Comparing Fly Ash Samples from Different Types of Incinerators for Their Potential as Storage Materials for Thermochemical Energy and CO2
Abstract
1. Introduction
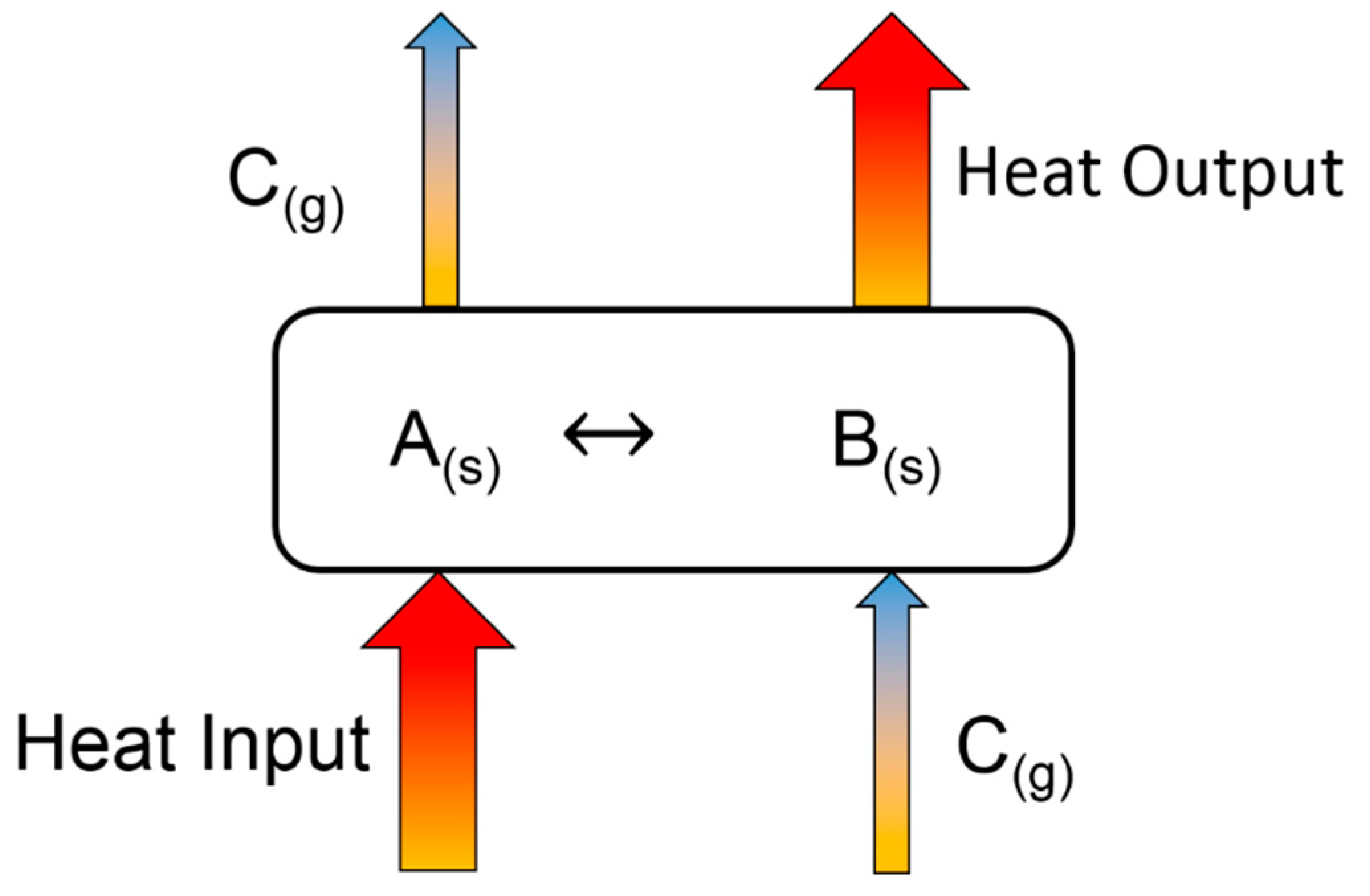
- Charging (endothermic reactions through thermal treatment)
- ○
- A(s) (fly ash/discharged) + heat ⇆ B(s) (fly ash/charged) + C(g)
- Discharging (exothermic reaction by reacting with reactive gases, such as H2O and CO2)
- ○
