Co-Polymers based on Poly(1,4-butylene 2,5-furandicarboxylate) and Poly(propylene oxide) with Tuneable Thermal Properties: Synthesis and Characterization
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Dimethyl 2,5-Furandicarboxylate Monomer
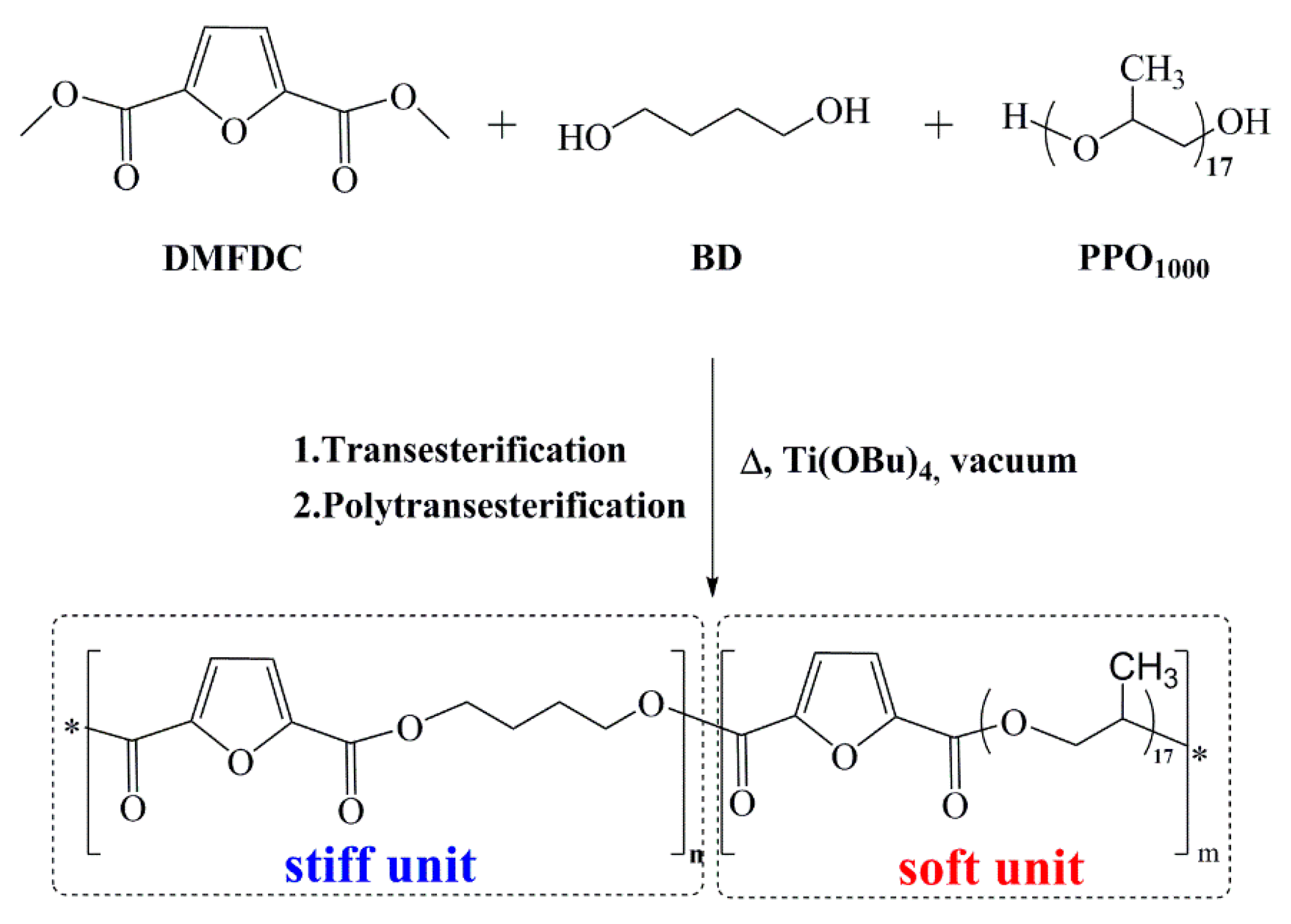
2.3. Syntheses of Poly(1,4-Butylene 2,5-Furandicarboxylate)-Co-Poly(Poly(Propylene Oxide) Co-Polymers and Poly(1,4-Butylene 2,5-Furandicarboxylate)
2.4. Characterization Methods
3. Results and Discussion
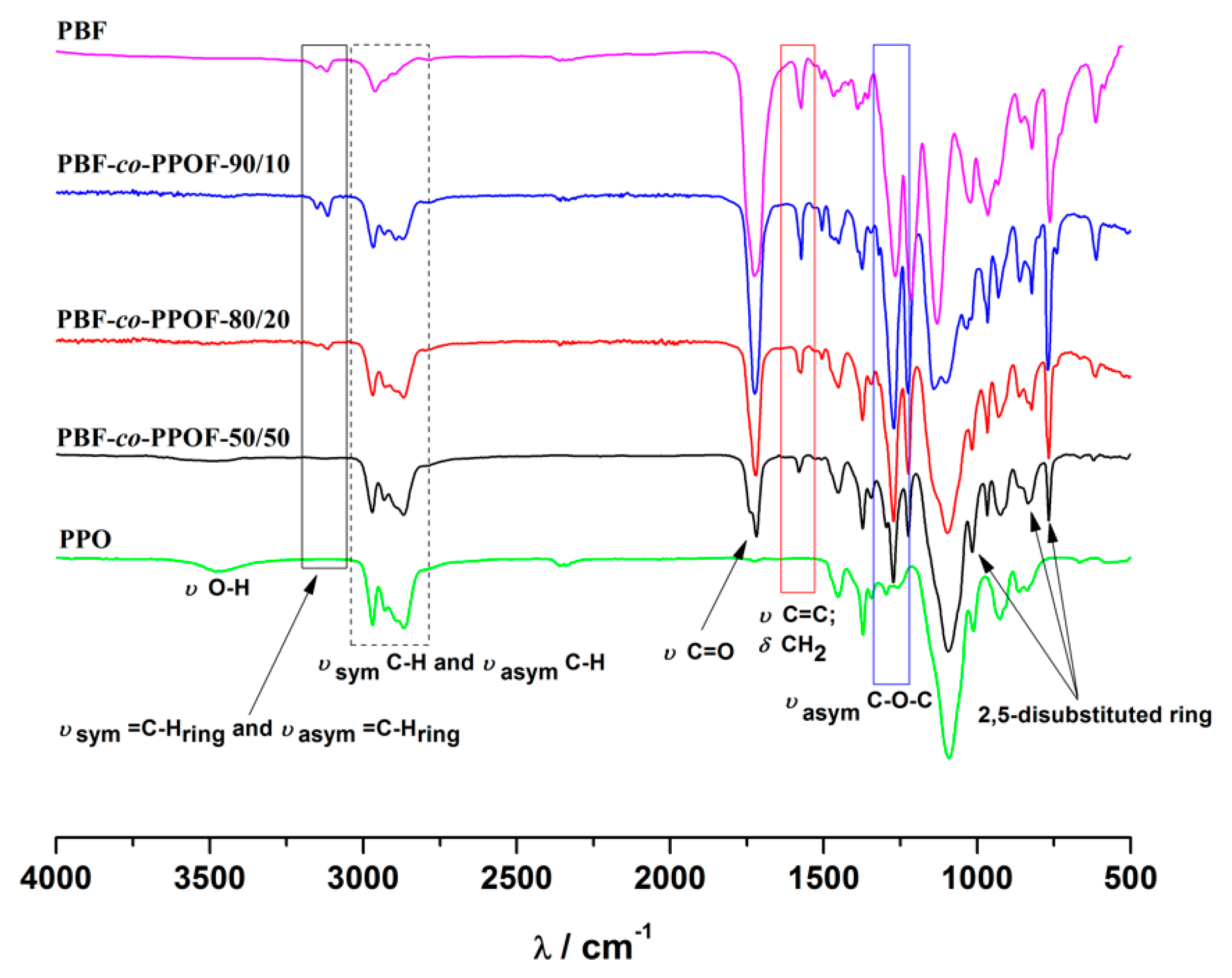
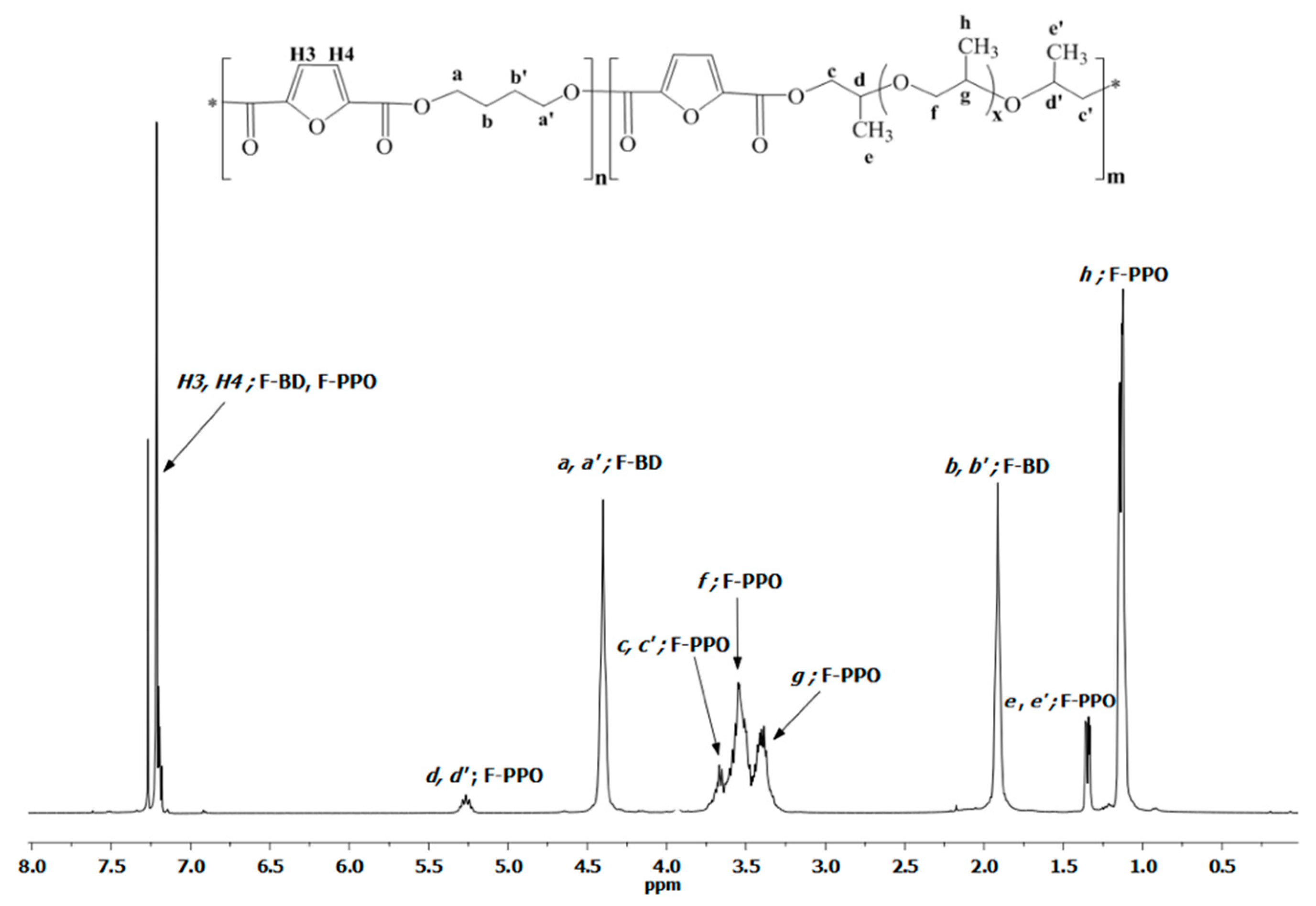
3.1. PBF-co-PPOF Co-Polyesters Synthesis and Structural Characterization
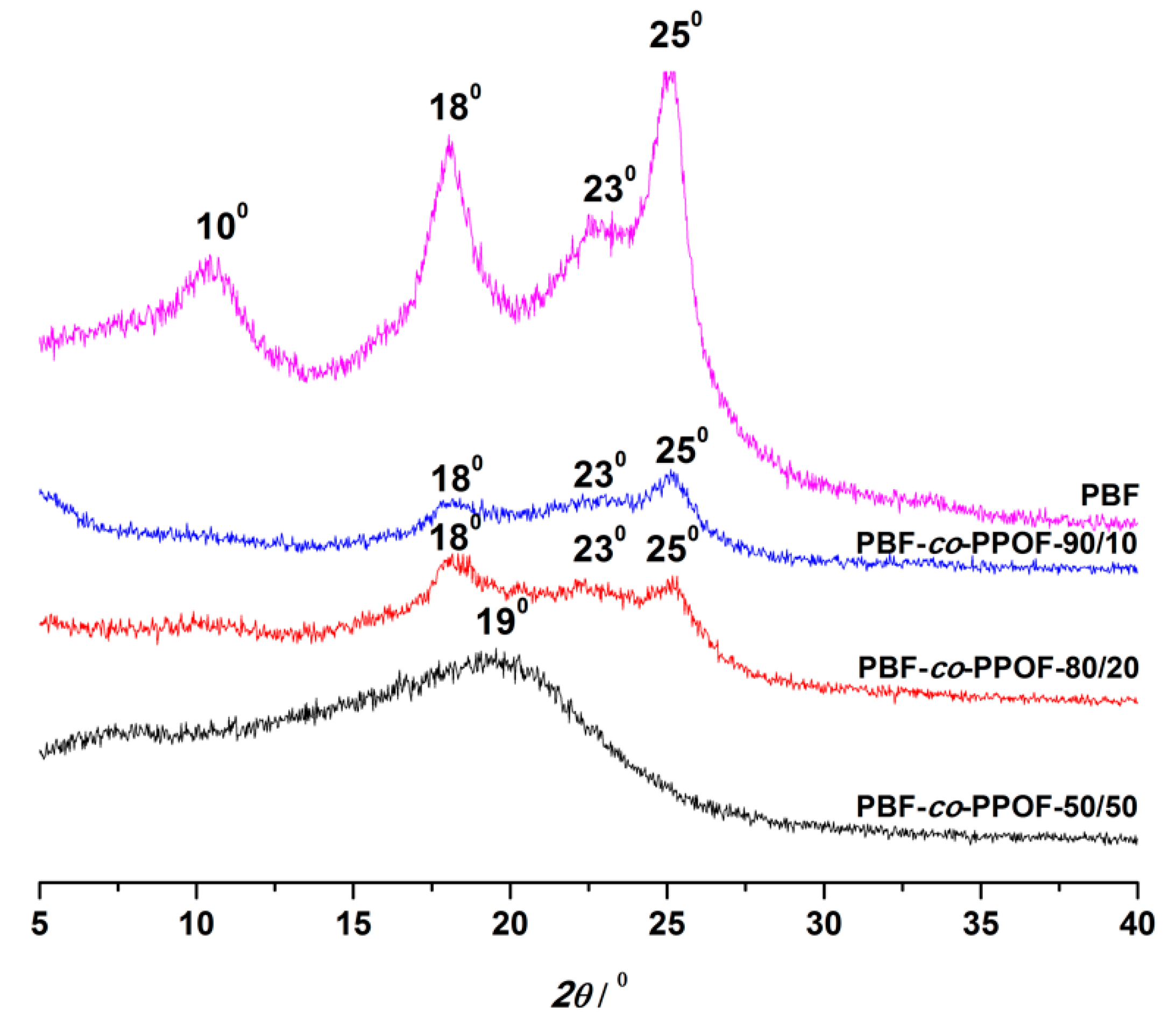
3.2. X-Ray Diffraction Analysis
3.3. Thermal Behaviur
3.4. Hydrolytic and Enzymatic Degradation Tests
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- De Jong, E.; Dam, M.A.; Sipos, L.; Gruter, G.-J. Furandicarboxylic acid (FDCA), a versatile building block for a very interesting class of polyesters. In Biobased Monomers, Polymers, and Materials; Smith, P.B., Gross, R.A., Eds.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2012; Volume 1105, pp. 1–13. ISBN 0-8412-2767-5. [Google Scholar]
- Jia, Z.; Wang, J.; Sun, L.; Zhu, J.; Liu, X. Fully bio-based polyesters derived from 2,5-furandicarboxylic acid (2,5-FDCA) and dodecanedioic acid (DDCA): From semicrystalline thermoplastic to amorphous elastomer. J. Appl. Polym. Sci. 2018, 135, 46076. [Google Scholar] [CrossRef]
