High Mechanical Performance Based on Physically Linked Double Network (DN) Hydrogels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
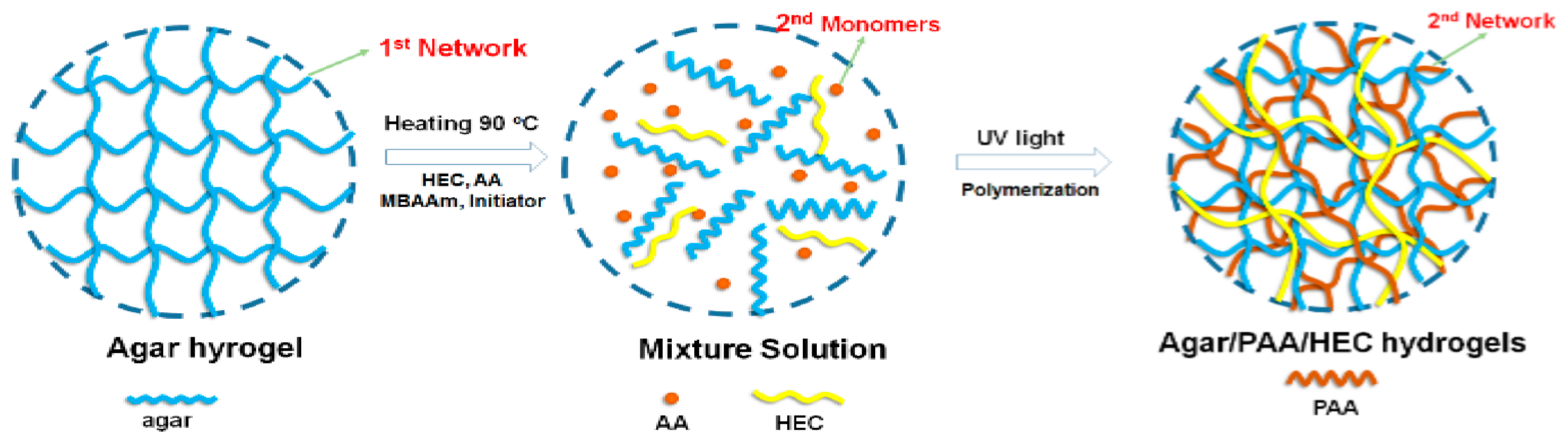
2.2. Synthesis of Agar/PAA/HEC Double Network (DN) Hydrogels
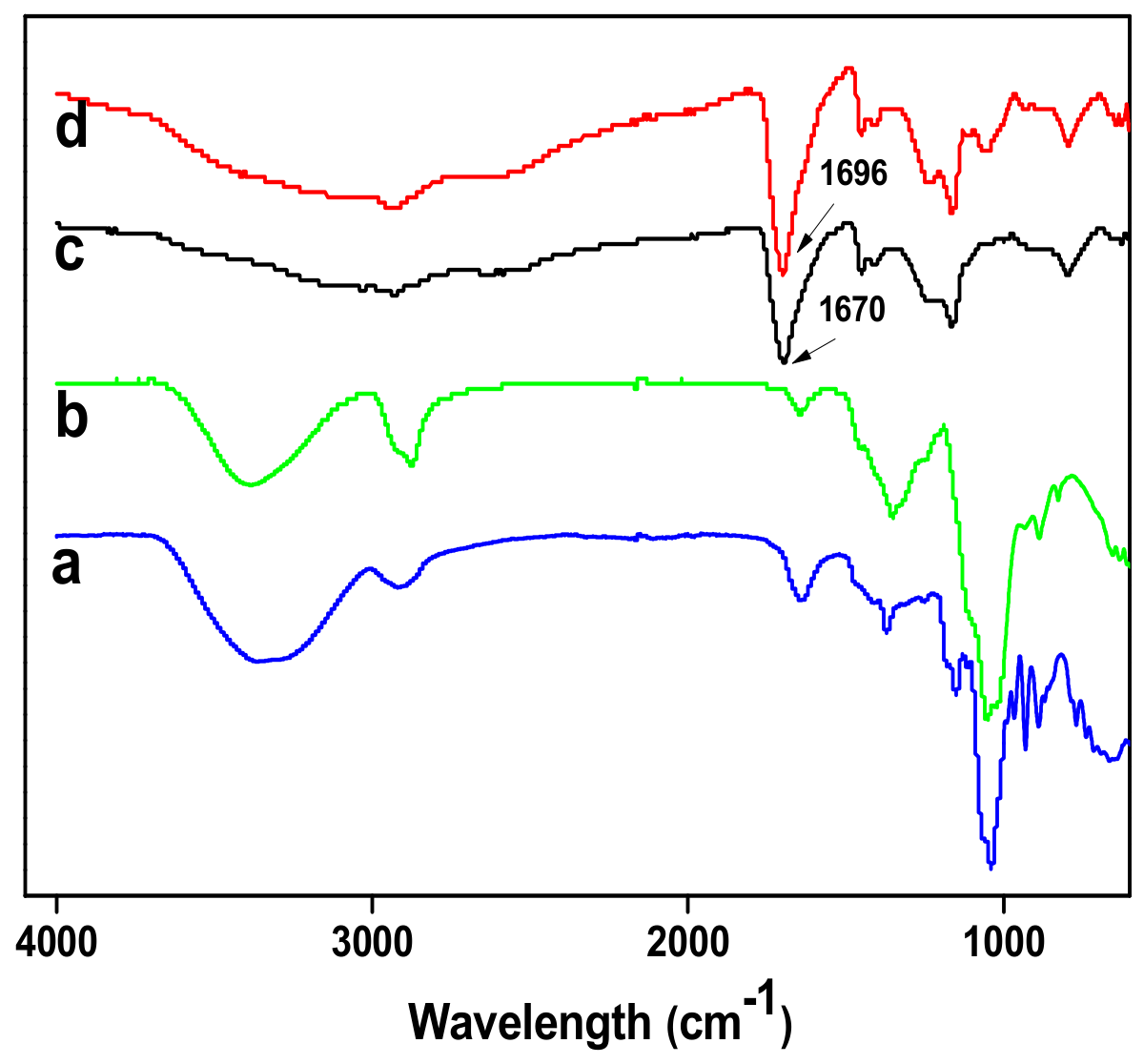
2.3. Fourier-Transform Infrared Spectroscopy (FTIR) Test
2.4. Morphology Characterization
2.5. Measurement of Mechanical Properties
2.6. Swelling Properties Test
3. Results and Discussion
3.1. Preparation of the Agar/PAA/HEC DN Hydrogels
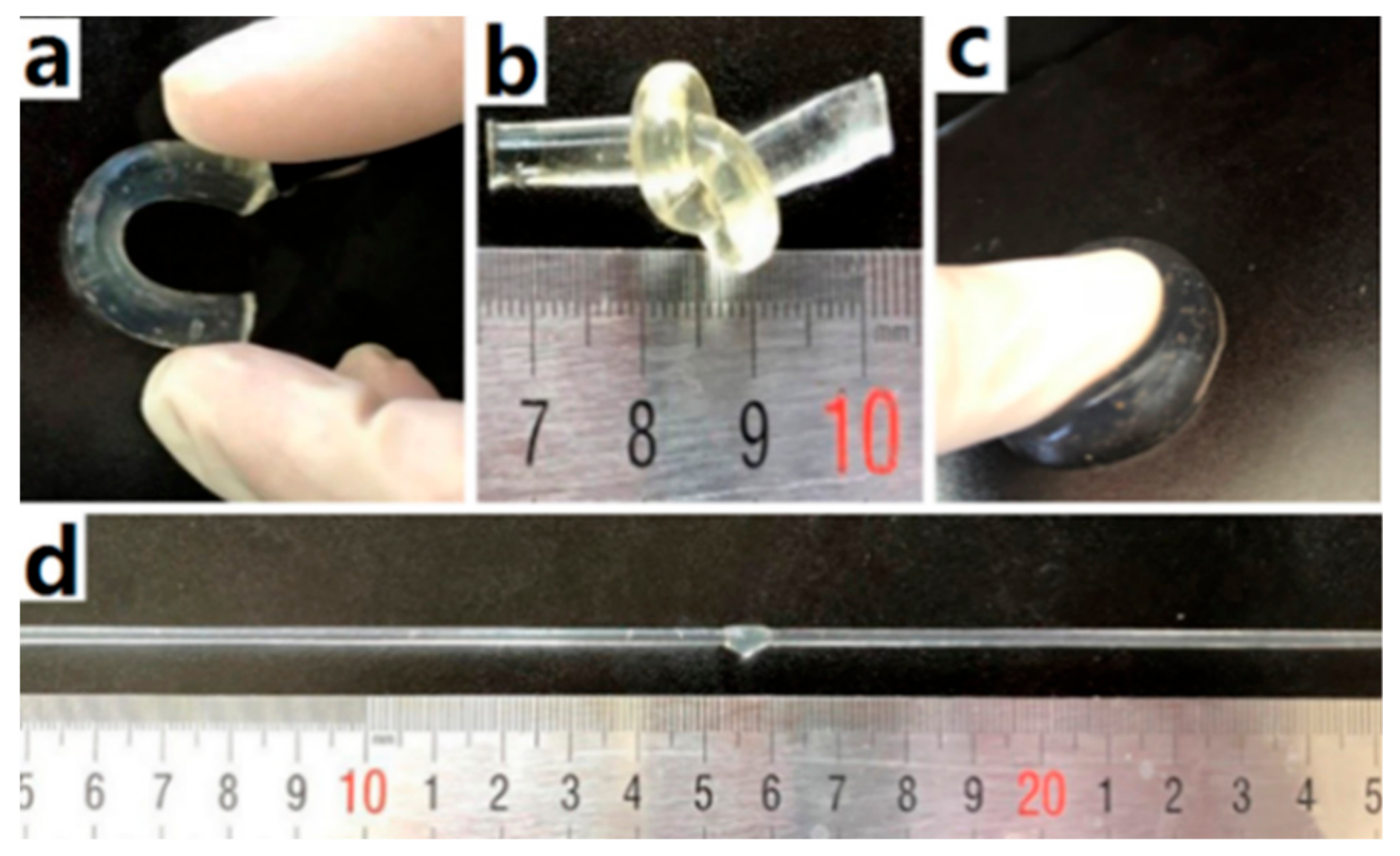
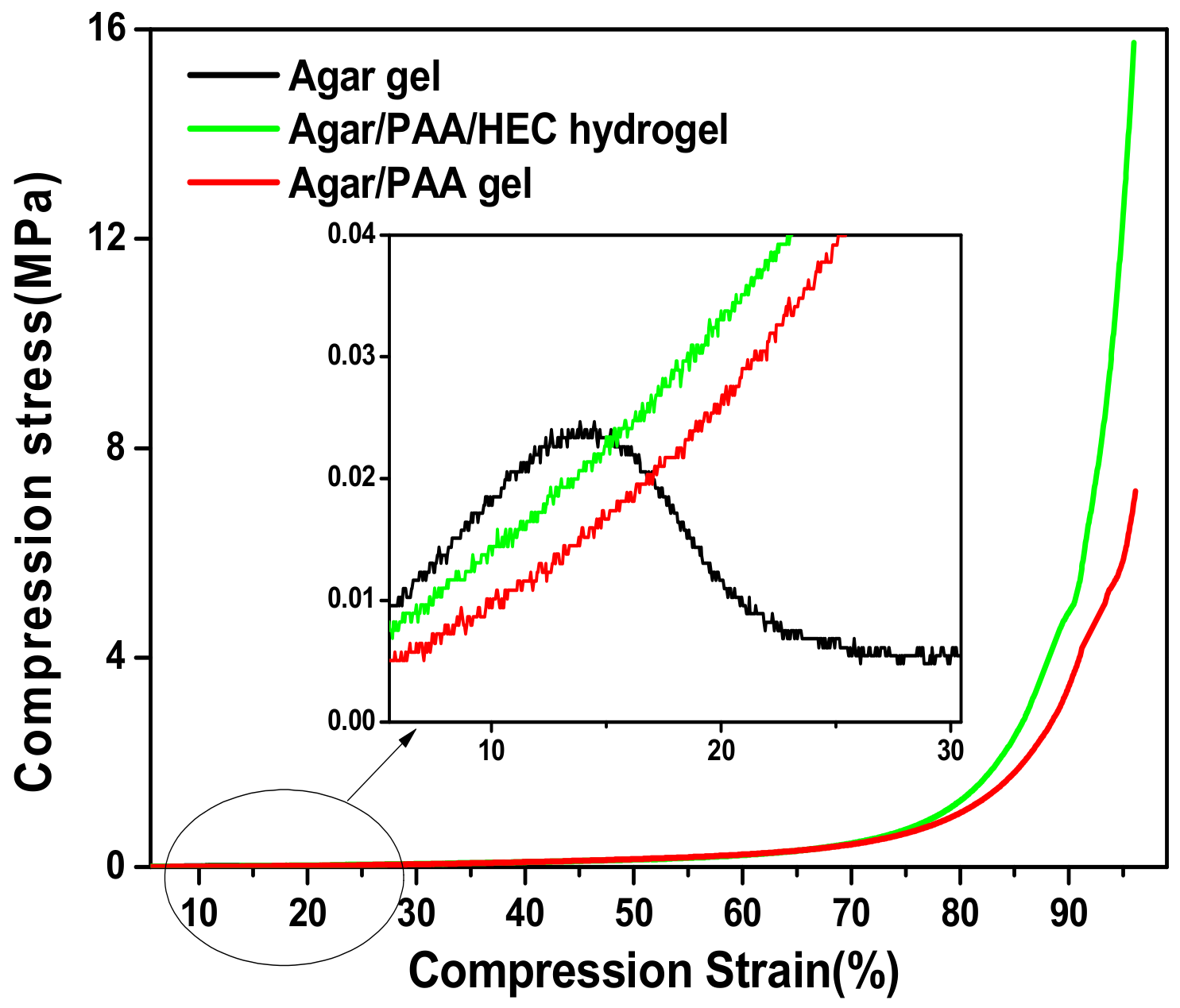
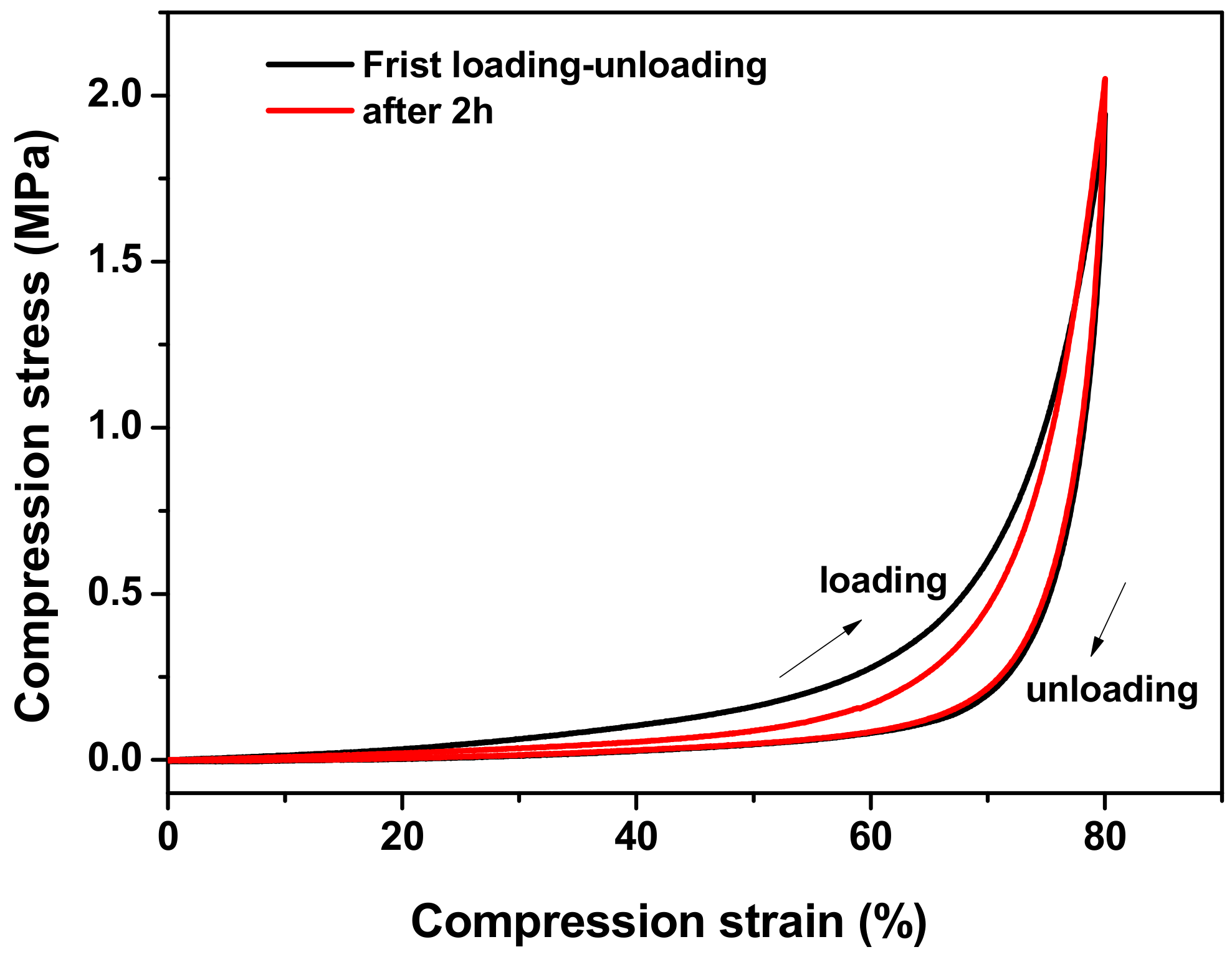
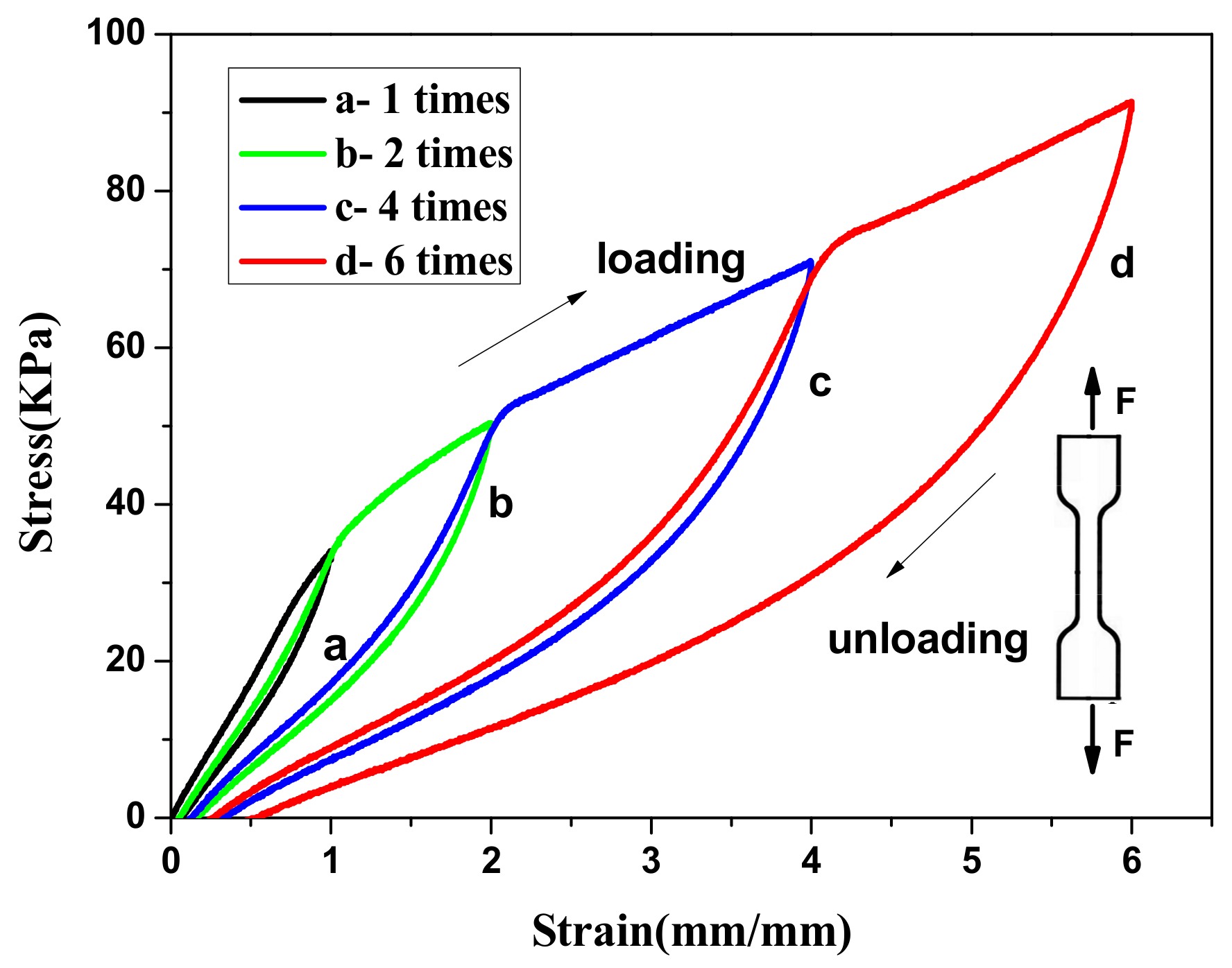
3.2. The Mechanical Properties of the Agar/PAA/HEC DN Hydrogels
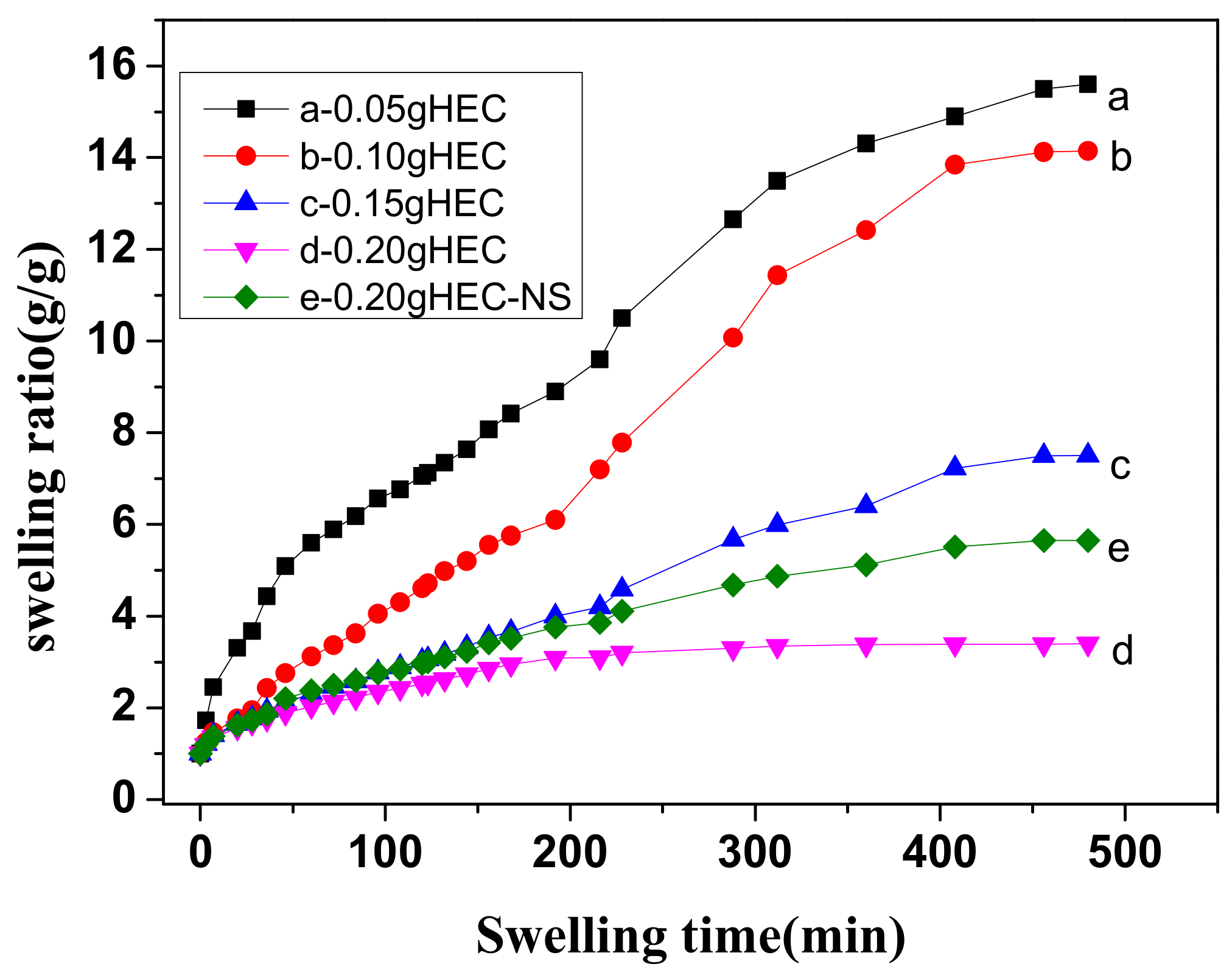
3.3. Swelling Properties of the Agar/PAA/HEC DN Hydrogels
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, L.; Pan, L.; Ma, Z.; Yan, K.; Cheng, W.; Shi, Y.; Yu, G. All Inkjet-Printed Amperometric Multiplexed Biosensors based on Nanostructured Conductive Hydrogel Electrodes. Nano Lett. 2018, 18, 3322–3327. [Google Scholar] [CrossRef]
- Sun, L.; Zhong, Y.; Gui, J.; Wang, X.; Zhuang, X.; Weng, J. A hydrogel biosensor for high selective and sensitive detection of amyloid-beta oligomers. Int. J. Nanomed. 2018, 13, 843–856. [Google Scholar] [CrossRef]