- B(s) (fly ash/charged) + C(g) ⇆ A(s) (fly ash/discharged) + heat
- Cycling stability test (various charging and discharging of heat)
2. Materials and Methods
2.1. Fly Ash Sampling and Waste Incineration Plants
2.2. Chemical and Physical Analysis of Fly Ash Samples
3. Results and Discussion
3.1. BET Surface Area
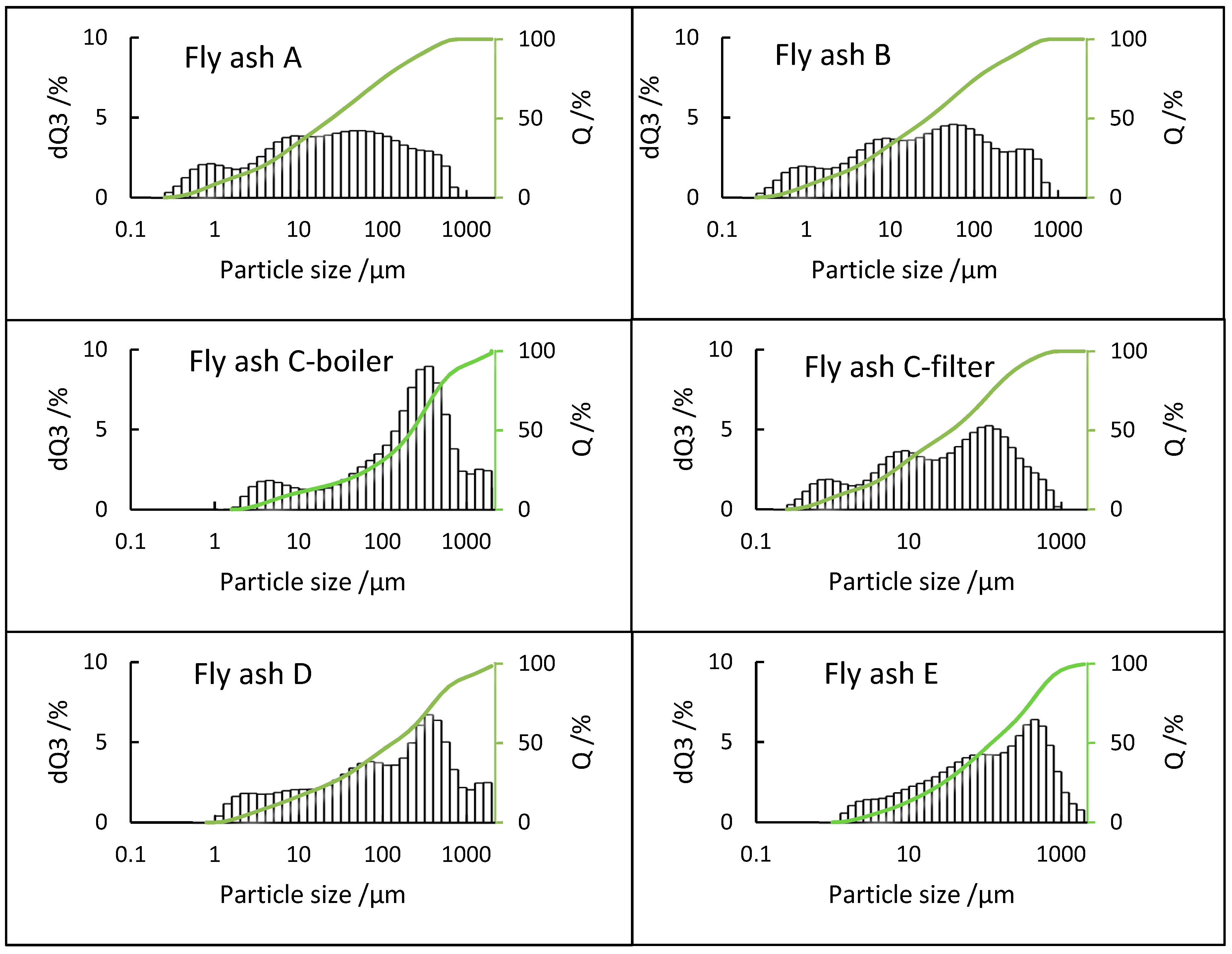
3.2. PSD Analysis
3.3. Chemical Composition of Fly Ash Samples
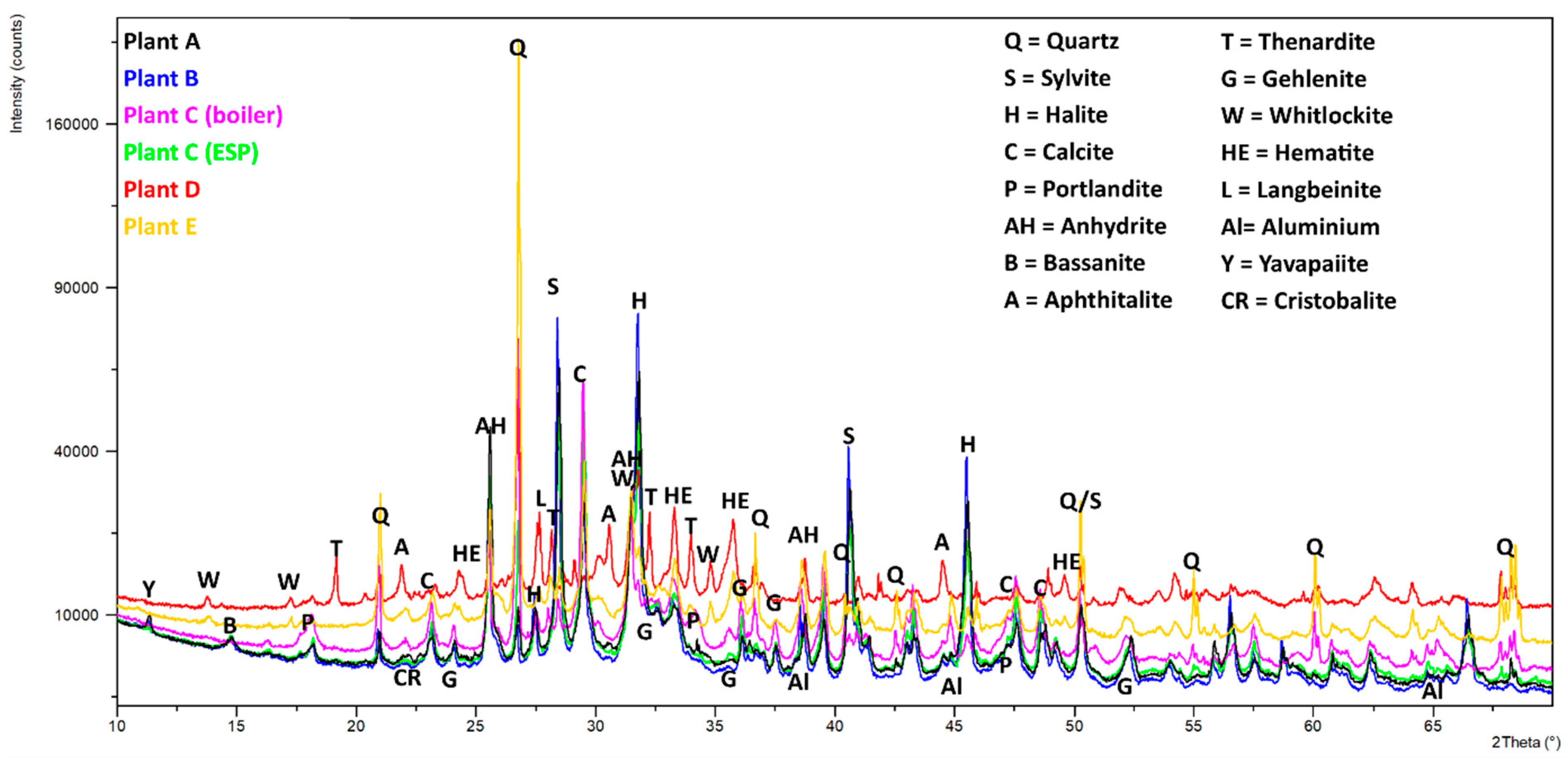
