1. Introduction
Alkali-activated slag (AAS) binders are produced by alkaline activation of water-cooled ground granulated blast furnace slag (GGBFS), which is an industrial by-product from steel production. Commonly used alkaline activators include sodium hydroxide, sodium silicates, sodium carbonate and sodium sulphate in solid state to use it as one-part alkali-activated based binder or in liquid form [
1].
AAS is considered as a sustainable alternative binder to Portland cement (PC) enabling a reduction in the CO
2 emission of up to 80% compared to traditional concretes [
2,
3]. The solidified binder matrixes based on alkali-activation usually have a finer pore structure, lower water permeability as well as lower ion and molecular diffusivities in comparison with PC based systems [
4,
5]. Alkali-activated slag systems usually show a rapid strength development and often a rapid setting. The resistance to chemical attack is in most cases very high [
6,
7,
8]. On the other hand, the AAS-based binder matrix has higher autogenous and drying shrinkage [
9,
10,
11]. The drying shrinkage in PC systems tends to increase with decreasing relative humidity [
12]. On the contrary, concretes based on AAS appeared to shrink more when exposed to a higher relative humidity. This was related to the reorganization of the C-A-S-H phase where alkali cations decrease the stacking regularity of the C-A-S-H layers and cause their collapse during drying [
13]. The mineralogical composition, the fineness of the slag and the type and dosage of the alkaline activator were indicated as the most critical factors controlling the dissolution rate and solidification processes [
14,
15]. The main hydration products of AAS systems are calcium silicate hydrates incorporating aluminium C-(A)-S-H, hydrotalcite with Mg/Al ratios between 2 and 3, AFm and strätlingite [
15,
16]. The presence of small amounts of crystalline phases and a higher MgO content, increased the compressive strength, whereas more of Al
2O
3 resulted in its decrease [
17].
The often observed efflorescence of alkali-activated binder systems is influenced by the pore structure and properties of the precursor [
18].
The increased MgO content in the GGBFS enhanced the soundness of the autoclaved Portland cement-slag blends when the MgO was present in non-reactive phases including for example merwinite. On the other hand, the presence of MgO in more reactive phases has shown negative effects [
19]. A higher MgO content of sodium silicate-activated slags resulted in a rapid strength development, higher ultimate compressive strength, lower porosity, and higher hydration heat [
17,
20]. Addition of a highly reactive MgO produced by calcination of MgCO
3 at temperatures below 1000 °C to sodium silicate-activated-slag paste accelerated the early stage reaction, reduced the drying shrinkage and increased the carbonation resistance [
21,
22]. Those effects were related to the formation of C-M-S-H, having more polymerized units, stronger bond strength and better carbonation resistance in comparison with the C-S-H [
23]. On the other hand, it also generated a severe cracking under drying condition [
21,
22]. The incorporation of MgO into AAS appeared to reduce the Al substitution in the C-S-H and resulted in a formation of more hydrotalcite phases [
20].
On the contrary, the dead-burned MgO naturally present in GGBFS lowered and formed at 1500–1600 °C lowered the hydration degree [
24]. Brucite (Mg(OH)
2) was not detected in MgO-alkali-activated slag system, therefore suggesting the interaction between MgO and broken Si-O and Al-O, which possibly forms magnesium silicate hydrate (MSH) and expansive hydrotalcite-like phase (HT), which decrease the drying shrinkage strains of both alkali-activated slag and OPC [
14].
Carbonation of C-S-H or (C-A-S-H) is essentially a chemical degradation process controlling the strength and durability of both AAS and OPC binders. The transformation of C-(A)-S-H into an amorphous silica gel and leaching of Ca from C-(A)-S-H results in a formation of an amorphous calcium carbonate (ACC) before its transformation into a more stable CaCO
3 polymorph [
24]. The MgO content in slag was observed to control the rate and the degree of the carbonation of AAS systems [
14]. A higher MgO content lead to the formation of a stable ACC in AAS and increased the amount of formed hydrotalcite-like phase Mg
6Al
2CO
3(OH)
16·4(H
2O) [
25]. The trend was especially pronounced in sodium hydroxide-activated systems. The Al uptake by the C-S-H was decreased resulting in a reduced carbonation of the C-(A)-S-H [
20,
22,
26]. Formation of a stable amorphous calcium carbonate lowered the solubility of the high MgO content slag by creating an alternative buffering system. The system reduced the decalcification, dehydration, and polymerization of C-A-S-H under accelerated carbonation conditions. The formed hydrotalcite-like phase would effectively bind CO
2 and therefore hinder later carbonation [
22]. Low-MgO AAS pastes formed amorphous calcium carbonate which crystallized quickly into calcite/vaterite, which is followed by further decalcification of the C-A-S-H gel [
26].
The reaction heat development of the SS activated BFS systems was affected by the sodium content and the alkali modulus (M
s) [
27]. Higher Na
2O content led to a more intensive breakage of the slag oxides bonds Ca-O, Mg-O, Si-O-Si, Al-O-Al, and Al-O-Si, which was correlated with the visible initial peaks. The second temperature rise occurred due to the formation of the Si-Al layers on the surface of the slag particles. In the final step, the reaction products formed through a condensation process. The MgO content of the slag seemed to effect the reaction kinetics especially with low concentrations of the alkali activator, while, the CaO content was more critical at higher concentrations [