- Peak, C.W.; Wilker, J.J.; Schmidt, G. A review on tough and sticky hydrogels. Colloid Polym. Sci. 2013, 291, 2031–2047. [Google Scholar] [CrossRef]
- Zhao, Z.G.; Xu, Y.C.; Fang, R.C.; Liu, M.J. Bioinspired Adaptive Gel Materials with Synergistic Heterostructures. Chin. J. Polym. Sci. 2018, 36, 683–696. [Google Scholar] [CrossRef]
- Qiu, Y.; Park, K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Deliv. Rev. 2001, 53, 321–339. [Google Scholar] [CrossRef]
- Ng, L.T.; Jönsson, S.; Swami, S.; Lindgren, K. Synthesis of hydrogel for drug delivery studies utilizing photoinitiator-free photopolymerization based on the donor/acceptor pair, N-vinylpyrrolidinone and hydroxypentyl maleimide. Polym. Int. 2018, 51, 1398–1403. [Google Scholar] [CrossRef]
- Yu, Y.; Shu, Y.; Ye, L. In situ crosslinking of poly (vinyl alcohol)/graphene oxide-glutamic acid nano-composite hydrogel as microbial carrier: Intercalation structure and its wastewater treatment performance. Chem. Eng. J. 2018, 336, 306–314. [Google Scholar] [CrossRef]
- El-Sherbiny, I.M.; Yacoub, M.H. Hydrogel scaffolds for tissue engineering: Progress and challenges. Glob. Cardiol. Sci. Pr. 2013, 2013, 316–342. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Z.; Chen, B.; Shao, J.; Guo, Z. Self-healing hydrogel of poly(vinyl alcohol)/graphite oxide with pH-sensitive and enhanced thermal properties. J. Appl. Polym. Sci. 2018, 135, 46143. [Google Scholar] [CrossRef]
