The Effect of the Synthetic Procedure of Acrylonitrile–Acrylic Acid Copolymers on Rheological Properties of Solutions and Features of Fiber Spinning
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
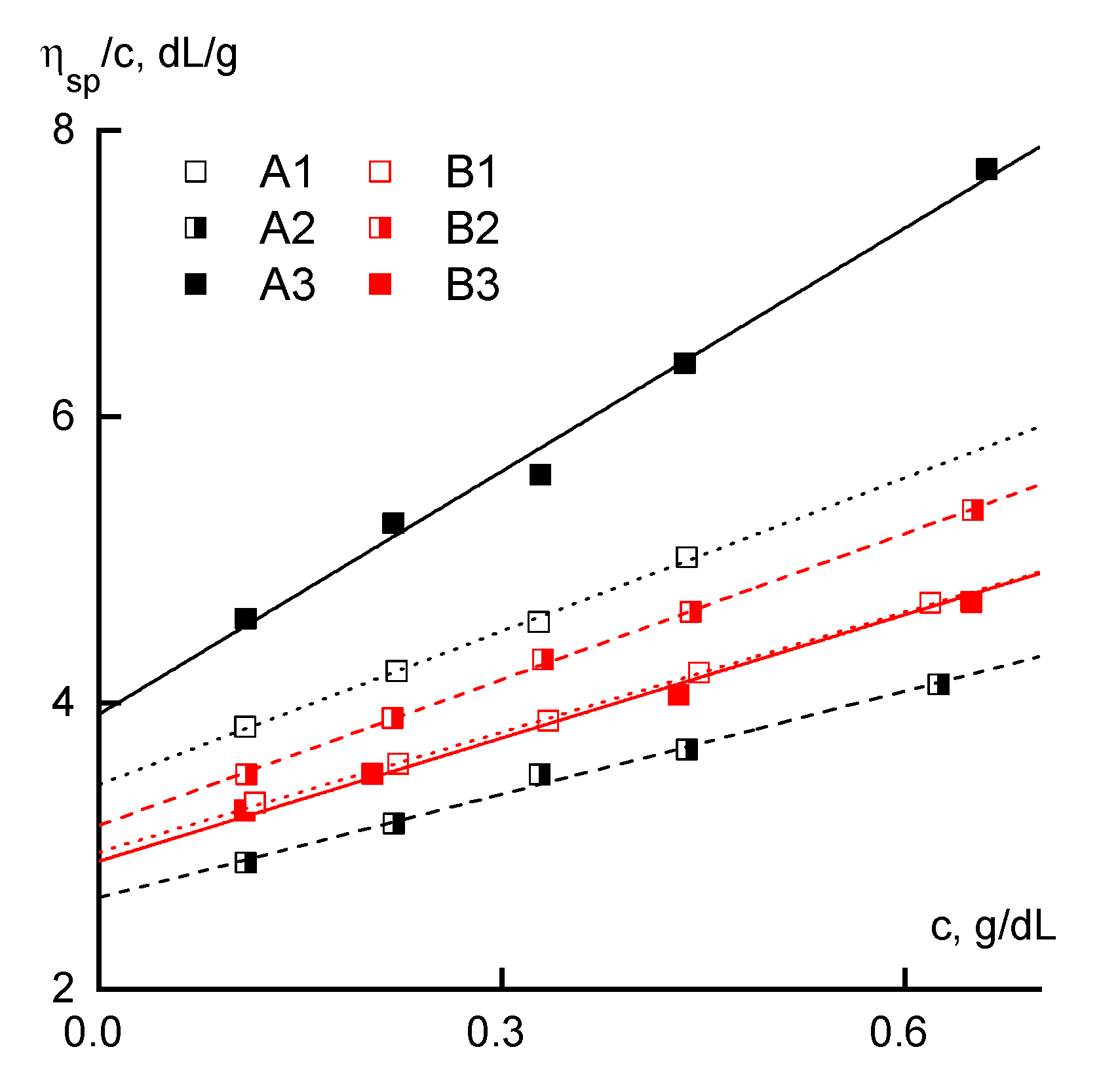
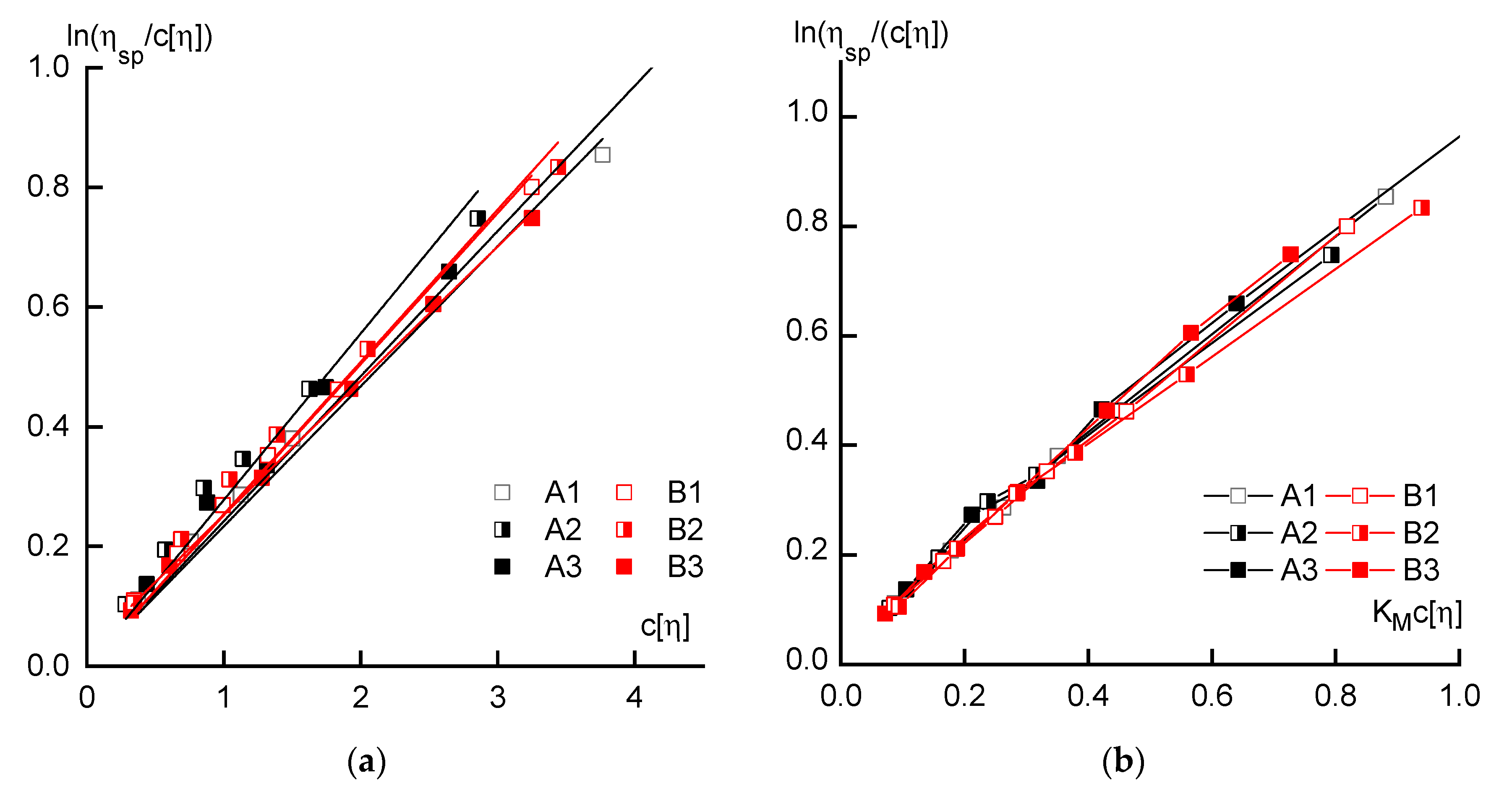
2.2.1. Rheology
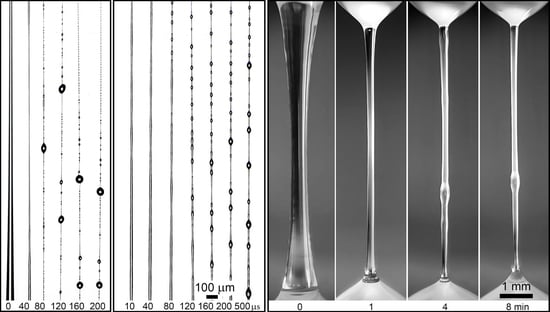
2.2.2. Modeling of Mechanotropic Spinning
- A solution drops with a volume of ~10 μL was squeezed from a syringe located at the bottom of the unit;
- By displacing the movable syringe, a drop was brought into contact with the upper plate of the optical fiber lens;
- The droplet was stretched at a constant speed of 65 mm/min by linearly moving the syringe to a distance of 11 mm. The duration of the process was approximately 10 s.
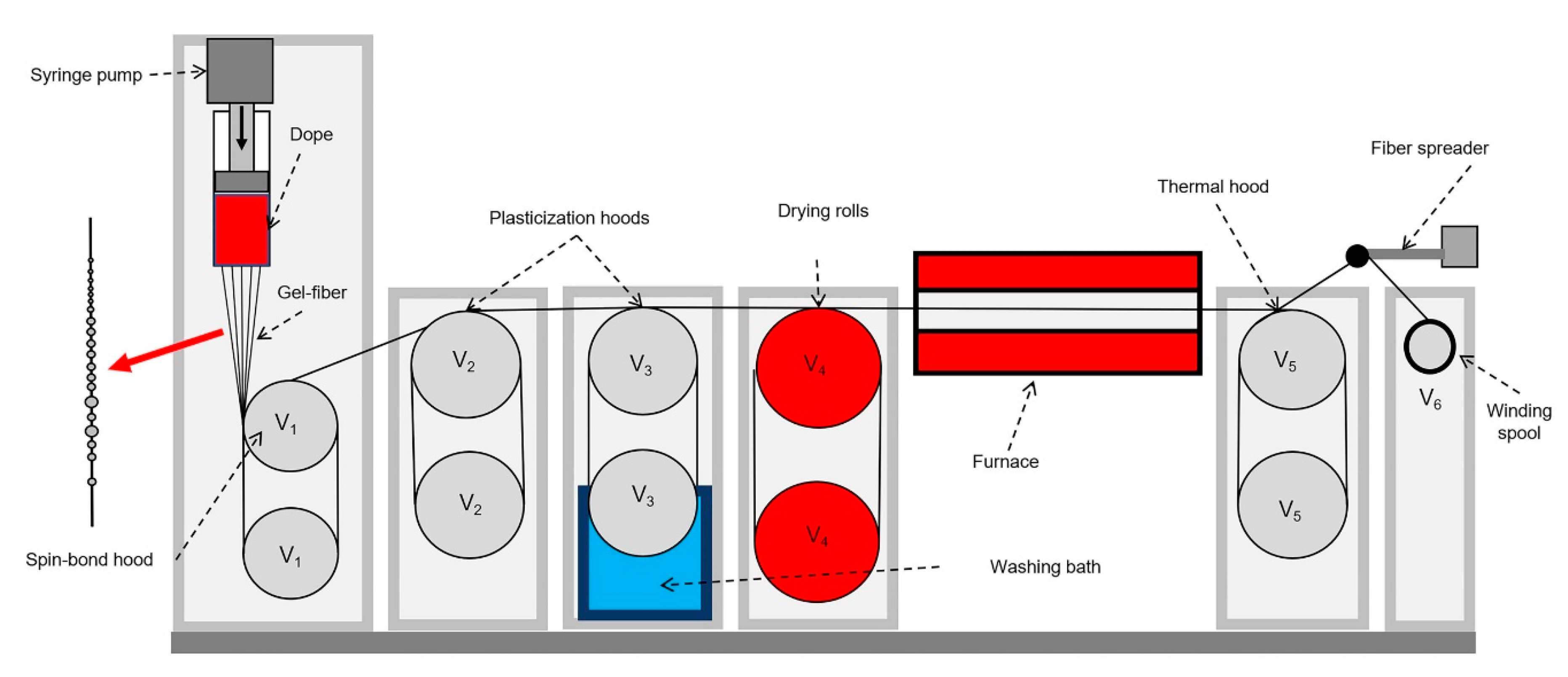
2.2.3. Fiber Spinning
2.2.4. Fiber Characterization
3. Results and Discussion
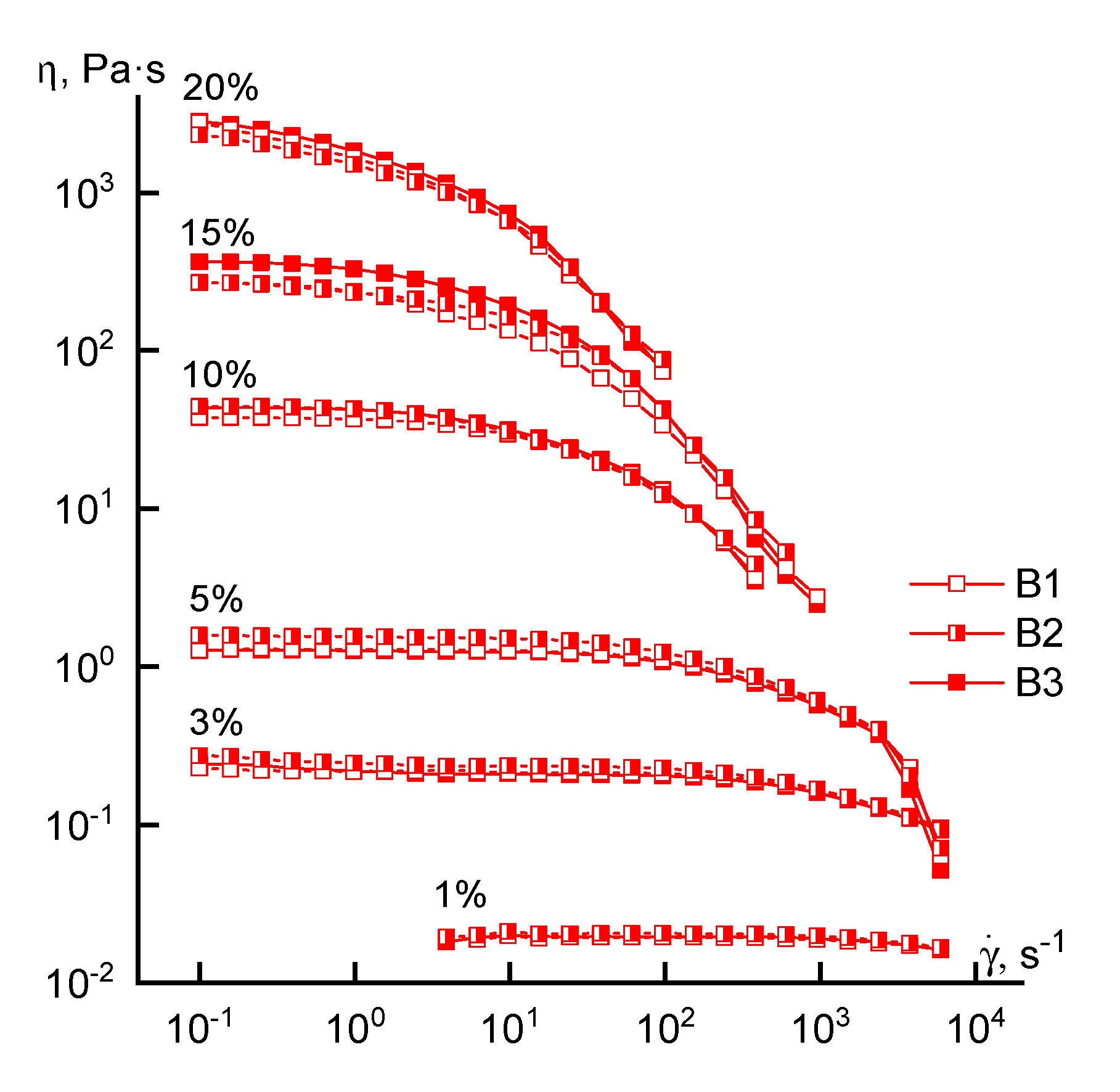
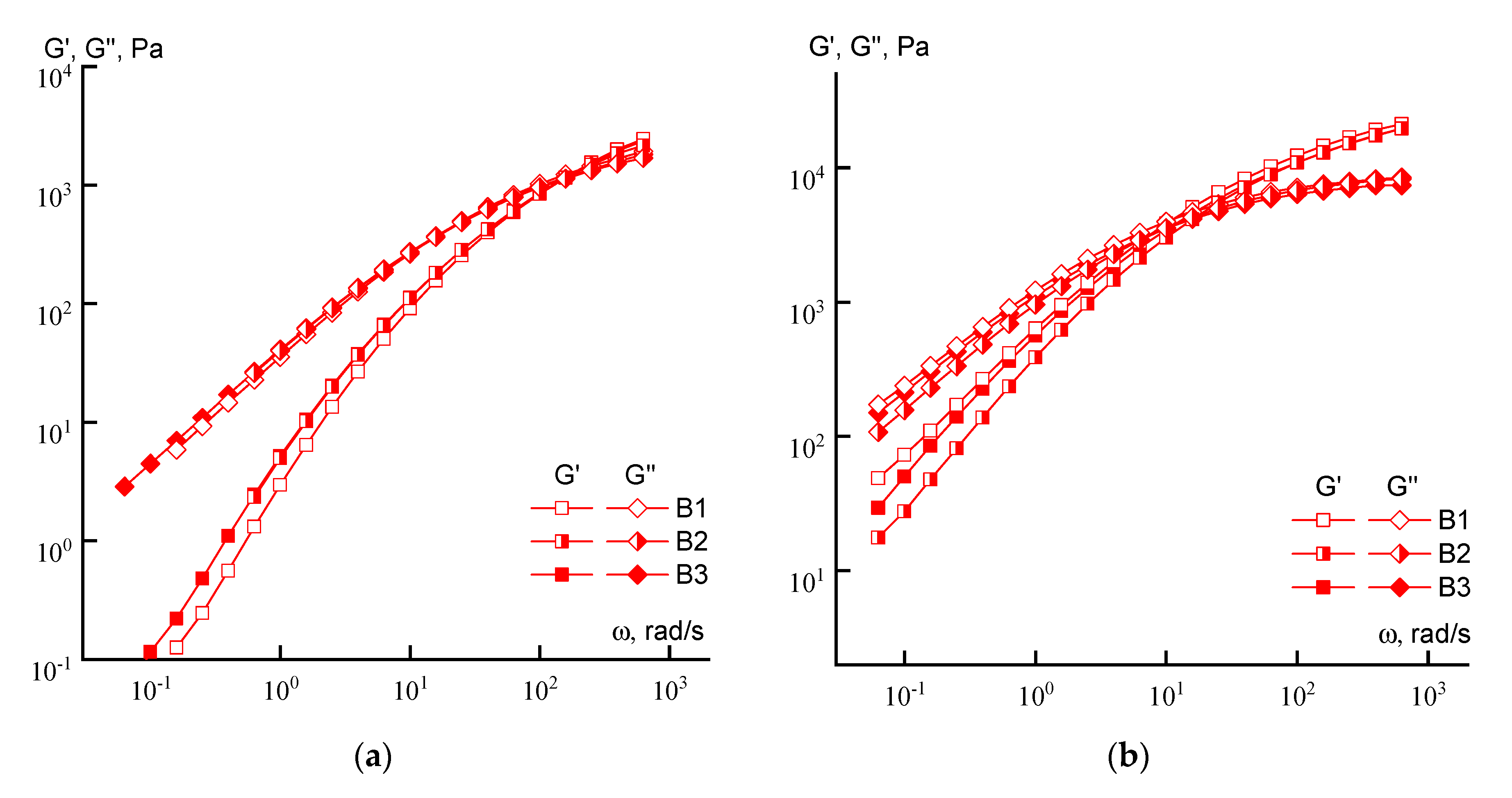
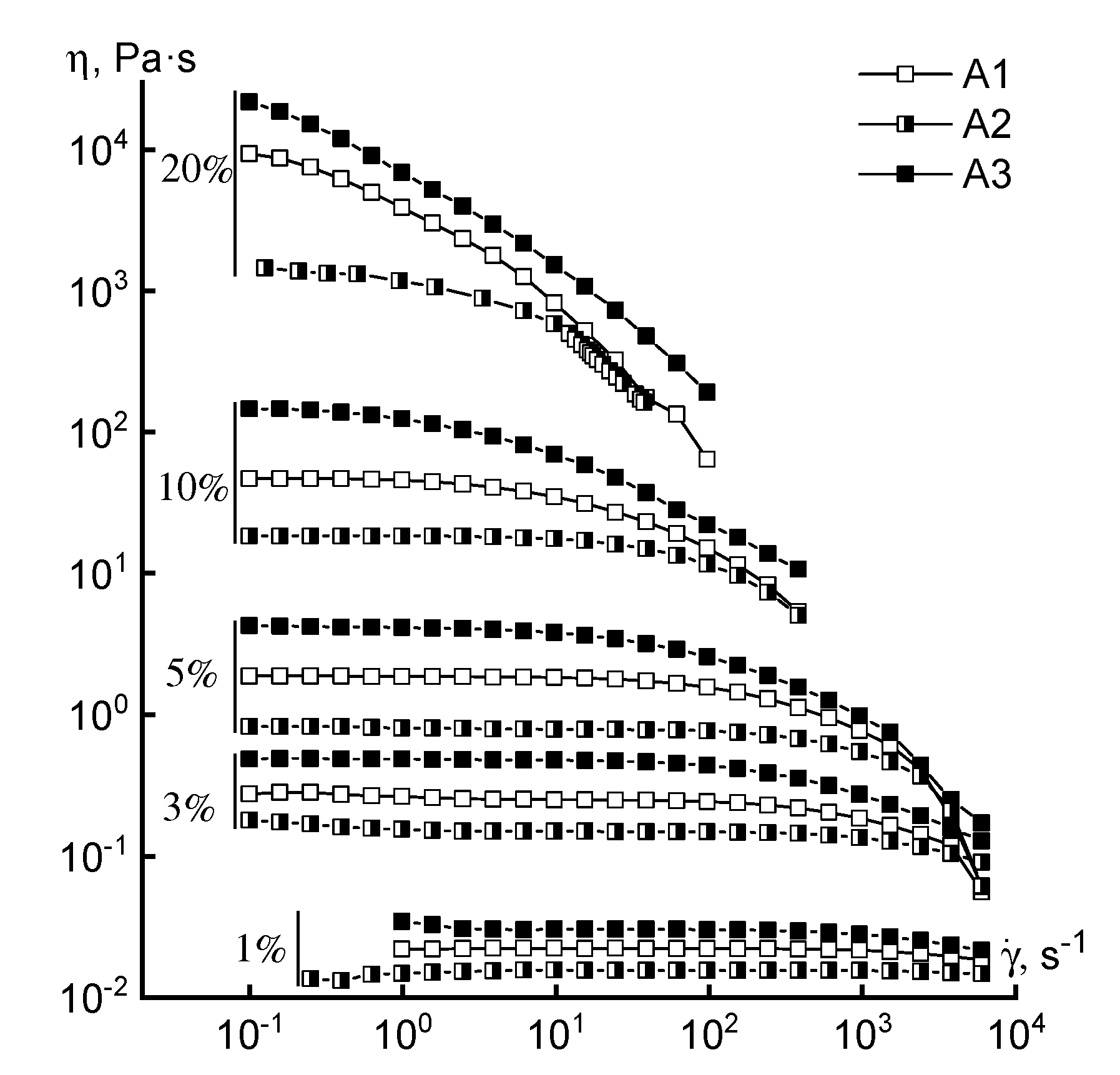
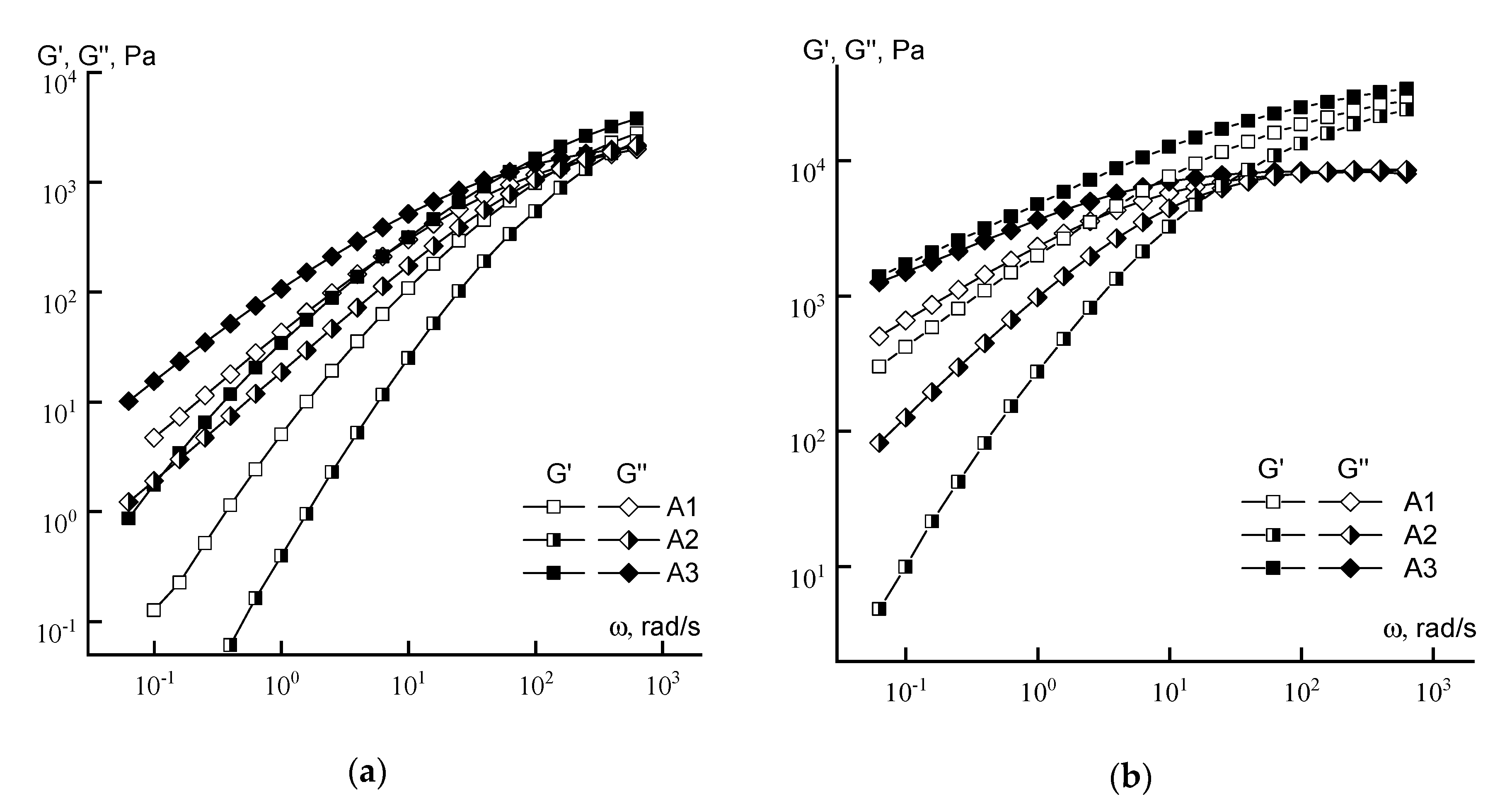
3.1. Rheology
3.2. Modeling of the Mechanotropic Spinning
3.3. Fiber Spinning
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Frank, E.; Ingildeev, D.; Buchmeiser, M.R. High-Performance PAN-Based Carbon Fibers and Their Performance Requirements. In Structure and Properties of High-Performance Fibers; Bhat, G., Ed.; Woodhead Publishing: Cambrige, MA, USA, 2016; pp. 7–30. ISBN 978-0-0810-0550-7. [Google Scholar]