28].
Summarising, the reaction kinetics appeared to be affected by the chemical composition and physical properties of the slag precursors as well as the used type and dose of the alkali activator [
28,
29]. However, the number of studies focusing on special slags containing for example higher MgO content is rather limited. This study fills this gap by determining the effects of chemical composition of slag, MgO content, and other basic raw materials properties on strength, microstructure, and chemical composition of alkali activated mortars based on GGBFS.
2. Materials and Methods
Three ground granulated blast furnace slags GGBSs with different MgO contents of 16.1 wt % (S
16), 12.1 wt % (S
12), and 7.78 wt % (S
8) were used. The S
16 and S
12 slags were Merit 5000 provided from Merox AB-Sweden, while the S
8 slag was provided from Thomas CEMENT/Bremen, Germany. Chemical composition, physical properties, basicity (K
b), and hydration modulus (HM) are shown in
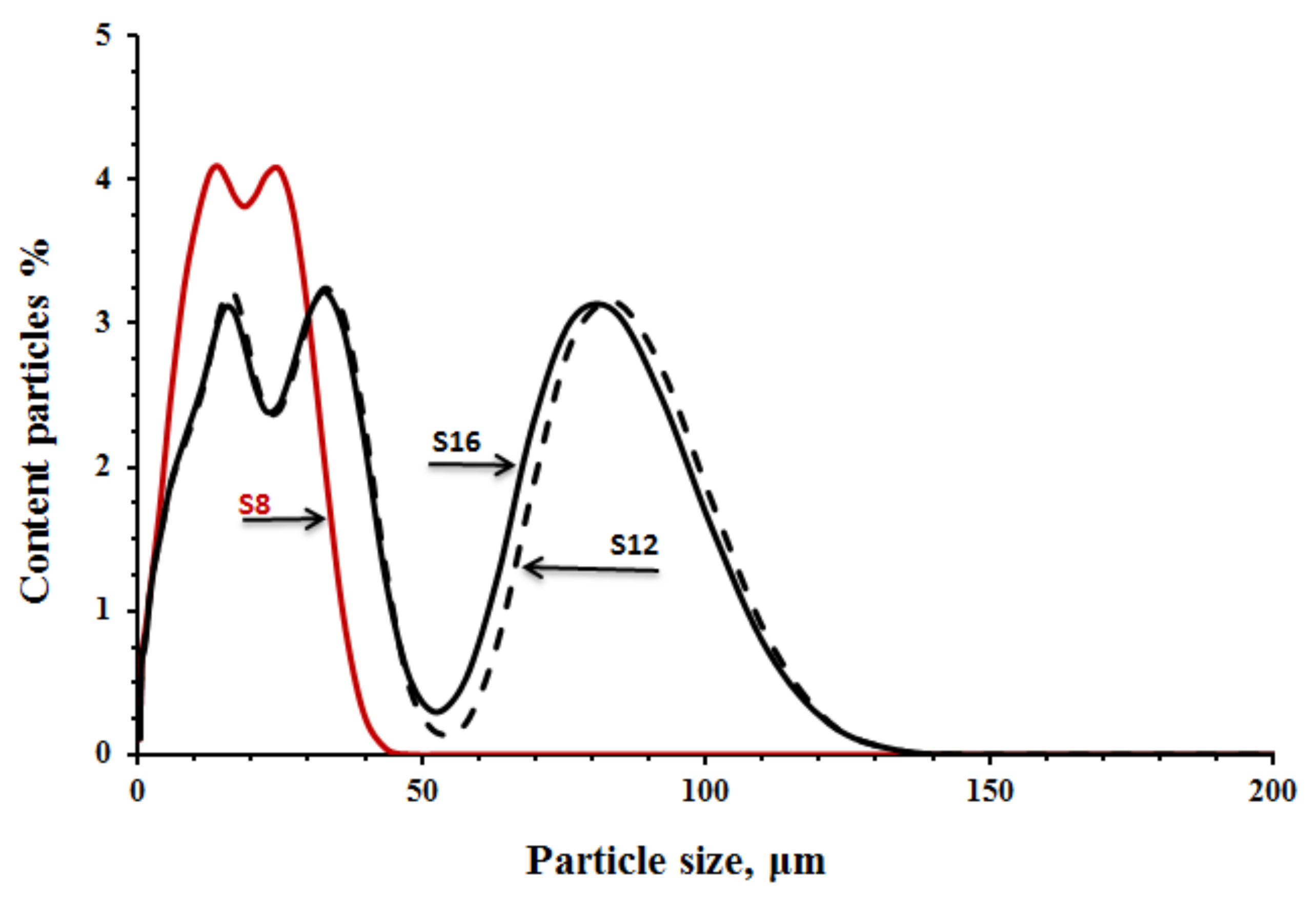
Table 1. The particle size distribution, shown in
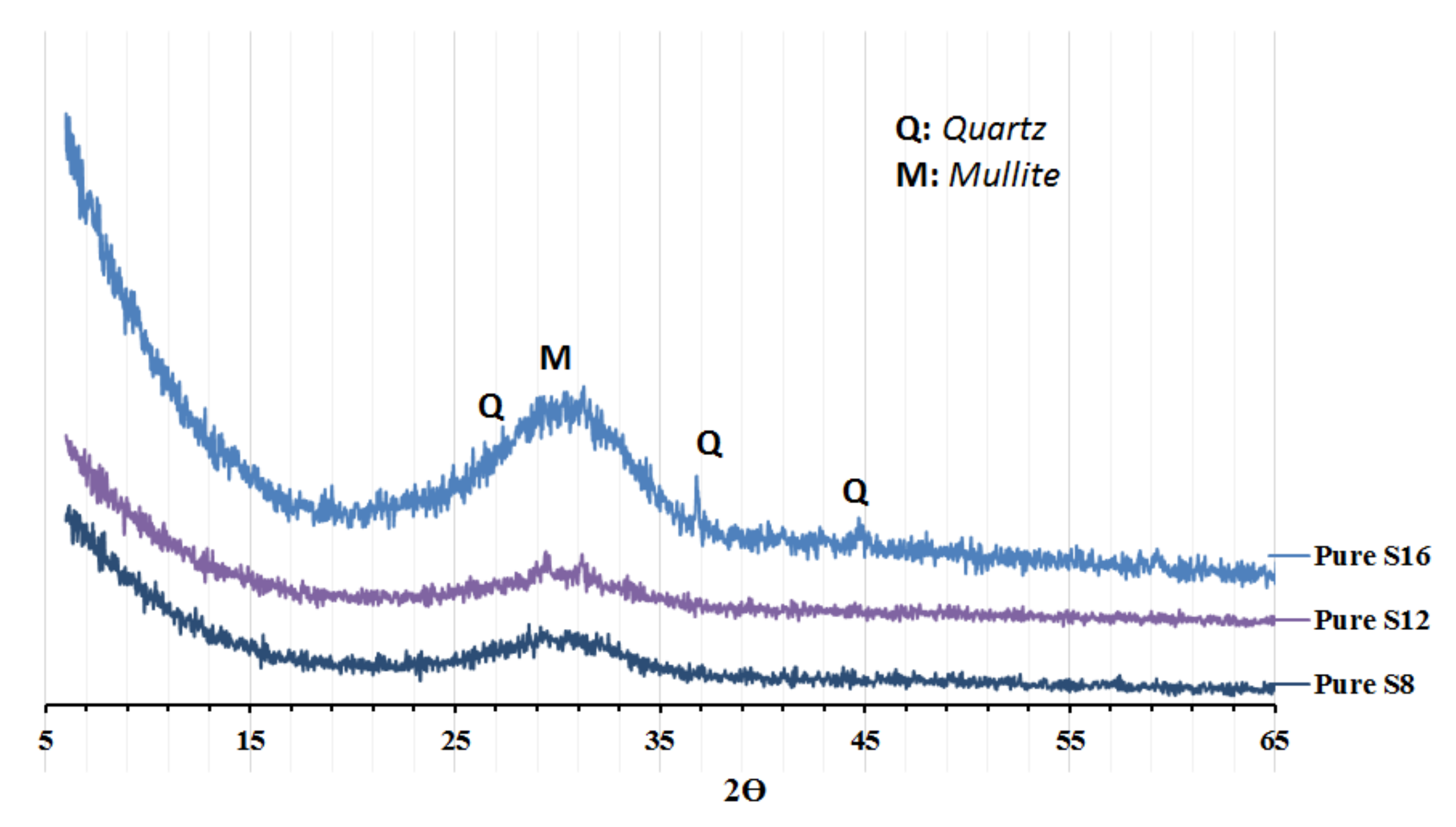
Figure 1, was determined with an LS 13 320 XR laser Particle Size Analyser (tested in Espoo, Finland). The XRD spectra of the untreated GGBFS are shown in
Figure 2.
The basicity modulus (Kb) and hydration modulus (HM) values were calculated for the three slags using the following equations; Kb = (CaO + MgO)/(SiO2 + Al2O3) and HM = (CaO + MgO + Al2O3)/SiO2).
Two alkaline activators were used: liquid sodium silicate (SS) Na2SiO3 provided by PQ Corporation (Karlstad, Sweden) and powder sodium carbonate (SC) Na2CO3 provided by CEICH SA (Warsaw, Poland). The supplied SS had an alkali modulus calculated as Ms = SiO2/Na2O (mass ratio) of 2.2, with 34.37 wt % SiO2, 15.6 wt % Na2O, and a solid content of 49.97 wt %. The Ms value was adjusted to 1.0 by adding sodium hydroxide pellets (98% purity), with 76.31 wt % Na2O. The alkali activator dosage was 10 wt % as a solid content of the binder weight for all mixes including pastes and mortars.
The water content of the water glass was taken into account in the mix design. The activators were dissolved in the mixing water the day before preparation of the mixes. The w/b ratio was 0.63 for pastes and 0.45 for mortars. Pastes and mortars for the determination of the setting time, efflorescence, carbonation, and heat development were prepared using a Hobart mixer. The dry materials were mixed for one minute, followed by the addition of the alkali activators dissolved in water and mixed for another 2 min. Mortars and pastes used to determine the mechanical properties were prepared in a small volume vacuum mixer type Ecovac-produced by Bredent/Senden, Germany. The mixing time was 2 min. The mortar had a w/b ratio of 0.45, with a binder/sand ratio of 1/1,
Table 2.
Initial and final setting times were determined using the Vicat apparatus on pastes (Form+Test Seidner&Co. Gmbh, Germany, tested in Sweden) following the ASTM C191-13 standard. A mini cone with dimensions of D
U = 31.0 mm, D
L = 44.5 mm and H = 32.5 mm was used to determine the workability of the produced mixes,
Figure 3. The slump flow diameter D was determined as an average of the two measured values D
1 and D
2.
The temperature development was measured and recorded by using a thermocouple apparatus connected with the TC-08 produced by Pico technology (test in Sweden) using the PicoLog6 software (PicoSDK 10.6.13). The measurements were performed on paste cubes having dimensions of 60 × 60 × 60 mm3. The specimens were casted into isolated boxes, which were built of three layers of 20 mm thick polystyrene sheets. The temperature was recorded every minute for 156 hours at room conditions at 21 ± 2 °C and 35 ± 5% RH.
The compressive strength values were determined using mortar beams 12 × 12 × 60 mm3 tested at 7 and 28 d. After casting, the beams were sealed in plastic bags and stored at 21 ± 2 °C and 35 ± 5% RH until solidification. In the next step, samples were de-moulded and kept sealed in the same conditions until testing. The compressive strength was determined using a WTKEHAM FARRANCE compression device (made in England, tested in Sweden) using Catman Easy software (version 5.2.1) to measure the strength values. The loading speed was 0.05 mm/min.
Efflorescence and carbonation depth were determined using mortar prisms having dimensions of 40 × 40 × 160 mm3. All samples were casted and sealed in plastic bags for four days followed by their storage in unsealed conditions at 20 ± 2 °C and 50 ± 10% RH. The visual examination of efflorescence was done two days after opening of the moulds. The carbonation depth was determined three months later using a Phenolphthalein Deep Purple Indicator (GI, Copenhagen, Denmark) sprayed onto the split concrete surfaces.
The microstructure and microchemistry of the hardened AAS mortar samples were studied using a scanning electron microscope (SEM) model JSM-IT100 combined with a QUANTAX EDX (Energy-dispersive X-ray spectrometer) produced by BRUKER (made in Japan, tested in Sweden) and the ESPRIT 2 software (version 2.1). Samples used for the SEM and the SEM-EDX analyses were stored in isopropyl alcohol for 48 h to stop ongoing reactions. In the next step, the samples were impregnated with a low viscosity epoxy resin. No heat drying was applied to any of the studied samples to limit a possibility for artificial cracking of the matrix. After curing for 24 h, the resin-impregnated samples were grinded and polished in steps using polishing spray containing 9, 3, and 1 μm synthetic diamond particles. A load of 35 N was applied to the polished samples. The SEM was operating with a 15.0 kV accelerating voltage and a probe current of 60–62 mA. Backscattered electron images (BSE) (model JSM-IT100, tested in Sweden) in low vacuum mode were obtained.