- Gandini, A.; Silvestre, A.J.D.; Neto, C.P.; Sousa, A.F.; Gomes, M. The furan counterpart of poly(ethylene terephthalate): An alternative material based on renewable resources. J. Polym. Sci. Polym. Chem. 2009, 47, 295–298. [Google Scholar] [CrossRef]
- Sousa, A.F.A.F.; Vilela, C.; Fonseca, A.C.; Matos, M.; Freire, C.S.R.; Gruter, G.-J.M.; Coelho, J.F.J.; Silvestre, A.J.D. Biobased polyesters and other polymers from 2,5-furandicarboxylic acid: A tribute to furan excellency. Polym. Chem. 2015, 6, 5961–5983. [Google Scholar] [CrossRef]
- Zhang, D.; Dumont, M.J. Advances in polymer precursors and bio-based polymers synthesized from 5-hydroxymethylfurfural. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 1478–1492. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Papageorgiou, D.G.; Terzopoulou, Z.; Bikiaris, D.N. Production of bio-based 2,5-furan dicarboxylate polyesters: Recent progress and critical aspects in their synthesis and thermal properties. Eur. Polym. J. 2016, 83, 202–229. [Google Scholar] [CrossRef]
- Gomes, M.; Gandini, A.; Silvestre, A.J.D.; Reis, B. Synthesis and characterization of poly(2,5-furan dicarboxylate)s based on a variety of diols. J. Polym. Sci. Polym. Chem. 2011, 49, 3759–3768. [Google Scholar] [CrossRef]