- Morgan, P. Carbon Fibers and Their Composites; CRC Press: Boca Raton, FL, USA, 2005; pp. 130–162. ISBN 0-8247-0983-7. [Google Scholar]
- Chernikova, E.V.; Poteryaeva, Z.A.; Belyaev, S.S.; Nifant’ev, I.E.; Shlyakhtin, A.V.; Kostina, Y.V.; Cherevan, A.S.; Efimov, M.N.; Bondarenko, G.N.; Sivtsov, E.V. Controlled synthesis of polyacrylonitrile via reversible addition-fragmentation chain-transfer pseudoliving radical polymerization and its thermal behavior. Polym. Sci. Ser. B+ 2011, 53, 391–403. [Google Scholar] [CrossRef]
- Hirao, A.; Goseki, R.; Ishizone, T. Advances in living anionic polymerization: From functional monomers, polymerization systems, to macromolecular architectures. Macromolecules 2014, 47, 1883–1905. [Google Scholar] [CrossRef]
- Cai, J.Y.; McDonnell, J.; Brackley, C.; O’Brien, L.; Church, J.S.; Millington, K.; Smith, S.; Phair-Sorensen, N. Polyacrylonitrile-based precursors and carbon fibers derived from advanced RAFT technology and conventional methods–The 1st comparative study. Mater. Today Commun. 2016, 9, 22–29. [Google Scholar] [CrossRef]
- Toms, R.V.; Balashov, M.S.; Shaova, A.A.; Gerval’d, A.Y.; Prokopov, N.I.; Plutalova, A.V.; Grebenkina, N.A.; Chernikova, E.V. Copolymers of Acrylonitrile and Acrylic Acid: Effect of Composition and Distribution of Chain Units on the Thermal Behavior of Copolymers. Polym. Sci. Ser. B 2020, 62, 102–115. [Google Scholar] [CrossRef]