The XRD analysis was done on powdered paste samples at the age of 28 d using a Panalytical Empyrean XRD (UK)unit with Cu Kα radiation, step size 0.0262° 2θ, the total scanning time for each sample was 16 min. The results were evaluated using the HighScore Plus software (v.4.0-4.7a).
The thermogravimetric analysis (TGA/DSC) (tested in Saraburi, Thailand) was done in a nitrogen gas atmosphere using a NETZSCH STA 409PC/PG TGA/DSC apparatus. The temperature range was set between 30 and 1000 °C, at heat rate of 10 °C/min was applied. Before the measurement, 28-day old samples were immersed in isopropyl alcohol for 48 h to stop ongoing reactions followed by grinding.
For the drying shrinkage test, two concrete cylinders the both having diameters of 100 mm and height of 200 mm were casted using 10 wt % SS added as solid materials. The binder content was 450 kg/m3, the w/b ratio 0.45, the maximum aggregate size was 8 mm and the fine aggregate content was 80% of the total aggregate amount, which was 1663 kg/m3. The specimens were sealed for three days, except for the S12 mix, and stored in laboratory conditions at 20 ± 2 °C and 50 ± 10%, then kept unsealed in laboratory conditions. Three pairs of stainless steel studs were glued with an epoxy resin to the specimen surfaces. Strain values were recorded using an electronic manual strain gauge DEMEC type. A reference concrete mix was produced using rapid hardened PC cement with fineness of 520 m2/kg and w/c ratio of 0.45 with 1% plasticizer.
3. Results and Discussion
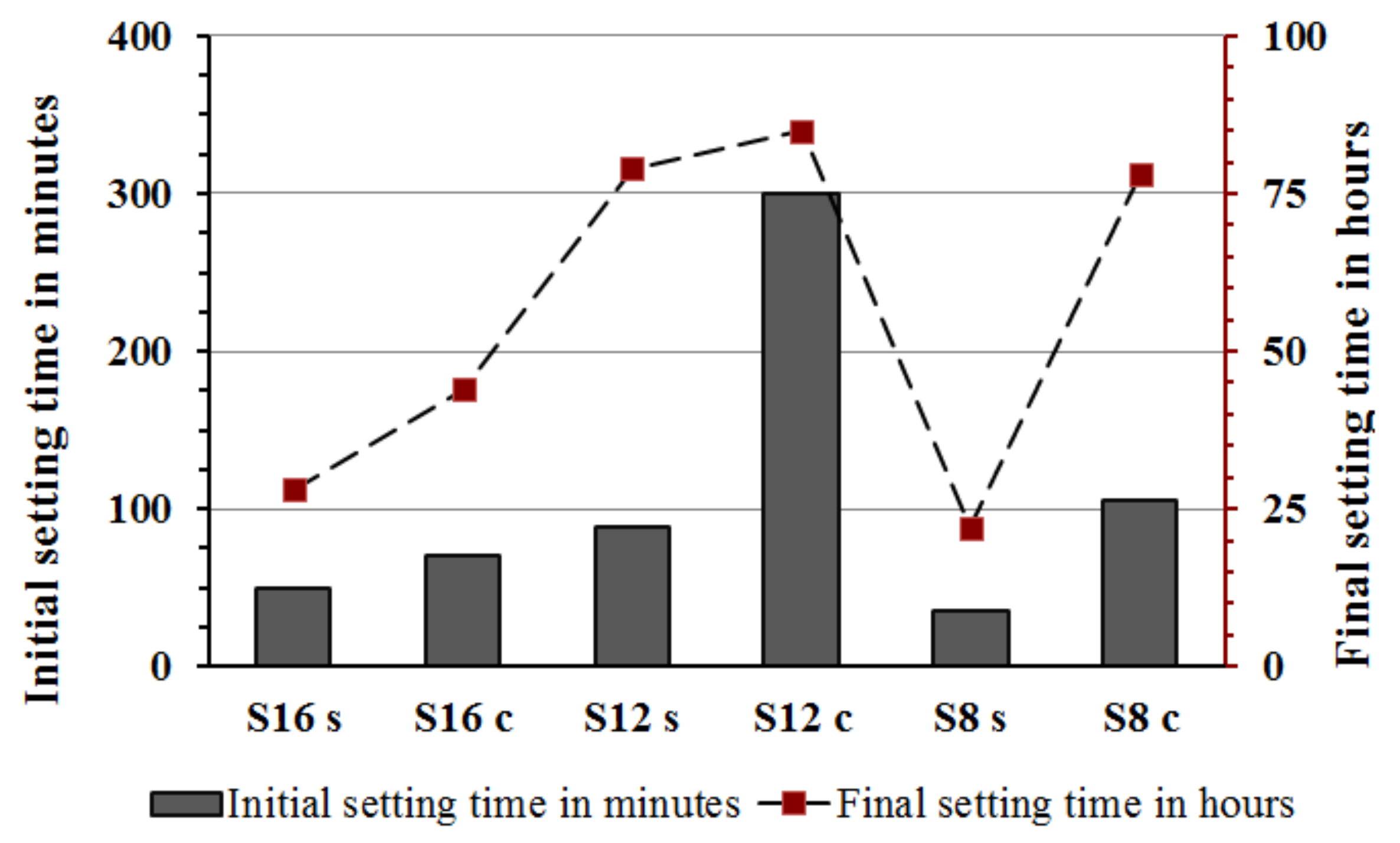
The measured setting times were affected by fineness and chemical composition of the used slag and alkali activator
Figure 4. The pastes activated with the SS showed generally shorter initial and final setting times in comparison with pastes activated with the SC. This is based on the higher pH values that led to a faster dissolution rate and increased reaction rate. On the contrary, the longest initial and final setting times were recorded for mixes activated with SC. The ultimate time length was strongly related to the fineness of the used GGBFS, which appeared to be the critical factor. The mix S
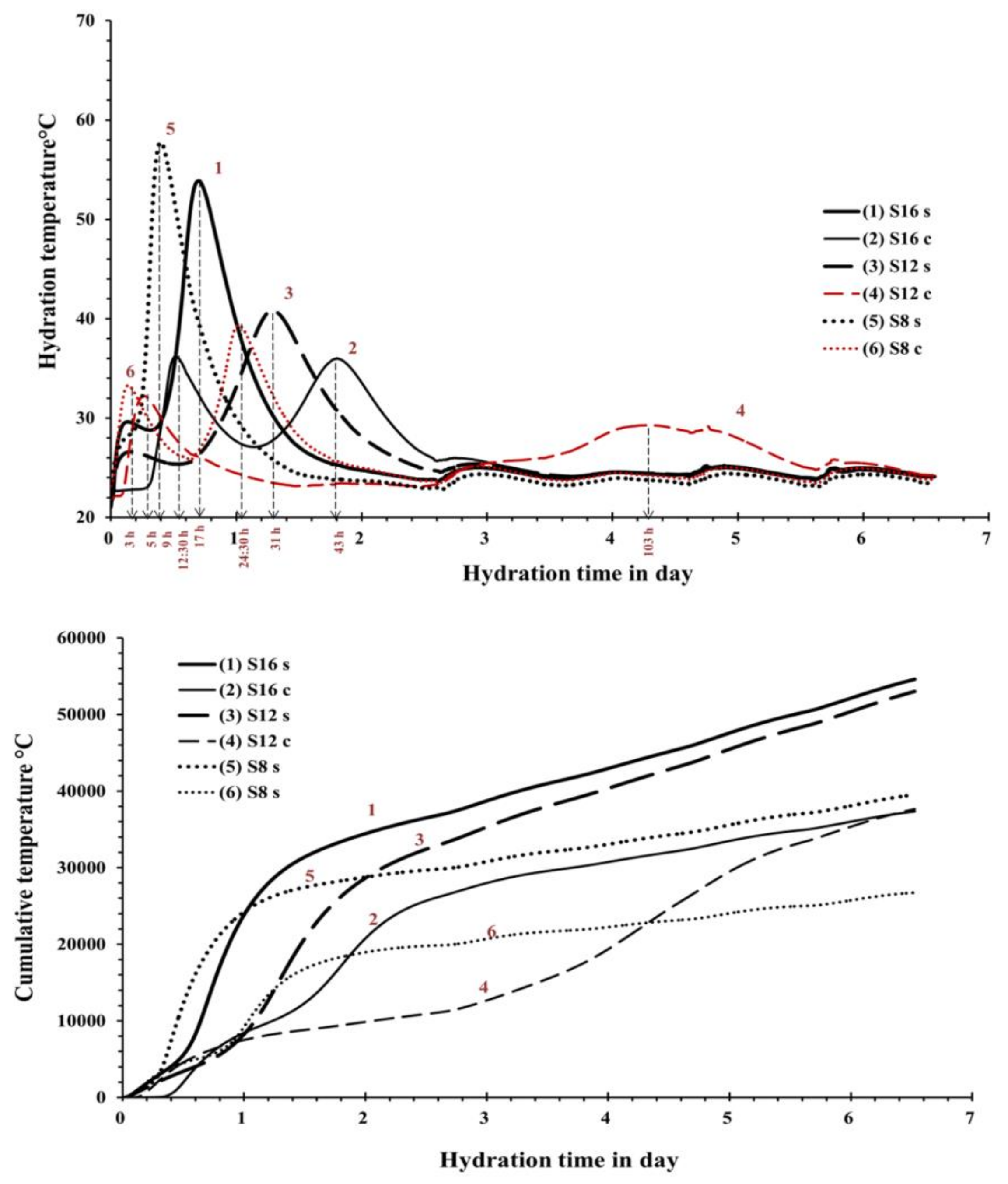
12C contained the coarsest GGBFS and was activated with the SC showed an initial setting time of 300 min. This result can be directly related to a lower developed reaction temperature and thus slower reaction as observed also in the performed semi adiabatic measurement,
Figure 5. Mixes with the longest initial setting times S
12s and S
12c showed also significantly lower maximum temperature as well as an elongated and delayed induction period. In addition to the coarsest particle sizes of this GGBFS, also the Al
2O
3 content was the lowest of all three tested slags
Table 1. Earlier studies indicated that both the MgO and the Al
2O
3 content tend to affect the reaction speed of the SS-activated slag [
20,
30]. Those results showed that increasing the MgO content increased the reaction heat and the amount of the formed hydrotalcite but decreased the Al uptake by the C-S-H. The present results indicate the same trend. For example, when comparing setting times of mixes S
16S and S
12S the higher MgO content accelerated the setting. Both slags had nearly the same particle size distribution,
Table 1 and
Figure 1. The mixes made with the S
8 slag, which has the lowest content of MgO, the highest SiO
2, CaO content and the highest specific surface area, showed the shortest setting times. The recorded temperature development was also the highest, independently of the used alkali activator
Figure 5 (curve 5 and 6).
