Biocarbon Meets Carbon—Humic Acid/Graphite Electrodes Formed by Mechanochemistry
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Chemical and Reagents
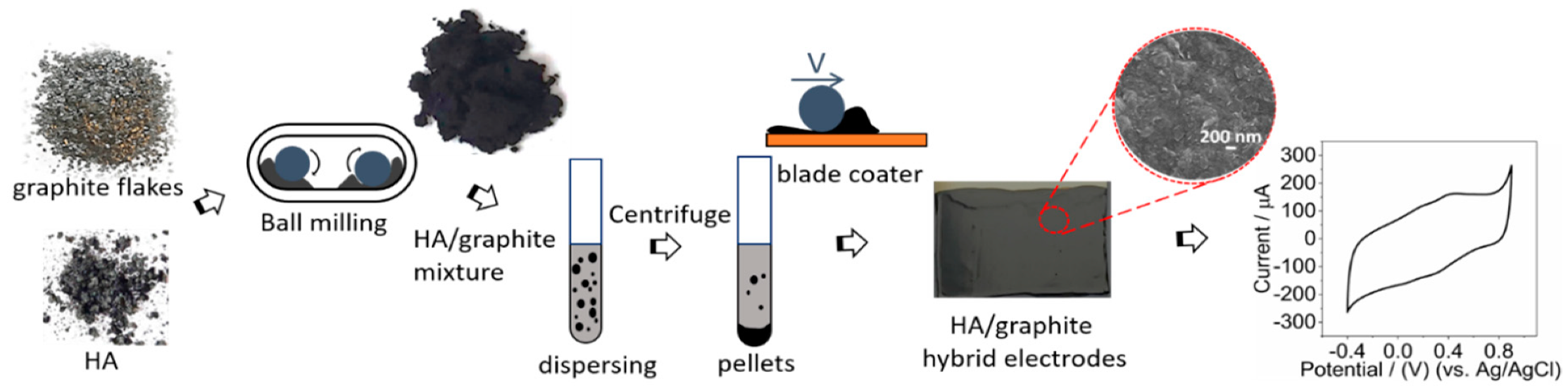
2.1.2. Mechanical Grinding
2.1.3. Electrode Preparation
2.2. Methods
2.2.1. Electrochemical Measurements
2.2.2. Thermogravimetric Analysis (TGA)
2.2.3. Fourier Transform Infrared Spectroscopy (FTIR)
2.2.4. Scanning Electron Microscopy (SEM).
2.2.5. Raman Spectroscopy
2.2.6. X-ray Diffraction (XRD)
3. Results and Discussions
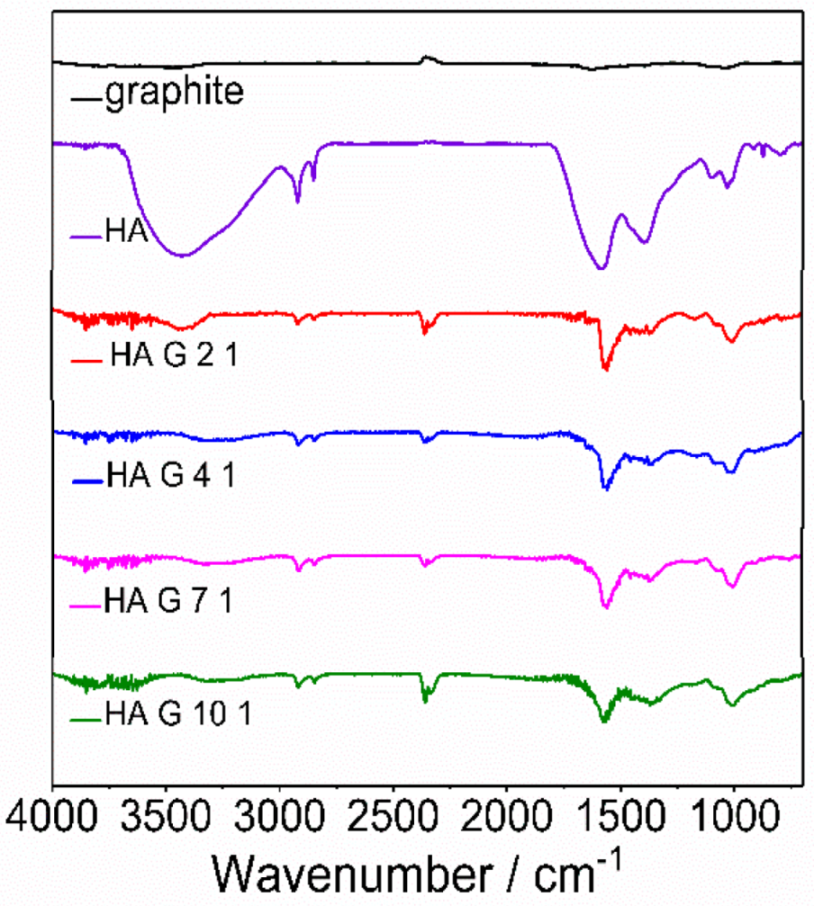
3.1. FTIR
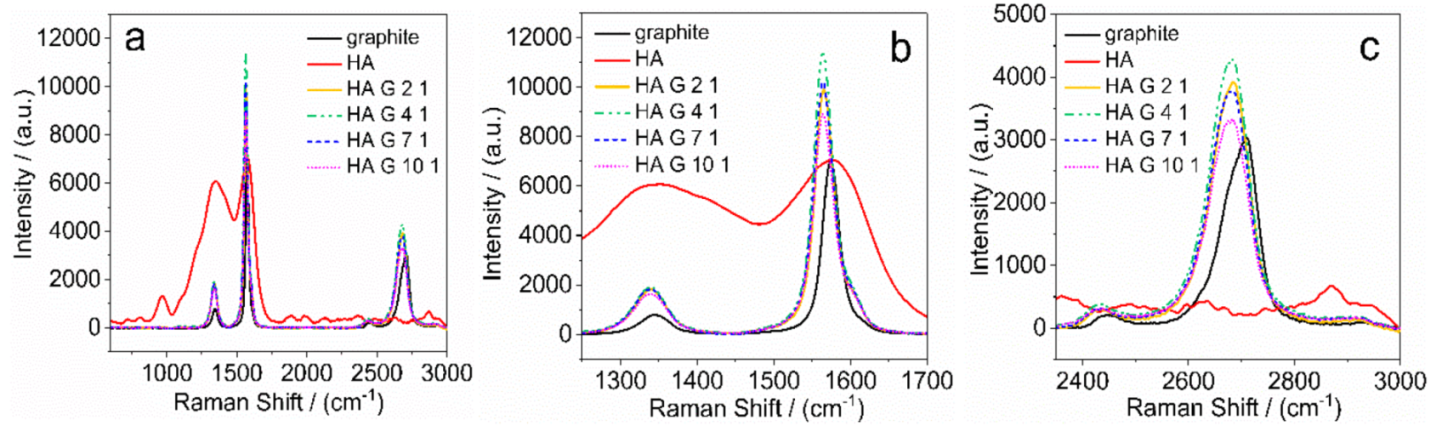
3.2. Raman
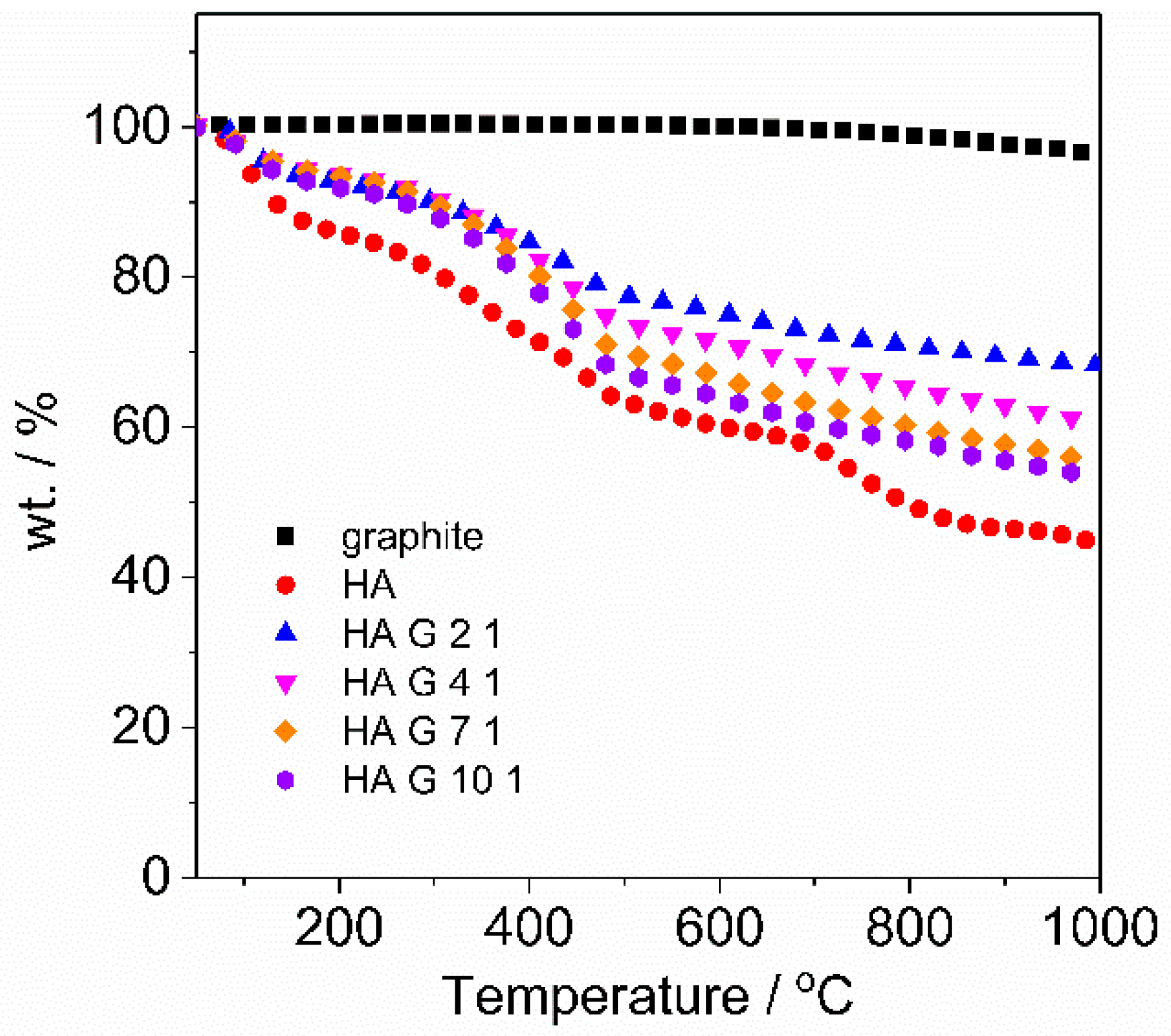
3.3. Thermal Gravimetric Analysis (TGA)
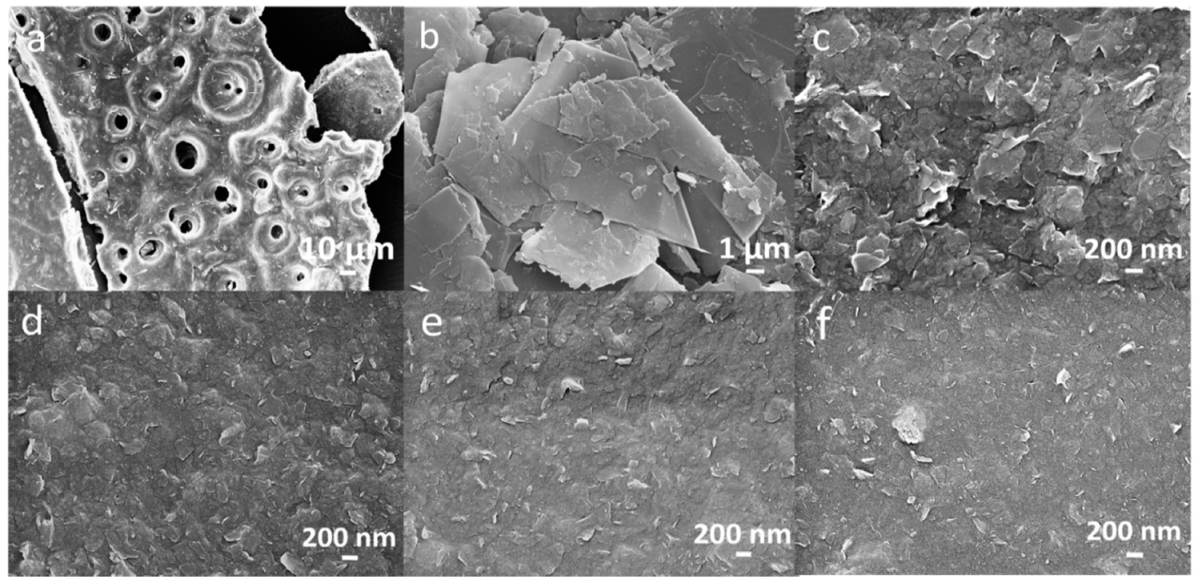
3.4. SEM
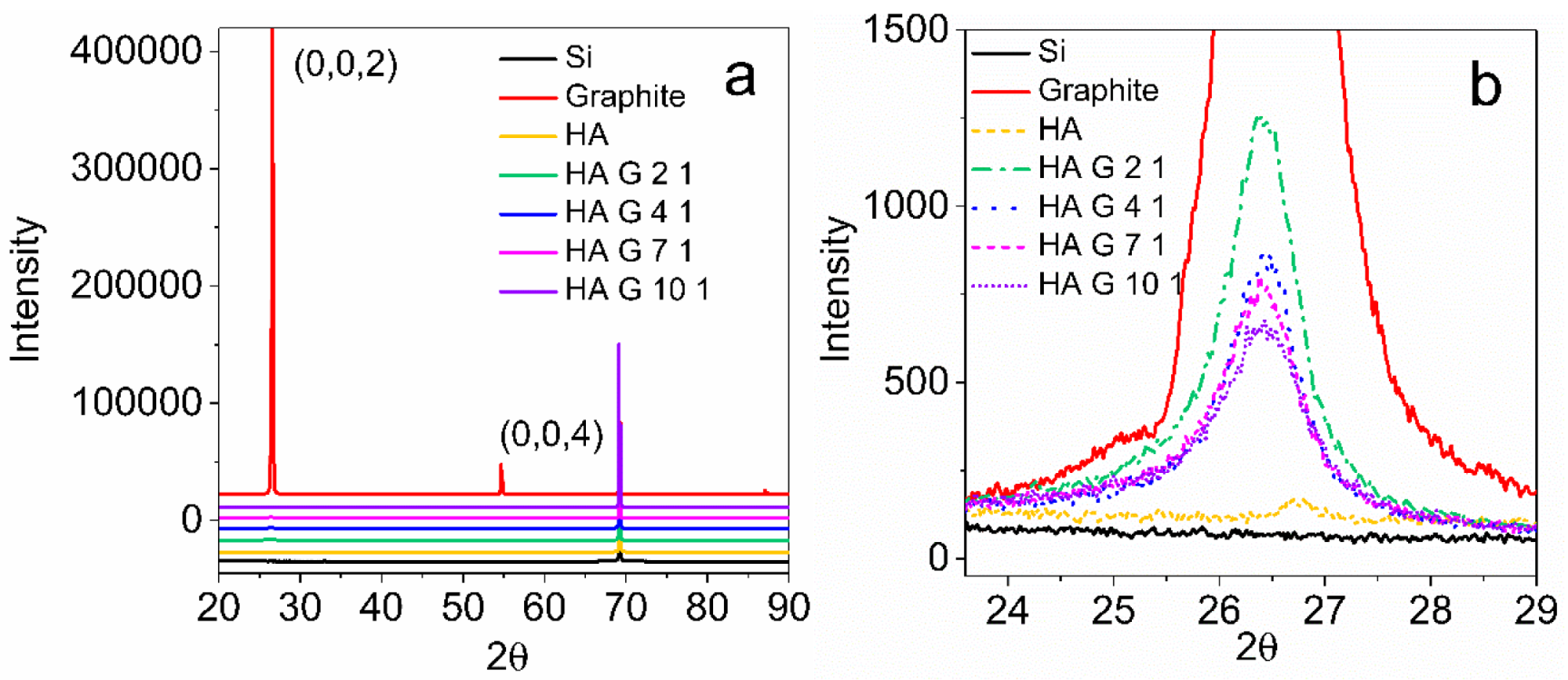
3.5. XRD
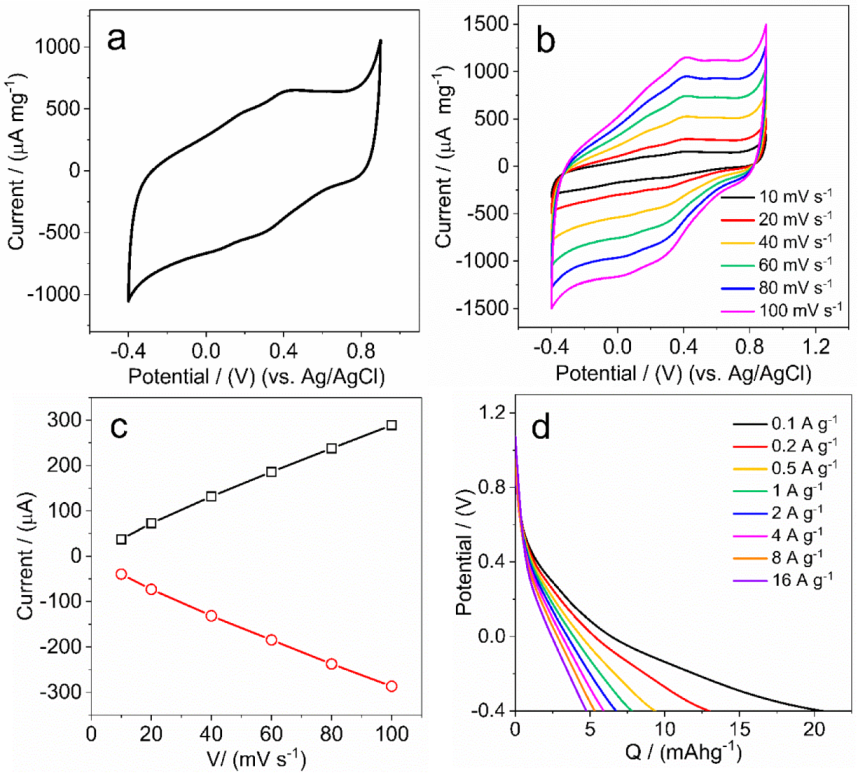
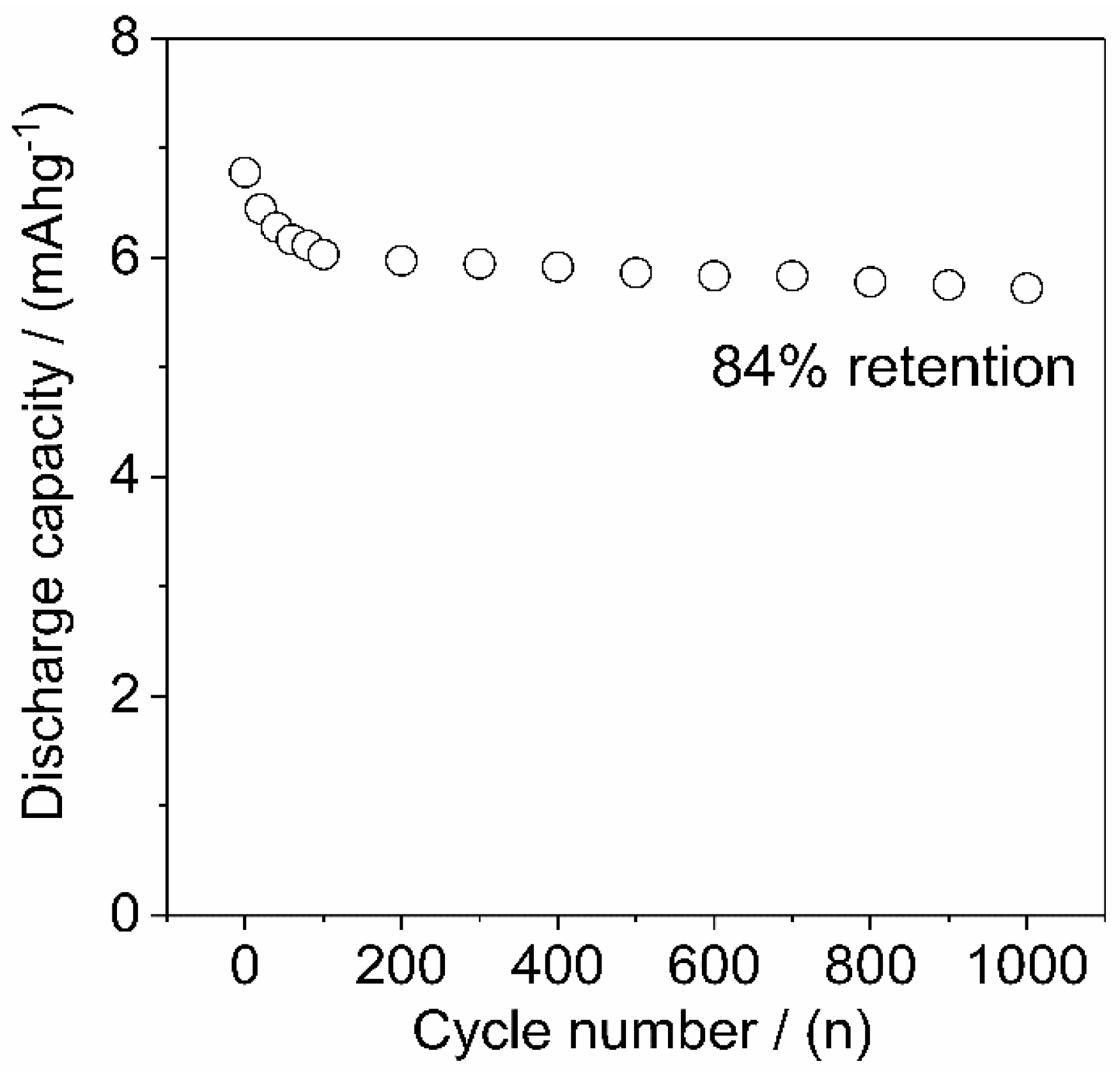
3.6. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Inganäs, O.; Admassie, S. 25th anniversary article: Organic photovoltaic modules and biopolymer supercapacitors for supply of renewable electricity: A perspective from Africa. Adv. Mater. 2014, 26, 830–848. [Google Scholar] [CrossRef] [PubMed]
- Milczarek, G.; Inganäs, O. Renewable cathode materials from biopolymer/conjugated polymer interpenetrating networks. Science 2012, 335, 1468–1471. [Google Scholar] [CrossRef] [PubMed]
