Synthesis and Post-Annealing of Cu2ZnSnS4 Absorber Layers Based on Oleylamine/1-dodecanethiol
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the CZTS Nanoparticles
2.3. Characterization of the CZTS Film: Methods and Instrumentations
3. Results and Discussion
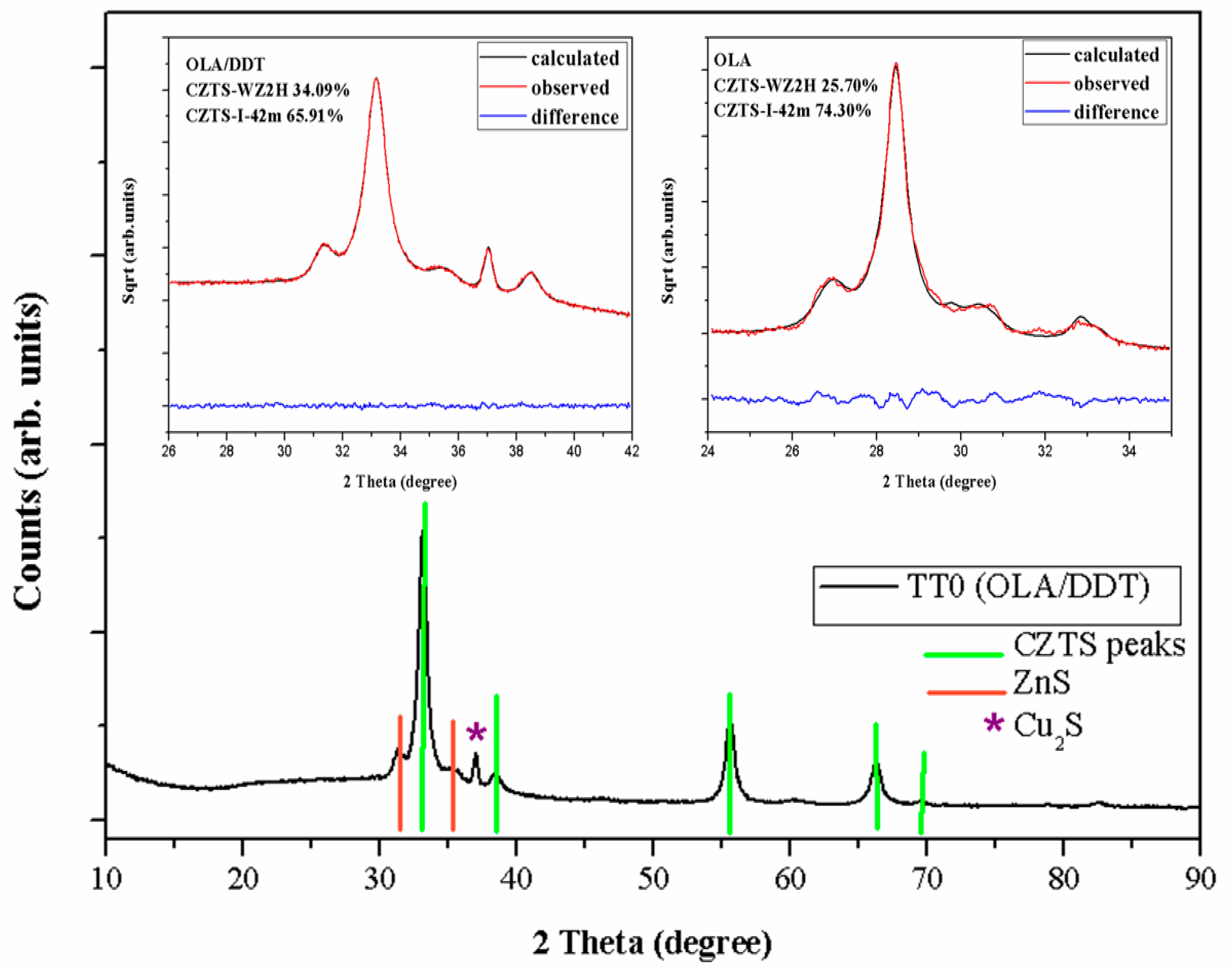
3.1. XRD and DLS
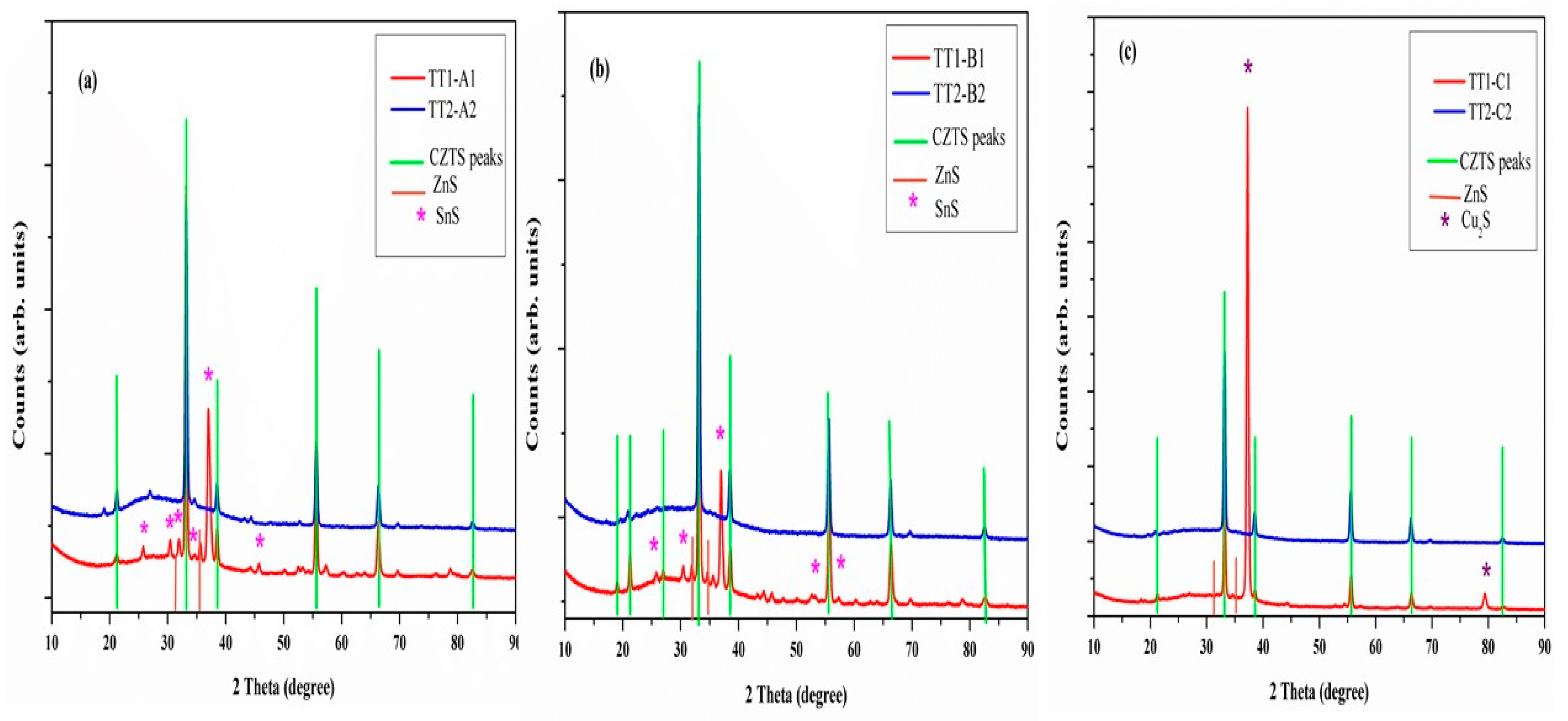
3.2. XRD
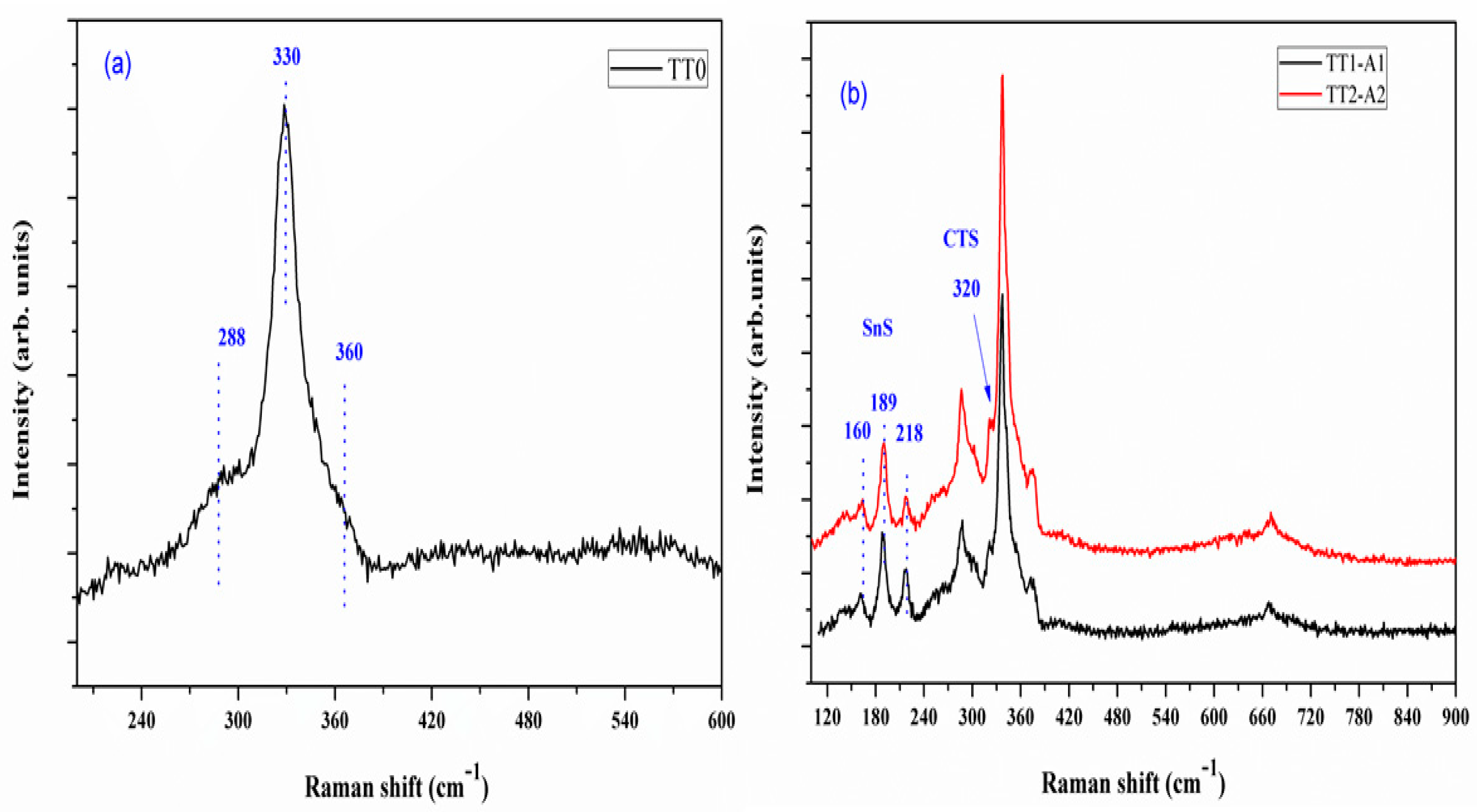
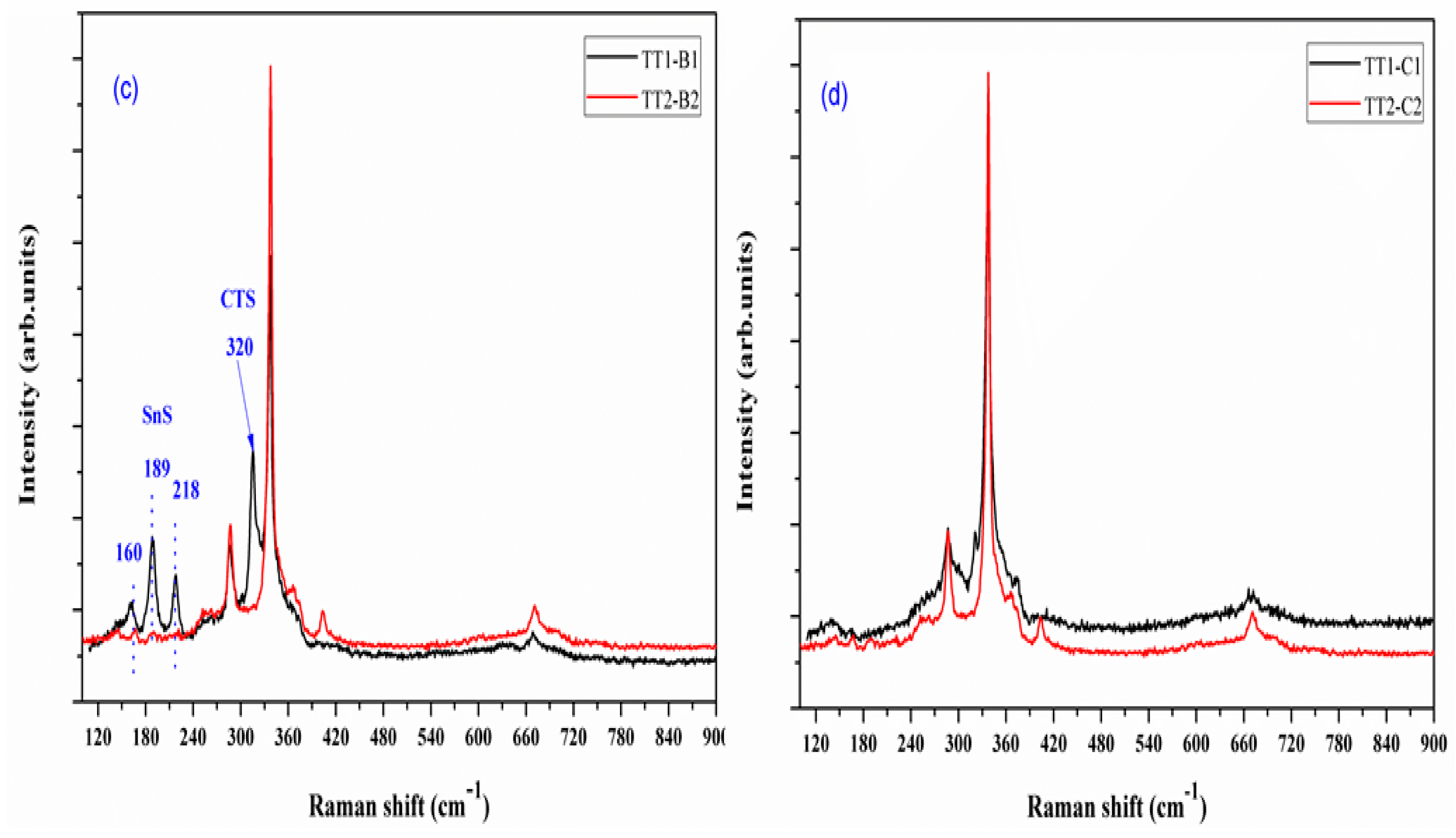
3.3. Raman Spectroscopy
3.4. SEM
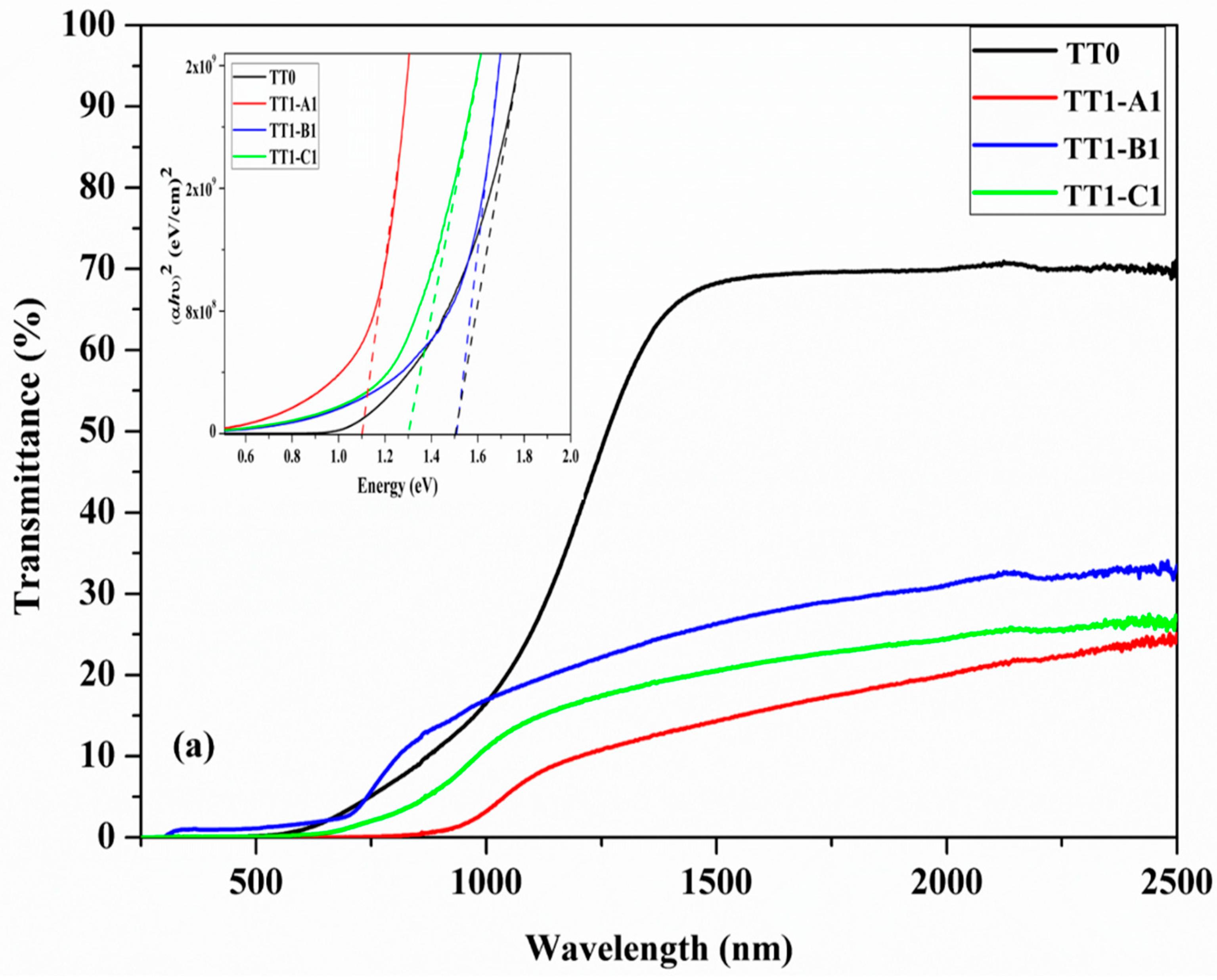
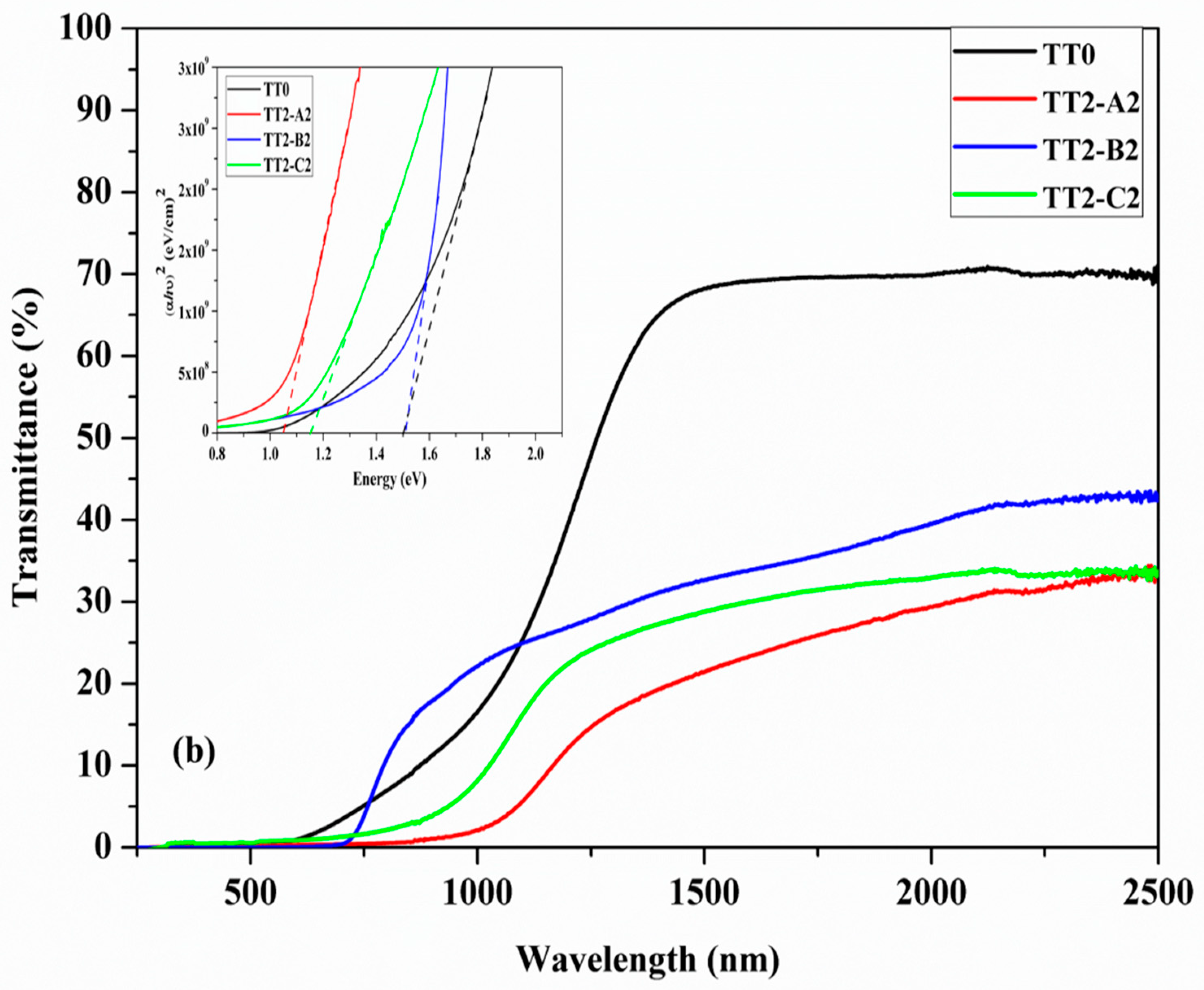
3.5. UV-Vis Spectroscopy and Resistivity Measurement
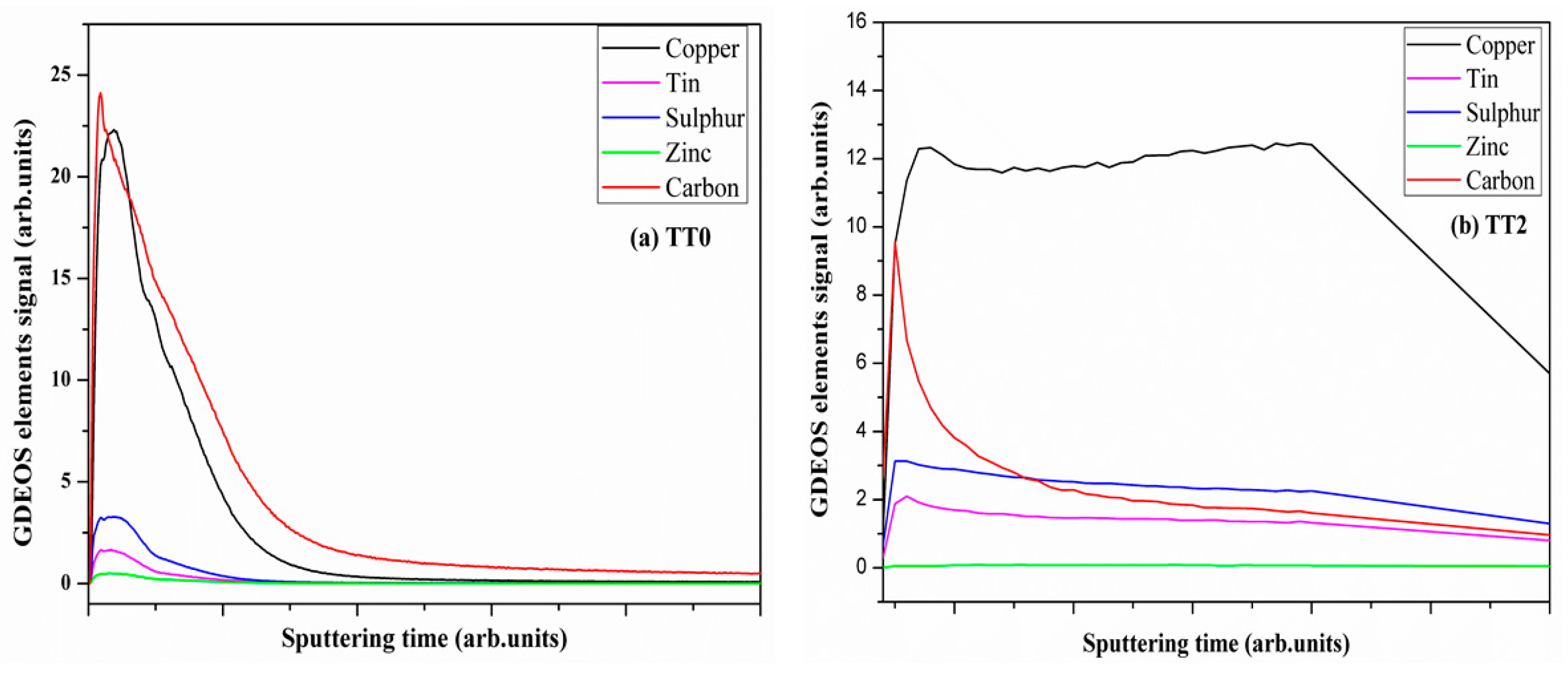
3.6. Carbon Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yan, C.; Sun, K.; Huang, J.; Johnston, S.; Liu, F.; Veettil, B.P.; Sun, K.; Pu, A.; Zhou, F.; Stride, J.A.; et al. Beyond 11% Efficient Sulfide Kesterite Cu2ZnxCd1-xSnS4 Solar Cells: Effects of cadmium Alloying. ACS Energy Lett. 2017, 2, 930–936. [Google Scholar] [CrossRef]
- Fairbrother, A.; Fontané, X.; Izquierdo-Roca, V.; Espindola-Rodriguez, M.; López-Marino, S.; Placidi, M.; López-García, J.; Pérez-Rodríguez, A.; Saucedo, E. Single-Step Sulfo-Selenization Method to Synthesize Cu2ZnSn(SySe1−y)4 Absorbers from Metallic Stack Precursors. ChemPhysChem 2013, 14, 1836–1843. [Google Scholar] [CrossRef] [PubMed]
