A Precautionary Approach to Guide the Use of Transition Metal-Based Nanotechnology to Prevent Orthopedic Infections
Abstract
:1. Introduction
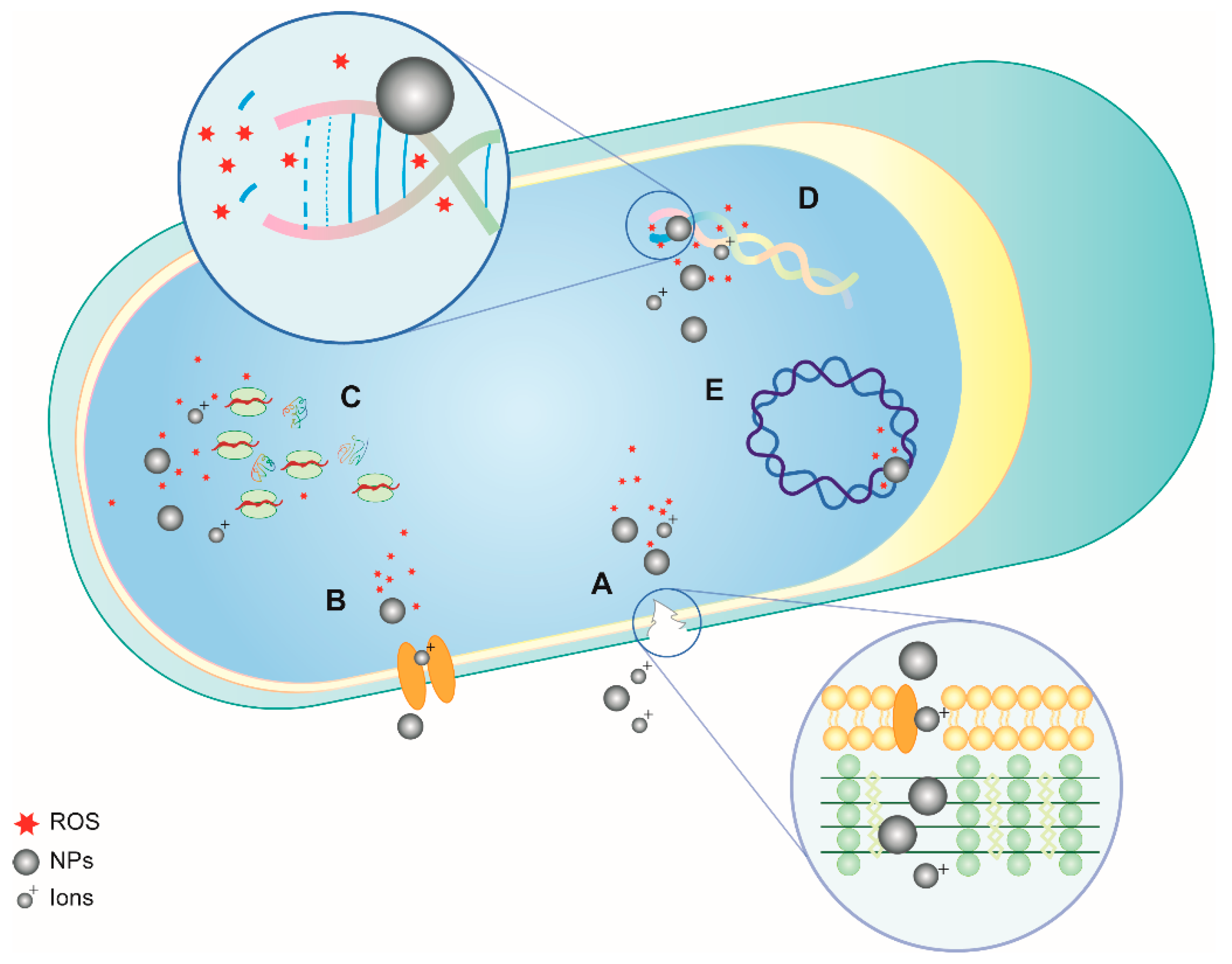
2. Antibacterial Properties of NPs: Mechanisms of Action
3. Transition Metal NPs with Antimicrobial Activity for Potential Use in Orthopedics
3.1. Silver
3.2. Gold
3.3. Copper
3.4. Titanium
3.5. Zinc
3.6. Zirconium
3.7. Iron
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pram. Ther. 2015, 40, 277–283. [Google Scholar]
- Pechère, J.C. Patients’ interviews and misuse of antibiotics. Clin. Infect. Dis. 2001, 15, S170–S173. [Google Scholar] [CrossRef] [PubMed]
- Norrby, S.R.; Nord, C.E.; Finch, R. European Society of Clinical Microbiology and Infectious Diseases. Lack of development of new antimicrobial drugs: A potential serious threat to public health. Lancet Infect. Dis. 2005, 5, 115–119. [Google Scholar] [CrossRef]
- Ribeiro, M.; Monteiro, F.J.; Ferraz, M.P. Infection of orthopedic implants with emphasis on bacterial adhesion process and techniques used in studying bacterial-material interactions. Biomatter 2012, 2, 176–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beyth, N.; Houri-Haddad, Y.; Domb, A.; Khan, W.; Hazan, R. Alternative antimicrobial approach: Nano-antimicrobial materials. Evid. Based Complement. Alternat. Med. 2015, 2015, 246012. [Google Scholar] [CrossRef] [PubMed]
- Peersman, G.; Laskin, R.; Davis, J.; Peterson, M. Infection in total knee replacement: A retrospective review of 6489 total knee replacements. Clin. Orthop. Relat. Res. 2001, 392, 15–23. [Google Scholar] [CrossRef]
- Marculescu, C.E.; Berbari, E.F.; Hanssen, A.D.; Steckelberg, J.M.; Harmsen, S.W.; Mandrekar, J.N.; Osmon, D.R. Outcome of prosthetic joint infections treated with debridement and retention of components. Clin. Infect. Dis. 2006, 42, 471–478. [Google Scholar] [CrossRef]
- Trampuz, A.; Zimmerli, W. Diagnosis and treatment of implant-associated septic arthritis and osteomyelitis. Curr. Infect. Dis. Rep. 2008, 10, 394–403. [Google Scholar] [CrossRef]
- Gristina, A.G.; Naylor, P.T.; Myrvik, Q. The race for the surface: Microbes, tissue cells, and biomaterials. In Molecular Mechanisms of Microbial Adhesion, 1st ed.; Switalski, L., Höök, M., Beachey, E., Eds.; Springer: New York, NY, USA, 1989; pp. 177–211. ISBN 978-1-4612-8169-6. [Google Scholar]
- Koseki, H.; Yonekura, A.; Shida, T.; Yoda, I.; Horiuchi, H.; Morinaga, Y.; Yanagihara, K.; Sakoda, H.; Osaki, M.; Tomita, M. Early staphylococcal biofilm formation on solid orthopaedic implant materials: In vitro study. PLoS ONE 2014, 9, e107588. [Google Scholar] [CrossRef]
- Kırmusaoğlu, S. Staphylococcal biofilms: Pathogenicity, mechanism and regulation of biofilm formation by quorum-sensing system and antibiotic resistance mechanisms of biofilm-embedded microorganisms. In Microbial Biofilms Importance and Applications, 1st ed.; Dhanasekaran, D., Ed.; IntechOpen: London, UK, 2016; ISBN 978-953-51-2436-8. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef]
- Santos, C.L.; Albuquerque, A.J.R.; Sampaio, F.C.; Keyson, D. Nanomaterials with Antimicrobial Properties: Applications in Health Sciences. In Microbial Pathogens and Strategies for Combating Them: Science, Technology and Education, 1st ed.; Méndez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2013; pp. 143–154. ISBN 978-84-939843-9-7. [Google Scholar]
- Li, X.; Robinson, S.M.; Gupta, A.; Saha, K.; Jiang, Z.; Moyano, D.F.; Sahar, A.; Riley, M.A.; Rotello, V.M. Functional gold nanoparticles as potent antimicrobial agents against multi-drug-resistant bacteria. ACS Nano 2014, 8, 10682–10686. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.M.; Fouad, S.A.; Elshoky, H.A.; Mohammed, G.M.; Salaheldin, T.A. Antibacterial effect of gold nanoparticles against Corynebacterium pseudotuberculosis. Int. J. Vet. Sci. Med. 2017, 5, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Markowska, K.; Grudniak, A.M.; Wolska, K.I. Silver nanoparticles as an alternative strategy against bacterial biofilms. Acta Biochim. Pol. 2013, 60, 523–530. [Google Scholar] [PubMed]