- Ajjan, F.; Casado, N.; Rębiś, T.; Elfwing, A.; Solin, N.; Mecerreyes, D.; Inganäs, O. High performance PEDOT/lignin biopolymer composites for electrochemical supercapacitors. J. Mater. Chem. A 2016, 4, 1838–1847. [Google Scholar] [CrossRef]
- Geng, X.; Zhang, Y.; Jiao, L.; Yang, L.; Hamel, J.; Giummarella, N.; Henriksson, G.; Zhang, L.; Zhu, H. Bioinspired Ultrastable Lignin Cathode via Graphene Reconfiguration for Energy Storage. Acs Sustain. Chem. Eng. 2017, 5, 3553–3561. [Google Scholar] [CrossRef]
- Yin, J.; Zhang, D.; Zhao, J.; Wang, X.; Zhu, H.; Wang, C. Meso-and micro-porous composite carbons derived from humic acid for supercapacitors. Electrochimica Acta 2014, 136, 504–512. [Google Scholar] [CrossRef]
- Wang, C.-F.; Fan, X.; Zhang, F.; Wang, S.-Z.; Zhao, Y.-P.; Zhao, X.-Y.; Zhao, W.; Zhu, T.-G.; Lu, J.-L.; Wei, X.-Y. Characterization of humic acids extracted from a lignite and interpretation for the mass spectra. RSC Adv. 2017, 7, 20677–20684. [Google Scholar] [CrossRef]
- Stevenson, F.J. Humus Chemistry: Genesis, Composition, Reactions; John Wiley & Sons: Hoboken, NJ, USA, 1994. [Google Scholar]
- De Melo, B.A.G.; Motta, F.L.; Santana, M.H.A. Humic acids: Structural properties and multiple functionalities for novel technological developments. Mater. Sci. Eng. C 2016, 62, 967–974. [Google Scholar] [CrossRef]
- Qiao, Z.-J.; Chen, M.-M.; Wang, C.-Y.; Yuan, Y.-C. Humic acids-based hierarchical porous carbons as high-rate performance electrodes for symmetric supercapacitors. Bioresour. Technol. 2014, 163, 386–389. [Google Scholar] [CrossRef]