- Toms, R.V.; Balashov, M.S.; Gerval’d, A.Y.; Prokopov, N.I.; Plutalova, A.V.; Berkovich, A.K.; Chernikova, E.V. The influence of the synthesis method on the properties of carbon fiber precursors based on copolymers of acrylonitrile and acrylic acid. Polym. Sci. Ser. B 2020. (In press)
- Khayyam, H.; Jazar, R.N.; Nunna, S.; Golkarnarenji, G.; Badii, K.; Fakhrhoseini, S.M.; Kumar, S.; Naebe, M. PAN Precursor Fabrication, Applications and Thermal Stabilization Process in Carbon Fiber Production: Experimental and Mathematical Modelling. Prog. Mater. Sci. 2020, 107, 100575. [Google Scholar] [CrossRef]
- Kulichikhin, V.G.; Skvortsov, I.Y.; Subbotin, A.V.; Kotomin, S.V.; Malkin, A.Y. A Novel Technique for Fiber Formation: Mechanotropic Spinning—Principle and Realization. Polymers 2018, 10, 856. [Google Scholar] [CrossRef]
- Mittal, J.; Mathur, R.B.; Bahl, O.P. Post spinning modification of PAN fibres—A review. Carbon 1997, 35, 1713–1721. [Google Scholar] [CrossRef]
- Bashir, Z. A critical review of the stabilization of polyacrylonitrile. Carbon 1991, 29, 1081–1090. [Google Scholar] [CrossRef]
- Rahaman, M.S.A.; Ismail, A.F.; Mustafa, A. A review of heat treatment on polyacrylonitrile fiber. Polym. Degrad. Stabil. 2007, 92, 1421–1432. [Google Scholar] [CrossRef]
- Yusof, N.; Ismail, A.F. Post spinning and pyrolysis processes of polyacrylonitrile (PAN)-based carbon fiber and activated carbon fiber: A review. J. Anal. Appl. Pyrol. 2012, 93, 1–13. [Google Scholar] [CrossRef]