- Wang, L.; Xu, M.-E.; Luo, L.; Zhou, Y.; Si, P. Iterative feedback bio-printing-derived cell-laden hydrogel scaffolds with optimal geometrical fidelity and cellular controllability. Sci. Rep. 2018, 8, 2802. [Google Scholar] [CrossRef]
- Kuo, C.K.; Ma, P.X. Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: Part 1. Structure, gelation rate and mechanical properties. Biomaterials 2001, 22, 511–521. [Google Scholar] [CrossRef]
- Li, H.; Lv, T.; Sun, H.; Qian, G.; Li, N.; Yao, Y.; Chen, T. Ultrastretchable and superior healable supercapacitors based on a double cross-linked hydrogel electrolyte. Nat. Commun. 2019, 10, 536. [Google Scholar] [CrossRef]
- Gao, H.; Xiao, F.; Ching, C.B.; Duan, H. High-Performance Asymmetric Supercapacitor Based on Graphene Hydrogel and Nanostructured MnO2. ACS Appl. Mater. Interfaces 2012, 4, 2801–2810. [Google Scholar] [CrossRef]
- Guo, Y.; Zheng, K.; Wan, P. A Flexible Stretchable Hydrogel Electrolyte for Healable All-in-One Configured Supercapacitors. Small 2018, 14, 1704497. [Google Scholar] [CrossRef]