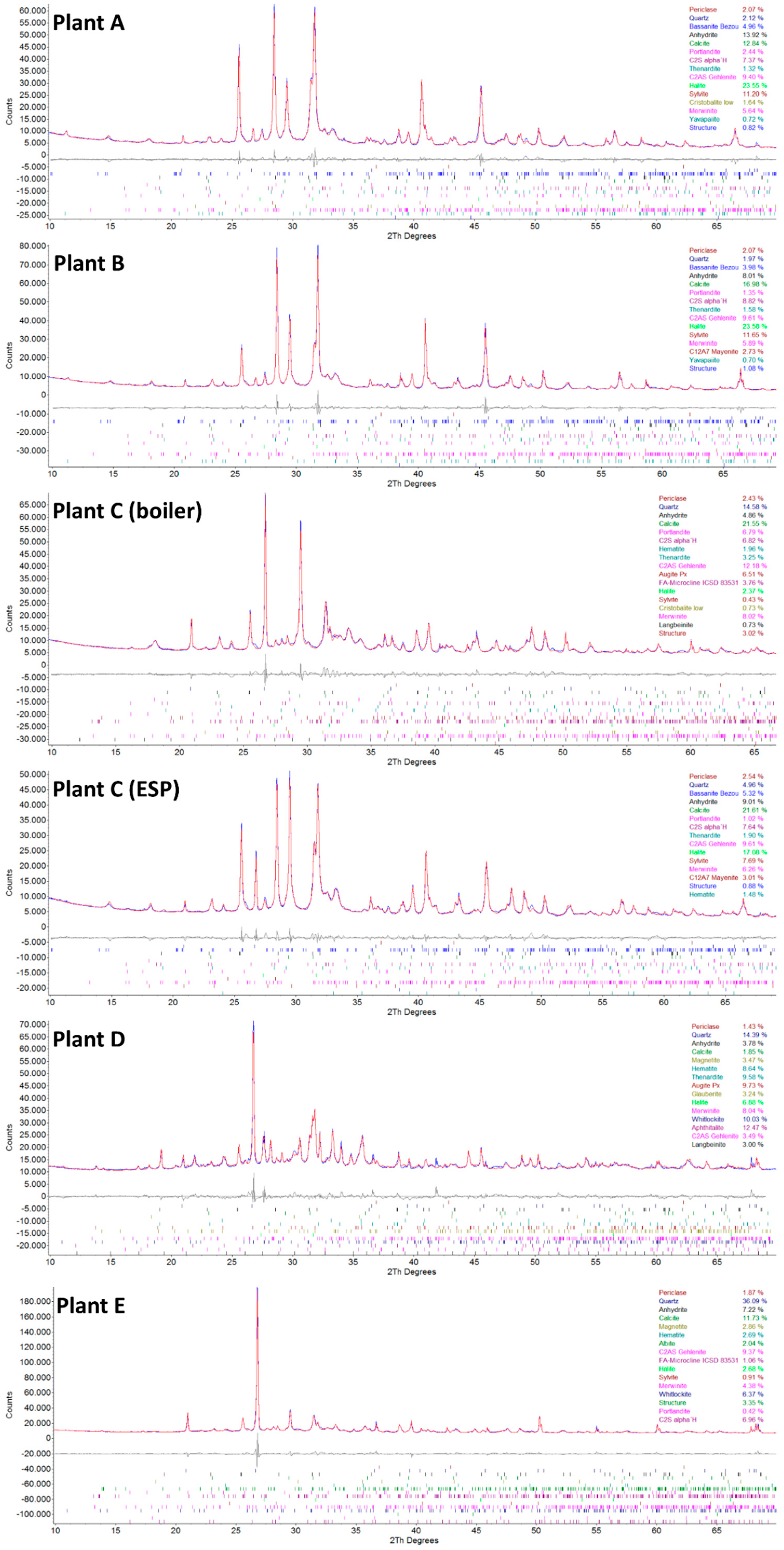
3.4. XRD Analysis
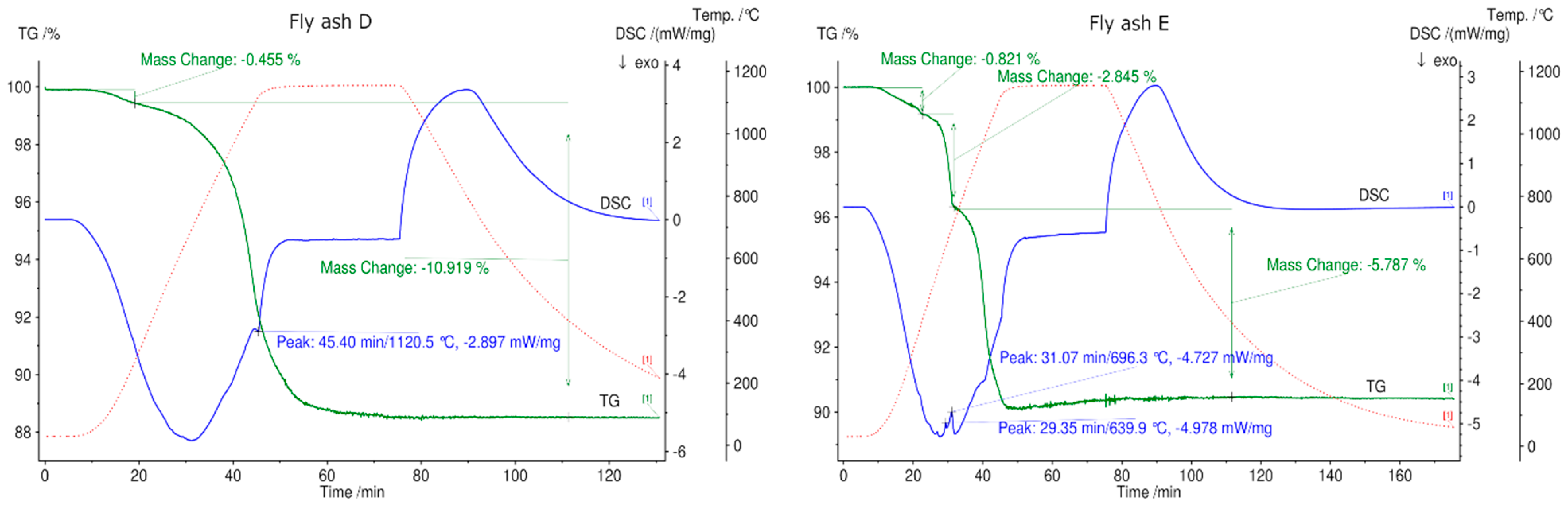
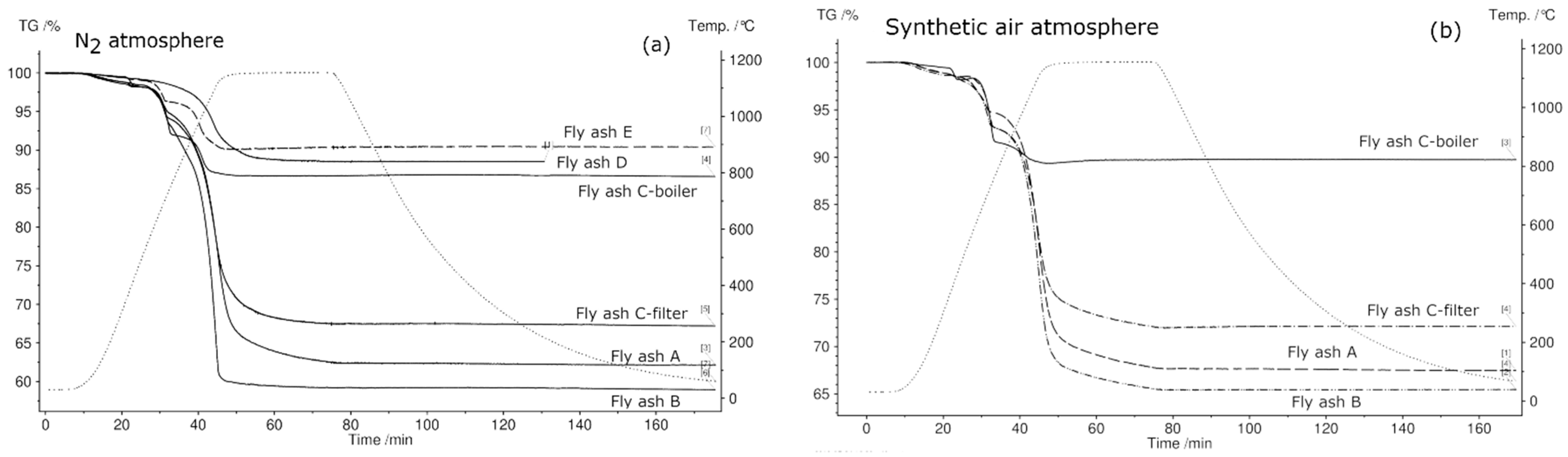
3.5. Thermogravimetric Analysis and Differential Scanning Calorimetry (TGA/DSC) of Fly Ash Samples
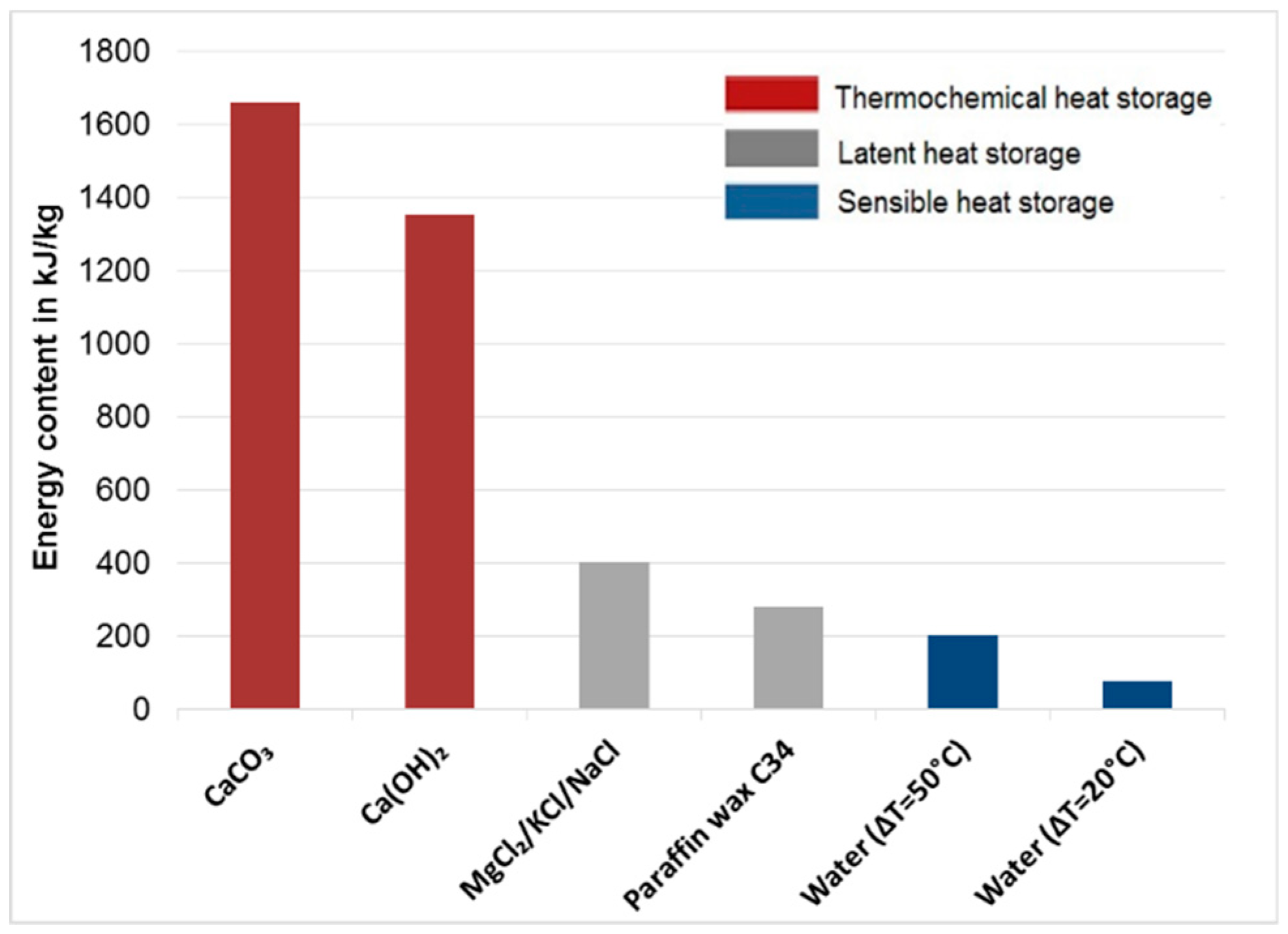
3.6. Energy Density
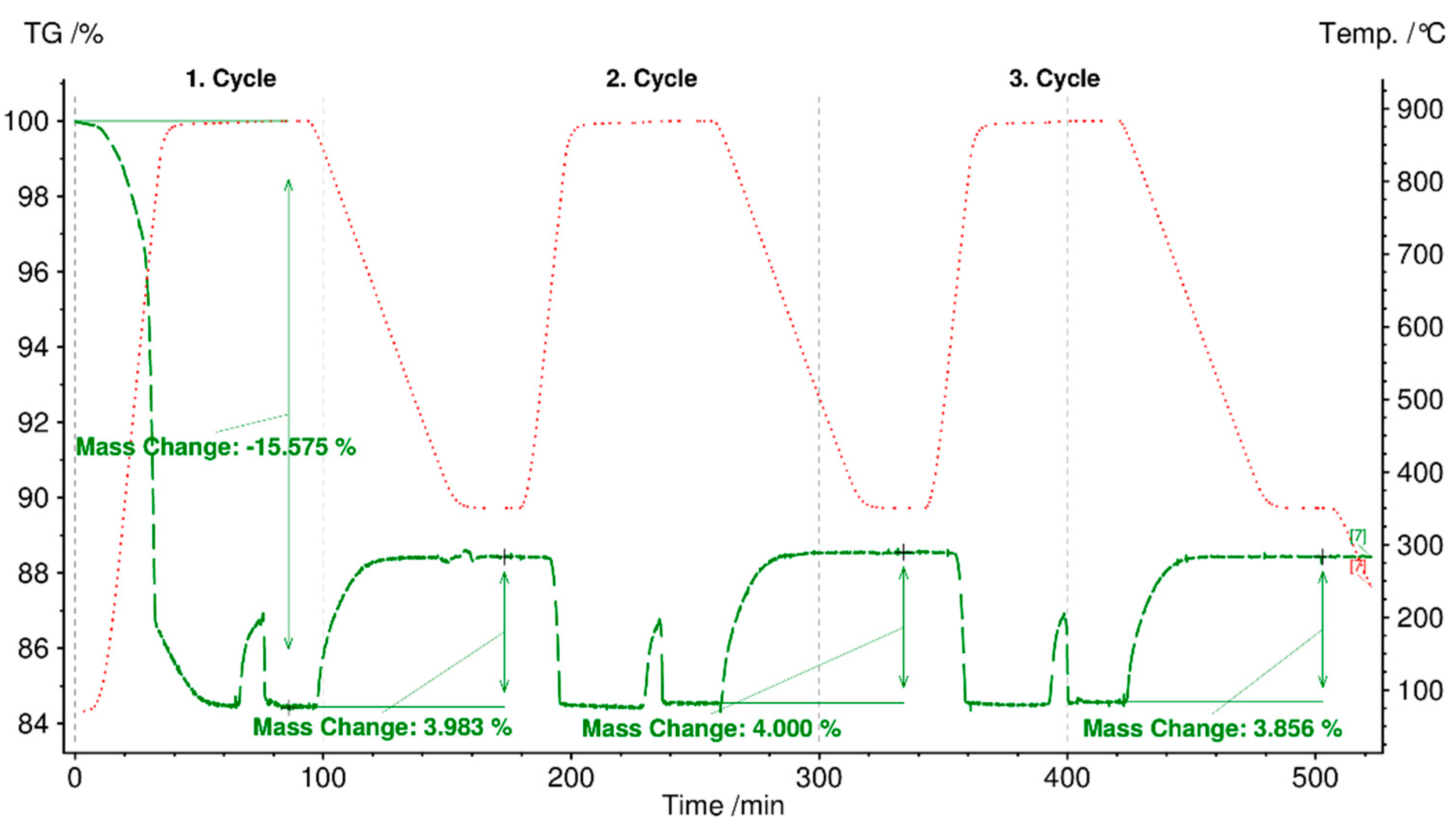
3.7. Cycling Stability Test
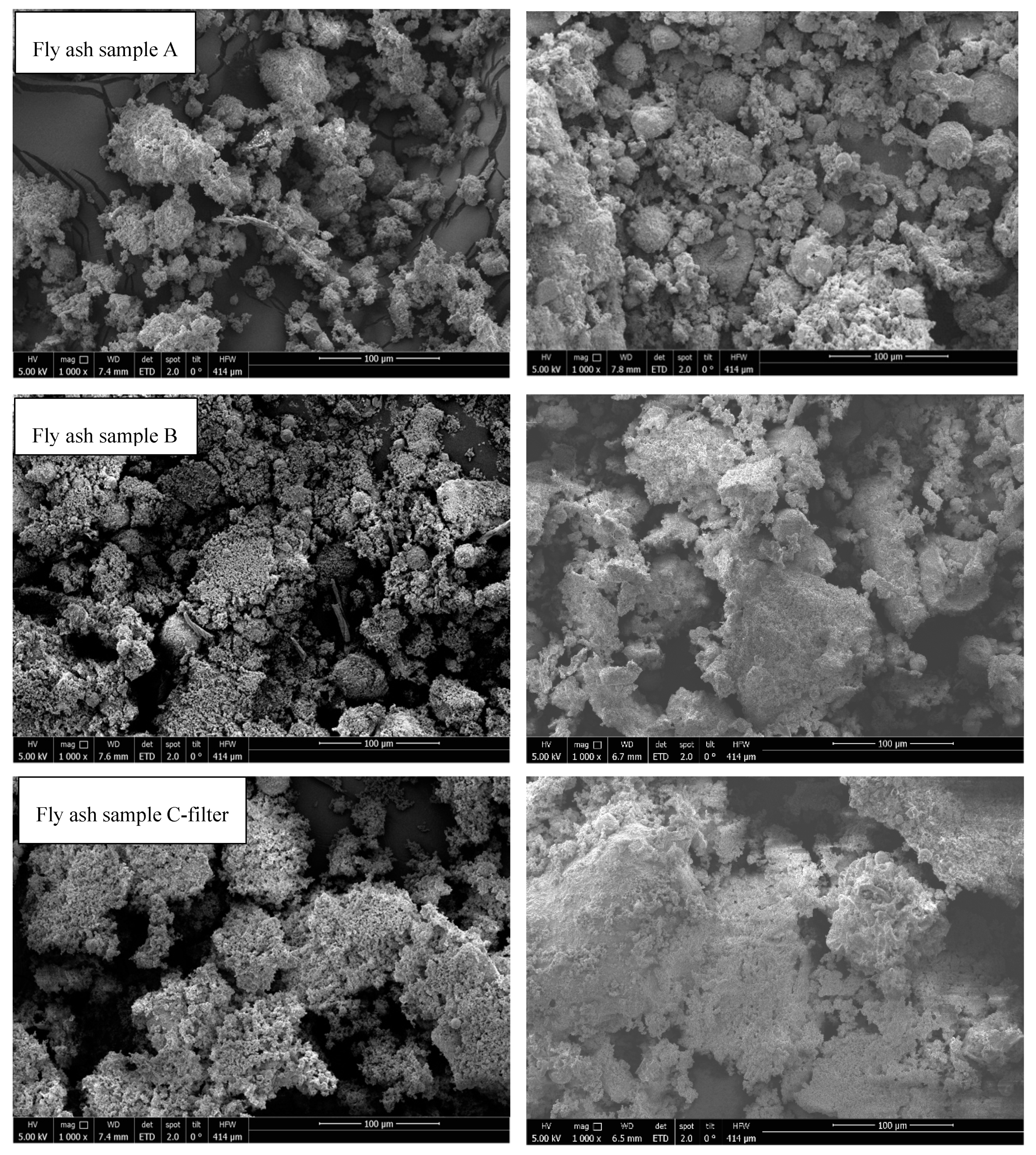
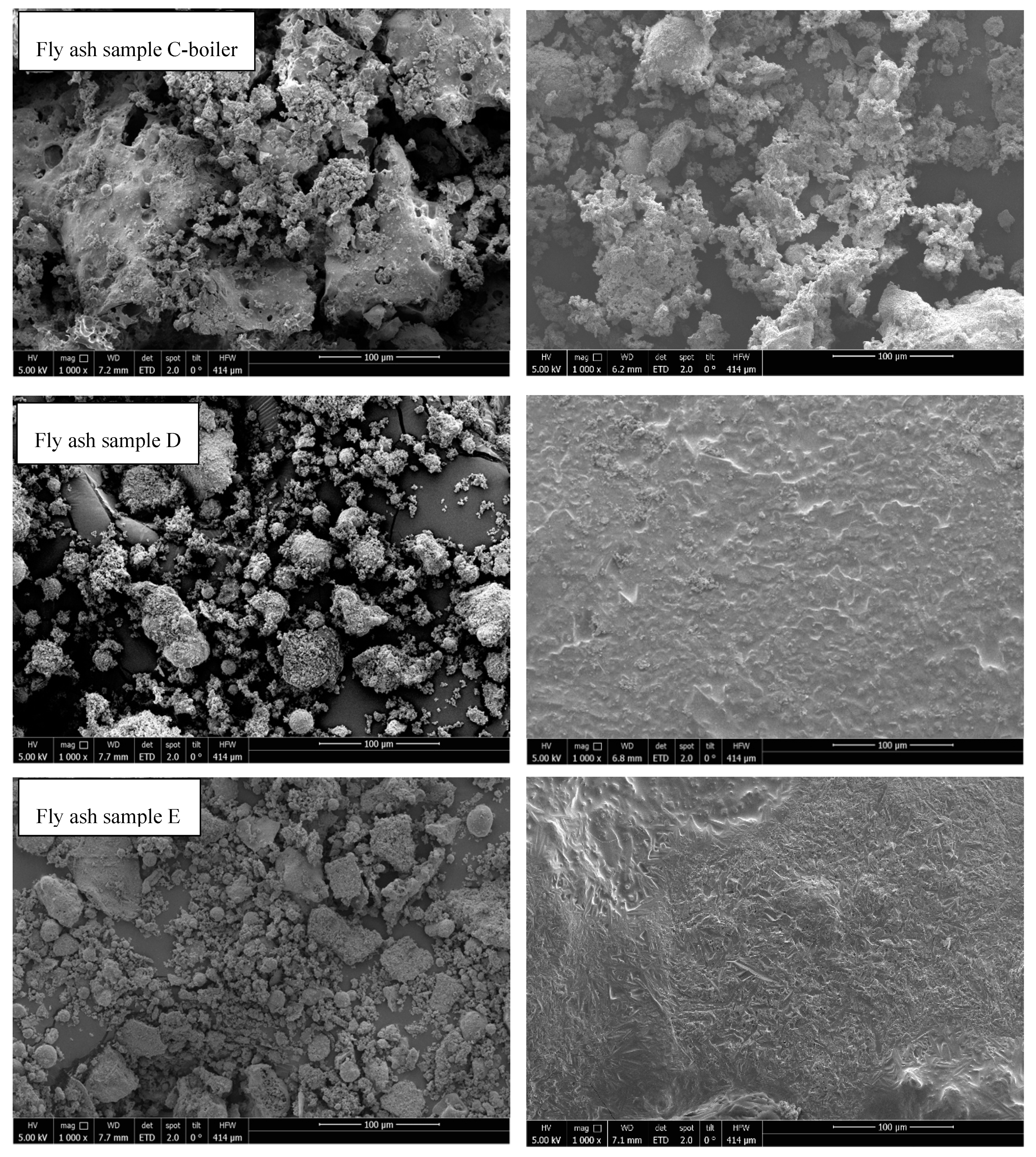
3.8. Scanning Electron Microscopy
3.9. Leaching Test
4. Conclusions
- Based on the SSA results obtained between 1.5 and 4.3 m2/g, no specific relation between their values and potential usability of fly ashes for TCES and CO2 storage was found. To determine the sorption potential, further analysis on the pore volume, pore size distribution, average pore diameter, and narrow microporosity parameters should be performed.