- Fairbrother, A.; García-Hemme, E.; Izquierdo-Roca, V.; Fontané, X.; Pulgarín-Agudelo, F.A.; Vigil-Galán, O.; Pérez-Rodríguez, A.; Saucedo, E. Development of a Selective Chemical Etch To Improve the Conversion Efficiency of Zn-Rich Cu2ZnSnS4 Solar Cells. J. Am. Chem. Soc. 2012, 134, 8018–8021. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Winkler, M.T.; Gunawan, O.; Gokmen, T.; Todorov, T.K.; Zhu, Y.; Mitzi, D.B. Device Characteristics of CZTSSe Thin-Film Solar Cells with 12.6% Efficiency. Adv. Energy Mater. 2014, 4, 1301465. [Google Scholar] [CrossRef]
- Chen, G.; Yuan, C.; Liu, J.; Deng, Y.; Jiang, G.; Liu, W.; Zhu, C. Low cost preparation of Cu2ZnSnS4 and Cu2ZnSn(SxSe1−x)4 from binary sulfide nanoparticles for solar cell application. J. Power Sources 2014, 262, 201–206. [Google Scholar] [CrossRef]
- Mitzi, D.B. Solution Processing of Inorganic Materials; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Yang, H.; Jauregui, L.A.; Zhang, G.; Chen, Y.P.; Wu, Y. Nontoxic and Abundant Copper Zinc Tin Sulfide Nanocrystals for Potential High-Temperature Thermoelectric Energy Harvesting. Nano Lett. 2012, 12, 540–545. [Google Scholar] [CrossRef]
- Habas, S.E.; Platt, H.A.S.; Hest, M.F.A.M.; Ginley, D.S. Low-Cost Inorganic Solar Cells: From Ink To Printed Device. Chem. Rev. 2010, 110, 6571–6594. [Google Scholar] [CrossRef]