- Yu, Q.; Li, J.; Zhang, Y.; Wang, Y.; Liu, L.; Li, M. Inhibition of gold nanoparticles (Au NPs) on pathogenic biofilm formation and invasion to host cells. Sci. Rep. 2016, 25, 26667. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Wang, C.; Hou, J.; Wang, P.; Ao, Y.; Li, Y.; Geng, N.; Yao, Y.; Lv, B.; Yang, Y.; et al. Aggregation and removal of copper oxide (CuO) nanoparticles in wastewater environment and their effects on the microbial activities of wastewater biofilms. Bioresour. Technol. 2016, 216, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 14, 1227–1249. [Google Scholar] [CrossRef] [PubMed]
- Mazaheri, M.; Eslahi, N.; Ordikhani, F.; Tamjid, E.; Simchi, A. Nanomedicine applications in orthopedic medicine: State of the art. Int. J. Nanomed. 2015, 28, 6039–6053. [Google Scholar] [CrossRef]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver nanoparticles as potential antibacterial agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef] [PubMed]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic Basis of Antimicrobial Actions of Silver Nanoparticles. Front. Microbiol. 2016, 16, 1–17. [Google Scholar] [CrossRef]
- Gottenbos, B.; Grijpma, D.W.; van der Mei, H.C.; Feijen, J.; Busscher, H.J. Antimicrobial effects of positively charged surfaces on adhering Gram-positive and Gram-negative bacteria. J. Antimicrob. Chemother. 2001, 48, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Lara, H.H.; Ayala-Nuñez, N.V.; Ixtepan-Turrent, L.; Rodriguez-Padilla, C. Bactericidal effect of silver nanoparticles against multidrug-resistant bacteria. World J. Microbiol. Biotechnol. 2010, 26, 615–621. [Google Scholar] [CrossRef]
- Qing, Y.; Cheng, L.; Li, R.; Liu, G.; Zhang, Y.; Tang, X.; Wang, J.; Liu, H.; Qin, Y. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int. J. Nanomed. 2018, 5, 3311–3327. [Google Scholar] [CrossRef] [PubMed]
- Flores, C.Y.; Miñán, A.G.; Grillo, C.A.; Salvarezza, R.C.; Vericat, C.; Schilardi, P.L. Citrate-capped silver nanoparticles showing good bactericidal effect against both planktonic and sessile bacteria and a low cytotoxicity to osteoblastic cells. ACS Appl. Mater. Interfaces 2013, 5, 3149–3159. [Google Scholar] [CrossRef] [PubMed]
- Clement, J.L.; Jarrett, P.S. Antibacterial silver. Met. Based Drugs 1994, 1, 467–482. [Google Scholar] [CrossRef] [PubMed]
- Panácek, A.; Kolár, M.; Vecerová, R.; Prucek, R.; Soukupová, J.; Krystof, V.; Hamal, P.; Zboril, R.; Kvítek, L. Antifungal activity of silver nanoparticles against Candida spp. Biomaterials 2009, 30, 6333–6340. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Braekling, T.; Streitbuerger, A.; Gosheger, G.; Boettner, F.; Nottrott, M.; Ahrens, H.; Dieckmann, R.; Guder, W.; Andreou, D.; Hauschild, G.; et al. Silver-coated megaprostheses: Review of the literature. Eur. J. Orthop. Surg. Traumatol. 2017, 27, 483–489. [Google Scholar] [CrossRef]
- Konop, M.; Damps, T.; Misicka, A.; Rudnicka, L. Certain Aspects of Silver and Silver Nanoparticles in Wound Care: A Minireview. J. Nanomater. 2016, 2016, 1–10. [Google Scholar] [CrossRef]
- Burdușel, A.C.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical Applications of Silver Nanoparticles: An Up-to-Date Overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef]
- Cavaliere, E.; De Cesari, S.; Landini, G.; Riccobono, E.; Pallecchi, L.; Rossolini, G.M.; Gavioli, L. Highly bactericidal Ag nanoparticle films obtained by cluster beam deposition. Nanomedicine 2015, 11, 1417–1423. [Google Scholar] [CrossRef] [Green Version]
- Benetti, G.; Cavaliere, E.; Brescia, R.; Salassi, S.; Ferrando, R.; Vantomme, A.; Pallecchi, L.; Pollini, S.; Boncompagni, S.; Fortuni, B.; et al. Tailored multi-elemental Nanoparticles for wide spectrum antibacterial coatings. Nanoscale 2019. [Google Scholar] [CrossRef]