- The PSDs of fly ash samples E (fluidized bed), C-boiler (grate furnace), and D (rotary kiln), showed unimodal, bimodal, and multimodal distributions, respectively. Fly ashes A and B from grate furnace technologies with the same combustion processes showed the same PSDs with modes at appropriately 1, 10, and 100 µm.
- The fly ash sample C-boiler differs in Ca amount compared to other fly ash samples via XRF analysis. According to XRD results, no free CaO was detectable. However, portlandite Ca(OH)2 and calcite (CaCO3), which are most likely the alteration products of free CaO, were present in most samples. Alteration and its effect on free CaO content and subsequently on its potential for TCES and CO2 capture should be investigated separately.
- Based on TGA results up to 1150 °C in N2 atmosphere, fly ashes A, B, C-filter, C-boiler (grate furnace), D (rotary kiln), and E (fluidized bed) achieved total mass losses of 37%, 40%, 32%, 13%, 11%, and 9%, respectively. Fly ash sample C-boiler had the highest energy content of 394 kJ/kg, and no endothermic peaks were detected for fly ash sample D. Fly ash samples A, B, C-filter, and E possessed energy contents of 94, 85, 98, and 50 kJ/kg, respectively. This showed that all fly ash samples, except fly ash sample D from a rotary kiln, met the first requirement of TCES under the selected experimental conditions, which is to store thermal heat through endothermic reactions.
- The second requirement for a TCES material (exothermic reaction by reaction with reactive gas components (H2O and CO2)) was met by fly ash sample C-boiler. The energy content in fly ash sample C-boiler obtained by thermal treatment up to 880 °C was approximately 226 kJ/kg, approximately 86 kJ/kg of which could be released under the selected operational conditions in STA.
- Cycling stability tests, i.e., various charging and discharging of heat, as the third requirement for TCES materials, were accomplished for fly ash sample C-boiler for three cycles in STA. The charging step occurred at a heating rate of 30 °C/min up to 880 °C for 30 min, followed by heat storage between 99 and 290 kJ/kg. Discharging started at a cooling rate of 10 °C/min from 880 °C to 350 °C, maintaining at 350 °C for 30 min in CO2 and H2O vapor atmospheres, and followed by heat release between 73 and 101 kJ/kg.
- Fly ash sample C-boiler could store CO2 with a storage capacity of 27 kg CO2/ton fly ash.
- According to ICP-OES analysis and leachability test, the fly ash C-boiler showed that this kind of fly ash may be considered to be nonhazardous, making this fly ash more feasible since it does not exceed the limits for disposal in standard above-ground landfills. However, before it can be considered to be a nonhazardous material such as total organic carbon, further analysis of its total dissolved solids needs to be performed.
- SEM analysis of all samples before and after thermal treatment by STA up to 1150 °C in N2 atmosphere revealed sintering, agglomeration, and melting of particles. However, fly ash samples were in compact powder form and easily removable from the crucible, except for fly ashes D (rotary kiln) and E (fluidized bed) because they melted.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bringezu, S. Towards sustainable resource management in the European Union. Wupp. Pap. 2002, 121, 50. [Google Scholar]
- Brunner, P.H.; Rechberger, H. Waste to energy--key element for sustainable waste management. Waste Manag. 2015, 37, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Chandler, A.J.; Eighmy, T.T.; Hartlén, J.; Hjelmar, O.; Kosson, D.S.; Sawell, S.E.; van der Sloot, H.A.; Vehlow, J. Municipal Solid Waste Incineration Residues. In Studies in Environmental Science, 1st ed.; Elsevier Science Press: Amsterdam, The Netherlands, 1997; Volume 67, pp. 1–973. [Google Scholar]
- Fellner, J.; Lederer, J.; Purgar, A.; Winterstetter, A.; Rechberger, H.; Winter, F.; Laner, D. Evaluation of resource recovery from waste incineration residues--the case of zinc. Waste Manag. 2015, 37, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Block, C.; Van Caneghem, J.; Van Brecht, A.; Wauters, G.; Vandecasteele, C. Incineration of Hazardous Waste: A Sustainable Process? Waste Biomass Valori. 2014, 6, 137–145. [Google Scholar] [CrossRef]
- Fytili, D.; Zabaniotou, A. Utilization of sewage sludge in EU application of old and new methods—A review. Renew. Sustain. Energy Rev. 2008, 12, 116–140. [Google Scholar] [CrossRef]
- Huber, F.; Blasenbauer, D.; Mallow, O.; Lederer, J.; Winter, F.; Fellner, J. Thermal co-treatment of combustible hazardous waste and waste incineration fly ash in a rotary kiln. Waste Manag. 2016, 58, 181–190. [Google Scholar] [CrossRef]
- Li, W.; Huang, Q.; Lu, S.; Wu, H.; Li, X.; Yan, J. Life Cycle Assessment of the Environmental Impacts of Typical Industrial Hazardous Waste Incineration in Eastern China. Aerosol Air Qual. Res. 2015, 15, 242–251. [Google Scholar] [CrossRef]
- Huber, F.; Laner, D.; Fellner, J. Comparative life cycle assessment of MSWI fly ash treatment and disposal. Waste Manag. 2018, 73, 392–403. [Google Scholar] [CrossRef]
- Ferreira, C.; Ribeiro, A.; Ottosen, L. Possible applications for municipal solid waste fly ash. J. Hazard Mater. 2003, B96, 201–2016. [Google Scholar] [CrossRef]
- Astrup, T. Management of APC residues from W-t-E Plants; ISWA-WG Thermal Treatment of Waste Press: Wien, Austria, 2008. [Google Scholar]
- del Valle-Zermeno, R.; Barreneche, C.; Cabeza, L.F.; Formosa, J.; Fernandez, A.I.; Chimenos, J.M. MSWI bottom ash for thermal energy storage: An innovative and sustainable approach for its reutilization. Renew. Energy 2016, 99, 431–436. [Google Scholar] [CrossRef]
- Gutierrez, A.; Miro, L.; Gil, A.; Rodriguez-Aseguinolaza, J.; Barreneche, C.; Calvet, N.; Py, X.; Fernandez, A.I.; Grageda, M.; Ushak, S.; et al. Advances in the valorization of waste and by-product materials as thermal energy storage (TES) materials. Renew. Sust. Energ. Rev. 2016, 59, 763–783. [Google Scholar] [CrossRef]
- Cherbański, R.; Molga, E. Sorption-enhanced steam-methane reforming with simultaneous sequestration of CO2 on fly ashes–Proof of concept and simulations for gas-solid-solid trickle flow reactor. Chem. Eng. Process Process Intensif. 2018, 124, 37–49. [Google Scholar] [CrossRef]