- Abermann, S. Non-vacuum processed next generation thin film photovoltaics: Towards marketable efficiency and production of CZTS based solar cells. Sol. Energy 2013, 94, 37–70. [Google Scholar] [CrossRef]
- Jiang, M.; Yan, X. Cu2ZnSnS4 Thin Film Solar Cells: Present Status and Future Prospects. In Solar Cells; Morales-Acevedo, A., Ed.; IntechOpen: Rijeka, Croatia, 2013. [Google Scholar]
- Aldakov, D.; Lefrançois, A.; Reiss, P. Ternary and quaternary metal chalcogenide nanocrystals: Synthesis, properties and applications. J. Mater. Chem. C 2013, 1, 3756–3776. [Google Scholar] [CrossRef]
- Ataollahi, N.; Malerba, C.; Cappelletto, E.; Ciancio, R.; Edla, R.; Maggio, R.D.; Scardi, P. Control of composition and grain growth in Cu2ZnSnS4 thin films from nanoparticle inks. Thin Solid Film. 2018, 674, 12–21. [Google Scholar] [CrossRef]
- Xia, D.; Lei, P.; Zheng, Y.; Zhou, B. Synthesis and characterization of Cu2ZnSnS4 nanocrystals by hot-injection method. J. Mater. Sci. Mater. Electron. 2015, 26, 5426–5432. [Google Scholar] [CrossRef]
- Singh, A.; Geaney, H.; Laffir, F.; Ryan, K.M. Colloidal Synthesis of Wurtzite Cu2ZnSnS4 Nanorods and Their Perpendicular Assembly. J. Am. Chem. Soc. 2013, 134, 2910–2913. [Google Scholar] [CrossRef] [PubMed]