- Yang, Y.; Guan, L.; Gao, G. A low-cost, rapidly-responsive, controllable and reversible photochromic hydrogel for display and storage. ACS Appl. Mater. Interfaces 2018, 10, 13975–13984. [Google Scholar] [CrossRef]
- Lopresti, C.; Vetri, V.; Ricca, M.; Foderà, V.; Tripodo, G.; Spadaro, G.; Dispenza, C. Pulsatile protein release and protection using radiation-crosslinked polypeptide hydrogel delivery devices. React. Funct. Polym. 2010, 71, 155–167. [Google Scholar] [CrossRef]
- Hennink, W.; Van Nostrum, C. Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 2012, 64, 223–236. [Google Scholar] [CrossRef]
- Pacelli, S.; Paolicelli, P.; Avitabile, M.; Varani, G.; Di Muzio, L.; Cesa, S.; Tirillò, J.; Bartuli, C.; Nardoni, M.; Petralito, S.; et al. Design of a tunable nanocomposite double network hydrogel based on gellan gum for drug delivery applications. Eur. Polym. J. 2018, 104, 184–193. [Google Scholar] [CrossRef]
- Lowe, T.L.; Tenhu, H.; Tylli, H. Effect of hydrophobicity of a drug on its release from hydrogels with different topological structures. J. Appl. Polym. Sci. 2015, 73, 1031–1039. [Google Scholar] [CrossRef]
- Haraguchi, K. Nanocomposite hydrogels. Curr. Opin. Solid State Mater. Sci. 2007, 11, 47–54. [Google Scholar] [CrossRef]
