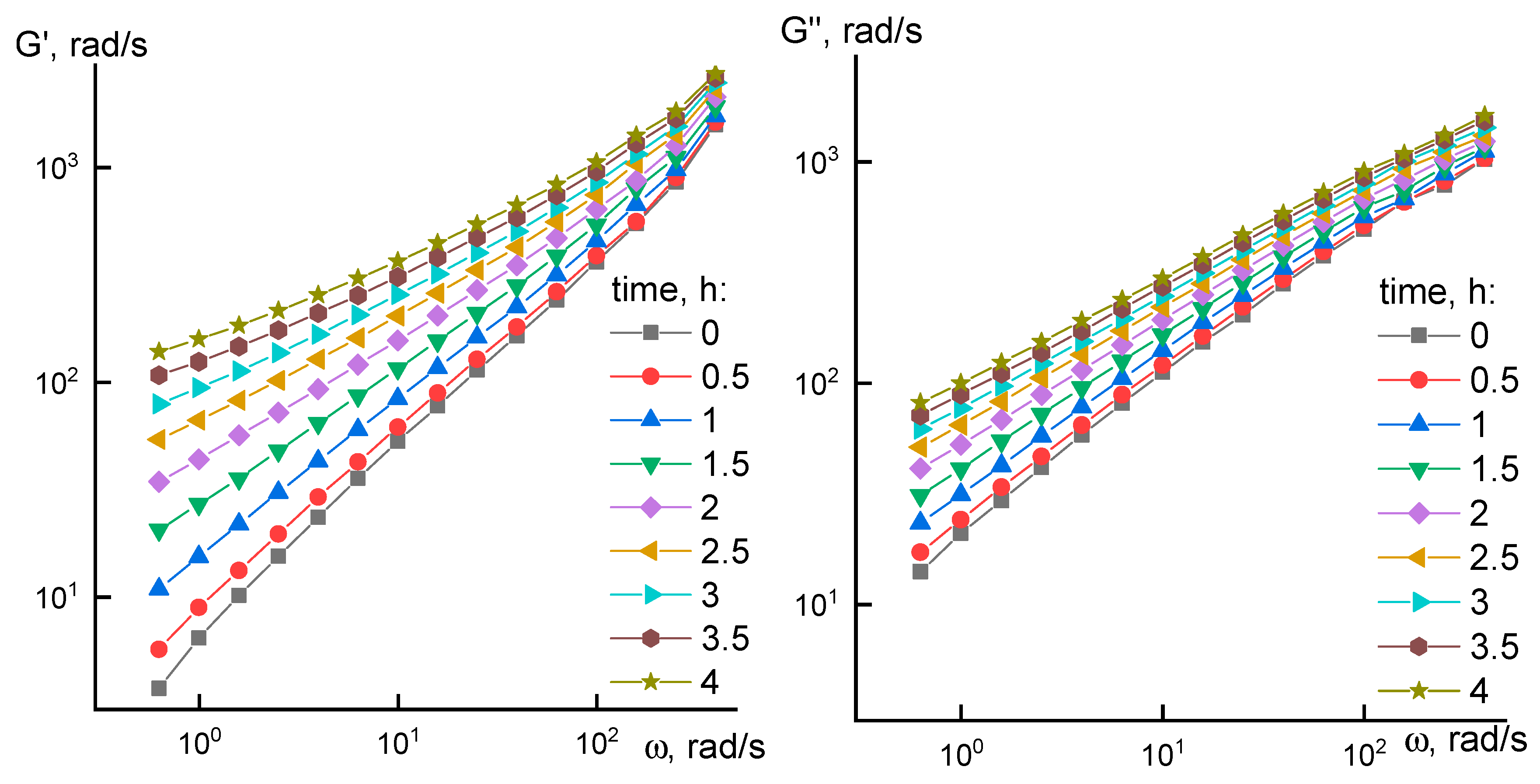
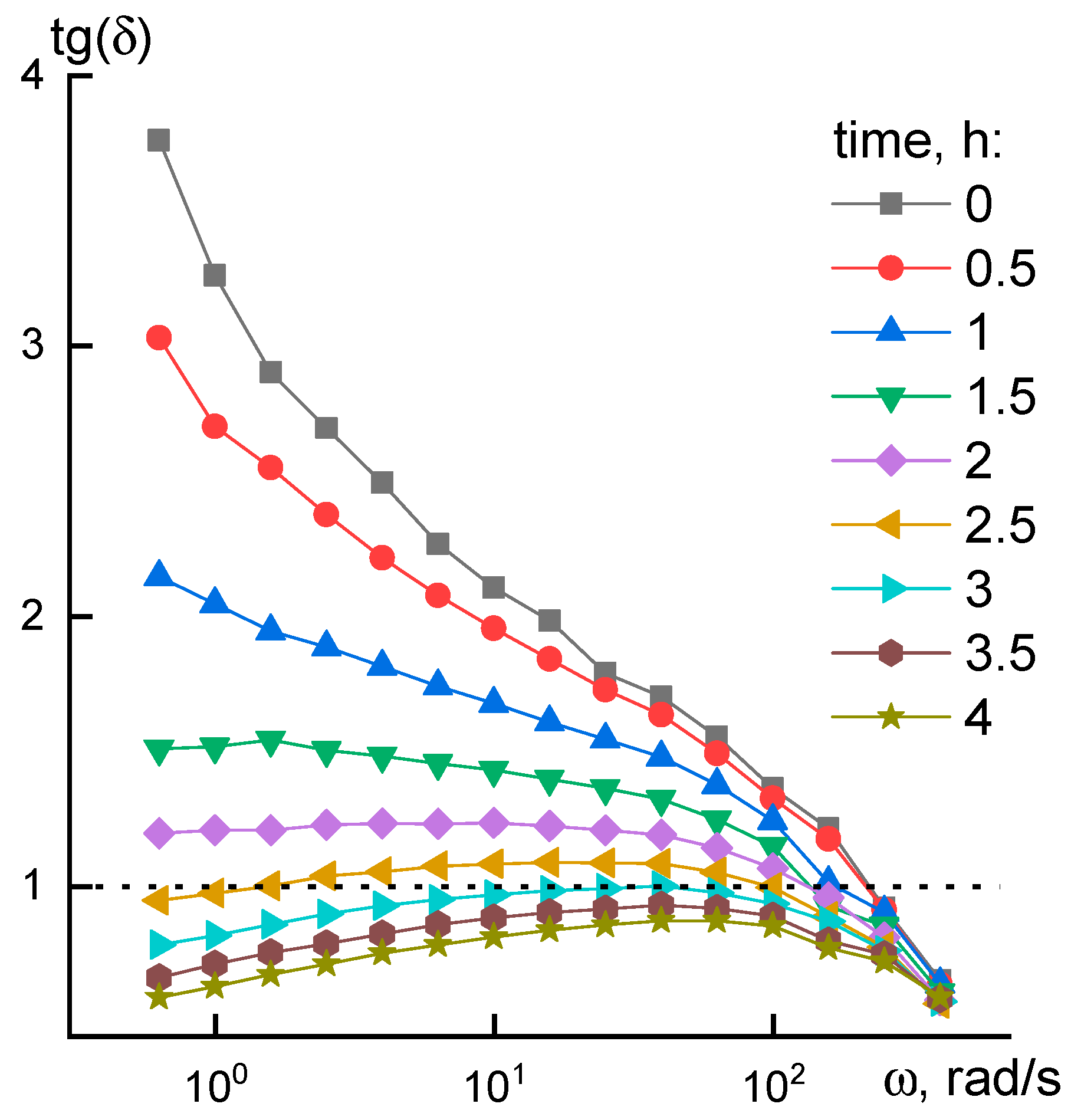
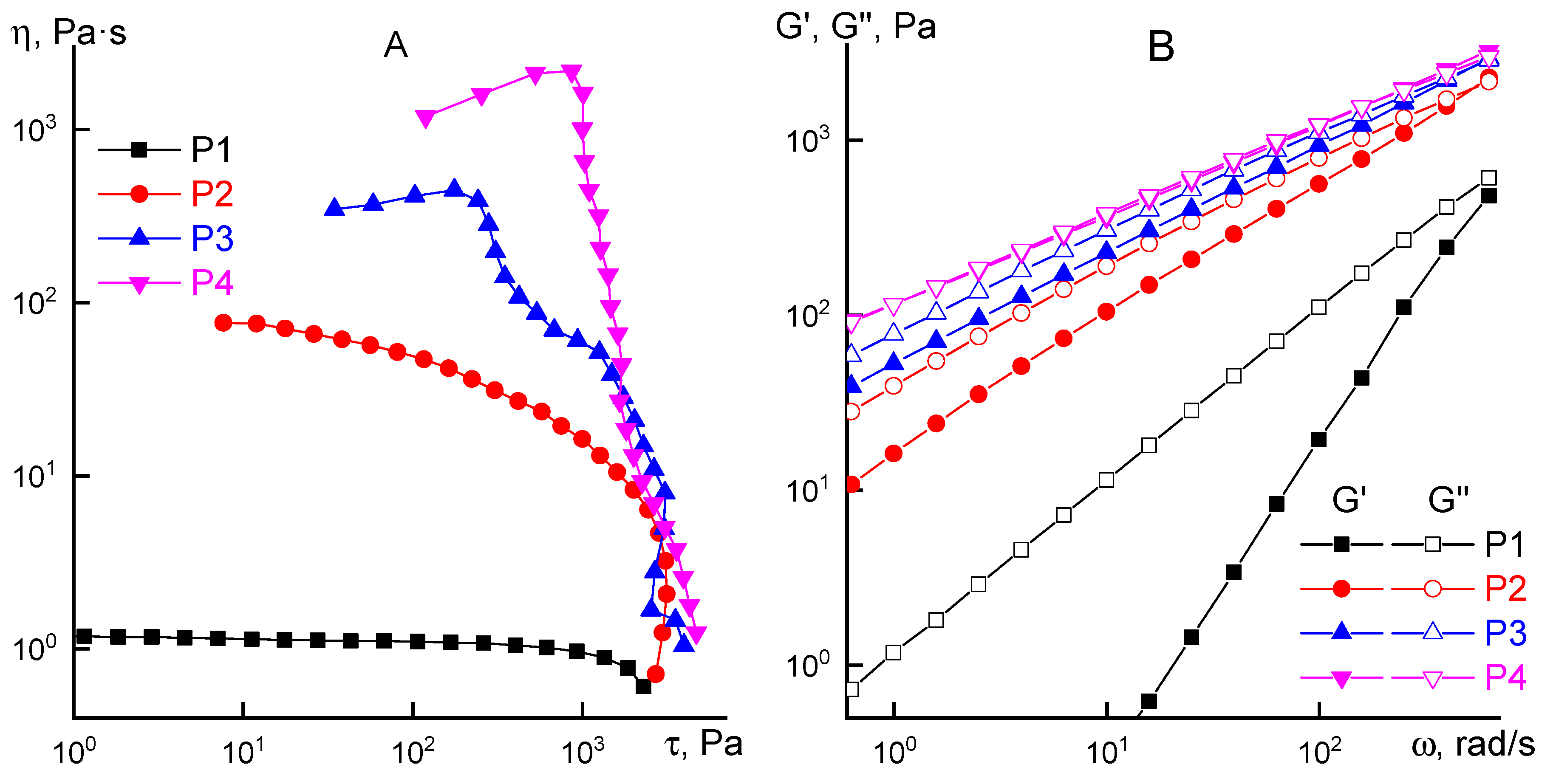
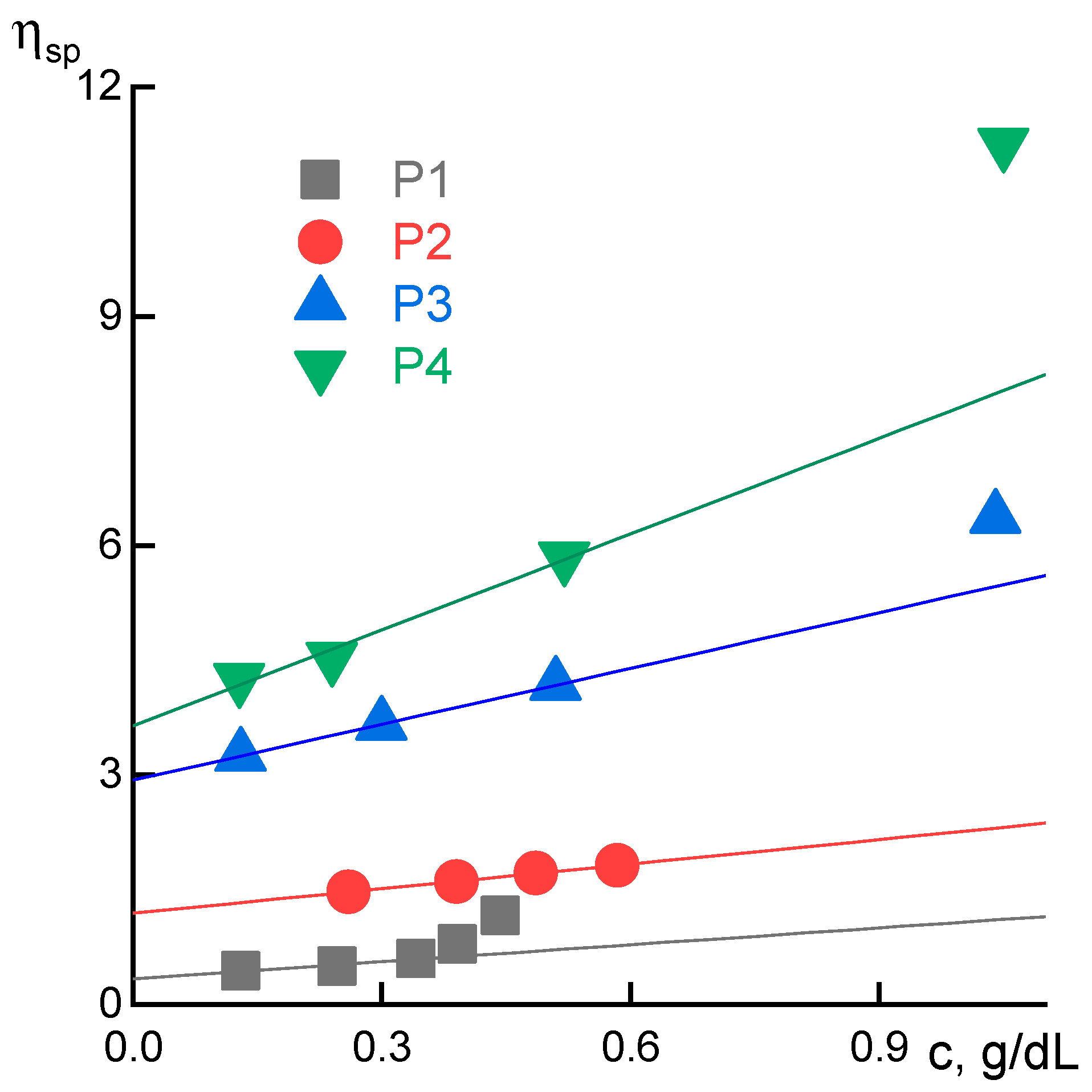
2.3. Rheology
2.4. Fiber Spinning
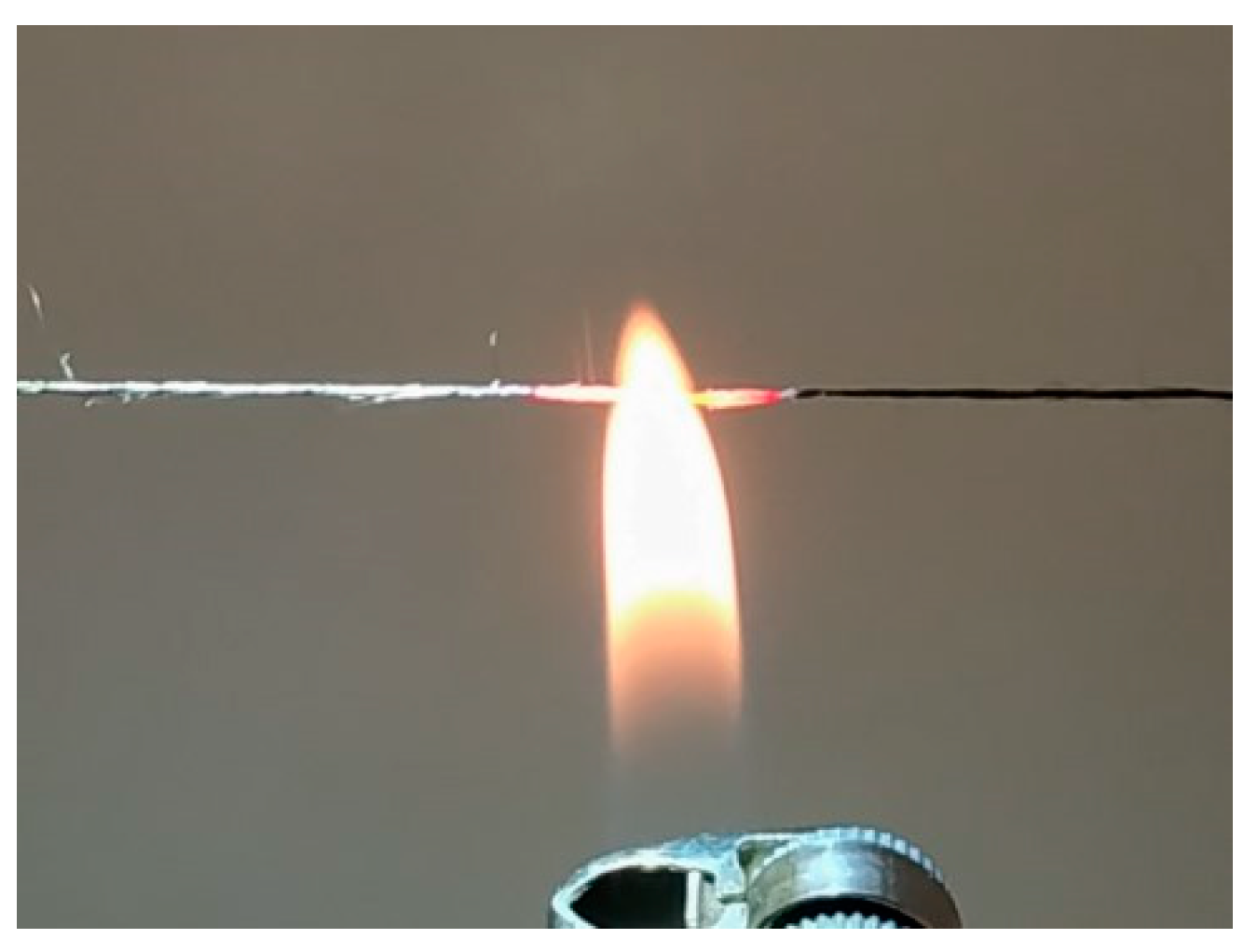
2.5. Fiber Characterization
3. Results and Discussion
3.1. Rheological Properties
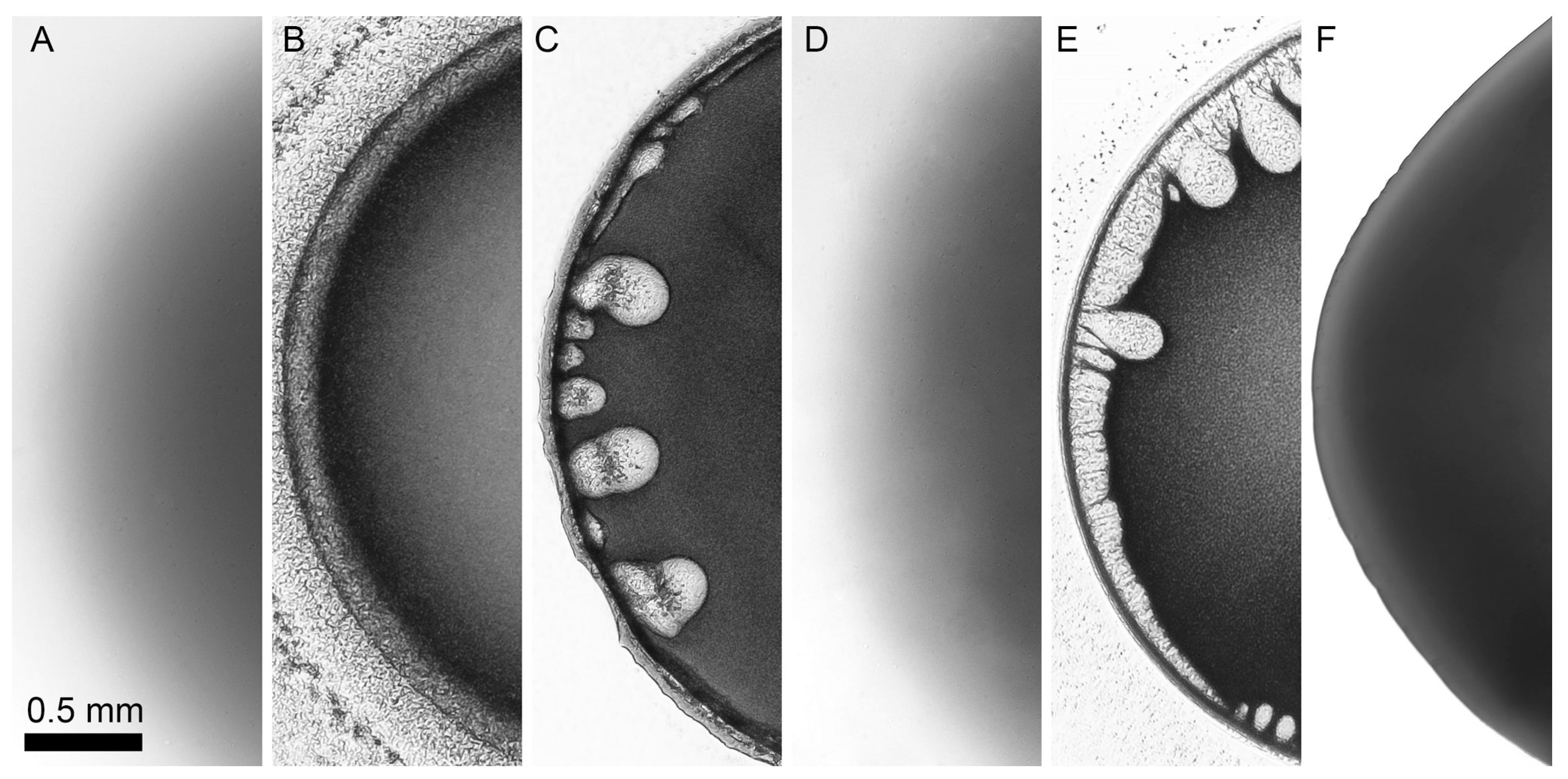
3.2. Selection of a Coagulant
3.3. Fiber Spinning
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Volokhina, A.V.; Shchetinin, A.M. Creation of High-Strength, Heat- and Fire-Resistant Synthetic Fibres. Fibre Chem. 2001, 33, 96–104. [Google Scholar] [CrossRef]
- Bühler, K.U. Spezialplaste; Akademie: Berlin, Germany, 1978. [Google Scholar]