- Tang, W.-W.; Zeng, G.-M.; Gong, J.-L.; Liang, J.; Xu, P.; Zhang, C.; Huang, B.-B. Impact of humic/fulvic acid on the removal of heavy metals from aqueous solutions using nanomaterials: A review. Sci. Total Environ. 2014, 468, 1014–1027. [Google Scholar] [CrossRef]
- Sun, Z.; Tang, B.; Xie, H. Treatment of waste gases by humic acid. Energy Fuels 2015, 29, 1269–1278. [Google Scholar] [CrossRef]
- Aeschbacher, M.; Sander, M.; Schwarzenbach, R.P. Novel electrochemical approach to assess the redox properties of humic substances. Environ. Sci. Technol. 2009, 44, 87–93. [Google Scholar] [CrossRef]
- Struyk, Z.; Sposito, G. Redox properties of standard humic acids. Geoderma 2001, 102, 329–346. [Google Scholar] [CrossRef]
- Scott, D.T.; McKnight, D.M.; Blunt-Harris, E.L.; Kolesar, S.E.; Lovley, D.R. Quinone moieties act as electron acceptors in the reduction of humic substances by humics-reducing microorganisms. Environ. Sci. Technol. 1998, 32, 2984–2989. [Google Scholar] [CrossRef]
- Zhu, H.; Yin, J.; Zhao, X.; Wang, C.; Yang, X. Humic acid as promising organic anodes for lithium/sodium ion batteries. Chem. Commun. 2015, 51, 14708–14711. [Google Scholar] [CrossRef]