- Sousa, A.F.; Matos, M.; Freire, C.S.R.; Silvestre, A.J.D.; Coelho, J.F.J. New copolyesters derived from terephthalic and 2,5-furandicarboxylic acids: A step forward in the development of biobased polyesters. Polymer 2013, 54, 513–519. [Google Scholar] [CrossRef]
- Matos, M.; Sousa, A.F.; Silvestre, A.J.D. Improving the thermal properties of poly(2,5-furandicarboxylate)s using cyclohexylene moieties: A comparative study. Macromol. Chem. Phys. 2017, 218, 1600492. [Google Scholar] [CrossRef]
- Soares, M.J.; Dannecker, P.-K.; Vilela, C.; Bastos, J.; Meier, M.A.R.; Sousa, A.F. Poly(1,20-eicosanediyl 2,5-furandicarboxylate), a biodegradable polyester from renewable resources. Eur. Polym. J. 2017, 90, 301–311. [Google Scholar] [CrossRef]
- Sousa, A.F.; Coelho, J.F.J.; Silvestre, A.J.D. Renewable-based poly((ether)ester)s from 2,5-furandicarboxylic acid. Polymer 2016, 98, 129–135. [Google Scholar] [CrossRef]
- Weinberger, S.; Canadell, J.; Quartinello, F.; Yeniad, B.; Arias, A.; Pellis, A.; Guebitz, G. Enzymatic degradation of poly(ethylene 2,5-furanoate) powders and amorphous films. Catalysts 2017, 7, 318. [Google Scholar] [CrossRef]