- Van Deusen, R.L. Benzimidazo-benzophenanthroline polymers. J. Polym. Sci. Part B Polym. Lett. 1966, 4, 211–214. [Google Scholar] [CrossRef]
- Ponomarev, I.I.; Ronova, I.A.; Lindeman, A.I.; Rusanov, A.L.; Vinogradova, S.V.; Struchkov, Y.T. The effect of isomeric composition on the equilibrium rigidity of polynaphthoylenebenzimidazole chains. Polym. Sci. 1992, 34, 354–358. [Google Scholar]
- Rusanov, A.L.; Serkov, B.B.; Bulycheva, E.G.; Kolosova, T.N.; Lekae, T.V.; Ponomarev, I.I.; Matvelashvili, N.G. Flame-resistant polynaphthoylenebenzimidazoles. In Makromolekulare Chemie. Macromolecular Symposia; Hüthig & Wepf Verlag: Basel, Switzerland, 1993; Volume 74, pp. 189–192. [Google Scholar] [CrossRef]
- Pavlova, S.S.A.; Timofeeva, G.I.; Ponomarev, I.I.; Rusanov, A.L.; Matvelashvili, N.G.; Vinogradova, S.V. Gidrodinamicheskiye svoystva poli(o-aminofenilen)naftoilenimidov. (Hydrodynamic properties of poly(o-aminophenylene)naphthoyleneimides.). Vysokomol. Soedin. B 1990, 32, 842–844. [Google Scholar]
- Kulichikhin, V.G.; Skvortsov, I.Y.; Mironova, M.I.; Ozerin, A.N.; Kurkin, T.S.; Berkovich, A.K.; Frenkin, E.I.; Malkin, A.Y. From polyacrylonitrile, its solutions, and filaments to carbon fibers II. Spinning PAN-precursors and their thermal treatment. Adv. Polym. Technol. 2018, 37, 1099–1113. [Google Scholar] [CrossRef]