- Matsunaga, T.; Sakai, T.; Akagi, Y.; Chung, U.I.; Shibayama, M. SANS and SLS Studies on Tetra-Arm PEG Gels in As-Prepared and Swollen States. Macromolecules 2009, 42, 6245–6252. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Qiu, C.; Li, J.; Cao, Z.; Ma, C.; Zheng, J.; Huang, G. Self-recovery magnetic hydrogel with high strength and toughness using nanofibrillated cellulose as a dispersing agent and filler. Carbohydr. Polym. 2018, 196, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, L.; Yu, X.; Wang, C.; Wang, Z. Synthesis and characterization of a novel double cross-linked hydrogel based on Diels-Alder click reaction and coordination bonding. Mater. Sci. Eng. c-Mater. Biol. Appl. 2018, 82, 299–309. [Google Scholar] [CrossRef]
- Chen, Y.; Shull, K.R. High-Toughness Polycation Cross-Linked Triblock Copolymer Hydrogels. Macromolecules 2017, 50, 3637–3646. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, L.; Wang, L.; Yu, J. Hydrogen-bonded Supramolecular Hydrogels Derived from 3,3’,4,4’-Benzophenonetetracarboxylic Acid and Hydroxylpyridines. Chin. J. Chem. 2010, 27, 2279–2283. [Google Scholar] [CrossRef]
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-Network Hydrogels with Extremely High Mechanical Strength. Adv. Mater. 2010, 15, 1155–1158. [Google Scholar] [CrossRef]
- Yan, X.; Chen, Q.; Zhu, L.; Chen, H.; Wei, D.; Chen, F.; Tang, Z.; Yang, J.; Zheng, J. High strength and self-healable gelatin/polyacrylamide double network hydrogels. J. Mater. Chem. B 2017, 5, 7683–7691. [Google Scholar] [CrossRef]