- Spörl, J.M.; Ota, A.; Beyer, R.; Lehr, T.; Müller, A.; Hermanutz, F.; Buchmeiser, M.R. Carbon Fibers Prepared from Tailored Reversible-Addition-Fragmentation Transfer Copolymerization-Derived Poly(Acrylonitrile)- Co -Poly(Methylmethacrylate). J. Polym. Sci. Polym. Chem. 2014, 52, 1322–1333. [Google Scholar] [CrossRef]
- Kulichikhin, V.G.; Skvortsov, I.Y.; Mironova, M.I.; Ozerin, A.N.; Kurkin, T.S.; Berkovich, A.K.; Frenkin, E.I.; Malkin, A.Y. From Polyacrylonitrile, Its Solutions, and Filaments to Carbon Fibers II. Spinning PAN-Precursors and Their Thermal Treatment. Adv. Polym. Tech. 2018, 37, 1099–1113. [Google Scholar] [CrossRef]
- Dalton, S.; Heatley, F.; Budd, P.M. Thermal stabilization of polyacrylonitrile fibres. Polymer 1999, 40, 5531–5543. [Google Scholar] [CrossRef]
- Ju, A.; Guang, S.; Xu, H. Effect of comonomer structure on the stabilization and spinnability of polyacrylonitrile copolymers. Carbon 2013, 54, 323–335. [Google Scholar] [CrossRef]
- Huang, X. Fabrication and properties of carbon fibers. Materials 2009, 2, 2369–2403. [Google Scholar] [CrossRef]
- Edie, D.D. The effect of processing on the structure and properties of carbon fibers. Carbon 1998, 36, 345–362. [Google Scholar] [CrossRef]
- Bajaj, P.; Paliwal, D.K.; Gupta, A.K. Acrylonitrile–acrylic acids copolymers. I. Synthesis and characterization. J. Appl. Polym. Sci. 1993, 49, 823–833. [Google Scholar] [CrossRef]
- Gupta, A.; Harrison, I.R. New aspects in the oxidative stabilization of PAN-based carbon fibers: II. Carbon 1997, 35, 809–818. [Google Scholar] [CrossRef]