- Skvortsov, I.Y.; Kalugina, A.D.; Litvinova, E.G.; Malkin, A.Y.; Khotimskiy, V.S.; Kulichikhin, V.G. Fibers spinning from poly(trimethylsilylpropyne) solutions. J. Appl. Polym. Sci. 2019. [Google Scholar] [CrossRef]
- Dong, X.G.; Wang, C.G.; Bai, Y.J.; Cao, W.W. Effect of DMSO/H2O coagulation bath on the structure and property of polyacrylonitrile fibers during wet-spinning. J. Appl. Polym. Sci. 2007, 105, 1221–1227. [Google Scholar] [CrossRef]
- Makarov, I.S.; Golova, L.K.; Kuznetsova, L.K.; Antonov, S.V.; Kotsyuk, A.V.; Ignatenko, V.Y.; Kulichikhin, V.G. Influence of Precipitation and Conditioning Baths on the Structure, Morphology, and Properties of Cellulose Films. Fibre Chem. 2016, 48, 298–305. [Google Scholar] [CrossRef]
- Kulichikhin, V.; Golova, L.; Makarov, I.; Bondarenko, G.; Makarova, V.; Ilyin, S.; Skvortsov, I.; Berkovich, A. Solutions of acrylonitrile copolymers in N-methylmorpholine-N-oxide: Structure, properties, fiber spinning. Eur. Polym. J. 2017, 92, 326–337. [Google Scholar] [CrossRef]
- Vinogradov, G.V. Fundamental Problems Concerning the Interrelation of the Structure of Polymers and their Rheological Properties in the Fluid State. Pure Appl. Chem. 1974, 39, 115–149. [Google Scholar] [CrossRef]
- Vinogradov, G.V. Ultimate regimes of deformation of linear flexible chain fluid polymers. Polymer 1977, 18, 1275–1285. [Google Scholar] [CrossRef]
- Malkin, A.Y.; Patlazhan, S.A.; Kulichikhin, V.G. Physicochemical phenomena leading to slip of a fluid along a solid surface. Russ. Chem. Rev. 2019, 88, 319–349. [Google Scholar] [CrossRef]