- Weinberger, S.; Haernvall, K.; Scaini, D.; Ghazaryan, G.; Zumstein, M.T.; Sander, M.; Pellis, A.; Guebitz, G.M. Enzymatic surface hydrolysis of poly(ethylene furanoate) thin films of various crystallinities. Green Chem. 2017, 19, 5381–5384. [Google Scholar] [CrossRef]
- Haernvall, K.; Zitzenbacher, S.; Amer, H.; Zumstein, M.T.; Sander, M.; McNeill, K.; Yamamoto, M.; Schick, M.B.; Ribitsch, D.; Guebitz, G.M. Polyol structure influences enzymatic hydrolysis of bio-based 2,5-furandicarboxylic acid (FDCA) polyesters. Biotechnol. J. 2017, 12, 1600741. [Google Scholar] [CrossRef] [PubMed]
- Gubbels, E.; Jasinska-Walc, L.; Koning, C.E. Synthesis and characterization of novel renewable polyesters based on 2,5-furandicarboxylic acid and 2,3-butanediol. J. Polym. Sci. Polym. Chem. 2013, 51, 890–898. [Google Scholar] [CrossRef]
- Thiyagarajan, S.; Vogelzang, W.; Knoop, R.J.; Frissen, A.E.; van Haveren, J.; van Es, D.S. Biobased furandicarboxylic acids (FDCAs): Effects of isomeric substitution on polyester synthesis and properties. Green Chem. 2014, 16, 1957. [Google Scholar] [CrossRef]
- Jiang, Y.; Woortman, A.J.J.; van Ekenstein, G.O.R.A.; Loos, K. A biocatalytic approach towards sustainable furanic-aliphatic polyesters. Polym. Chem. 2015, 6, 5198–5211. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Kasmi, N.; Tsanaktsis, V.; Doulakas, N.; Bikiaris, D.N.; Achilias, D.S.; Papageorgiou, G.Z. Synthesis and characterization of bio-based polyesters: Poly(2-methyl-1,3-propylene-2,5-furanoate), Poly(isosorbide-2,5-furanoate), Poly(1,4-cyclohexanedimethylene-2,5-furanoate). Materials 2017, 10, 801. [Google Scholar] [CrossRef]