- Soosaimanickam, A.; Jeyagopal, R.; Hayakawa, Y.; Sridharan, M.B. Synthesis of oleylamine-capped Cu2 ZnSn(S,Se)4 nanoparticles using 1-dodecanethiol as sulfur source. Jpn. J. Appl. Phys. 2015, 54, 08KA10. [Google Scholar] [CrossRef]
- Soares, J.A. Introduction to Optical Characterization of Materials. In Practical Materials Characterization; Springer: New York, NY, USA, 2014; pp. 43–92. [Google Scholar]
- Ingle, J.; Crouch, S. Spectrochemical Analysis; Prentice Hall: Englewood Cliffs, NJ, USA, 1988. [Google Scholar]
- Isotta, E.; Pugno, N.; Scardi, P. Nanostructured kesterite (Cu2ZnSnS4) for applications in thermoelectric devices. Powder Diffr. 2019, 34, 1–6. [Google Scholar] [CrossRef]
- Isotta, E.; Fanciulli, C.; Pugno, N.; Scardi, P. Effect of order-disorder transition on seebeck coefficient nanostructured thermoelectric Cu2ZnSnS4. Nanomaterials 2019, 9, 762. [Google Scholar] [CrossRef] [PubMed]
- Kapusta, K.; Drygas, M.; Janik, J.; Jelen, P.; Bucko, M.; Olejniczak, Z. From magnetic cubic pre-kesterite to semiconducting tetragonal kesterite Cu2ZnSnS4 nanopowders via the mechanochemically assisted route. J. Alloys Compd. 2019, 770, 981–988. [Google Scholar] [CrossRef]
- Coelho, A.A. TOPAS and TOPAS-Academic: An optimization program integrating computer algebra and crystallographic objects written in C++. J. Appl. Crystallogr. 2018, 51, 210–218. [Google Scholar] [CrossRef]