- Zhang, M.; Ogale, A.A. Carbon fibers from dry-spinning of acetylated softwood kraft lignin. Carbon 2014, 69, 626–629. [Google Scholar] [CrossRef]
- Kulichikhin, V.; Skvortsov, I.; Subbotin, A.; Kotomin, S.; Malkin, A. A Novel Technique for Fiber Formation: Mechanotropic Spinning—Principle and Realization. Polymers 2018, 10, 856. [Google Scholar] [CrossRef] [PubMed]





| Properties | P1 | P2 | P3 | P4 |
|---|---|---|---|---|
| Concentration, % mass. | 11.6 | 11.6 | 13.0 | 13.0 |
| Intrinsic viscosity,* dL/g | 0.4 | 1.2 | 2.8 | 3.8 |
| Sample | Strength, MPa | Elongation at break, % | Modulus of elasticity, GPa | Diameter, µm |
|---|---|---|---|---|
| P1 | 32 ± 40 | 10 ± 20 | 0.6 ± 0.3 | 34 ± 5 |
| P2 | 132 ± 15 | 47 ± 10 | 1.3 ± 0.2 | 24 ± 3 |
| P3 | 144 ± 20 | 19 ± 7 | 1.5 ± 0.1 | 23 ± 4 |
| P4 | 325 ± 50 | 19 ± 6 | 2.8 ± 0.4 | 19 ± 5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ponomarev, I.I.; Skvortsov, I.Y.; Volkova, Y.A.; Ponomarev, I.I.; Varfolomeeva, L.A.; Razorenov, D.Y.; Skupov, K.M.; Kuzin, M.S.; Serenko, O.A. New Approach to Preparation of Heat-Resistant “Lola-M” Fiber. Materials 2019, 12, 3490. https://doi.org/10.3390/ma12213490
Ponomarev II, Skvortsov IY, Volkova YA, Ponomarev II, Varfolomeeva LA, Razorenov DY, Skupov KM, Kuzin MS, Serenko OA. New Approach to Preparation of Heat-Resistant “Lola-M” Fiber. Materials. 2019; 12(21):3490. https://doi.org/10.3390/ma12213490
Chicago/Turabian StylePonomarev, Igor I., Ivan Y. Skvortsov, Yulia A. Volkova, Ivan I. Ponomarev, Lydia A. Varfolomeeva, Dmitry Y. Razorenov, Kirill M. Skupov, Mikhail S. Kuzin, and Olga A. Serenko. 2019. "New Approach to Preparation of Heat-Resistant “Lola-M” Fiber" Materials 12, no. 21: 3490. https://doi.org/10.3390/ma12213490
APA StylePonomarev, I. I., Skvortsov, I. Y., Volkova, Y. A., Ponomarev, I. I., Varfolomeeva, L. A., Razorenov, D. Y., Skupov, K. M., Kuzin, M. S., & Serenko, O. A. (2019). New Approach to Preparation of Heat-Resistant “Lola-M” Fiber. Materials, 12(21), 3490. https://doi.org/10.3390/ma12213490