- Setoodeh Jahromy, S.; Jordan, C.; Azam, M.; Werner, A.; Harasek, M.; Winter, F. Fly Ash from Municipal Solid Waste Incineration as a Potential Thermochemical Energy Storage Material. Energy Fuels 2019, 33, 5810–5819. [Google Scholar] [CrossRef]
- André, L.; Abanades, S.; Flamant, G. Screening of thermochemical systems based on solid-gas reversible reactions for high temperature solar thermal energy storage. Renew. Sustain. Energy Rev. 2016, 64, 703–715. [Google Scholar] [CrossRef]
- Pardo, P.; Deydier, A.; Anxionnaz-Minvielle, Z.; Rouge, S.; Cabassud, M.; Cognet, P. A review on high temperature thermochemical heat energy storage. Renew. Sustain. Energy Rev. 2014, 32, 591–610. [Google Scholar] [CrossRef]
- André, L.; Abanades, S.; Cassayre, L. Mixed Metal Oxide Systems Applied to Thermochemical Storage of Solar Energy: Benefits of Secondary Metal Addition in Co and Mn Oxides and Contribution of Thermodynamics. Appl. Sci. 2018, 8, 2618. [Google Scholar] [CrossRef]
- Deutsch, M. A Systematic Approach to Identify New Thermochemical Energy Storage Systems; TU Wien Press: Wien, Austria, 2017. [Google Scholar]
- Deutsch, M.; Müller, D.; Aumeyr, C.; Jordan, C.; Gierl-Mayer, C.; Weinberger, P.; Winter, F.; Werner, A. Systematic search algorithm for potential thermochemical energy storage systems. Appl. Energy 2016, 183, 113–120. [Google Scholar] [CrossRef]
- Setoodeh Jahromy, S.; Birkelbach, F.; Jordan, C.; Huber, C.; Harasek, M.; Werner, A.; Winter, F. Impact of Partial Pressure, Conversion, and Temperature on the Oxidation Reaction Kinetics of Cu2O to CuO in Thermochemical Energy Storage. Energies 2019, 12, 508. [Google Scholar] [CrossRef]
- Müller, D.; Knoll, C.; Gravogl, G.; Artner, W.; Welch, J.M.; Eitenberger, E.; Friedbacher, G.; Schreiner, M.; Harasek, M.; Hradil, K.; et al. Tuning the performance of MgO for thermochemical energy storage by dehydration–From fundamentals to phase impurities. Appl. Energy 2019, 253, 113562. [Google Scholar] [CrossRef]
- Agrafiotis, C.; Roeb, M.; Schmücker, M.; Sattler, C. Exploitation of thermochemical cycles based on solid oxide redox systems for thermochemical storage of solar heat. Part 1: Testing of cobalt oxide-based powders. Sol. Energy 2014, 102, 189–211. [Google Scholar] [CrossRef]
- Block, T.; Schmücker, M. Metal oxides for thermochemical energy storage: A comparison of several metal oxide systems. Sol. Energy 2016, 126, 195–207. [Google Scholar] [CrossRef]
- Müller, D.; Knoll, C.; Ruh, T.; Artner, W.; Welch, J.M.; Peterlik, H.; Eitenberger, E.; Friedbacher, G.; Harasek, M.; Blaha, P.; et al. Calcium Doping Facilitates Water Dissociation in Magnesium Oxide. Adv. Sustain. Syst. 2018, 2, 1700096. [Google Scholar] [CrossRef]
- Chen, X.; Jin, X.; Liu, Z.; Ling, X.; Wang, Y. Experimental investigation on the CaO/CaCO3 thermochemical energy storage with SiO2 doping. Energy 2018, 155, 128–138. [Google Scholar] [CrossRef]
- Carrillo, A.J.; Moya, J.; Bayón, A.; Jana, P.; de la Peña O’Shea, V.A.; Romero, M.; Gonzalez-Aguilar, J.; Serrano, D.P.; Pizarro, P.; Coronado, J.M. Thermochemical energy storage at high temperature via redox cycles of Mn and Co oxides: Pure oxides versus mixed ones. Sol. Energy Mater. Sol. Cells 2014, 123, 47–57. [Google Scholar] [CrossRef]
- Carrillo, A.J.; Serrano, D.P.; Pizarro, P.; Coronado, J.M. Improving the Thermochemical Energy Storage Performance of the Mn2O3 /Mn3O4 Redox Couple by the Incorporation of Iron. ChemSusChem 2015, 8, 1947–1954. [Google Scholar] [CrossRef]
- Barker, R. The reversibility of the reaction CaCO3 ⇄ CaO+CO2. Chem. Technol. Biotechnol. 1973, 23, 733–742. [Google Scholar] [CrossRef]
- Erans, M.; Manovic, V.; Anthony, E.J. Calcium looping sorbents for CO2 capture. Appl. Energy 2016, 180, 722–742. [Google Scholar] [CrossRef]
- Lu, S.; Wu, S. Calcination–carbonation durability of nano CaCO3 doped with Li2SO4. Chem. Eng. J. 2016, 294, 22–29. [Google Scholar] [CrossRef]
- Yanase, I.; Maeda, T.; Kobayashi, H. The effect of addition of a large amount of CeO2 on the CO2 adsorption properties of CaO powder. Chem. Eng. J. 2017, 327, 548–554. [Google Scholar] [CrossRef]
- Jing, J.-Y.; Li, T.-Y.; Zhang, X.-W.; Wang, S.-D.; Feng, J.; Turmel, W.A.; Li, W.-Y. Enhanced CO2 sorption performance of CaO/Ca3Al2O6 sorbents and its sintering-resistance mechanism. Appl. Energy 2017, 199, 225–233. [Google Scholar] [CrossRef]
- SolidHeat, Thermochemical energy storage consortium. Wien. 2015. Available online: http://solidheat.project.tuwien.ac.at/about-the-project/solidheat-basic/ (accessed on 10 October 2019).
- Wee, J.-H. A review on carbon dioxide capture and storage technology using coal fly ash. Appl. Energy 2013, 106, 143–151. [Google Scholar] [CrossRef]
- Dindi, A.; Quang, D.V.; Vega, L.F.; Nashef, E.; Abu-Zahra, M.R.M. Applications of fly ash for CO2 capture, utilization, and storage. J. CO2 Util. 2019, 29, 82–102. [Google Scholar] [CrossRef]
- Ecke, H.; Menad, N.; Lagerkvist, A. Treatment-oriented characterization of dry scrubber residue from municipal solid waste incineration. J. Mater. Cycles Waste Manag. 2002, 4, 117–126. [Google Scholar] [CrossRef]
- Holger, E.; Nourreddine, M.; Anders, L. Carbonation of Municipal Solid Waste Incineration Fly Ash and the Impact on Metal Mobility. J. Environ. Eng. 2003, 129. [Google Scholar] [CrossRef]
- Bertos, M.F.; Li, X.; Simons, S.J.R.; Hills, C.D.; Carey, P.J. Investigation of accelerated carbonation for the stabilisation of MSW incinerator ashes and the sequestration of CO2. Green Chem. 2004, 6, 428–436. [Google Scholar] [CrossRef]
- Li, X.; Bertos, M.F.; Hills, C.D.; Carey, P.J.; Simon, S. Accelerated carbonation of municipal solid waste incineration fly ashes. Waste Manag. 2007, 27, 1200–1206. [Google Scholar] [CrossRef]
- De Boom, A.; Aubert, J.E.; Degrez, M. Carbonation of municipal solid waste incineration electrostatic precipitator fly ashes in solution. Waste Manag. Res. 2014, 32, 406–413. [Google Scholar] [CrossRef]
- Degen, T.T.; Sadki, M.; Bron, E.; König, U.; Nénert, G. The HighScore suite. Powder Diffr. 2014, 29, 13–18. [Google Scholar] [CrossRef]
- Bruker-AXS TOPAS V4.2 General profile and structure analysis software for powder diffraction data User´s manual. 2009. Available online: http://algol.fis.uc.pt/jap/TOPAS%204-2%20Users%20Manual.pdf (accessed on 11 October 2019).