- Wasiński, K.; Walkowiak, M.; Lota, G. Humic acids as pseudocapacitive electrolyte additive for electrochemical double layer capacitors. J. Power Sources 2014, 255, 230–234. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, N.; Cui, Y. Promises and challenges of nanomaterials for lithium-based rechargeable batteries. Nat. Energy 2016, 1, 1–12. [Google Scholar] [CrossRef]
- Xu, Z.L.; Park, J.; Yoon, G.; Kim, H.; Kang, K. Graphitic Carbon Materials for Advanced Sodium-Ion Batteries. Small Methods 2019, 3, 1800227. [Google Scholar] [CrossRef]
- Zhou, M.; Chi, M.; Luo, J.; He, H.; Jin, T. An overview of electrode materials in microbial fuel cells. J. Power Sources 2011, 196, 4427–4435. [Google Scholar] [CrossRef]
- Le, T.X.H.; Bechelany, M.; Cretin, M. Carbon felt based-electrodes for energy and environmental applications: A review. Carbon 2017, 122, 564–591. [Google Scholar]
- Švancara, I.; Vytřas, K.; Kalcher, K.; Walcarius, A.; Wang, J. Carbon paste electrodes in facts, numbers, and notes: A review on the occasion of the 50-years jubilee of carbon paste in electrochemistry and electroanalysis. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2009, 21, 7–28. [Google Scholar]
- Krueger, A. Carbon Materials and Nanotechnology; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Ciesielski, A.; Samorì, P. Graphene via sonication assisted liquid-phase exfoliation. Chem. Soc. Rev. 2014, 43, 381–398. [Google Scholar] [CrossRef]
- Sampath, S.; Basuray, A.N.; Hartlieb, K.J.; Aytun, T.; Stupp, S.I.; Stoddart, J.F. Direct exfoliation of graphite to graphene in aqueous media with diazaperopyrenium dications. Adv. Mater. 2013, 25, 2740–2745. [Google Scholar] [CrossRef]
