Utilization of Several Industrial Wastes as Raw Material for Calcium Sulfoaluminate Cement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials and Sample Preparation for CSA Clinker
2.2. Analytical Methods
2.2.1. Energy Dispersive X-ray Fluorescence (EDXRF)
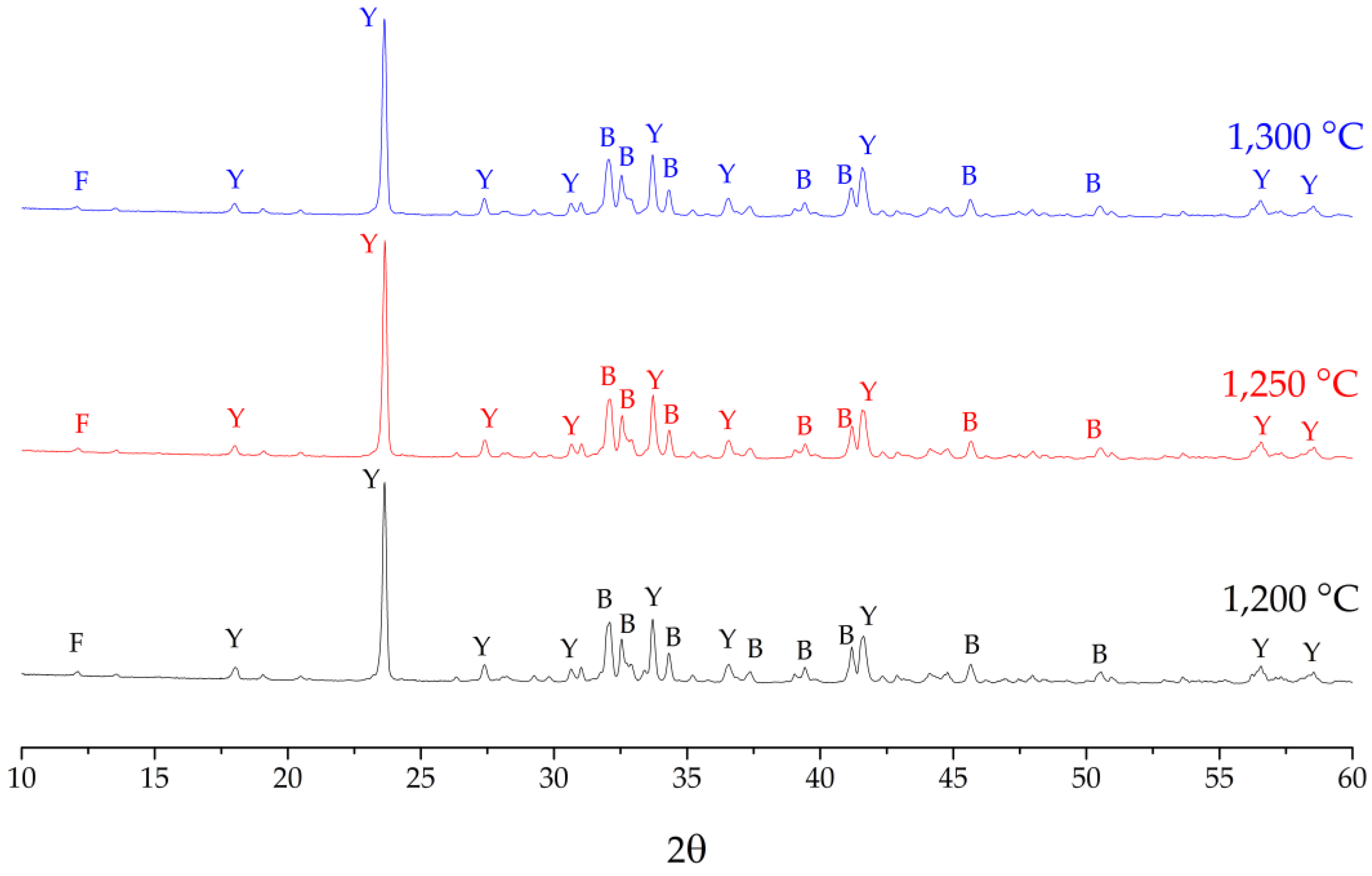