- Huang, C.; Chan, Y.; Liu, F.; Tang, D.; Yang, J.; Lai, Y.; Li, J.; Liu, Y. Synthesis and characterization of multicomponent Cu2 (Fex Zn1−x) SnS4 nanocrystals with tunable band gap and structure. J. Mater. Chem. A 2013, 1, 5402–5407. [Google Scholar] [CrossRef]
- Kheraj, V.; Patel, K.K.; Patel, S.J.; Shah, D.V. Synthesis and characterisation of Copper Zinc Tin Sulphide (CZTS) compound for absorber material in solar-cells. J. Cryst. Growth 2013, 362, 174–177. [Google Scholar] [CrossRef]
- Kannan, A.G.; Manjulavalli, T.E.; Chandrasekaran, J. Influence of Solvent on the Properties of CZTS Nanoparticles. Procedia Eng. 2016, 141, 15–22. [Google Scholar] [CrossRef]
- Diwate, K.; Mohite, K.; Shinde, M.; Rondiya, S.; Pawbake, A.; Date, A.; Pathan, H.; Jadkar, S. Synthesis and Characterization of Chemical Spray Pyrolysed CZTS Thin Films for Solar Cell Applications. Energy Procedia 2017, 110, 180–187. [Google Scholar] [CrossRef]
- Fernandes, P.A.; Salomé, P.M.P.; Cunha, A.F. Study of polycrystalline Cu2ZnSnS4 films by Raman scattering. J. Alloys Compd. 2011, 509, 7600–7606. [Google Scholar] [CrossRef]
- Suarez, H.; Correa, J.M.; Cruz, S.D.; Otalora, C.A.; Hurtado, M.; Gordillo, G. Synthesis and Study of Properties of CZTS Thin Films Grown Using a Novel Solution-Based Chemical Route. In Proceedings of the 2013 IEEE 39th Photovoltaic Specialists Conference (PVSC), Tampa, FL, USA, 16–21 June 2013. [Google Scholar]