- Tsanaktsis, V.; Terzopoulou, Z.; Exarhopoulos, S.; Bikiaris, D.N.; Achilias, D.S.; Papageorgiou, D.G.; Papageorgiou, G.Z. Sustainable, eco-friendly polyesters synthesized from renewable resources: Preparation and thermal characteristics of poly(dimethyl-propylene furanoate). Polym. Chem. 2015, 6, 8284–8296. [Google Scholar] [CrossRef]
- Genovese, L.; Lotti, N.; Siracusa, V.; Munari, A. Poly(neopentyl glycol furanoate): A member of the furan-based polyester family with smart barrier performances for sustainable food packaging applications. Materials 2017, 10, 1028. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, Y.; Xu, Y.; Wang, P.; Gao, L.; Zhang, W.; Ji, J. Synthesis and characterization of bio-based poly(butylene furandicarboxylate)-b-poly(tetramethylene glycol) copolymers. Polym. Degrad. Stab. 2014, 109, 21–26. [Google Scholar] [CrossRef]
- Sousa, A.F.; Guigo, N.; Pozycka, M.; Delgado, M.; Soares, J.; Mendonça, P.V.; Coelho, J.F.J.; Sbirrazzuoli, N.; Silvestre, A.J.D. Tailored design of renewable copolymers based on poly(1,4-butylene 2,5-furandicarboxylate) and poly(ethylene glycol) with refined thermal properties. Polym. Chem. 2018, 9, 722–731. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, R.; Sousa, A.; Long, Y.Y.; Ying, W.B.; Wang, J.; Zhu, J. Bio-based poly(butylene 2,5-furandicarboxylate)-b-poly(ethylene glycol) copolymers with adjustable degradation rate and mechanical properties: Synthesis and characterization. Eur Polym J 2018, 106, 42–52. [Google Scholar] [CrossRef]
- Chi, D.; Liu, F.; Na, H.; Chen, J.; Hao, C.; Zhu, J. Poly(neopentyl glycol 2,5-furandicarboxylate): A Promising Hard Segment for the Development of Bio-based Thermoplastic Poly(ether-ester) Elastomer with High Performance. ACS Sustain. Chem. Eng. 2018, 6, 9893–9902. [Google Scholar] [CrossRef]
- Deschamps, A.A.; Claase, M.B.; Sleijster, W.J.; Bruijn, J.D.; Grijpma, D.W.; Feijen, J. Design of segmented poly(ether ester) materials and structures. J. Control. Release 2002, 78, 175–186. [Google Scholar] [CrossRef]
- Buitinga, M.; Truckenmüller, R.; Engelse, M.A.; Moroni, L.; Ten Hoopen, H.W.; van Blitterswijk, C.A.; de Koning, E.J.; van Apeldoorn, A.A.; Karperien, M. Microwell Scaffolds for the Extrahepatic Transplantation of Islets of Langerhans. PLoS ONE 2013, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Octoplus PolyActiveTM A Biodegradable Polymer-Based Drug Delivery System. Available online: http://www.octoplus.nl/nl/Contact/News (accessed on 3 December 2018).