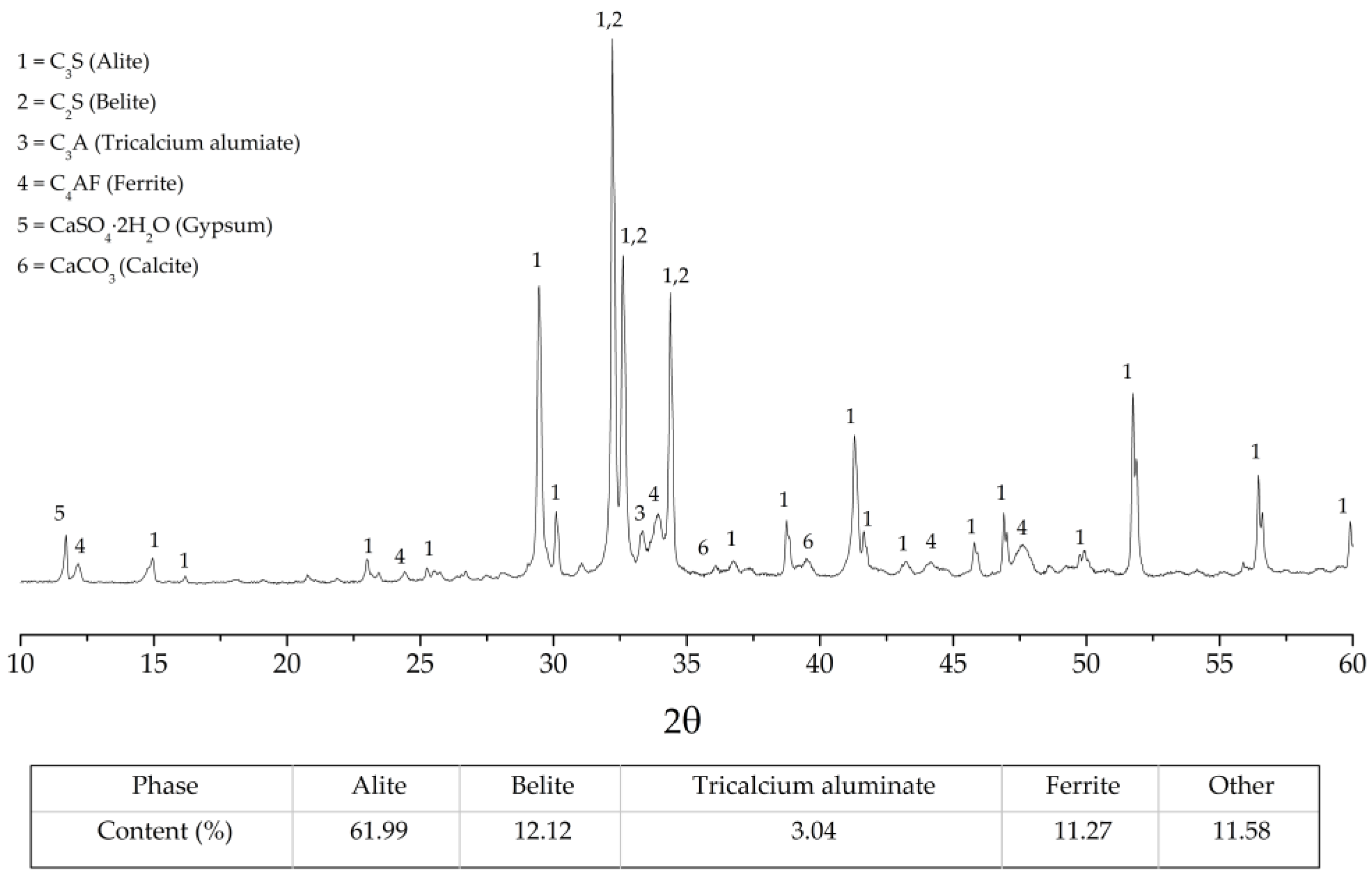
2.2.2. X-ray Diffraction (XRD)
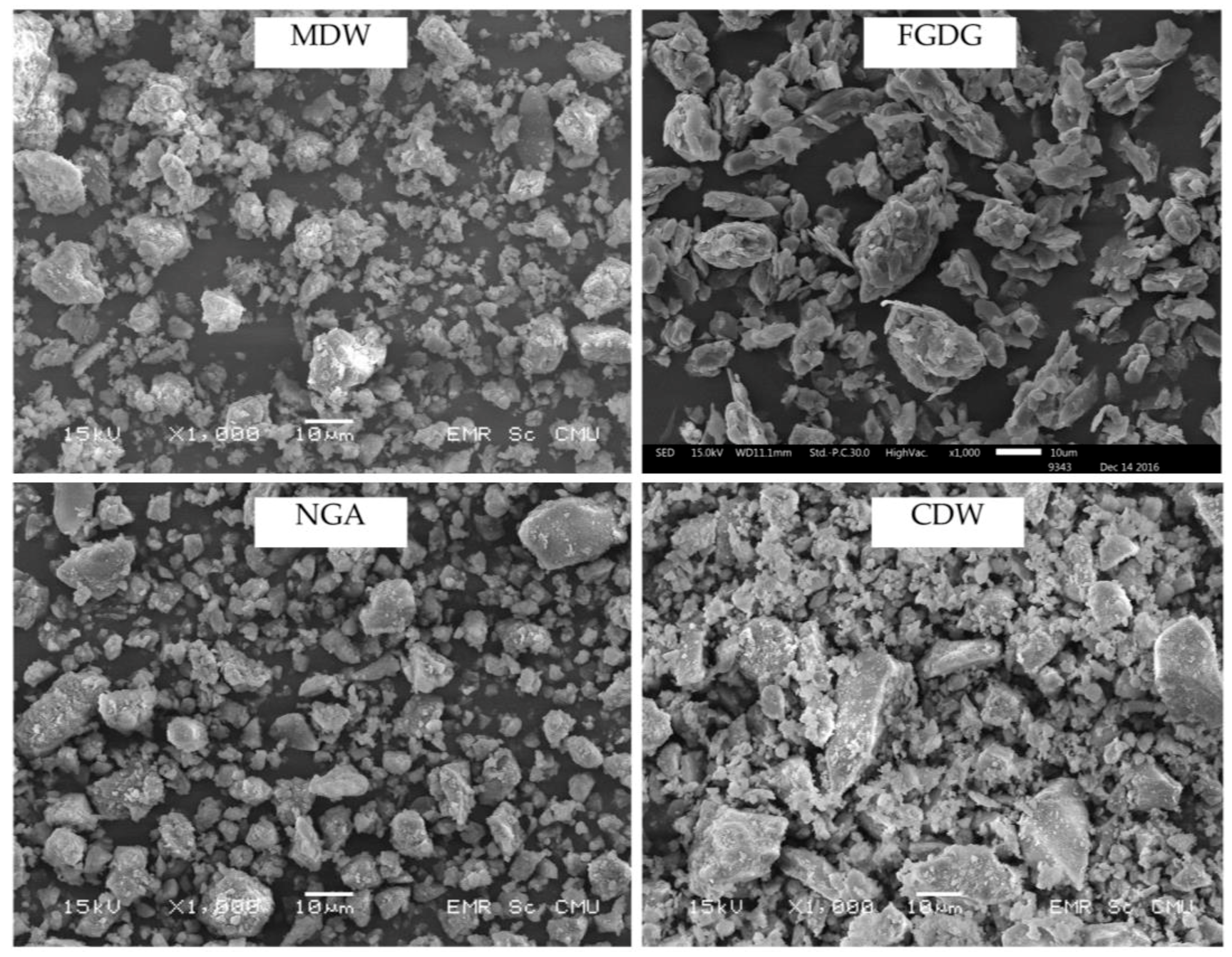
2.2.3. Scanning Electron Microscopy (SEM)
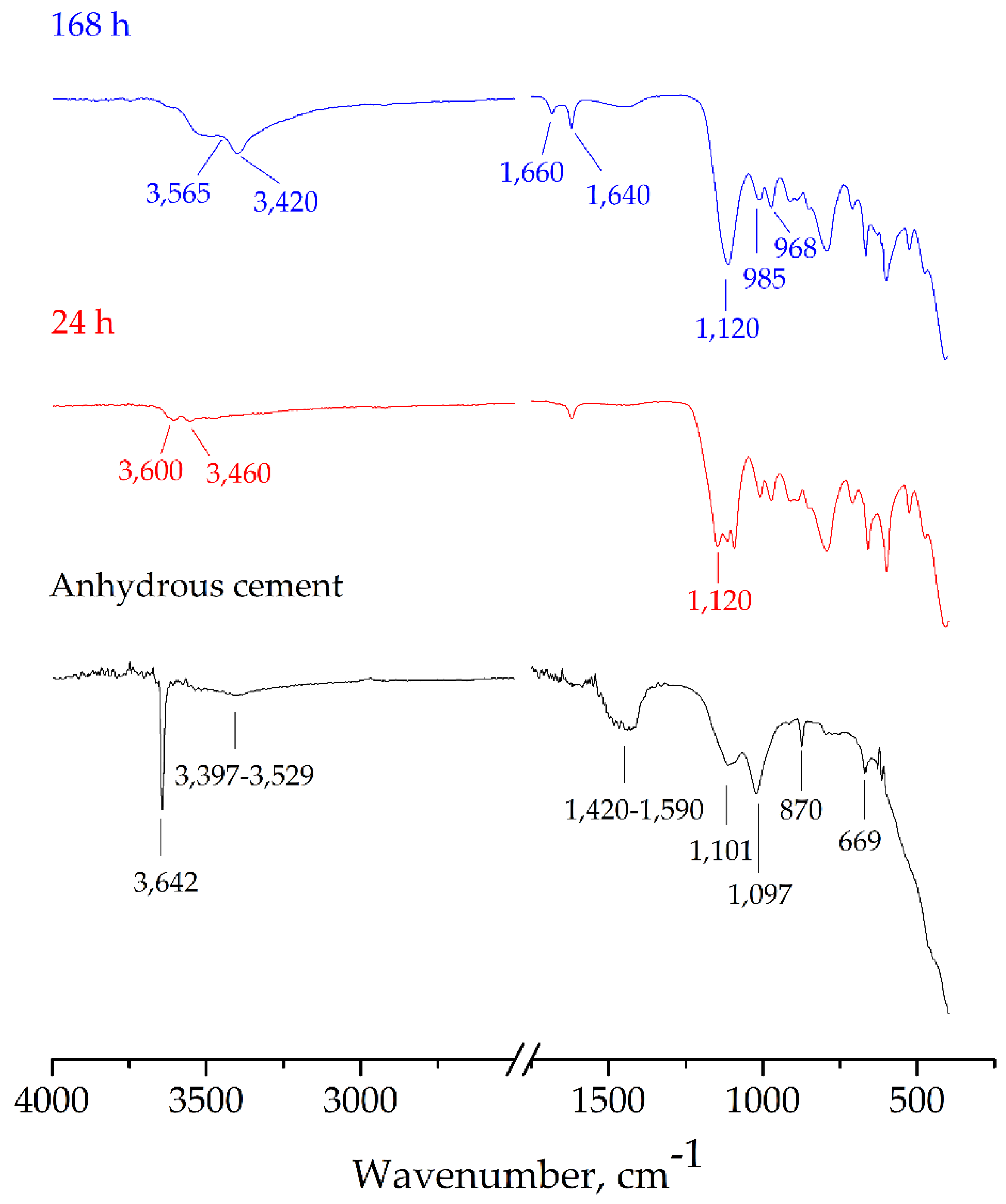
2.2.4. Fourier-transform Infrared Spectroscopy (FTIR)
2.2.5. Water Requirement, Setting Time and Compressive Strength of Various CSA Blends
3. Results and Discussion
3.1. Characterization of Raw Materials
3.2. Characterization of CSA Clinkers
3.3. Characterization of Hydrated Paste
3.4. Engineering Properties of OPC-CSA Blended Paste
4. Conclusions
- The particular industrial wastes show potential for application as raw material for CSA cement such as marble dust waste, flue gas desulfurization gypsum, and napier grass ash. CSA clinker with desired phase composition (i.e., C2S, C4A3$ and C4AF) can be successfully synthesized with the appropriate mixed proportion.