- Gordillo, G.; Calderón, C.; Bartolo-Pérez, P. XPS analysis and structural and morphological characterization of Cu2ZnSnS4 thin films grown by sequential evaporation. Appl. Surf. Sci. 2014, 305, 506–514. [Google Scholar] [CrossRef]
- Xu, Q.; Huang, B.; Zhao, Y.; Yan, Y.; Noufi, R.; Wei, S.-H. Crystal and electronic structures of CuxS solar cell absorbers. Appl. Phys. Lett. 2012, 100, 061906. [Google Scholar] [CrossRef]
- Sharma, P.; Sharma, I.; Katyal, S.C. Physical and optical properties of binary amorphous selenium-antimony thin films. J. Appl. Phys. 2009, 105, 053509. [Google Scholar] [CrossRef]
- Madelung, O.; Rössler, U.; Schulz, M. Tin Sulfide (SnS) Band Structure, Energy Gaps: Datasheet from Landolt-Börnstein–Group III. In Condensed Matter Volume 41C: “Non-Tetrahedrally Bonded Elements and Binary Compounds I” in Springermaterials; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar] [CrossRef]
- Oztas, M. Influence of grain size on electrical and optical properties of InP films. Chin. Phys. Lett. 2008, 25, 4090–4092. [Google Scholar] [CrossRef]
- Tiong, V.; Zhang, Y.; Bell, J.; Wang, H. Carbon concentration dependent grain growth of Cu2ZnSnS4 thin films. RSC Adv. 2015, 5, 20178–20185. [Google Scholar] [CrossRef]