- Ouyang, Q.; Cheng, L.; Wang, H.; Li, K. Mechanism and kinetics of the stabilization reactions of itaconic acid-modified polyacrylonitrile. Polym. Degrad. Stabil. 2008, 93, 1415–1421. [Google Scholar] [CrossRef]
- Beltz, L.A.; Gustafson, R.R. Cyclization kinetics of poly (acrylonitrile). Carbon 1996, 34, 561–566. [Google Scholar] [CrossRef]
- Wang, Y.; Tong, Y.; Zhang, B.; Su, H.; Xu, L. Formation of Surface Morphology in Polyacrylonitrile (PAN) Fibers during Wet-Spinning. J. Eng. Fiber. Fabr. 2018, 13, 52–57. [Google Scholar] [CrossRef]
- Makarov, I.S.; Golova, L.K.; Vinogradov, M.I.; Levin, I.S.; Sorokin, S.E. Structure of Polyacrylonitrile Fibers Produced from N-Methylmorpholine-N-Oxide Solutions. Fibre Chem. 2019, 50, 508–513. [Google Scholar] [CrossRef]
- Dong, R.; Zhao, J.; Zhang, Y.; Pan, D. Morphology control of polyacrylonitrile (PAN) fibers by phase separation technique. J. Polym. Sci. Pol. Phys. 2009, 47, 261–275. [Google Scholar] [CrossRef]
- Ponomarev, I.I.; Skvortsov, I.Y.; Volkova, Y.A.; Ponomarev, I.I.; Varfolomeeva, L.A.; Razorenov, D.Y.; Skupov, K.M.; Kuzin, M.S.; Serenko, O.A. New Approach to Preparation of Heat-Resistant “Lola-M” Fiber. Materials 2019, 12, 3490. [Google Scholar] [CrossRef] [PubMed]
- Kotomin, S.V.; Malkin, A.Y.; Skvortsov, I.Y. Electrospinning and Mechanotropic Phenomena in Polymer Solutions. Macromol. Symp. 2020, 389, 1900091. [Google Scholar] [CrossRef]
- ASTM D2857-16. Standard Practice for Dilute Solution Viscosity of Polymers; ASTM International: West Conshohocken, PA, USA, 2016. [Google Scholar] [CrossRef]