The initial as well as the final setting times and the hydration temperature were comparable between mixes S
8s and S
16s activated with SS,
Figure 4 and
Figure 5. The same mixes but activated with SC showed longer initial setting times for mix using a finer slag S8. This could be related to a low pH of the SC solution (<12), which lead to a slow dissolution of the slag. Moreover, the Ca
2+ released from the dissolved slag could react with the CO
32− released from the activator (SC) to form carbonate salts like calcite and gaylussite, see XRD test results,
Figure 6. This reaction takes place before the precipitation of C-(A)-S-H gel, consuming the Ca
2+ released by the slag, and the later step of the reaction mechanism is similar to that of a NaOH-activated slag [
31].
The XRD analysis showed the presence of calcite, gaylussite, as well as a larger amount of portlandite to be present in the SC-activated mixes,
Figure 6. The recorded final setting times followed rather closely the same trends observed for the initial setting,
Figure 5. The longest times were observed for mixes activated with SC and based on slags with the lowest Al
2O
3 content. Earlier studies also indicated an elongation of the final setting times for SC-activated slag pastes when compared with SS- or hydroxide-activated slag systems [
7,
32,
33].
The semi-adiabatic calorimetry test results showed a significant discrepancy between mixes activated with SS and SC. The SS activated mixes showed only one main peak 9, 17, and 31 h after mixing for the mixes S
8s, S
16s, and S
12s respectively. The location and the height of the peak can be directly related with the fineness of the slags,
Table 1 and
Figure 1.
An earlier and more extensive heat development corresponded to higher fineness of the slag. Others indicated also that higher Na
2O concentration could also increase the amount of the developed heat but it cannot be confirmed in the present studies [
23,
34]. An interesting difference was observed before the development of the main peak. Mixes S
16s and S
12s had similar pattern during the first 40 h with a smaller peak forming indicating a following slight temperature drop. On the contrary, the mix S
8c did no develop any early age temperature rise. The difference could be related to the MgO content, which was significantly higher for mixes S
16s and S
12s. A higher MgO content, as described earlier, tends to facilitate the formation of hydrotalcite, which could be visible as those early age temperature increase [
22,
30].
Mixes activated with the SC developed two main temperature peaks. The first peak occurred after 3, 5 and 12.5 h for the mixes S
8c, S
12c, and S
16c respectively. The second peak developed after 24, 103 and 43 h for mixes and S
8C, S
12C, and S
16c, respectively. The second peak appeared to be larger in all cases indicating a predominant precipitation of carbonate salts including calcite and gaylussite in the first hours of the reaction. The following increase of the pH through the release of hydroxide ions resulted in the formation of C-S-H-like phases [
35].
The observed late formation of a wide peak in the case of the Mix S
12C could be related to a lower fineness and a lower MgO content. Similar results were obtained earlier by others [
35]. In that case, long induction periods were detected in SC-activated slag pastes and were strongly related to the chemical composition of the used slags. The second peak of the mix S
8c formed earlier and was the highest indicating a rapid temperature development and intensive chemical reactions. The cumulative developed temperature measured for up to 6 d were significantly higher for the SS-activated slag pastes S
16S, S
12S, and S
8S. The highest total heat released during the hardening process appeared to develop in the case of the mix S
16s based on slag with the highest MgO% content. Enhanced hydration kinetics during early ages related to the formation of calcite and gaylussite [
20].
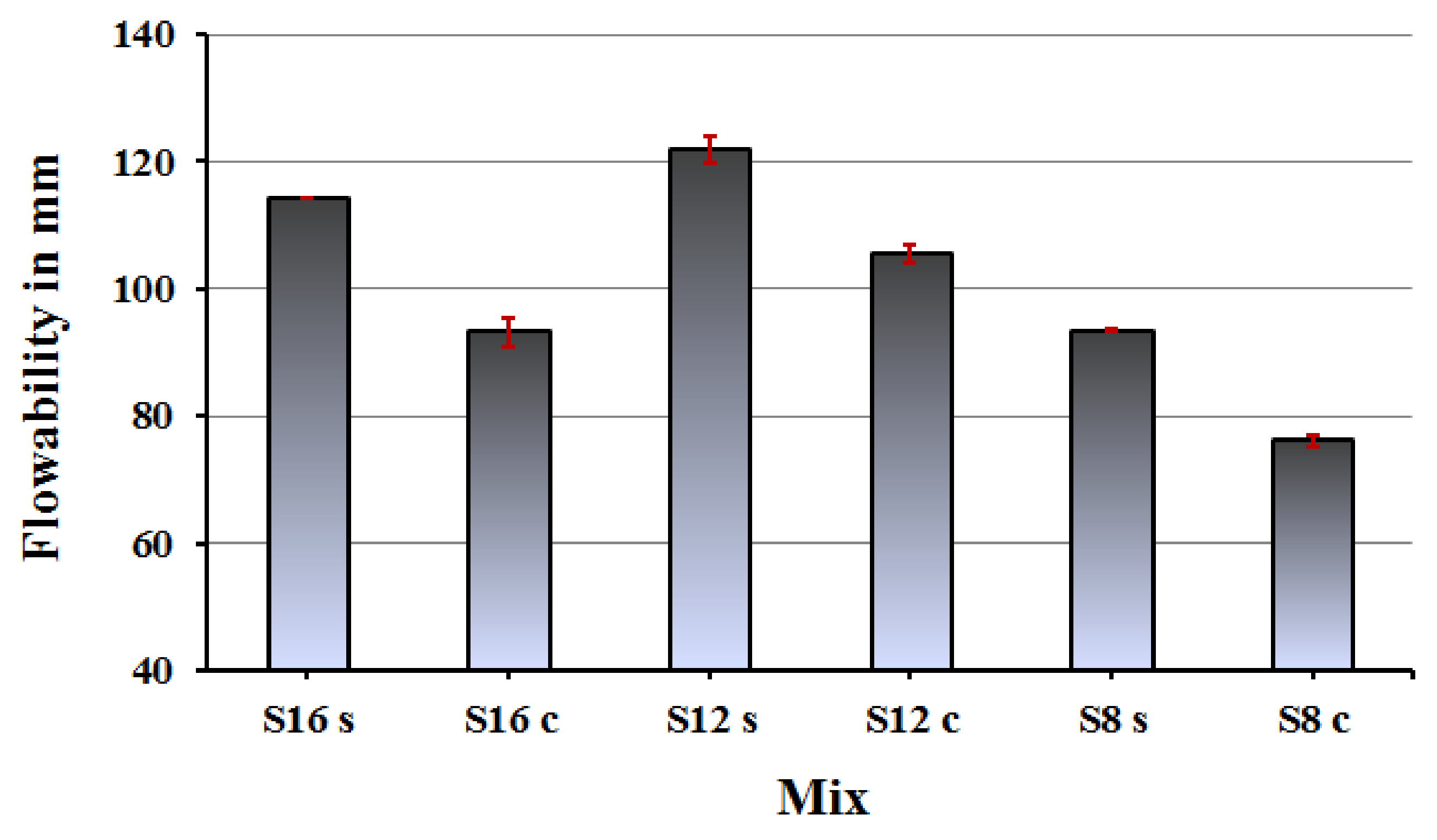
The workability of fresh mixes was generally better when water glass was used as alkali activator, which is related to a lubrication effect
Figure 7. Finer slags tended to lower the flowability as expected due to increase the partials surface area.