- Yang, F.; Tadepalli, V.; Wiley, B.J. 3D Printing of a Double Network Hydrogel with a Compression Strength and Elastic Modulus Greater than those of Cartilage. ACS Biomater. Sci. Eng. 2017, 3, 863–869. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, X.; Yang, F.; Shen, H.; Wu, D. A Universal Soaking Strategy to Convert Composite Hydrogels into Extremely Tough and Rapidly Recoverable Double-Network Hydrogels. Adv. Mater. 2016, 28, 7178–7184. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, L. Improvement of Permeability of Poly (vinyl alcohol) Hydrogel by Using Poly(ethylene glycol) as Porogen. Polym. Technol. Eng. 2011, 50, 776–782. [Google Scholar] [CrossRef]
- Zhu, L.; Qiu, J.; Sakai, E. A high modulus hydrogel obtained from hydrogen bond reconstruction and its application in vibration damper. RSC Adv. 2017, 7, 43755–43763. [Google Scholar] [CrossRef] [Green Version]
- Yuan, N.; Xu, L.; Zhang, L.; Ye, H.; Zhao, J.; Liu, Z.; Rong, J. Superior hybrid hydrogels of polyacrylamide enhanced by bacterial cellulose nanofiber clusters. Mater. Sci. Eng. C 2016, 67, 221–230. [Google Scholar] [CrossRef]