- CSA clinker fired at 1250 °C showed most similar phase content compared to designed composition. This clinker was used to study the hydrated pastes at various curing periods by using the FTIR technique.
- The replacement of OPC cement by CSA cement increased the water requirement for normal consistency, and shortened the initial and final setting times.
- Adding of the synthesized CSA cement to OPC cement is very helpful to improve compressive strength in the early age of hydration. However, the long-term compressive strength of synthesized CSA-OPC blended pastes were lower than that of the OPC paste.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ioannou, S.; Reig, L.; Paine, K.; Quillin, K. Properties of a ternary calcium sulfoaluminate–calcium sulfate–fly ash cement. Cem. Concr. Res. 2014, 56, 75–83. [Google Scholar] [CrossRef]
- Zhang, L.; Su, M.Z.; Wang, Y.M. Development of the use of sulfo- and ferroaluminate cements in China. Adv. Cem. Res. 1999, 11, 15–21. [Google Scholar] [CrossRef]
- Gartner, E. Are there any practical alternatives to the manufacture of Portland cement clinker? In Proceedings of the 11th International Conference on Non-conventional Materials and Technologies NOCMAT, Bath, UK, 6–9 September 2009; University of Bath: Bath, UK, 2009. [Google Scholar]
- Hanein, T.; Elhoweris, A.; Galan, I.; Glasser, F.P.; Campbell Bannerman, M.N. Thermodynamic data of ye’elemite (C4A3$) for cement clinker equilibrium calculations. In Proceedings of the 35th Cement & Concrete Science Conference, Aberdeen, UK, 26–28 August 2015; University of Aberdeen: Aberdeen, UK, 2015. [Google Scholar]
- Wang, J.; Baco, I.; Morin, V.; Walenta, G.; Damidot, D.; Gartner, E. Hydration mechanism of cements based on low-CO2 clinkers containing belite, ye’elimite and calcium alumino-ferrite. In Proceedings of the 7th International Symposium on Cement & Concrete, Jinan, China, 9–12 May 2010; Foreign Languages Press: Beijing, China, 2010. [Google Scholar]
- Galan, I.; Glasser, F.P.; Elhoweris, A.; Tully, S.; Murdoch, A. Novel process for calcium sulfoaluminate cement production. In Proceedings of the 34th Cement and Concrete Science Conference, Sheffield, UK, 14–17 September 2014; University of Sheffield: Sheffield, UK, 2014. [Google Scholar]
- Sharp, J.H.; Lawrence, C.D.; Yang, R. Calcium sulfoaluminate cements—Low-energy cements, special cements or what? Adv. Cem. Res. 1999, 11, 3–13. [Google Scholar] [CrossRef]
- Georgin, J.; Ambroise, J.; Pera, J.; Reynouard, J. Development of self-leveling screed based on calcium sulfoaluminate cement: Modelling of curling due to drying. Cem. Concr. Compos. 2008, 30, 769–778. [Google Scholar] [CrossRef]
- Winnefeld, F.; Lothenbach, B. Hydration of calcium sulfoaluminate cements—Experimental findings and thermodynamic modelling. Cem. Concr. Res. 2010, 40, 1239–1247. [Google Scholar] [CrossRef]
- Sirtoli, D.; Wyrzykowski, M.; Riva, P.; Tortelli, S.; Marchi, M.; Lura, P. Shrinkage and creep of high-performance concrete based on calcium sulfoaluminate cement. Cem. Concr. Compos. 2019, 98, 61–73. [Google Scholar] [CrossRef]
- Glasser, F.; Zhang, L. High-performance cement matrices based on calcium sulfoaluminate–belite compositions. Cem. Concr. Res. 2001, 31, 1881–1886. [Google Scholar] [CrossRef]
- Mehdipour, I.; Khayat, K.H. Enhancing the performance of calcium sulfoaluminate blended cements with shrinkage reducing admixture or lightweight sand. Cem. Concr. Compos. 2018, 87, 29–43. [Google Scholar] [CrossRef]