- James, S.L.; Adams, C.J.; Bolm, C.; Braga, D.; Collier, P.; Friščić, T.; Grepioni, F.; Harris, K.D.; Hyett, G.; Jones, W. Mechanochemistry: Opportunities for new and cleaner synthesis. Chem. Soc. Rev. 2012, 41, 413–447. [Google Scholar] [CrossRef]
- Liu, L.; Xiong, Z.; Hu, D.; Wu, G.; Chen, P. Production of high quality single-or few-layered graphene by solid exfoliation of graphite in the presence of ammonia borane. Chem. Commun. 2013, 49, 7890–7892. [Google Scholar] [CrossRef]
- León, V.; Rodriguez, A.M.; Prieto, P.; Prato, M.; Vázquez, E. Exfoliation of graphite with triazine derivatives under ball-milling conditions: Preparation of few-layer graphene via selective noncovalent interactions. ACS Nano 2014, 8, 563–571. [Google Scholar] [CrossRef]
- Bondavalli, P.; Delfaure, C.; Legagneux, P.; Pribat, D. Supercapacitor electrode based on mixtures of graphite and carbon nanotubes deposited using a dynamic air-brush deposition technique. J. Electrochem. Soc. 2013, 160, A601–A606. [Google Scholar] [CrossRef]
- Barbieri, O.; Hahn, M.; Herzog, A.; Kötz, R. Capacitance limits of high surface area activated carbons for double layer capacitors. Carbon 2005, 43, 1303–1310. [Google Scholar] [CrossRef]
- Frackowiak, E.; Beguin, F. Electrochemical storage of energy in carbon nanotubes and nanostructured carbons. Carbon 2002, 40, 1775–1787. [Google Scholar] [CrossRef]