- Liu, R.; Liang, S.; Tang, X.-Z.; Yan, D.; Li, X.; Yu, Z.-Z. Tough and highly stretchable graphene oxide/polyacrylamide nanocomposite hydrogels. J. Mater. Chem. 2012, 22, 14160–14167. [Google Scholar] [CrossRef]
- Ye, X.; Li, X.; Shen, Y.; Chang, G.; Yang, J.; Gu, Z. Self-healing pH-sensitive cytosine- and guanosine-modified hyaluronic acid hydrogels via hydrogen bonding. Polymer 2017, 108, 348–360. [Google Scholar] [CrossRef]
- Zhao, J.; Zheng, K.; Nan, J.; Tang, C.; Chen, Y.; Hu, Y. Synthesis and characterization of lignosulfonate- graft -poly (acrylic acid)/hydroxyethyl cellulose semi-interpenetrating hydrogels. React. Funct. Polym. 2017, 115, 28–35. [Google Scholar] [CrossRef]
- Li, X.; Yang, Q.; Zhao, Y.; Long, S.; Zheng, J. Dual Physically Crosslinked Double Network Hydrogels with High Toughness and Self-Healing Properties. Soft Matter 2017, 13, 911–920. [Google Scholar] [CrossRef]
- Chen, Q.; Zhu, L.; Zhao, C.; Wang, Q.; Zheng, J. A Robust, One-Pot Synthesis of Highly Mechanical and Recoverable Double Network Hydrogels Using Thermoreversible Sol-Gel Polysaccharide. Adv. Mater. 2013, 25, 4171–4176. [Google Scholar] [CrossRef]


| Sample | Synthesis | Tensile Test | |||
|---|---|---|---|---|---|
| HEC/AA/H2O (g/g/g) | HEC viscosity (mpa.s, 25 °C) | Strain at fracture (mm/mm) | Stress at fracture (kPa) | Tensile modulus (kPa) | |
| Gel-1 | 0.05/2/10 | 5000–6400 | 15.1 | 125.5 | 4.5 |