- Belsky, A.; Hellenbrandt, M.; Karen, V.L.; Luksch, P. New developments in the Inorganic Crystal Structure database (ICSD): accessibility in support of materials and design. Acta Cryst. 2002, B58, 364–369. [Google Scholar] [CrossRef]
- Girón, R.P.; Ruiz, B.; Fuente, E.; Gil, R.R.; Suárez-Ruiz, I. Properties of fly ash from forest biomass combustion. Fuel 2013, 114, 71–77. [Google Scholar] [CrossRef]
- Osan, J.; Alföldy, B.; Törok, S.; Grieken, R.V. Characterisation of wood combustion particles using electron probe microanalysis. Atmos. Env. 2002, 36, 2207–2214. [Google Scholar] [CrossRef]
- Schlumberger, S. Neue Technologien und Möglichkeiten der Behandlung von Rauchgasreinigungsrückständen im Sinne eines nachhaltigen Ressourcenmanagements. KVA-Rückstände in der Schweiz Der Rohstoff mit Mehrwert 2010, 194–202. [Google Scholar]
- Weibel, G.; Eggenberger, U.; Schlumberger, S.; Mader, U.K. Chemical associations and mobilization of heavy metals in fly ash from municipal solid waste incineration. Waste Manag. 2017, 62, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Schlumberger, S.; Bühler, J. Metallrückgewinnung aus Filterstäuben der thermischen Abfallbehandlung nach dem FLUREC-Verfahren. In Flaschen–Schlacken–Stäube–aus Abfallverbrennung und Metallurgie; TK Verlag Karl Thomé -Kozmiensky: Neuruppin, Germany, 2013; pp. 377–398. [Google Scholar]
- Yang, Y.; Xiao, Y.; Wilson, N.; Voncken, J.H.L. Thermal behaviour of ESP ash from municipal solid waste incinerators. J. Hazard Mater. 2009, 166, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Haiying, Z.; Youcai, Z.; Jingyu, Q. Thermal characterization of fly ash from one municipal solid waste incinerator (MSWI) in Shanghai. Process. Saf. Env. 2010, 88, 269–275. [Google Scholar] [CrossRef]
- Nowak, B.; Frías Rocha, S.; Aschenbrenner, P.; Rechberger, H.; Winter, F. Heavy metal removal from MSW fly ash by means of chlorination and thermal treatment: Influence of the chloride type. Chem. Eng. J. 2012, 179, 178–185. [Google Scholar] [CrossRef]
- Li, M.; Xiang, J.; Hu, S.; Sun, L.-S.; Su, S.; Li, P.-S.; Sun, X.-X. Characterization of solid residues from municipal solid waste incinerator. Fuel 2004, 83, 1397–1405. [Google Scholar] [CrossRef]
- Koukouzas, N.; Ketikidis, C.; Itskos, G. Heavy metal characterization of CFB-derived coal fly ash. Fuel Process. Technol. 2011, 92, 441–446. [Google Scholar] [CrossRef]
- Ordinance by the Federal Minister for the Environment on waste disposal sites. Wien, Austria. 1996, Volume 164, p. 49. Available online: https://www.ecolex.org/details/legislation/landfill-ordinance-lex-faoc033823/ (accessed on 11 October 2019).




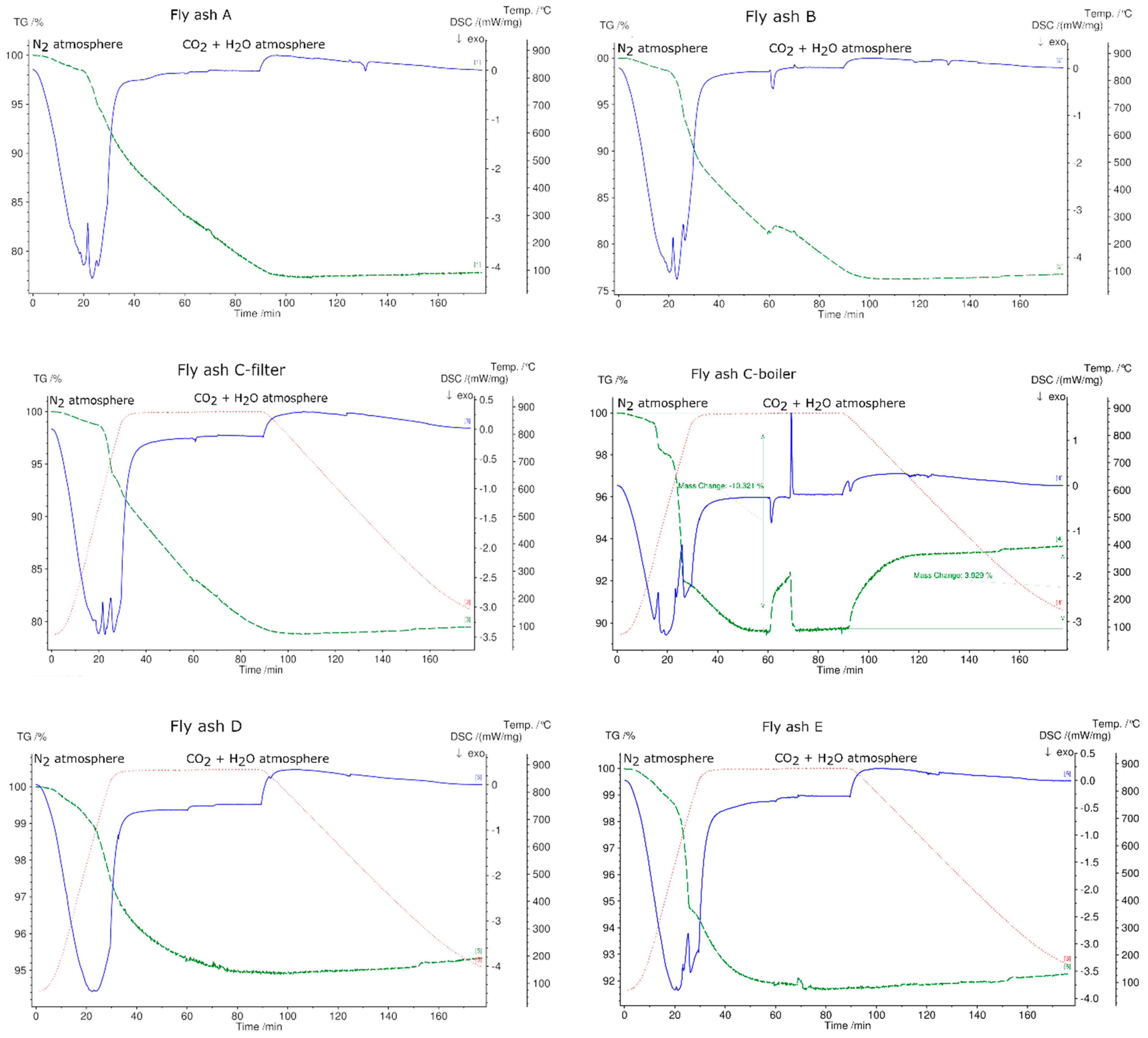
| Atmosphere | Fly Ash Sample A | Fly Ash Sample B | Fly Ash Sample C-filter | Fly Ash Sample C-boiler | Fly Ash Sample D | Fly Ash Sample E |
|---|---|---|---|---|---|---|
| N2 | x | x | x | x | x | x |
| O2 | x | x | x | x | – | – |
| CO2 | x | x | x | x | x | x |
| H2O | x | x | x | x | – | – |
| H2O/CO2 | x | x | x | x | x | x |
| Fly Ash Samples | A | B | C-boiler | C-filter | D | E |
|---|---|---|---|---|---|---|
| BET surface area | 3.69 | 4.36 | 1.61 | 2.15 | 1.79 | 1.56 |
| Elements | Fly Ash A | Fly Ash B | Fly Ash C-boiler | Fly Ash C-filter | Plant D | Plant E |
|---|---|---|---|---|---|---|
| Na2O | +++ | +++ | + | +++ | +++ | + |
| MgO | + | + | + | + | + | + |
| Al2O3 | ++ | ++ | +++ | ++ | + | +++ |
| SiO2 | +++ | +++ | ++++ | +++ | +++ | +++++ |
| P2O5 | + | + | + | + | + | + |
| SO3 | ++ | ++ | ++ | ++ | +++ | + |
| Cl | +++ | +++ | + | +++ | + | + |
| K2O | ++ | ++ | + | ++ | + | + |
| CaO | ++++ | ++++ | +++++ | ++++ | +++ | ++++ |
| TiO2 | + | + | + | + | + | + |
| Fe2O3 | + | + | + | + | ++ | + |
| Elements | Plant A | Plant B | Plant C-boiler | Plant C-filter | Plant D | Plant E |
|---|---|---|---|---|---|---|
| Sb | 742 | 635 | 302 | 1316 | 656 | 127 |
| As | 37 | 32 | 13 | 37 | 83 | 7 |
| Ba | 927 | 984 | 1745 | 1048 | 183 | 809 |
| Pb | 4883 | 4394 | 1100 | 7016 | 12948 | 1254 |
| Cd | 347 | 321 | 38 | 375 | 321 | 14 |
| Cr | 245 | 259 | 276 | 242 | 780 | 234 |
| Co | 24 | 27 | 38 | 35 | 111 | 27 |
| Cu | 1008 | 1004 | 1009 | 1901 | 3315 | 3608 |
| Mn | 574 | 676 | 1050 | 816 | 816 | 676 |
| Mo | 24 | 20 | 22 | 24 | 191 | 10 |
| Ni | 59 | 60 | 152 | 95 | 615 | 109 |
| Hg | 12.50 | 34.79 | 0.34 | 4.34 | 0.21 | <LOQ |
| Ag | 49 | 44 | 14 | 47 | 126 | 12 |
| Zn | 20722 | 18371 | 5684 | 21613 | 38108 | 3870 |
| Sn | 948 | 835 | 177 | 865 | 1154 | 147 |
| Compound | Chemical Formula | A | B | C-Filter | C-Boiler | D | E |
|---|---|---|---|---|---|---|---|
| Aluminum | Al | 1 | 1 | 1 | 3 | 3 | 3 |
| Quartz | SiO2 | 2 | 2 | 5 | 15 | 14 | 36 |
| Cristobalite | SiO2 | 2 | 1 | ||||
| Hematite | Fe2O3 | 1 | 2 | 9 | 3 | ||
| Magnetite | Fe3O4 | 3 | 3 | ||||