- Kim, S.K.; Kim, Y.K.; Lee, H.; Lee, S.B.; Park, H.S. Superior Pseudocapacitive Behavior of Confined Lignin Nanocrystals for Renewable Energy-Storage Materials. ChemSusChem 2014, 7, 1094–1101. [Google Scholar] [CrossRef]
- Chaleawlert-umpon, S.; Berthold, T.; Wang, X.; Antonietti, M.; Liedel, C. Kraft Lignin as Electrode Material for Sustainable Electrochemical Energy Storage. Adv. Mater. Interfaces 2017, 4, 1700698. [Google Scholar] [CrossRef]
- Huang, G.; Kang, W.; Geng, Q.; Xing, B.; Liu, Q.; Jia, J.; Zhang, C. One-step green hydrothermal synthesis of few-layer graphene oxide from humic acid. Nanomaterials 2018, 8, 215. [Google Scholar] [CrossRef]
- El-shazly, M.D.; Henderson, B.; Beall, G.W. Reduced humic acid nanosheets and its uses as nanofiller. J. Phys. Chem. Solids 2015, 85, 86–90. [Google Scholar]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J.L. Raman spectrum of graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef]
- Snowdon, M.R.; Mohanty, A.K.; Misra, M. A study of carbonized lignin as an alternative to carbon black. ACS Sustain. Chem. Eng. 2014, 2, 1257–1263. [Google Scholar] [CrossRef]
- Raccichini, R.; Varzi, A.; Chakravadhanula, V.S.K.; Kübel, C.; Balducci, A.; Passerini, S. Enhanced low-temperature lithium storage performance of multilayer graphene made through an improved ionic liquid-assisted synthesis. J. Power Sources 2015, 281, 318–325. [Google Scholar] [CrossRef]