- Deschamps, A.A.; Grijpma, D.W.; Feijen, J. Poly(ethylene oxide)/poly(butylene terephthalate) segmented block copolymers: The effect of copolymer composition on physical properties and degradation behavior. Polymer 2001, 42, 9335–9345. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Jia, Z.; Liu, Y.; Sun, L.; Zhu, J. Synthesis of bio-based poly(ethylene 2,5-furandicarboxylate) copolyesters: Higher glass transition temperature, better transparency, and good barrier properties. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 3298–3307. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Zhu, J.; Jiang, Y. Copolyesters based on 2,5-furandicarboxylic acid (FDCA): Effect of 2,2,4,4-tetramethyl-1,3-cyclobutanediol units on their properties. Polymers 2017, 9, 305. [Google Scholar] [CrossRef]
- Gandini, A.; Coelho, D.; Gomes, M.; Reis, B.; Silvestre, A. Materials from renewable resources based on furan monomers and furan chemistry: Work in progress. J. Mater. Chem. 2009, 19, 8656. [Google Scholar] [CrossRef]
- Jiang, M.; Liu, Q.; Zhang, Q.; Ye, C.; Zhou, G. A series of furan-aromatic polyesters synthesized via direct esterification method based on renewable resources. J. Polym. Sci. Polym. Chem. 2012, 50, 1026–1036. [Google Scholar] [CrossRef]
- Ma, J.; Yu, X.; Xu, J.; Pang, Y. Synthesis and crystallinity of poly(butylene 2,5-furandicarboxylate). Polymer 2012, 53, 4145–4151. [Google Scholar] [CrossRef]
- Yao, C.; Yang, G. Crystallization, and morphology of poly(trimethylene terephthalate)/poly(ethylene oxide terephthalate) segmented block copolymers. Polymer 2010, 51, 1516–1523. [Google Scholar] [CrossRef]
- Szymczyk, A. Structure and properties of new polyester elastomers composed of poly(trimethylene terephthalate) and poly(ethylene oxide). Eur. Polym. J. 2009, 45, 2653–2664. [Google Scholar] [CrossRef]
- Allen, G.; Crossley, H.G. Polypropylene oxide IV-Preparation and properties of polyether networks. Polymer 1964, 5, 553–557. [Google Scholar] [CrossRef]
- Vesterinen, A.; Lipponen, S.; Rich, J.; Seppälä, J. Effect of block composition on thermal properties and melt viscosity of poly[2-(dimethylamino)ethyl methacrylate], poly(ethylene oxide) and poly(propylene oxide) block co-polymers. Express Polym. Lett. 2011, 5, 754–765. [Google Scholar] [CrossRef]
- Chao, G.T.; Fan, L.Y.; Jia, W.J.; Qian, Z.Y.; Gu, Y.C.; Liu, C.B.; Ni, X.P.; Li, J.; Deng, H.X.; Gong, C.Y.; et al. Synthesis, characterization and hydrolytic degradation of degradable poly(butylene terephthalate)/poly(ethylene glycol) (PBT/PEG) copolymers. J. Mater. Sci. Mater. Med. 2007, 18, 449–455. [Google Scholar] [CrossRef]

| (co)polymer | BD/PPOfeed 1 (mol%) | Yield (%) 2 | Mw3 | Đ4 |
|---|---|---|---|---|
| PBF | 100/0 | 71.0 | - | - 5 |
| PBF-co-PPOF-90/10 | 90/10 | 64.8 | 36 700 | 2.2 |
| PBF-co-PPOF-80/20 | 80/20 | 68.1 | 41 600 | 2.2 |
| PBF-co-PPOF-50/50 | 50/50 | 71.4 | 48 500 | 2.1 |
| δ/ppm | Assignment | Diads | Integration Area | ||||
|---|---|---|---|---|---|---|---|
| PBF | PBF-co-PPOF | PPO | |||||
| 90/10 | 80/20 | 50/50 | |||||
| 7.21 | H3 and H4; CH | F-BD; FDCA-PPO | 1.00 | 1.00 | 1.00 | 1.00 | |