- Cavaliere, E.; Benetti, G.; Van Bael, M.; Winckelmans, N.; Bals, S.; Gavioli, L. Exploring the Optical and Morphological Properties of Ag and Ag/TiO₂ Nanocomposites Grown by Supersonic Cluster Beam Deposition. Nanomaterials 2017, 13, 442. [Google Scholar] [CrossRef] [PubMed]
- Benetti, G.; Caddeo, C.; Melis, C.; Ferrini, G.; Giannetti, C.; Winckelmans, N.; Bals, S.; Van Bael, M.J.; Cavaliere, E.; Gavioli, L.; et al. Bottom-Up Mechanical Nanometrology of Granular Ag Nanoparticles Thin Films. J. Phys. Chem. C 2017, 121, 22434–22441. [Google Scholar] [CrossRef]
- Benetti, G.; Cavaliere, E.; Canteri, A.; Landini, G.; Rossolini, G.M.; Pallecchi, L.; Chiodi, M.; Van Bael, M.J.; Winckelmans, N.; Bals, S.; et al. Direct synthesis of antimicrobial coatings based on tailored bi-elemental nanoparticles. APL Mater. 2017, 5, 036105. [Google Scholar] [CrossRef] [Green Version]
- Peli, S.; Cavaliere, E.; Benetti, G.; Gandolfi, M.; Chiodi, M.; Cancellieri, C.; Giannetti, C.; Ferrini, G.; Gavioli, L.; Banfi, F. Mechanical Properties of Ag Nanoparticle Thin Films Synthesized by Supersonic Cluster Beam Deposition. J. Phys. Chem. C 2016, 120, 4673–4681. [Google Scholar] [CrossRef]
- Karak, N. Silver Nanomaterials and Their Polymer Nanocomposites. In Nanomaterials and Polymer Nanocomposites, 1st ed.; Karak, N., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 47–89. ISBN 978-0-12-814615-6. [Google Scholar]
- Tran, Q.H.; Nguyen, V.Q.; Le, A.T. Silver nanoparticles: Synthesis, properties, toxicology, applications and perspectives. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4, 033001. [Google Scholar] [CrossRef]
- Raza, M.A.; Kanwal, Z.; Rauf, A.; Sabri, A.N.; Riaz, S.; Naseem, S. Size- and shape-dependent antibacterial studies of silver nanoparticles synthesized by wet chemical routes. Nanomaterials 2016, 6, 74. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the Gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 95–101. [Google Scholar] [CrossRef]
- Naqvi, S.Z.; Kiran, U.; Ali, M.I.; Jamal, A.; Hameed, A.; Ahmed, S.; Ali, N. Combined efficacy of biologically synthesized silver nanoparticles and different antibiotics against multidrug-resistant bacteria. Int. J. Nanomed. 2013, 8, 3187–3195. [Google Scholar] [CrossRef]
- Randall, C.P.; Gupta, A.; Jackson, N.; Busse, D.; O’Neill, A.J. Silver resistance in Gram-negative bacteria: A dissection of endogenous and exogenous mechanisms. J. Antimicrob. Chemother. 2015, 70, 1037–1046. [Google Scholar] [CrossRef]
- Gupta, A.; Matsui, K.; Lo, J.F.; Silver, S. Molecular basis for resistance to silver cations in Salmonella. Nat. Med. 1999, 5, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Panáček, A.; Kvítek, L.; Smékalová, M.; Večeřová, R.; Kolář, M.; Röderová, M.; Dyčka, F.; Šebela, M.; Prucek, R.; Tomanec, O.; et al. Bacterial resistance to silver nanoparticles and how to overcome it. Nat. Nanotechnol. 2017, 13, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Cameron, S.J.; Hosseinian, F.; Willmore, W.G. A Current Overview of the Biological and Cellular Effects of Nanosilver. Int. J. Mol. Sci. 2018, 12, 2030. [Google Scholar] [CrossRef] [PubMed]
- Albers, C.E.; Hofstetter, W.; Siebenrock, K.A.; Landmann, R.; Klenke, F.M. In vitro cytotoxicity of silver nanoparticles on osteoblasts and osteoclasts at antibacterial concentrations. Nanotoxicology 2013, 7, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Castiglioni, S.; Cazzaniga, A.; Locatelli, L.; Maier, J.A.M. Silver Nanoparticles in Orthopedic Applications: New Insights on Their Effects on Osteogenic Cells. Nanomaterials 2017, 27, 124. [Google Scholar] [CrossRef]
- Flores-López, L.Z.; Espinoza-Gómez, H.; Somanathan, R. Silver nanoparticles: Electron transfer, reactive oxygen species, oxidative stress, beneficial and toxicological effects. Mini review. J. Appl. Toxicol. 2019, 39, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Politano, A.D.; Campbell, K.T.; Rosenberger, L.H.; Sawyer, R.G. Use of silver in the prevention and treatment of infections: Silver review. Surg. Infect. 2013, 14, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhao, Y.; Tian, Y.; Zhang, W.; Lü, X.; Jiang, X. The molecular mechanism of action of bactericidal gold nanoparticles on Escherichia coli. Biomaterials 2012, 33, 2327–2333. [Google Scholar] [CrossRef]