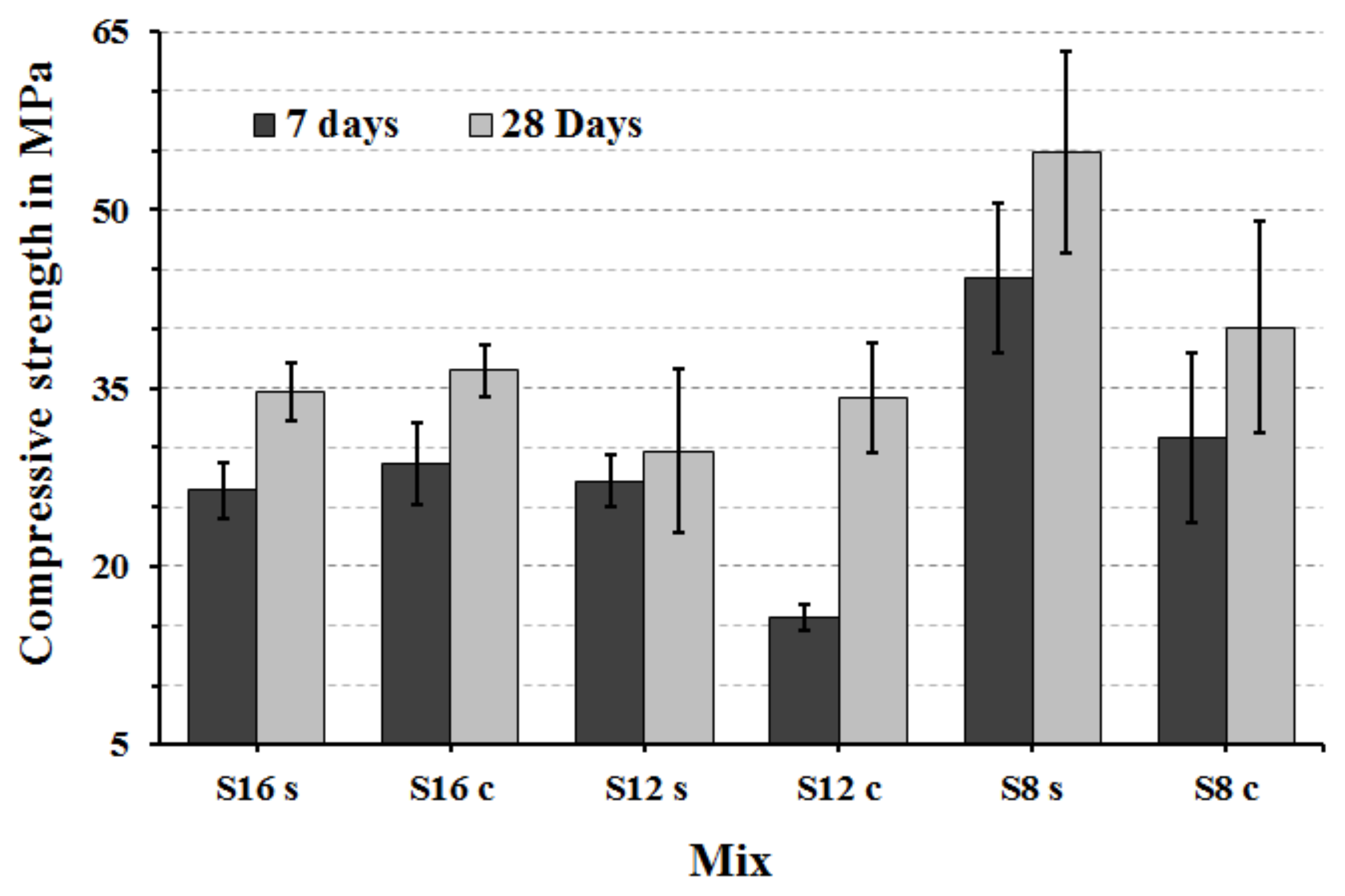
The highest measured 7 and 28 d compressive strength values were achieved by mixes based on fine S8 slag activated with sodium silicate,
Figure 8. which complied with earlier results [
7]. The lowest 7-day compressive strength values of the mix S
12C are related to the slow hydration process and a long induction period
Figure 5. At later stages that mix showed a rapid strength development and reached eventually nearly 35 MPa after 28 days. The highest compressive strength at both measured ages 7 and 28 d are recorded for mixes using fine slag S
8 and reached 44 and 55 MPa, respectively. The high strength values can be also related to higher contents of SiO
2, CaO and MgO. The MgO/Al
2O
3 ratio for this particular slag was also the lowest of all used material,
Table 1. The high recorded compressive strength is in agreement with a previous study, which tested mixes containing 30 wt % of Portland cement blended with different proportion of GGBFS based mortars. Those results indicated that mixes with the lowest MgO/Al
2O
3 ratio and high Al
2O
3 produced matrixes having the highest compressive strength but also a high amount of ettringite and some hydrotalcite [
17].
The efflorescence is caused by migration of alkalis (Na
+) in the pore solution to the surface, their reaction with atmospheric CO
2 producing the Na
2CO
3. The extent of efflorescence in this study was determined by visual observation 2 d after removal of the seal. The used specimens were initially kept in sealed-moulds for four days, then opened and kept in the ambient conditions at 20 ± 2 °C and 50 ± 10% RH. The AAS mortar mixes based on the S
12 slag and activated with both SS and SC (Mixes S
12S and S
12C) showed the most severe efflorescence. The observed trend can be related to the slow reaction rate of mixes containing the S
12 GGBFS,
Figure 5. The development of more porous microstructure especially at early age facilitated the migration of alkali to the surface. The efflorescence as such is structurally harmless [
36]. Furthermore, the solubility of Na
+ and leaching are limited, but the high mobility is due to the high dose of activator or the inherent neutralization of Al(OH)
4 in pore solution, or both [