- De Souza, F.; Bragança, S.R. Extraction and characterization of humic acid from coal for the application as dispersant of ceramic powders. J. Mater. Res. Technol. 2018, 7, 254–260. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, Y.; Wang, S. Enhancing thermoelectric properties of organic composites through hierarchical nanostructures. Sci. Rep. 2013, 3, 3448. [Google Scholar] [CrossRef]
- Agarwal, S.P.; Anwer, M.K.; Khanna, R.; Ali, A.; Sultana, Y. Humic acid from Shilajit–a physico-chemical and spectroscopic characterization. J. Serb. Chem. Soc. 2010, 75, 413–422. [Google Scholar] [CrossRef]
- Liu, C.; Liu, X.; Tan, J.; Wang, Q.; Wen, H.; Zhang, C. Nitrogen-doped graphene by all-solid-state ball-milling graphite with urea as a high-power lithium ion battery anode. J. Power Sources 2017, 342, 157–164. [Google Scholar] [CrossRef]
- Iwashita, N.; Park, C.R.; Fujimoto, H.; Shiraishi, M.; Inagaki, M. Specification for a standard procedure of X-ray diffraction measurements on carbon materials. Carbon 2004, 42, 701–714. [Google Scholar] [CrossRef]
- Mabbott, G.A. An introduction to cyclic voltammetry. J. Chem. Educ. 1983, 60, 697. [Google Scholar] [CrossRef]
| Carbon Electrodes | Charge Storage Performance |
|---|---|
| HA/graphite hybrid material electrodes | 60 F·g−1 (20 mAhg−1) |
| Graphite electrode [28] | 5 F·g−1 |
| Carbon black [29] | 12.6 F·g−1 |
| Single-walled carbon nanotube [30] | 18 F·g−1 |