- Al Horr, Y.; Elhoweris, A.; Elsarrag, E. The development of a novel process for the production of calcium sulfoaluminate. Int. J. Sustain. Built Environ. 2017, 6, 734–741. [Google Scholar] [CrossRef]
- Chen, I.A.; Hargis, C.W.; Juenger, M.C. Understanding expansion in calcium sulfoaluminate–belite cements. Cem. Concr. Res. 2012, 42, 51–60. [Google Scholar] [CrossRef]
- Jeong, Y.; Hargis, C.W.; Chun, S.; Moon, J. Effect of Calcium Carbonate Fineness on Calcium Sulfoaluminate-Belite Cement. Materials 2017, 10, 900. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; Hargis, C.W.; Chun, S.-C.; Moon, J. The effect of water and gypsum content on strätlingite formation in calcium sulfoaluminate-belite cement pastes. Constr. Build. Mater. 2018, 166, 712–722. [Google Scholar] [CrossRef]
- Schneider, M.; Romer, M.; Tschudin, M.; Bolio, H. Sustainable cement production—Present and future. Cem. Concr. Res. 2011, 41, 642–650. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, M.; Zhang, Y. Preparation and properties of self-pulverizing calcium sulfoaluminate cement. Constr. Build. Mater. 2012, 34, 107–113. [Google Scholar] [CrossRef]
- Juenger, M.; Winnefeld, F.; Provis, J.; Ideker, J. Advances in alternative cementitious binders. Cem. Concr. Res. 2011, 41, 1232–1243. [Google Scholar] [CrossRef]
- Gartner, E. Industrially interesting approaches to “low-CO2” cements. Cem. Concr. Res. 2004, 34, 1489–1498. [Google Scholar] [CrossRef]
- Chen, I.A.; Juenger, M.C. Incorporation of coal combustion residuals into calcium sulfoaluminate-belite cement clinkers. Cem. Concr. Compos. 2012, 34, 893–902. [Google Scholar] [CrossRef]
- Bouregba, A.; Elghattas, B.; Guedira, T. Influence of Fluorine on Clinker burnability and mechanical properties of CPA Moroccan cement. MATEC Web Conf. 2018, 149, 01075. [Google Scholar] [CrossRef]
- Djerdi, I. Rietveld Refinement in the Characterization of Crystalline Materials, Printed Edition of the Special Issue Published in Crystals; MDPI: Basel, Switzerland, 2019; pp. 1–11. [Google Scholar]
- Le Saoût, G.; Kocaba, V.; Scrivener, K. Application of the Rietveld method to the analysis of anhydrous cement. Cem. Concr. Res. 2011, 41, 133–148. [Google Scholar] [CrossRef]
- Chaunsali, P.; Mondal, P. Physico-chemical interaction between mineral admixtures and OPC–calcium sulfoaluminate (CSA) cements and its influence on early-age expansion. Cem. Concr. Res. 2016, 80, 10–20. [Google Scholar] [CrossRef]
- Trauchessec, R.; Mechling, J.-M.; LeComte, A.; Roux, A.; Le Rolland, B. Hydration of ordinary Portland cement and calcium sulfoaluminate cement blends. Cem. Concr. Compos. 2015, 56, 106–114. [Google Scholar] [CrossRef]
- Telesca, A.; Marroccoli, M.; Pace, M.; Tomasulo, M.; Valenti, G.; Monteiro, P. A hydration study of various calcium sulfoaluminate cements. Cem. Concr. Compos. 2014, 53, 224–232. [Google Scholar] [CrossRef]
- Yeung, J.S.; Yam, M.C.; Wong, Y. 1-Year development trend of concrete compressive strength using Calcium Sulfoaluminate cement blended with OPC, PFA and GGBS. Constr. Build. Mater. 2019, 198, 527–536. [Google Scholar] [CrossRef]
- ASTM C187-16, Standard Test Method for Amount of Water Required for Normal Consistency of Hydraulic Cement Paste; ASTM International: West Conshohocken, PA, USA, 2016.