| Portlandite | Ca(OH)2 | 2 | 1 | 1 | 7 | 0 | |
| Periclase | MgO | 2 | 2 | 3 | 2 | 1 | 2 |
| Calcite | CaCO3 | 13 | 17 | 22 | 22 | 2 | 12 |
| Anhydrite | CaSO4 | 14 | 8 | 9 | 5 | 4 | 7 |
| Bassanite | CaSO4x0.5H2O | 5 | 4 | 5 | |||
| Aphthitalite | K3Na(SO4)2 | 12 | |||||
| Thenardite | Na2SO4 | 1 | 2 | 2 | 3 | 10 | |
| Glauberite | CaNa2(SO4)2 | 3 | |||||
| Ca-Langbeinite | K2Ca2(SO4)3 | 1 | 3 | ||||
| Yavapaiite | KFe3+(SO4)2 | 1 | 1 | ||||
| Halite | NaCl | 24 | 24 | 17 | 2 | 7 | 3 |
| Sylvite | KCl | 11 | 12 | 8 | 0 | 1 | |
| Belite | 2CaO SiO2 | 7 | 9 | 8 | 7 | 7 | |
| Merwinite | Ca3Mg(SiO4)2 | 6 | 6 | 6 | 8 | 8 | 4 |
| Mayenite | Ca12Al14O33 | 3 | 3 | ||||
| Gehlenite | Ca2Al [AlSiO7] | 9 | 10 | 10 | 12 | 3 | 9 |
| Feldspar | (Ca,Na,K)(Al,Si)4O8 | 4 | 3 | ||||
| Augite Pyroxene | (Ca,Mg,Fe)Si2O6 | 7 | 10 | ||||
| Whitlockite | Ca3(PO4)2 | 10 | 6 | ||||
| Sum | 100 | 100 | 100 | 100 | 100 | 100 |
| TR /°C | A % | RT | B % | RT | C-Fil | RT | TR /°C | C-Boiler % | RT | TR/°C | D % | RT | TR /°C | E % | RT |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 40–530 | 1.4 | – | 1.8 | – | 1.7 | – | 45–315 | 0.4 | – | 45–350 | 0.5 | – | 40–500 | 0.8 | – |
| – 530–750 | – 3.5 | – Endo | – 4.7 | – Endo | – 3.9 | – Endo | 315–550 | 1.1 | Endo | – – | – – | – – | – 500–750 | – 2.8 | – – |
| 550–810 | 6.3 | Endo | |||||||||||||
| 750–1150 | 32.8 | Endo | 34.1 | Endo | 26.8 | Endo | 810–1150 | 5.2 | Endo and Exo | 350–1150 | 10.8 | Exo | 750–1150 | 5.7 | Endo |
| Sum | 37.7 | 40.6 | 32.4 | 13 | 11.3 | 9.3 |
| Fly Ash | A | B | C-filter | C-boiler | D | E |
|---|---|---|---|---|---|---|
| Energy density | 94 | 85 | 98 | 394 | n.d. | 50 |
| Energy Content in kJ/kg | 1. Cycle | 2. Cycle | 3. Cycle |
|---|---|---|---|
| Charging (heating to 880 °C, N2 atmosphere) | 290 | 99 | 105 |
| Discharging (cooling from 880 °C to 350 °C, CO2, and H2O atmosphere) | 73 | 101 | 100 |
| Element Components | Limits Value | A | B | C-Filter | C-Boiler | D | E |
|---|---|---|---|---|---|---|---|
| Al | 1.9 | 2.1 | 1.51 | 1.61 | 3 | 1471 | |
| Sb | 0.7 (2.1) | 0.05 | 0.07 | 0.06 | 0.05 | 0.21 | 0.2 |
| As | 2 | 0.116 | 0.07 | 0.13 | 0.05 | - | 0.05 |
| Ba | 100 (300) | 5.61 | 4.7 | 5.75 | 4.8 | 1.5 | 74 |
| Pb | 10 (30) | 294 | 304 | 52.55 | 16.7 | 24 | 2.03 |
| Cd | 1 | 0.12 | 0.21 | 0.18 | 0.18 | 144 | 0.1 |
| Cr | 10 (20) | 2.3 | 2.6 | 7.8 | 6.5 | 1.1 | 0.9 |
| Co | 5 | 0.1 | 0.17 | 0.18 | 0.18 | 2.7 | 0.1 |
| Fe | 0.15 | 0.4 | 0.24 | 0.55 | 0.7 | 0.12 | |
| Cu | 50 | 0.1 | 0.21 | 0.2 | 0.166 | 1.3 | 0.18 |
| Mn | 0.1 | 0.17 | 0.18 | 0.166 | 27 | 0.1 | |
| Ni | 10 | 8.9 | 5.8 | 1.7 | 4.8 | 7.2 | 1.3 |
| Hg | 0.1 | 0.11 | 0.18 | 0.22 | 0.183 | 0.01 | 0.1 |
| Ag | 1 | 0.01 | 0.01 | 0.01 | 0.01 | 0.6 | 0.01 |
| Zn | 50 (100) | 0.07 | 0.05 | 0.05 | 0.05 | 2.1 | 0.05 |
| Sn | 20 | 0.1 | 0.15 | 0.18 | 0.18 | 0.4 | 0.1 |
| NH4 | 300 | 0.1 | 0.17 | 0.18 | 0.18 | 2.2 | 0.1 |
| Cr(VI) | 9.166 | 5.6 | 2.7 | 5.05 | 0.5 | ||
| F | 150 | 1.7 | 0.6 | 5.5 | 5.05 | 162 | 0.08 |
| NO2–N | 15 | 1 | 1 | 1 | 1 | 1 | 1 |
| PO4 | 50 | 37.5 | 43.7 | 33.48 | 18.3 | 2.7 | 5.26 |
| SO4 | 1 | 1 | 1 | 1 | 147 | 1 | |
| PH | 11.9 | 11.8 | 12.3 | 11.1 | 8.8 | 11.28 | |
| Electrical conductivity (mS/cm) | 40.5 | 43.35 | 16.71 | 37.43 | 31 | 67 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Setoodeh Jahromy, S.; Azam, M.; Huber, F.; Jordan, C.; Wesenauer, F.; Huber, C.; Naghdi, S.; Schwendtner, K.; Neuwirth, E.; Laminger, T.; et al. Comparing Fly Ash Samples from Different Types of Incinerators for Their Potential as Storage Materials for Thermochemical Energy and CO2. Materials 2019, 12, 3358. https://doi.org/10.3390/ma12203358
Setoodeh Jahromy S, Azam M, Huber F, Jordan C, Wesenauer F, Huber C, Naghdi S, Schwendtner K, Neuwirth E, Laminger T, et al. Comparing Fly Ash Samples from Different Types of Incinerators for Their Potential as Storage Materials for Thermochemical Energy and CO2. Materials. 2019; 12(20):3358. https://doi.org/10.3390/ma12203358
Chicago/Turabian StyleSetoodeh Jahromy, Saman, Mudassar Azam, Florian Huber, Christian Jordan, Florian Wesenauer, Clemens Huber, Shaghayegh Naghdi, Karolina Schwendtner, Erich Neuwirth, Thomas Laminger, and et al. 2019. "Comparing Fly Ash Samples from Different Types of Incinerators for Their Potential as Storage Materials for Thermochemical Energy and CO2" Materials 12, no. 20: 3358. https://doi.org/10.3390/ma12203358
APA StyleSetoodeh Jahromy, S., Azam, M., Huber, F., Jordan, C., Wesenauer, F., Huber, C., Naghdi, S., Schwendtner, K., Neuwirth, E., Laminger, T., Eder, D., Werner, A., Harasek, M., & Winter, F. (2019). Comparing Fly Ash Samples from Different Types of Incinerators for Their Potential as Storage Materials for Thermochemical Energy and CO2. Materials, 12(20), 3358. https://doi.org/10.3390/ma12203358