18]. The EDX analysis of the observed efflorescence salts indicated only the presence of sodium carbonate. On the contrary, the efflorescence products in the OPC system is composed mainly of CaCO
3 [
36]. The Na-O bond is weakly connected with water molecules in the pore solution, on the contrary to the Na-O bond in the N-A-S-H [
18]. The intensive micro-crack formation was observed in the case of S
16S and S
8C.
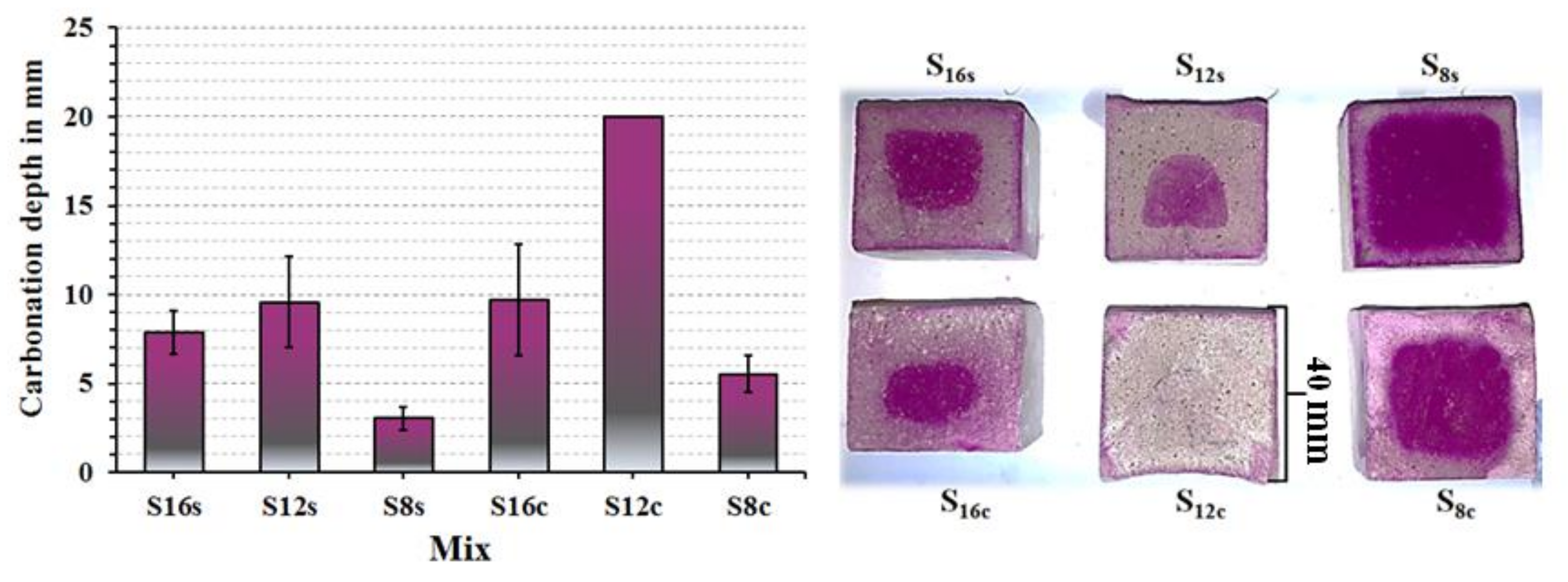
The carbonation of the studied mixes was determined on 3 months old specimens stored in ambient laboratory conditions at 20 ± 2 °C and 50 ± 10% RH. The highest carbonation developed in SC activated mixes based on the slag S
12,
Figure 9. While, the SS activated mortars based on the slag S
8 showed the least extensive carbonation. The high carbonation resistance can be related to a higher SiO
2, Al
2O
3 content and fineness, which led to faster reactions resulting in denser microstructures and higher amounts of formed C-(A)-S-H phases. Comparing mixes based on coarser slags S
12 and S
16, the results indicated that an increasing MgO content improved the carbonation resistance which is in agreement with earlier studies [
14]. The higher carbonation resistance of the S
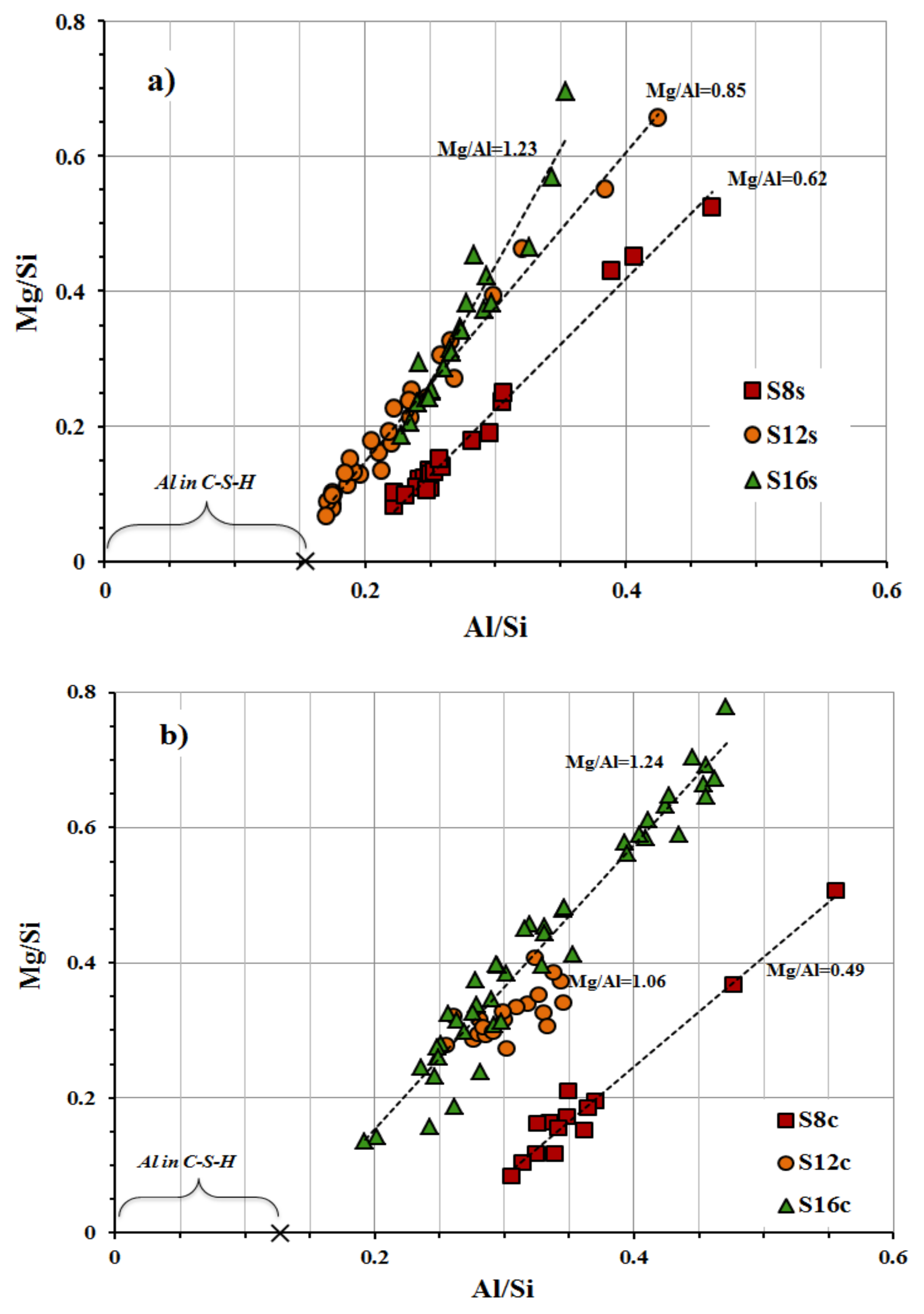
8 mix having the lowest MgO content can be related to its higher fineness. The MgO content effected the chemical composition of the formed phases. The calculated Al/Si vs Mg/Si ratios varied between 0.49 and 1.24 for SC-activated mixes and 0.62 and 1.23 for mixes activated with SS,
Figure 10. According to earlier studies, those results indicate the formation of hydrotalcite and as well as less incorporation of Al into the C-S-H phase [
20]. The incorporation of Al in the C-S-H decreased with an increased MgO content and resulted in a lower 28-day compressive strength indicating better mechanical properties of the C-A-S-H phase in comparison with hydrotalcite or gaylussite,
Figure 10.
The EDX analysis indicated that all mixes contained C-S-H phases. The Ca/Si ratio was between 1.0 and 1.55 for all mixes except for the mix S
12c that had the Ca/Si ratio of 3.2,
Table 2). The very high Ca/Si ratio of the mix S
12c indicated the presence of silicate chain units Q
1 and portlandite, which could be related to its high carbonation degree [