- ASTM C191-19, Standard Test Methods for Time of Setting of Hydraulic Cement by Vicat Needle; ASTM International: West Conshohocken, PA, USA, 2019.
- Rungchet, A.; Poon, C.; Chindaprasirt, P.; Pimraksa, K. Synthesis of low-temperature calcium sulfoaluminate-belite cements from industrial wastes and their hydration: Comparative studies between lignite fly ash and bottom ash. Cem. Concr. Compos. 2017, 83, 10–19. [Google Scholar] [CrossRef]
- Li, C.; Wu, M.; Yao, W. Effect of coupled B/Na and B/Ba doping on hydraulic properties of belite-ye’elimite-ferrite cement. Constr. Build. Mater. 2019, 208, 23–35. [Google Scholar] [CrossRef]
- Winnefeld, F.; Barlag, S. Calorimetric and thermogravimetric study on the influence of calcium sulfate on the hydration of ye’elimite. J. Therm. Anal. Calorim. 2010, 101, 949–957. [Google Scholar] [CrossRef]
- Cuesta, A.; Álvarez-Pinazo, G.; Sanfélix, S.; Peral, I.; Aranda, M.A.; De La Torre, A.; Alonso, I.P. Hydration mechanisms of two polymorphs of synthetic ye’elimite. Cem. Concr. Res. 2014, 63, 127–136. [Google Scholar] [CrossRef]
- Álvarez-Pinazo, G.; Santacruz, I.; Aranda, M.A.G.; De La Torre, Á.G. Hydration of belite–ye’elimite–ferrite cements with different calcium sulfate sources. Adv. Cem. Res. 2016, 28, 1–15. [Google Scholar] [CrossRef]
- Tang, S.; Zhu, H.; Li, Z.; Chen, E.; Shao, H. Hydration stage identification and phase transformation of calcium sulfoaluminate cement at early age. Constr. Build. Mater. 2015, 75, 11–18. [Google Scholar] [CrossRef]
- Horgnies, M.; Chen, J.J.; Bouillon, C. Overview about the use of Fourier transform infrared spectroscopy to study cementitious materials. In Proceedings of the 6th International Conference on Computational Methods and Experiments in Materials Characterization, Siena, Italy, 4–6 June 2013; WIT Transactions on Engineering Sciences: Southampton, UK, 2013; pp. 251–262. [Google Scholar]
- Khachani, M.; El Hamidi, A.; Halim, M.; Arsalane, S. Non-isothermal kinetic and thermodynamic studies of the dehydroxylation process of synthetic calcium hydroxide Ca(OH)2. J. Mater. Environ. Sci. 2014, 5, 615–624. [Google Scholar]
- Fernndez-Carrasco, L.; Torrens-Martn, D.; Morales, L.; Martnez-Ramrez, S.; Fernández-Carrasco, D.T.-M.L. Infrared Spectroscopy in the Analysis of Building and Construction Materials. In Infrared Spectroscopy—Materials Science, Engineering and Technology; IntechOpen: London, UK, 2012; pp. 369–382. [Google Scholar]
- Zhang, J.; Li, G.; Yang, X.; Ren, S.; Song, Z. Study on a high strength ternary blend containing calcium sulfoaluminate cement/calcium aluminate cement/ordinary Portland cement. Constr. Build. Mater. 2018, 191, 544–553. [Google Scholar] [CrossRef]

| Sample | OPC (wt.%) | CSA Clinker (wt.%) | Gypsum (wt.%) | CSA Cement (wt.%) |
|---|---|---|---|---|
| OPC | 100 | - | - | - |
| CSA20 | 80 | 16 | 4 | 20 |