- Zhang, Y.; Shareena Dasari, T.P.; Deng, H.; Yu, H. Antimicrobial Activity of Gold Nanoparticles and Ionic Gold. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2015, 33, 286–327. [Google Scholar] [CrossRef]
- Grzelczak, M.; Pérez-Juste, J.; Mulvaney, P.; Liz-Marzán, L.M. Shape control in gold nanoparticles synthesis. Chem. Soc. Rev. 2008, 37, 1783–1791. [Google Scholar] [CrossRef]
- Wani, I.A.; Ahmad, T.; Manzoor, N. Size and shape dependant antifungal activity of gold nanoparticles: A case study of Candida. Colloids Surf. B Biointerfaces 2013, 1, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Norman, R.S.; Stone, J.W.; Gole, A.; Murphy, C.J.; Sabo-Attwood, T.L. Targeted photothermal lysis of the pathogenic bacteria, Pseudomonas aeruginosa, with gold nanorods. Nano Lett. 2008, 8, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Nirmala, G.A.; Pandian, K. Antibacterial efficacy of aminoglycosidic antibiotics protected gold nanoparticles-A brief study. Colloids Surf. A Physicochem. Eng. Asp. 2007, 297, 63–70. [Google Scholar] [CrossRef]
- Zawrah, M.F.; El-Moez, S.I.A. Antimicrobial activities of gold nanoparticles against major foodborne pathogens. Life Sci. J. 2011, 8, 37–44. [Google Scholar]
- Tsai, S.W.; Liaw, J.W.; Kao, Y.C.; Huang, M.Y.; Lee, C.Y.; Rau, L.R.; Huang, C.Y.; Wei, K.C.; Ye, T.C. Internalized gold nanoparticles do not affect the osteogenesis and apoptosis of MG63 osteoblast-like cells: A quantitative, in vitro study. PLoS ONE 2013, 8, e76545. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, D.; Zhang, J.; Fong, C.; Yang, M. Gold nanoparticles stimulate differentiation and mineralization of primary osteoblasts through the ERK/MAPK signaling pathway. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 42, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, J.J.; Zhang, J.; Wang, X.; Kawazoe, N.; Chen, G. Gold nanoparticle size and shape influence on osteogenesis of mesenchymal stem cells. Nanoscale 2016, 8, 7992–8007. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Qian, S.; Qiao, Y.; Liu, X. Cytocompatibility and antibacterial activity of titania nanotubes incorporated with gold nanoparticles. Colloids Surf. B Biointerfaces 2016, 1, 597–606. [Google Scholar] [CrossRef]
- Liu, R.; Memarzadeh, K.; Chang, B.; Zhang, Y.; Ma, Z.; Allaker, R.P.; Ren, L.; Yang, K. Antibacterial effect of copper-bearing titanium alloy (Ti-Cu) against Streptococcus mutans and Porphyromonas gingivalis. Sci. Rep. 2016, 6, 29985. [Google Scholar] [CrossRef] [Green Version]
- Goudouri, O.M.; Kontonasaki, E.; Lohbauer, U.; Boccaccini, A.R. Antibacterial properties of metal and metalloid ions in chronic periodontitis and peri-implantitis therapy. Acta Biomater. 2014, 10, 3795–3810. [Google Scholar] [CrossRef]
- Su, Y.; Zheng, X.; Chen, Y.; Li, M.; Liu, K. Alteration of intracellular protein expressions as a key mechanism of the deterioration of bacterial denitrification caused by copper oxide nanoparticles. Sci. Rep. 2015, 5, 15824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.; Zhou, Y.; Xu, M.; Han, P.; Chen, L.; Chang, J.; Xiao, Y. Copper-containing mesoporous bioactive glass scaffolds with multifunctional properties of angiogenesis capacity, osteostimulation and antibacterial activity. Biomaterials 2013, 34, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, E.; Ahmed, R.A. Synthesis of Copper Nanoparticles with Various Sizes and Shapes: Application as a Superior Non-Enzymatic Sensor and Antibacterial Agent. Int. J. Electrochem. Sci. 2016, 11, 4712–4723. [Google Scholar] [CrossRef]
- EL-Mekkawia, D.M.; Selima, M.M.; Nehad Hamdib, M.M.; Hassanc, S.A.; Ezzatc, A. Studies on the influence of the physicochemical characteristics of nanostructured copper, zinc and magnesium oxides on their antibacterial activities. J. Environ. Chem. Eng. 2018, 6, 5608–5615. [Google Scholar] [CrossRef]