- Shin, S.; Pawar, S.; Park, C.; Yun, J.; Moon, J.; Kim, J.; Lee, J. Studies on Cu2ZnSnS4 (CZTS) absorber layer using different stacking orders in precursor thin films. Sol. Energy Mater. Sol. Cells. 2011, 95, 3202–3206. [Google Scholar] [CrossRef]
- Syafiq, U.; Ataollahi, N.; Maggio, R.D.; Scardi, P. Solution-Based Synthesis and Characterization of Cu2ZnSnS4 (CZTS) Thin Films. Molecules 2019, 24, 3454. [Google Scholar] [CrossRef]
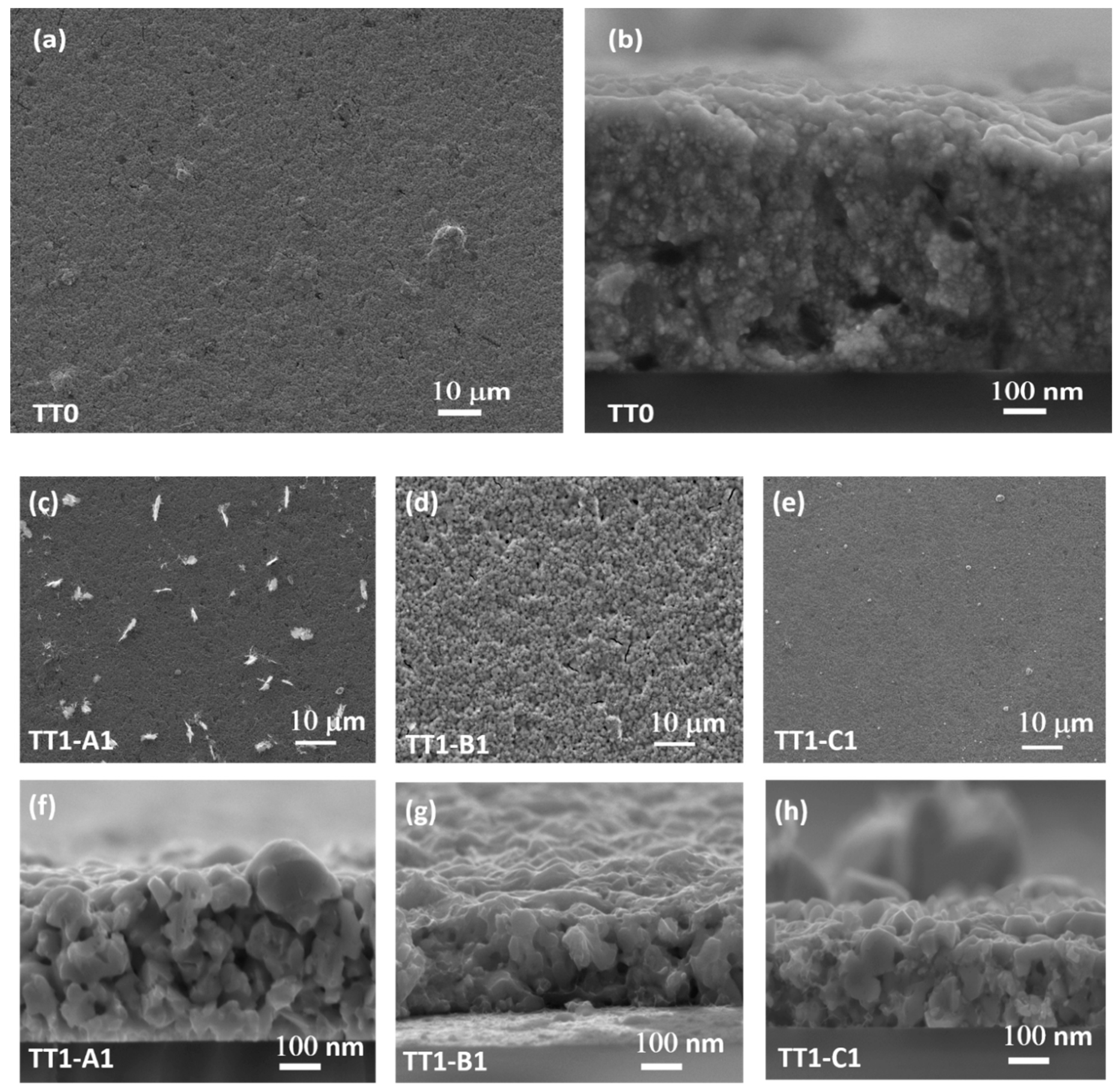
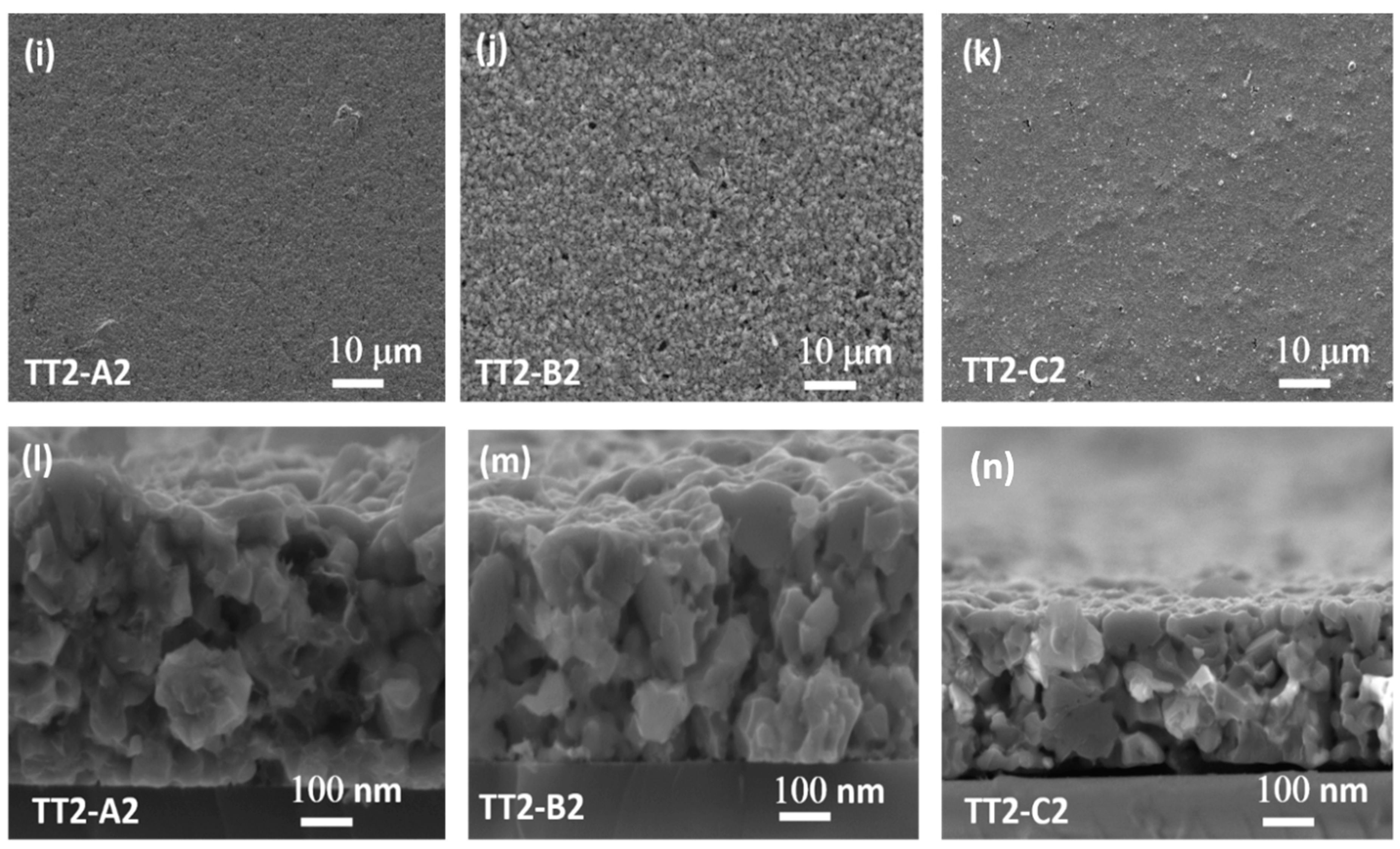
| Recipe | Sample/TT1 | Sample/TT2 | Ref. |
|---|---|---|---|
| A | A1: 560 °C, 20 min; 20 °C/min | A2: 560 °C, 2 h, 20 °C/min; S (20 mg) | [5] |
| B | B1: 500 °C, 2 h, 3 °C/min | B2: 600 °C, 2 h, 3 °C/min; S (20 mg) | this work |
| C | C1: 600 °C, 2 h, 3°C/min | C2: 600 °C, 2 h, 3 °C/min; S (20 mg) | this work |
| Sample | X-ray Fluorescence (XRF) (Assuming S = 4) | Cumulant Size (nm) | Polydispersity Index (PI) | Note | ||||
|---|---|---|---|---|---|---|---|---|
| Ideal composition | Cu 1.81 | Zn 1.21 | Sn 0.98 | Cu/Sn 1.75–1.90 | Zn/Sn 1.20 | – | – | – |
| CZTS film | 1.79 | 1.20 | 1.01 | 1.78 | 1.20 | 24.1 | 0.05 | Monodisperse |
| Sample | TT1 Temperature (°C) | TT1 Secondary Phases | Eg (TT1) (eV) | T (TT1)(at 1500 nm) | TT2 Temp (°C) | TT2 Secondary Phases | Eg (TT2)(eV) | T (TT2) (at 1500 nm) | Resistivity (ohm.cm) |
|---|---|---|---|---|---|---|---|---|---|