24]. The XRD analysis did not detect hydrotalcite despite indications from earlier experimental results and thermodynamic modelling.
The EDX analysis results showed the formation of hydrotalcite which increased by increasing the MgO content and decreased at lower Na
2O% concentrations [
17,
25,
26,
35].
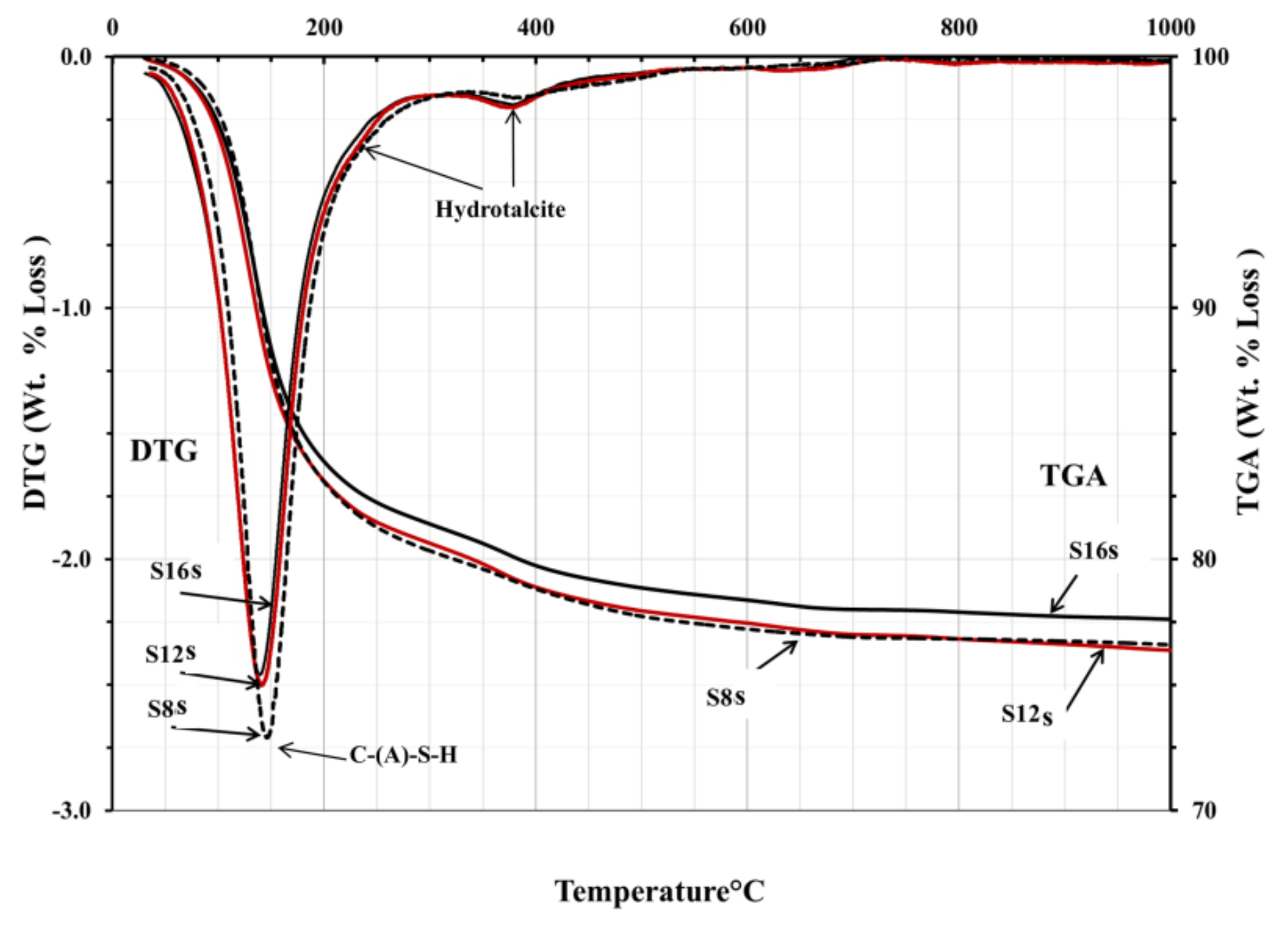
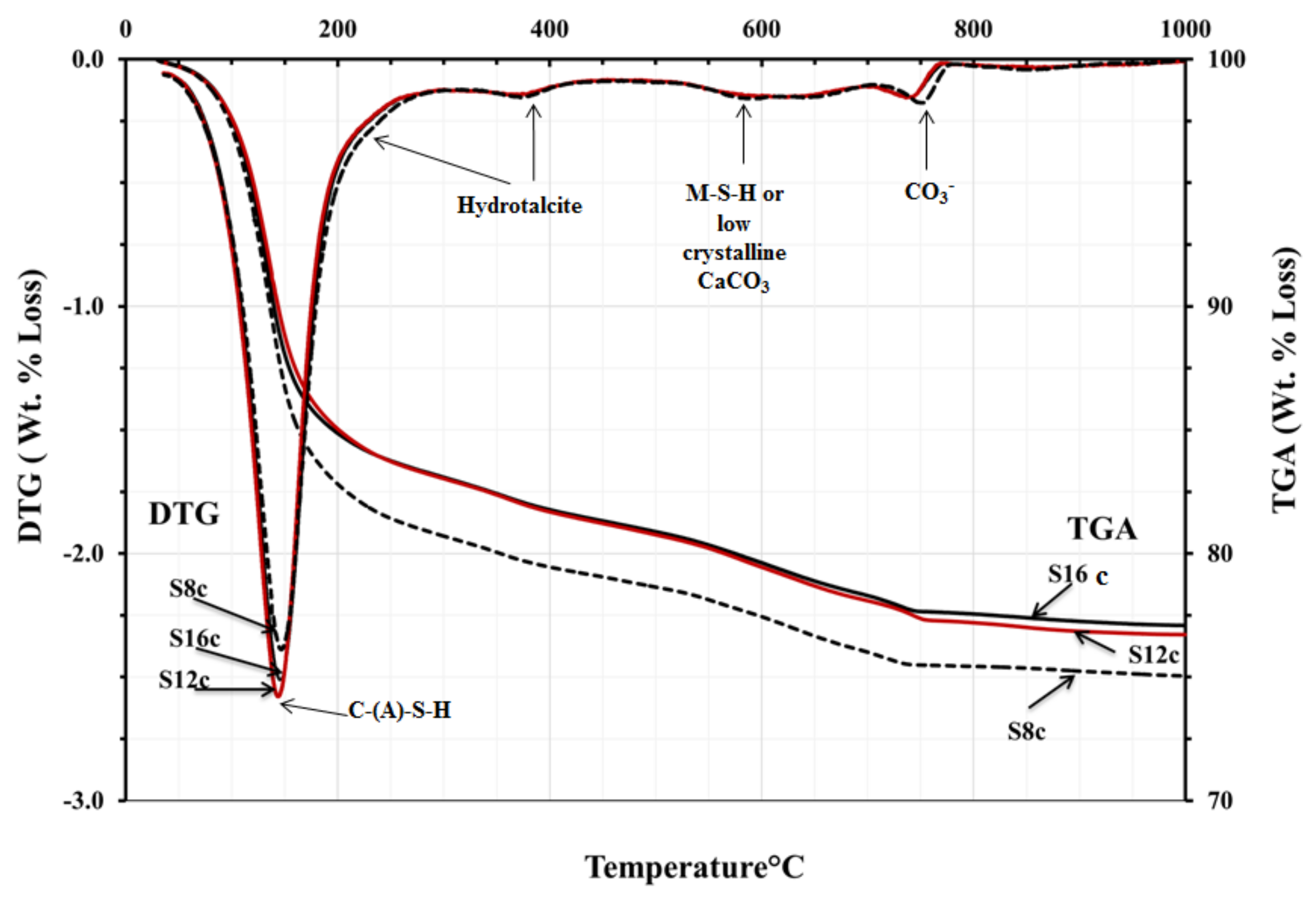
The TGA/DTG analysis showed the decomposition peaks between 200 and 400 °C, which could be identified as hydrotalcite-like phases,
Figure 11 and
Figure 12. Consequently, neither presence nor absence of hydrotalcite could be confirmed based on the obtained tests results. However, based on earlier results and taking into account the high MgO content, such trend should be considered as very likely. The TGA test results were very similar for all mixes. All mixes showed peaks related directly to the decomposition of C-S-H related phases located between 100 and 200 °C. An exception were mixes activated with SC carbonate where calcite or magnesium silicate hydrate M-S-H were identified as possible phases,
Figure 12. The peak observed at 500–600 °C was presumably related either due the dehydration of thomsonite (NaCa
2Al
5Si
5O
20·6H
2O) [
37], M-S-H gel [
21], or the decomposition of a poorly crystallised CaCO
3 [
38].
Other oxides including especially CaO and Al
2O
3 were shown by others to affect the mechanical properties of alkali-activated binders. For example decreasing CaO and Al
2O
3 contents of slag caused a strength reduction [
39]. The present results complied with this finding in the case of the CaO but were contradicting in the case of Al
2O
3, which could be related to the significantly finer particles size distribution of the S
8 slag.
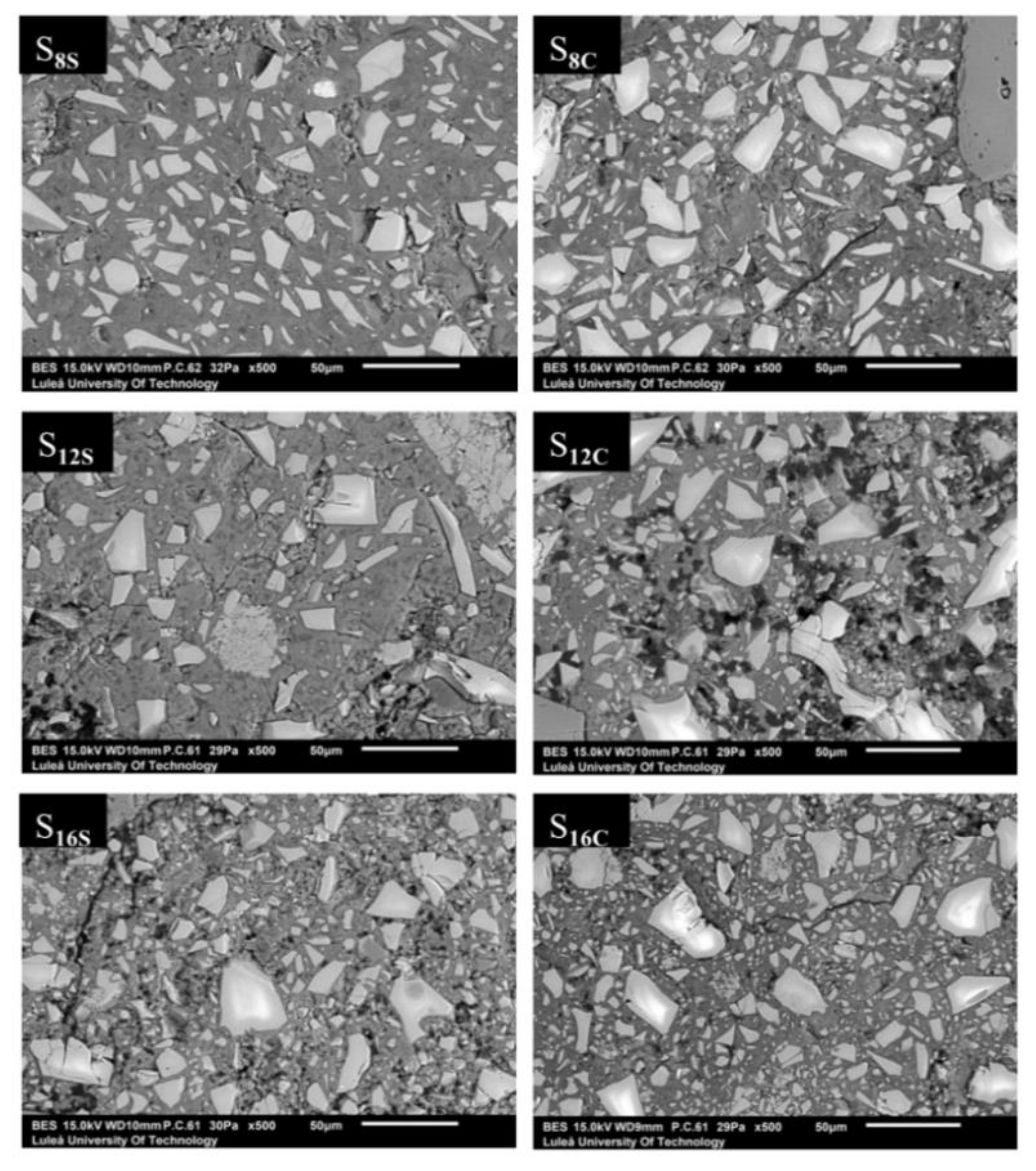
The SEM/BSE analysis of 28-day mortars showed a significant variation in porosity,
Figure 13. The lowest porosity and the most homogenous binder matrix was observed in mortars made of S
8 slag which complies with the highest develop strength results. The other two slags S
12 and S
16 being coarser and with higher MgO content, their microstructure appeared visibly more porous. The extreme case was the mix S
12 activated with SC, which also had the lowest 7-day compressive strength.