| Gel-2 | 0.10/2/10 | 5000–6400 | 16.7 | 147.6 | 18.5 |
| Gel-3 | 0.15/2/10 | 5000–6400 | 19.9 | 160.2 | 21.9 |
| Gel-4 | 0.2/2/10 | 5000–6400 | 14.6 | 99.3 | 9.3 |
| Sample | Synthesis | Tensile Test | |||
|---|---|---|---|---|---|
| HEC/AA/H2O (g/g/g) | HEC viscosity (mpa.s, 25 °C) | Strain at fracture (mm/mm) | Stress at fracture (kPa) | Tensile modulus (kPa) | |
| Gel-5 | 0.15/2/10 | 80–125 | 8.6 | 138.0 | 27.4 |
| Gel-6 | 0.15/2/10 | 1000–1500 | 12.0 | 131.4 | 22.1 |
| Gel-7 | 0.15/2/10 | 5000–6400 | 19.9 | 160.2 | 21.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, L.; Zhang, Y.; Shen, L.; Sheng, Q.; Fu, S.; Chen, S.; Du, Y.; Chen, Y.; Liu, Y. High Mechanical Performance Based on Physically Linked Double Network (DN) Hydrogels. Materials 2019, 12, 3333. https://doi.org/10.3390/ma12203333
Niu L, Zhang Y, Shen L, Sheng Q, Fu S, Chen S, Du Y, Chen Y, Liu Y. High Mechanical Performance Based on Physically Linked Double Network (DN) Hydrogels. Materials. 2019; 12(20):3333. https://doi.org/10.3390/ma12203333
Chicago/Turabian StyleNiu, Li, Yutao Zhang, Liyu Shen, Qiuyue Sheng, Shuai Fu, Shiyan Chen, Yun Du, Ying Chen, and Yupeng Liu. 2019. "High Mechanical Performance Based on Physically Linked Double Network (DN) Hydrogels" Materials 12, no. 20: 3333. https://doi.org/10.3390/ma12203333