- Ren, G.; Hu, D.; Cheng, E.W.C.; Vargas-Reus, M.A.; Reip, P.; Allaker, R.P. Characterisation of copper oxide nanoparticles for antimicrobial applications. Int. J. Antimicrob. Agents 2009, 33, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Eshed, M.; Lellouche, J.; Matalon, S.; Gedanken, A.; Banin, E. Sonochemical coatings of ZnO and CuO nanoparticles inhibit Streptococcus mutans biofilm formation on teeth model. Langmuir 2012, 28, 12288–12295. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, J.; Wang, X.; Wang, Y.; Hang, R.; Huang, X.; Tang, B.; Chu, P.K. Effects of copper nanoparticles in porous TiO2 coatings on bacterial resistance and cytocompatibility of osteoblasts and endothelial cells. Mater. Sci. Eng. C 2018, 1, 110–120. [Google Scholar] [CrossRef]
- Shi, F.; Liu, Y.; Zhi, W.; Xiao, D.; Li, H.; Duan, K.; Qu, S.; Weng, J. The synergistic effect of micro/nano-structured and Cu2+-doped hydroxyapatite particles to promote osteoblast viability and antibacterial activity. Biomed. Mater. 2017, 12, 035006. [Google Scholar] [CrossRef]
- Itabashi, T.; Narita, K.; Ono, A.; Wada, K.; Tanaka, T.; Kumagai, G.; Yamauchi, R.; Nakane, A.; Ishibashi, Y. Bactericidal and antimicrobial effects of pure titanium and titanium alloy treated with short-term, low-energy UV irradiation. Bone Jt. Res. 2017, 6, 108–112. [Google Scholar] [CrossRef] [Green Version]
- Koseki, H.; Asahara, T.; Shida, T.; Yoda, I.; Horiuchi, H.; Baba, K.; Osaki, M. Clinical and histomorphometrical study on titanium dioxide-coated external fixation pins. Int. J. Nanomed. 2013, 8, 593–599. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Yang, F.; Yang, X. Excellent antimicrobial properties of mesoporous anatase TiO2 and Ag/TiO2 composite films. Microporous Mesoporous Mater. 2008, 114, 431–439. [Google Scholar] [CrossRef]
- Foster, H.A.; Ditta, I.B.; Varghese, S.; Steele, A. Photocatalytic disinfection using titanium dioxide: Spectrum and mechanism of antimicrobial activity. Appl. Microbiol. Biotechnol. 2011, 90, 1847–1868. [Google Scholar] [CrossRef] [PubMed]
- Visai, L.; De Nardo, L.; Punta, C.; Melone, L.; Cigada, A.; Imbriani, M.; Arciola, C.R. Titanium oxide antibacterial surfaces in biomedical devices. Int. J. Artif. Organs 2011, 34, 929–946. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, K.; Mori, M.; Yoshida, M.; Oikawa, S.; Kawanishi, S. Photo-irradiated titanium dioxide catalyzes site specific DNA damage via generation of hydrogen peroxide. Free Radic. Res. 2004, 38, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.J.; Lewallen, E.A.; Trousdale, W.H.; Xu, W.; Thaler, R.; Salib, C.G.; Reina, N.; Abdel, M.P.; Lewallen, D.G.; van Wijnen, A.J. Local cellular responses to titanium dioxide from orthopedic implants. Biores. Open Access 2017, 6, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Hanley, C.; Thurber, A.; Hanna, C.; Punnoose, A.; Zhang, J.; Wingett, D.G. The Influences of Cell Type and ZnO Nanoparticle Size on Immune Cell Cytotoxicity and Cytokine Induction. Nanoscale Res. Lett. 2009, 4, 1409–1420. [Google Scholar] [CrossRef] [PubMed]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nanomicro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blencowe, D.K.; Morby, A.P. Zn(II) metabolism in prokaryotes. FEMS Microbiol. Rev. 2003, 27, 291–311. [Google Scholar] [CrossRef] [Green Version]
- Hobman, J.L.; Crossman, L.C. Bacterial antimicrobial metal ion resistance. J. Med. Microbiol. 2015, 64, 471–497. [Google Scholar] [CrossRef] [Green Version]
- Jones, N.; Ray, B.; Ranjit, K.T.; Manna, A.C. Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol. Lett. 2008, 279, 71–76. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; He, Y.; Irwin, P.L.; Jin, T.; Shi, X. Antibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylobacter jejuni. Appl. Environ. Microbiol. 2011, 77, 2325–2331. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.M.; Feris, K.; Bell, J.; Wingett, D.G.; Hanley, C.; Punnoose, A. Selective toxicity of zinc oxide nanoparticles to prokaryotic and eukaryotic systems. Appl. Phys. Lett. 2007, 24, 2139021–2139023. [Google Scholar] [CrossRef] [PubMed]