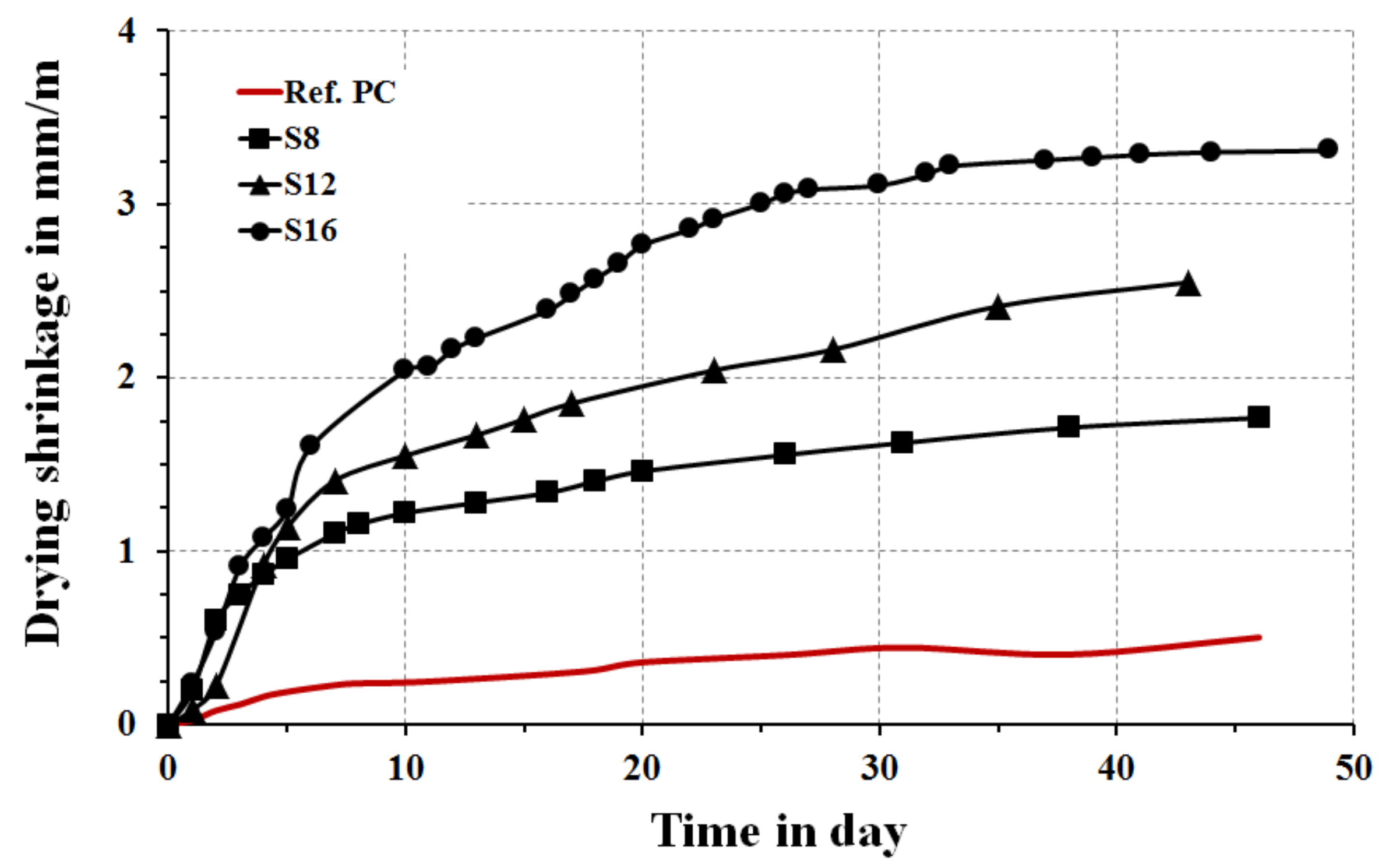
The shrinkage values were measured for concrete samples activated with 10 wt % SS to verify the effects of the slag,
Figure 14. As expected, shrinkage values were higher in comparison with the reference concrete base on the Portland cement. The lowest values in between the alkali-activated mortars were measured for the S
8 mix, which could be attributed to the fastest reaction rates, which led to form a stronger binder structure having higher resistance to shrinkage. Higher MgO content increased the drying shrinkage.
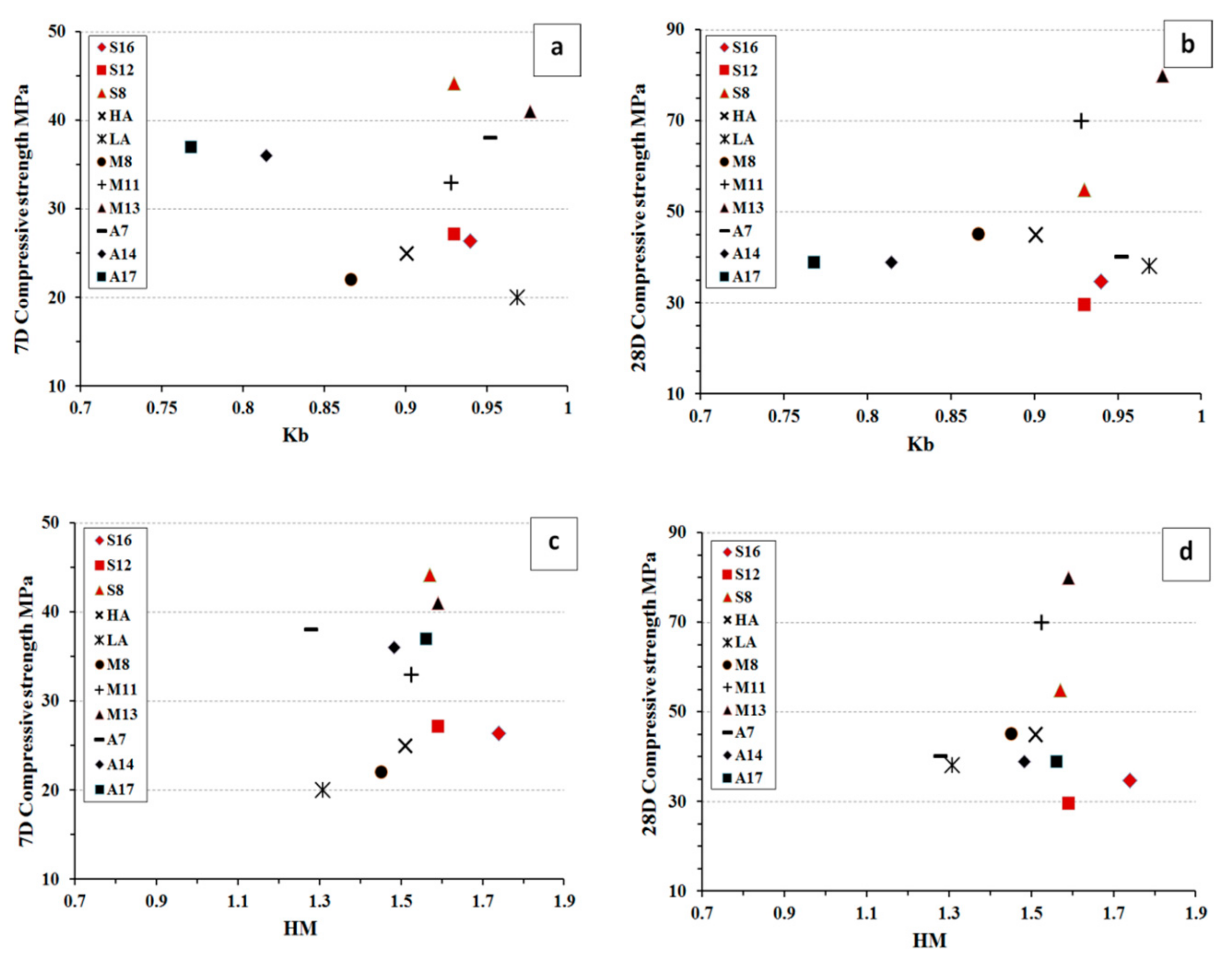
The comparative analysis of basicity modulus (K
b) and the hydration modulus (HM) versus the 7 and 28 d compressive strength are shown in,
Figure 15. The Kb value was calculated as K
b = (CaO + MgO)/(SiO
2 + Al
2O
3) and the HM as HM = (CaO + MgO + Al
2O
3)/SiO
2). The data included values for the three types of slag used in this study and 8 other slags used by others [
23,
30,
40,
41]. All slags were activated with liquid sodium silicate SS and had fineness values showed in
Table 3. The analysis showed that the fines has a stronger correlation on the developed strength values that the calculated Kb and HM values. There was not significant correlation find between Kb and HM values and the 7 or 28 d compressive strength values. The situation was the same for the studied in this research slags and the data adopted from others.