| A1-A2 | 560 | SnS + CTS | 1.16 | 15% | 560 | SnS, CTS | 1.20 | 20% | 0.012 |
| B1-B2 | 500 | SnS + CTS | 1.50 | 25% | 600 | Undetected | 1.50 | 30% | 1.05 |
| C1-C2 | 600 | Cu2S | 1.30 | 20% | 600 | Undetected | 1.20 | 30% | 0.016 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ataollahi, N.; Bazerla, F.; Malerba, C.; Chiappini, A.; Ferrari, M.; Di Maggio, R.; Scardi, P. Synthesis and Post-Annealing of Cu2ZnSnS4 Absorber Layers Based on Oleylamine/1-dodecanethiol. Materials 2019, 12, 3320. https://doi.org/10.3390/ma12203320
Ataollahi N, Bazerla F, Malerba C, Chiappini A, Ferrari M, Di Maggio R, Scardi P. Synthesis and Post-Annealing of Cu2ZnSnS4 Absorber Layers Based on Oleylamine/1-dodecanethiol. Materials. 2019; 12(20):3320. https://doi.org/10.3390/ma12203320
Chicago/Turabian StyleAtaollahi, Narges, Francesca Bazerla, Claudia Malerba, Andrea Chiappini, Maurizio Ferrari, Rosa Di Maggio, and Paolo Scardi. 2019. "Synthesis and Post-Annealing of Cu2ZnSnS4 Absorber Layers Based on Oleylamine/1-dodecanethiol" Materials 12, no. 20: 3320. https://doi.org/10.3390/ma12203320
APA StyleAtaollahi, N., Bazerla, F., Malerba, C., Chiappini, A., Ferrari, M., Di Maggio, R., & Scardi, P. (2019). Synthesis and Post-Annealing of Cu2ZnSnS4 Absorber Layers Based on Oleylamine/1-dodecanethiol. Materials, 12(20), 3320. https://doi.org/10.3390/ma12203320