- Memarzadeh, K.; Sharili, A.S.; Huang, J.; Rawlinson, S.C.; Allaker, R.P. Nanoparticulate zinc oxide as a coating material for orthopedic and dental implants. J. Biomed. Mater. Res. A 2015, 103, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Sasidharan, A.; Divya Rani, V.V.; Menon, D.; Nair, S.; Manzoor, K.; Raina, S. Role of size scale of ZnO nanoparticles and microparticles on toxicity toward bacteria and osteoblast cancer cells. J. Mater. Sci. Mater. Med. 2009, 20, S235–S241. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, A.; Banerjee, S.L.; Pramanik, N.; Jana, P.; Mitra, T.; Gnanamani, A.; Das, M.; Kundu, P.P. Organically modified clay supported chitosan/hydroxyapatite-zinc oxide nanocomposites with enhanced mechanical and biological properties for the application in bone tissue engineering. Int. J. Biol. Macromol. 2018, 106, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Gowri, S.; Gandhi, R.R.; Sundrarajan, M. Structural, optical, antibacterial and antifungal properties of zirconia nanoparticles by biobased protocol. J. Mater. Sci. Mater. Med. 2014, 30, 782–790. [Google Scholar] [CrossRef]
- Clarke, I.C.; Green, D.D.; Pezzoti, G.; Donaldson, D. 20 Year Experience of Zirconia Total Hip Replacements. In Bioceramics and Alternative Bearings in Joint Arthroplasty, 1st ed.; D’Antonio, J.A., Dietrich, M., Eds.; Steinkopff: Dresden, Germany, 2005; pp. 67–78. ISBN 978-3-7985-1518-5. [Google Scholar]
- Oetzel, C.; Clasen, R. Preparation of zirconia dental crowns via electrophoretic deposition. J. Mater. Sci. Mater. 2006, 41, 8130–8137. [Google Scholar] [CrossRef]
- Assal, P.A. The osseointegration of zirconia dental implants. Schweiz Monatsschr. Zahnmed. 2013, 123, 644–654. [Google Scholar] [PubMed]
- Gaihrea, B.; Jayasuriya, A.C. Comparative investigation of porous nano-hydroxyapaptite/chitosan, nano-zirconia/chitosan and novel nano-calcium zirconate/chitosan composite scaffolds for their potential applications in bone regeneration. Mater. Sci. Eng. C 2018, 91, 330–339. [Google Scholar] [CrossRef]
- Jangra, S.L.; Stalin, K.; Dilbaghi, N.; Kumar, S.; Tawale, J.; Singh, S.P.; Pasricha, R. Antimicrobial activity of zirconia (ZrO2) nanoparticles and zirconium complexes. J. Nanosci. Nanotechnol. 2012, 12, 7105–7112. [Google Scholar] [CrossRef]
- Fathima, J.B.; Pugazhendhi, A.; Venis, R. Synthesis and characterization of ZrO2 nanoparticles-antimicrobial activity and their prospective role in dental care. Microb. Pathog. 2017, 110, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, K.; Prithviraj, M.; Augustine, N.; Pradeep, S.P.; Thiagarajan, P. Analytical characterization and antimicrobial activity of nano zirconia particles. J. Chem. Pharm. Sci. 2016, 9, 1186–1190. [Google Scholar]
- Dusad, A.; Chakkalakal, D.A.; Namavar, F.; Haider, H.; Hanisch, B.; Duryee, M.J.; Diaz, A.; Rensch, A.; Zhang, Y.; Hess, R.; et al. Titanium implant with nanostructured zirconia surface promotes maturation of peri-implant bone in osseointegration. Proc. Inst. Mech. Eng. H 2013, 227, 510–522. [Google Scholar] [CrossRef] [PubMed]
- Waldvogel-Abramowski, S.; Waeber, G.; Gassner, C.; Buser, A.; Frey, B.M.; Favrat, B.; Tissot, J.D. Physiology of iron metabolism. Transfus. Med. Hemother. 2014, 41, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Abraham, R.; Walton, J.; Russell, L.; Wolman, R.; Wardley-Smith, B.; Green, J.R.; Mitchell, A.; Reeve, J. Dietary determinants of post-menopausal bone loss at the lumbar spine: A possible beneficial effect of iron. Osteoporos. Int. 2006, 17, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Heo, D.N.; Kung, H.M.; Gi, H.C.; Il, K.K.; Kinam, P.; Sang, C.L. Scale-Up Production of Theranostic Nanoparticles. In Cancer Theranostics; Chen, X., Wong, S., Eds.; Academic Press: Burlington, MA, USA, 2014; pp. 457–470. ISBN 978-0-12-407722-5. [Google Scholar]