- Malkin, A.Y.; Semakov, A.V.; Skvortsov, I.Y.; Zatonskikh, P.V.; Kulichikhin, V.G.; Subbotin, A.V.; Semenov, A.N. Spinnability of dilute polymer solutions. Macromolecules 2017, 50, 8231–8244. [Google Scholar] [CrossRef]
- Skvortsov, I.Y.; Toms, R.V.; Prokopov, N.I.; Chernikova, E.V.; Kulichikhin, V.G. Rheological Properties of Acrylonitrile Terpolymer Solutions Synthesized by Different Methods. Polym. Sci. Ser. A+ 2018, 60, 894–901. [Google Scholar] [CrossRef]
- Malkin, A.Y.; Isayev, A.I. Rheology: Concepts, Methods, and Applications, 3rd ed.; ChemTec Publishing: Toronto, ON, Canada, 2017; ISBN 978-1-927885-22-2. [Google Scholar]
- Cox, W.P.; Merz, E.H. Correlation of dynamic and steady flow viscosities. J. Polym. Sci. 1958, 28, 619–622. [Google Scholar] [CrossRef]
- Ferry, J.D. Viscoelastic Properties of Polymers, 3rd ed.; John Wiley & Sons, Inc.: Toronto, ON, Canada, 1980; ISBN 0-471-04894-1. [Google Scholar]
- Semakov, A.V.; Skvortsov, I.Y.; Kulichikhin, V.G.; Malkin, A.Y. From capillary to elastic instability of jets of polymeric liquids: Role of the entanglement network of macromolecules. JETP Lett. 2015, 101, 690–692. [Google Scholar] [CrossRef]
- Wang, Y.X.; Wang, C.G.; Bai, Y.J.; Bo, Z. Effect of the drawing process on the wet spinning of polyacrylonitrile fibers in a system of dimethyl sulfoxide and water. J. Appl. Polym. Sci. 2007, 104, 1026–1037. [Google Scholar] [CrossRef]


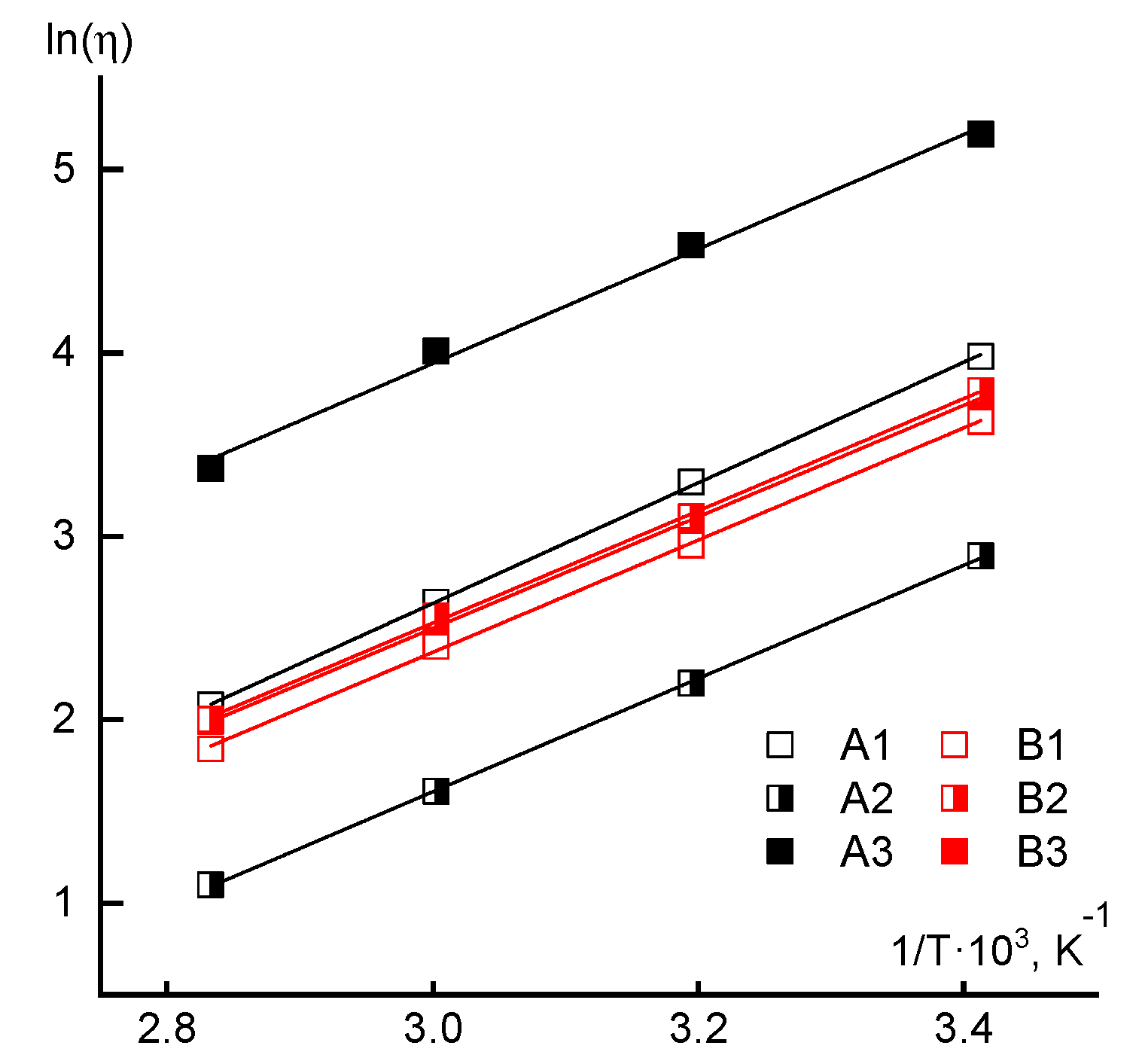
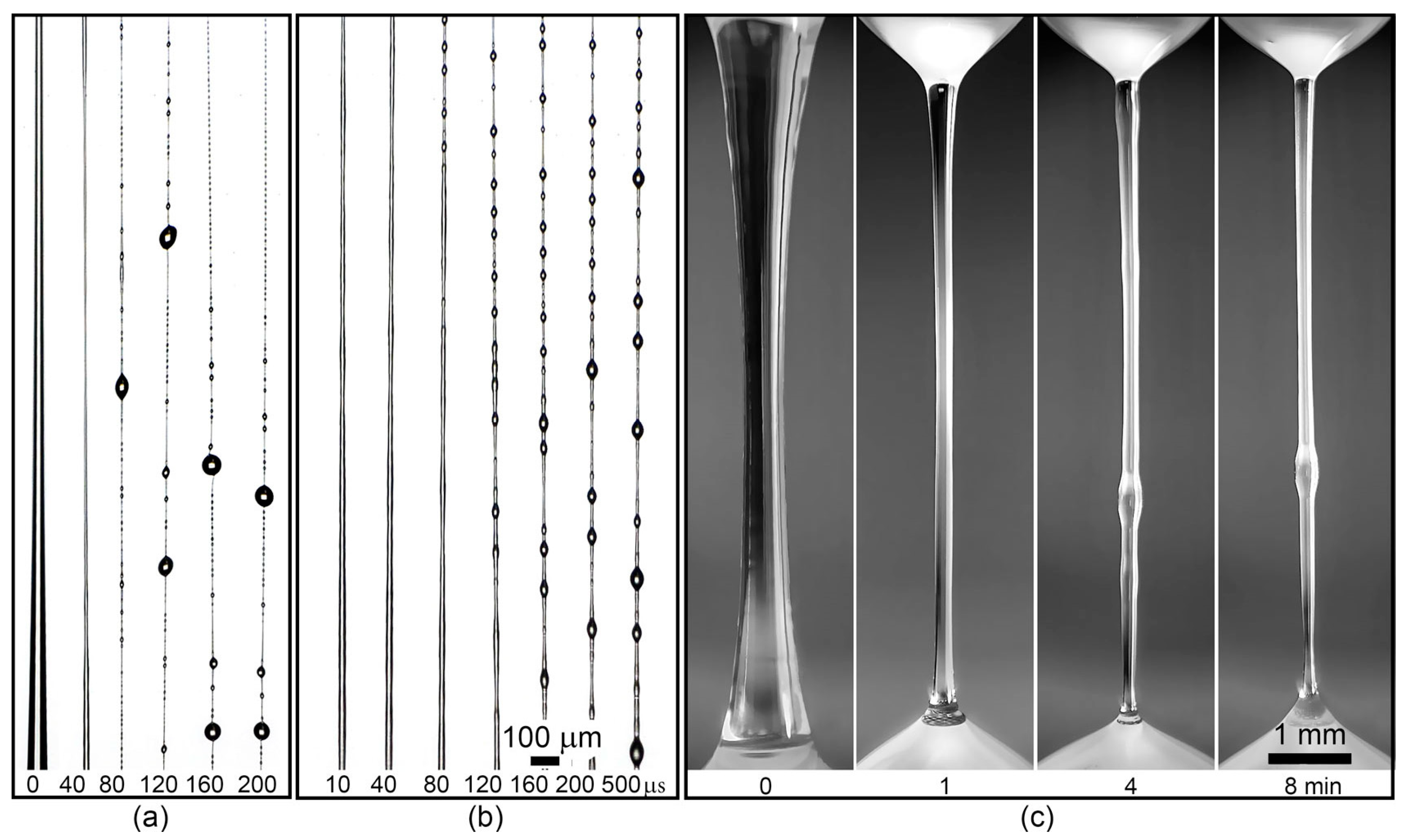