| Poly(3,4-ethylenedioxythiophene) (PEDOT)/Lignin hybrid materials [3] | 34 mAhg−1 |
| Lignin/reduced graphene oxides hybrid materials [31] | 72 mAhg−1 |
| Kraft lignin/conductive carbon materials [32] | 80 mAhg−1 |
| Thickness/(µm) | Conductivity/(S·m−1) | Discharge Capacity/(mAhg−1) | Mass Ratio of HA/Graphite in Pellets | |
|---|---|---|---|---|
| HA/graphite (2/1) 1 | 4.1 ± 0.1 | 159 ± 12 | 16.7 ± 0.5 | 1.17 |
| HA/graphite (4/1) | 4.2 ± 0.1 | 49 ± 2 | 20.7 ± 2.0 | 2.21 |
| HA/graphite (7/1) | 3.0 ± 0.1 | 6.3 ± 0.4 | 20.1 ± 0.9 | 3.78 |
| HA/graphite (10/1) | 15.9 ± 0.3 | 0.34 ± 0.05 | 18.9 ± 1.6 | 4.85 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Solin, N.; Inganäs, O. Biocarbon Meets Carbon—Humic Acid/Graphite Electrodes Formed by Mechanochemistry. Materials 2019, 12, 4032. https://doi.org/10.3390/ma12244032
Liu L, Solin N, Inganäs O. Biocarbon Meets Carbon—Humic Acid/Graphite Electrodes Formed by Mechanochemistry. Materials. 2019; 12(24):4032. https://doi.org/10.3390/ma12244032
Chicago/Turabian StyleLiu, Lianlian, Niclas Solin, and Olle Inganäs. 2019. "Biocarbon Meets Carbon—Humic Acid/Graphite Electrodes Formed by Mechanochemistry" Materials 12, no. 24: 4032. https://doi.org/10.3390/ma12244032
APA StyleLiu, L., Solin, N., & Inganäs, O. (2019). Biocarbon Meets Carbon—Humic Acid/Graphite Electrodes Formed by Mechanochemistry. Materials, 12(24), 4032. https://doi.org/10.3390/ma12244032