- Wang, Y.X.; Xuan, S.; Port, M.; Idee, J.M. Recent advances in superparamagnetic iron oxide nanoparticles for cellular imaging and targeted therapy research. Curr. Pharm. Des. 2013, 19, 6575–6593. [Google Scholar] [CrossRef]
- Lovati, A.B.; Vianello, E.; Talò, G.; Recordati, C.; Bonizzi, L.; Galliera, E.; Broggini, M.; Moretti, M. Biodegradable microcarriers as cell delivery vehicle for in vivo transplantation and magnetic resonance monitoring. J. Biol. Regul. Homeost. Agents 2011, 25, S63–S74. [Google Scholar]
- Barzan, E.; Mehrabian, S.; Irian, S. Antimicrobial and Genotoxicity Effects of Zero-valent Iron Nanoparticles. Jundishapur J. Microbiol. 2014, 7, e10054. [Google Scholar] [CrossRef]
- Auffan, M.; Achouak, W.; Rose, J.; Roncato, M.A.; Chanéac, C.; Waite, D.T.; Masion, A.; Woicik, J.C.; Wiesner, M.R.; Bottero, J.Y. Relation between the redox state of iron-based nanoparticles and their cytotoxicity toward Escherichia coli. Environ. Sci. Technol. 2008, 42, 6730–6735. [Google Scholar] [CrossRef]
- Ismail, R.A.; Sulaiman, G.M.; Abdulrahman, S.A.; Marzoog, T.R. Antibacterial activity of magnetic iron oxide nanoparticles synthesized by laser ablation in liquid. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 53, 286–297. [Google Scholar] [CrossRef]
- Margabandhu, M.; Sendhilnathan, S.; Maragathavalli, S.; Karthikeyan, V.; Annadurai, B. Synthesis characterization and antibacterial activity of iron oxide nanoparticles. Glob. J. Bio Sci. Biotechnol. 2015, 4, 335. [Google Scholar]
- Soenen, S.J.; De Cuyper, M.; De Smedt, S.C.; Braeckmans, K. Investigating the toxic effects of iron oxide nanoparticles. Methods Enzymol. 2012, 509, 195–224. [Google Scholar] [CrossRef] [PubMed]
- Jarockyte, G.; Daugelaite, E.; Stasys, M.; Statkute, U.; Poderys, V.; Tseng, T.C.; Hsu, S.H.; Karabanovas, V.; Rotomskis, R. Accumulation and Toxicity of Superparamagnetic Iron Oxide Nanoparticles in Cells and Experimental Animals. Int. J. Mol. Sci. 2016, 17, 1193. [Google Scholar] [CrossRef] [PubMed]
- Yin, G.; Huang, Z.; Deng, M.; Zeng, J.; Gu, J. Preparation and cell response of bio-mineralized Fe3O4 nanoparticles. J. Colloid Interface Sci. 2011, 363, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.P.; Ma, B.Y.; Wei, X.W.; Qian, Z.Y. The in vitro and in vivo toxicity of gold nanoparticles. Chin. Chem. Lett. 2017, 28, 691–702. [Google Scholar] [CrossRef]
- Petersen, S.; Barcikowski, S. In Situ Bioconjugation: Single Step Approach to Tailored Nanoparticle-Bioconjugates by Ultrashort Pulsed Laser Ablation. Adv. Funct. Mater. 2009, 19, 1167–1172. [Google Scholar] [CrossRef]
- Schröfel, A.; Kratošová, G.; Šafařík, I.; Šafaříková, M.; Raška, I.; Shor, L.M. Applications of biosynthesized metallic nanoparticles—A review. Acta Biomater. 2014, 10, 4023–4042. [Google Scholar] [CrossRef] [PubMed]
- Hemeg, H.A. Nanomaterials for alternative antibacterial therapy. Int. J. Nanomed. 2017, 12, 8211–8225. [Google Scholar] [CrossRef]
- Vaseeharan, B.; Ramasamy, P.; Chen, J.C. Antibacterial activity of silver nanoparticles (Ag NPs) synthesized by tea leaf extracts against pathogenic Vibrio harveyi and its protective efficacy on juvenile Feneropenaeus indicus. Lett. Appl. Microbiol. 2010, 50, 352–356. [Google Scholar] [CrossRef]
- MubarakAli, D.; Thajuddin, N.; Jeganathan, K.; Gunasekaran, M. Plant extract mediated synthesis of silver and gold nanoparticles and its antibacterial activity against clinically isolated pathogens. Colloids Surf. B Biointerfaces 2011, 85, 360–365. [Google Scholar] [CrossRef]
- Park, Y. New paradigm shift for the green synthesis of antibacterial silver nanoparticles utilizing plant extracts. Toxicol. Res. 2014, 30, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Zewde, B.; Ambaye, A.; Stubbs, J., III; Dharmara, R. A Review of Stabilized Silver Nanoparticles—Synthesis, Biological Properties, Characterization, and Potential Areas of Applications. JSM Nanotechnol. Nanomed. 2016, 4, 1043. [Google Scholar]