| Sample | The Method of AA Addition | Mw × 10−3 | Ð | FAA, mol.% |
|---|---|---|---|---|
| A1 | Continuous | 176 | 1.80 | 10.8 |
| A2 | Semi-batch | 120 | 1.54 | 7.8 |
| A3 | Batch | 152 | 1.62 | 9.8 |
| B1 | Continuous | 153 | 2.02 | 12.4 |
| B2 | Semi-batch | 175 | 2.40 | 13.3 |
| B3 | Batch | 145 | 2.14 | 14.8 |
| Sample | [η], dL/g | KM | KH | Mw × 10−3 g/mole |
|---|---|---|---|---|
| A1 | 3.4 | 0.23 | 0.30 | 176 |
| A2 | 2.6 | 0.28 | 0.36 | 120 |
| A3 | 4.0 | 0.24 | 0.33 | 152 |
| B1 | 3.0 | 0.25 | 0.32 | 153 |
| B2 | 3.1 | 0.25 | 0.34 | 175 |
| B3 | 2.9 | 0.23 | 0.34 | 145 |
| Solution Concentration | Crossover Frequency, rad/s | |||||
|---|---|---|---|---|---|---|
| A1 | A2 | A3 | B1 | B2 | B3 | |
| 10% | 158 | 400 | 63 | 180 | 158 | 158 |
| 20% | 2.5 | 20 | 0.06 | 10 | 17 | 10 |
| Sample | V0, m/min | V1, m/min | V2 m/min | V3 m/min | V4 m/min at 100 °C | V5–V6, m/min |
|---|---|---|---|---|---|---|
| A1 | 0.001 | 3.8 | 4 | 4.5 | 5 | 10.2 |
| A2 | 2.2 | 2.9 | 3.7 | 4.3 | 6.8 | |
| B1 | 3.8 | 9.1 | 9.6 | 10 | 20.7 | |
| B2 | 1.6 | 14.3 | 16.3 | 17.3 | 31.5 | |
| B3 | 2.2 | 10.6 | 12.9 | 14.9 | 28.5 |
| Sample | Strength, MPa | Elongation at Break, % | Modulus of Elasticity, GPa | Diameter, µm | Draw Ratio |
|---|---|---|---|---|---|
| A1 | 500 ± 42 | 28 ± 42 | 4.5 ± 0.3 | 16 ± 1.5 | 2.7 |
| A2 | 560 ± 31 | 22 ± 1 | 7.3 ± 0.4 | 12.6 ± 1 | 3.1 |
| A3 | Spinning unstable | ||||
| B1 | 640 ± 35 | 27 ± 2.5 | 4.5 ± 0.2 | 16 ± 1 | 5.4 |
| B2 | 550 ± 23 | 23 ± 1.9 | 7.1 ± 0.4 | 8 ± 1 | 19.7 |