| 5.26 | d, d′; CHCH3 | F-PPO | – | 0.14 | 0.37 | 0.92 | |
| 4.40 | a, a′; C(O)OCH2CH2 | F-BD | 2.01 | 1.66 | 1.28 | 0.46 | |
| 3.67 | c, c′; C(O)OCH2 | F-PPO | – | 0.30 | 0.84 | 1.84 | |
| 3.54 | f; OCH2 | PPO-PPO | – | 1.91 | 5.14 | 17.89 | 2.01 |
| 3.40 | g; OCH | PPO-PPO | – | 1.03 | 2.77 | 9.46 | 1.00 |
| 1.91 | b, b′; C(O)OCH2CH2 | F-BD | 2.01 | 1.66 | 1.41 | 0.48 | |
| 1.34 | e, e′; CHCH3 | F-PPO | 0.44 | 1.30 | 3.04 | ||
| 1.15 | h; OCHCH3 | PPO-PPO | – | 3.10 | 8.60 | 28.50 | 3.02 |
| (co)polymer | PBF/PPOFfeed 1 | PBF/PPOFreal 2 | Ln,BF3 |
|---|---|---|---|
| PBF | 100/0 | 100/0 | – |
| PBF-co-PPOF-90/10 | 90/10 | 85/15 | 6.5 |
| PBF-co-PPOF-80/20 | 80/20 | 76/24 | 4.2 |
| PBF-co-PPPOF-50/50 | 50/50 | 20/80 | 1.3 |
| Assignment | Diads | Integration Area | ||||
|---|---|---|---|---|---|---|
| PBF | PBF-co-PPOF | PPO | ||||
| 90/10 | 80/20 | 50/50 | ||||
| 2-CO and 5-CO; C(O)O | F-BD; F-PPO | 158.0 | 158.0 | 158.0 | 158.0 | - |
| C2 and C5; C-C(O)O | F-BD; F-PPO | 146.8 | 146.8 | 146.8 | 147.0 | - |
| C3 and C4; C-H | F-BD; F-PPO | 118.5 | 118.5 | 118.5 | 118.2 | - |
| f; OCH2 | PPO-PPO | - | 75.4 | 75.3 | 75.3 | 75.3 |
| g; OCH | PPO-PPO | - | 73.4 | 73.4 | 73.4 | 74.4 |
| c, c′; C(O)OCH2 | F-PPO | - | 72.9 | 72.9 | 72.9 | - |
| d, d′; CHCH3 | F-PPO | - | 71.7 | 71.7 | 71.3 | - |
| a, a′; C(O)OCH2CH2 | F-BD | 64.9 | 64.9 | 64.9 | 64.8 | - |
| b, b′; C(O)OCH2CH2 | F-BD | 25.4 | 25.4 | 25.3 | 25.0 | - |
| h ; OCHCH3 | PPO-PPO | - | 17.3 | 17.3 | 17.3 | 17.3 |
| e, e′; CHCH3 | F-PPO | - | 16.9 | 16.8 | 16.8 | - |
| co(polymer) | DSC 1 | DMTA 2 | TGA 3 | ||
|---|---|---|---|---|---|
| Tm/°C | Tg/°C | Tm/°C | Td,5%/°C | Td,max/°C | |
| PBF | 170.0 | 75.6 | 166.9 | 348.7 | 380.5 |
| PBF-co-PPOF-90/10 | 137.7 | −32.6 | 147.6 | 284.7 | 347.2 |
| PBF-co-PPOF-80/20 | 124.1 | −37.2 | 124.2 | 288.9 | 340.3 |
| PBF-co-PPOF-50/50 | - | −42.3 | - 3 | 308.1 | 365.2 |
| PPO | −67.0–72.0 5 | - | 283.0 | 323.4 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matos, M.; Sousa, A.F.; Mendonça, P.V.; Silvestre, A.J.D. Co-Polymers based on Poly(1,4-butylene 2,5-furandicarboxylate) and Poly(propylene oxide) with Tuneable Thermal Properties: Synthesis and Characterization. Materials 2019, 12, 328. https://doi.org/10.3390/ma12020328
Matos M, Sousa AF, Mendonça PV, Silvestre AJD. Co-Polymers based on Poly(1,4-butylene 2,5-furandicarboxylate) and Poly(propylene oxide) with Tuneable Thermal Properties: Synthesis and Characterization. Materials. 2019; 12(2):328. https://doi.org/10.3390/ma12020328
Chicago/Turabian StyleMatos, Marina, Andreia F. Sousa, Patrícia V. Mendonça, and Armando J. D. Silvestre. 2019. "Co-Polymers based on Poly(1,4-butylene 2,5-furandicarboxylate) and Poly(propylene oxide) with Tuneable Thermal Properties: Synthesis and Characterization" Materials 12, no. 2: 328. https://doi.org/10.3390/ma12020328
APA StyleMatos, M., Sousa, A. F., Mendonça, P. V., & Silvestre, A. J. D. (2019). Co-Polymers based on Poly(1,4-butylene 2,5-furandicarboxylate) and Poly(propylene oxide) with Tuneable Thermal Properties: Synthesis and Characterization. Materials, 12(2), 328. https://doi.org/10.3390/ma12020328