| CSA25 | 75 | 20 | 5 | 25 |
| CSA30 | 70 | 24 | 6 | 30 |
| CSA35 | 65 | 28 | 7 | 35 |
| CSA40 | 60 | 32 | 8 | 40 |
| Raw Materials | Mix Proportions (wt.%.) | CaO | SiO2 | Al2O3 | Fe2O3 | SO3 | K2O | P2O5 |
|---|---|---|---|---|---|---|---|---|
| MDW | 41 | 100 | - | - | - | - | - | - |
| NGA | 10 | 3.58 | 69.92 | 8.42 | 7.74 | - | 7.23 | 1.18 |
| CDW | 10 | - | 68.65 | 15.62 | 5.37 | - | 9.62 | - |
| FGDG | 14 | 49.34 | - | - | - | 49.25 | - | 1.28 |
| Al2O3 | 25 | - | - | 100 | - | - | - | - |
| Target chemical composition of raw mixture | 48.57 | 13.94 | 27.48 | 1.32 | 6.72 | 1.70 | 0.33 | |
| Measured chemical composition of calcined clinker at 1250 °C | 49.13 | 14.20 | 24.83 | 2.14 | 7.01 | 0.43 | 0.09 | |
| Phase | Target Phase Composition (wt.%.) | Clinker Phase Composition Fired at 1200 °C (wt.%.) | Clinker Phase Composition Fired at 1250 °C (wt.%.) | Clinker Phase Composition Fired at 1300 °C (wt.%.) |
|---|---|---|---|---|
| Ye’elimite (C4A3$) | 50 | 46.25 | 48.11 | 47.41 |
| β-Belite (C2S) | 40 | 43.80 | 41.95 | 42.79 |
| Brownmillerite (C4AF) | 10 | 4.54 | 4.90 | 4.36 |
| Mayenite | 1.50 | 0.07 | 0.36 | |
| Bassanite | 0.25 | 0.05 | 0.27 | |
| Good of fitness | 3.26 | 3.25 | 3.24 |
| Sample | % CSA | w/b to Normal Consistency | Initial Setting Time (minutes) | Final Setting Time (minutes) |
|---|---|---|---|---|
| OPC | 0 | 0.272 | 116 | 195 |
| CSA20 | 20 | 0.318 | 38 | 90 |
| CSA25 | 25 | 0.328 | 28 | 60 |
| CSA30 | 30 | 0.336 | 22 | 55 |
| CSA35 | 35 | 0.369 | 17 | 35 |
| CSA40 | 40 | 0.405 | 17 | 30 |
| Sample | % CSA | 6 h (ksc) | 12 h (ksc) | 24 h (ksc) | 72 h (ksc) | 168 h (ksc) | 336 h (ksc) | 672 h (ksc) |
|---|---|---|---|---|---|---|---|---|
| OPC | 0 | - | 171 | 413 | 519 | 697 | 817 | 850 |
| CSA20 | 20 | 43 | 156 | 310 | 524 | 610 | 627 | 649 |
| CSA25 | 25 | 54 | 212 | 434 | 476 | 549 | 594 | 600 |
| CSA30 | 30 | 59 | 244 | 428 | 444 | 526 | 536 | 582 |
| CSA35 | 35 | 101 | 274 | 439 | 442 | 456 | 481 | 493 |
| CSA40 | 40 | 239 | 305 | 367 | 402 | 424 | 448 | 455 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Julphunthong, P.; Joyklad, P. Utilization of Several Industrial Wastes as Raw Material for Calcium Sulfoaluminate Cement. Materials 2019, 12, 3319. https://doi.org/10.3390/ma12203319
Julphunthong P, Joyklad P. Utilization of Several Industrial Wastes as Raw Material for Calcium Sulfoaluminate Cement. Materials. 2019; 12(20):3319. https://doi.org/10.3390/ma12203319
Chicago/Turabian StyleJulphunthong, Phongthorn, and Panuwat Joyklad. 2019. "Utilization of Several Industrial Wastes as Raw Material for Calcium Sulfoaluminate Cement" Materials 12, no. 20: 3319. https://doi.org/10.3390/ma12203319
APA StyleJulphunthong, P., & Joyklad, P. (2019). Utilization of Several Industrial Wastes as Raw Material for Calcium Sulfoaluminate Cement. Materials, 12(20), 3319. https://doi.org/10.3390/ma12203319