| B3 | 710 ± 38 | 22 ± 1.1 | 6.7 ± 0.5 | 8 ± 1 | 13 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skvortsov, I.Y.; Chernikova, E.V.; Kulichikhin, V.G.; Varfolomeeva, L.A.; Kuzin, M.S.; Toms, R.V.; Prokopov, N.I. The Effect of the Synthetic Procedure of Acrylonitrile–Acrylic Acid Copolymers on Rheological Properties of Solutions and Features of Fiber Spinning. Materials 2020, 13, 3454. https://doi.org/10.3390/ma13163454
Skvortsov IY, Chernikova EV, Kulichikhin VG, Varfolomeeva LA, Kuzin MS, Toms RV, Prokopov NI. The Effect of the Synthetic Procedure of Acrylonitrile–Acrylic Acid Copolymers on Rheological Properties of Solutions and Features of Fiber Spinning. Materials. 2020; 13(16):3454. https://doi.org/10.3390/ma13163454
Chicago/Turabian StyleSkvortsov, Ivan Y., Elena V. Chernikova, Valery G. Kulichikhin, Lydia A. Varfolomeeva, Mikhail S. Kuzin, Roman V. Toms, and Nikolay I. Prokopov. 2020. "The Effect of the Synthetic Procedure of Acrylonitrile–Acrylic Acid Copolymers on Rheological Properties of Solutions and Features of Fiber Spinning" Materials 13, no. 16: 3454. https://doi.org/10.3390/ma13163454
APA StyleSkvortsov, I. Y., Chernikova, E. V., Kulichikhin, V. G., Varfolomeeva, L. A., Kuzin, M. S., Toms, R. V., & Prokopov, N. I. (2020). The Effect of the Synthetic Procedure of Acrylonitrile–Acrylic Acid Copolymers on Rheological Properties of Solutions and Features of Fiber Spinning. Materials, 13(16), 3454. https://doi.org/10.3390/ma13163454