- Pulido, L.; Ghanem, E.; Joshi, A.; Purtill, J.J.; Parvizi, J. Periprosthetic Joint Infection: The incidence, timing, and predisposing factors. Clin. Orthop. Relat. Res. 2008, 466, 1710–1715. [Google Scholar] [CrossRef] [PubMed]
- De Jong, W.H.; Borm, P.J. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef]
- Hajipour, M.J.; Fromm, K.M.; Ashkarran, A.A.; Jimenez de Aberasturi, D.; de Larramendi, I.R.; Rojo, T.; Serpooshan, V.; Parak, W.J.; Mahmoudi, M. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012, 30, 499–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimić, I.; Cvijović-Alagić, I.; Rakin, M.; Bugarski, B. Analysis of metal ion release from biomedical implants. Metall. Mater. Eng. 2013, 19, 167–176. [Google Scholar]
- Polyzois, I.; Nikolopoulos, D.; Michos, I.; Patsouris, E.; Theocharis, S. Local and systemic toxicity of nanoscale debris particles in total hip arthroplasty. J. Appl. Toxicol. 2012, 32, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Sivolella, S.; Stellini, E.; Brunello, G.; Gardin, C.; Ferroni, L.; Bressan, E.; Zavan, B. Silver nanoparticles in alveolar bone surgery devices. J. Nanomater. 2012, 2012, 15. [Google Scholar] [CrossRef]
- Biopharma Navigator. Available online: https://www.biopharmanavigator.com/bpn/#login (accessed on 6 November 2018).
- Wallace, D.R. Nanotoxicology and metalloestrogens: Possible involvement in breast cancer. Toxics 2015, 3, 390–413. [Google Scholar] [CrossRef]
- Brandt, O.; Mildner, M.; Egger, A.E.; Groessl, M.; Rix, U.; Posch, M.; Keppler, B.K.; Strupp, C.; Mueller, B.; Stingl, G. Nanoscalic silver possesses broad-spectrum antimicrobial activities and exhibits fewer toxicological side effects than silver sulfadiazine. Nanomedicine 2012, 8, 478–488. [Google Scholar] [CrossRef]
- Vik, H.; Andersen, K.J.; Julshamn, K.; Todnem, K. Neuropathy caused by silver absorption from arthroplasty cement. Lancet 1985, 1, 872. [Google Scholar] [CrossRef]
- Sudmann, E.; Vik, H.; Rait, M.; Todnem, K.; Andersen, K.J.; Julsham, K.; Flesland, O.; Rungby, J. Systemic and local silver accumulation after total hip replacement using silver-impregnated bone cement. Med. Prog. Technol. 1994, 20, 179–184. [Google Scholar] [PubMed]
- Qin, H.; Cao, H.; Zhao, Y.; Zhu, C.; Cheng, T.; Wang, Q.; Peng, X.; Cheng, M.; Wang, J.; Jin, G.; et al. In vitro and in vivo anti-biofilm effects of silver nanoparticles immobilized on titanium. Biomaterials 2014, 35, 9114–9125. [Google Scholar] [CrossRef] [PubMed]
- James, L.R.; Xu, Z.Q.; Sluyter, R.; Hawksworth, E.L.; Kelso, C.; Lai, B.; Paterson, D.J.; de Jonge, M.D.; Dixon, N.E.; Beck, J.L.; et al. An investigation into the interactions of gold nanoparticles and anti-arthritic drugs with macrophages, and their reactivity towards thioredoxin reductase. J. Inorg. Biochem. 2015, 142, 28–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharya, P.; Mukherjee, P. Biological properties of naked metal nanoparticles. Adv. Drug Deliv. Rev. 2008, 60, 1289–1306. [Google Scholar] [CrossRef] [PubMed]
- Connor, E.E.; Mwamuka, J.; Gole, A.; Murphy, C.J.; Wyatt, M.D. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small 2005, 1, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef]
- Villiers, C.L.; Freitas, H.; Couderc, R.; Villiers, M.B.; Marche, P.N. Analysis of the toxicity of gold nano particles on the immune system: Effect on dendritic cell functions. J. Nanopart. Res. 2010, 12, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Osredkar, J.; Sustar, N. Copper and Zinc, Biological Role and Significance of Copper/Zinc Imbalance. J. Clin. Toxicol. 2011, 001. [Google Scholar] [CrossRef]
- Chen, P.; Bornhorst, J.; Diana Neely, M.; Avila, D.S. Mechanisms and Disease Pathogenesis Underlying Metal-Induced Oxidative Stress. Oxid. Med. Cell. Longev. 2018, 2018, 7612172. [Google Scholar] [CrossRef]
- Buracco, S.; Peracino, B.; Andreini, C.; Bracco, E.; Bozzaro, S. Differential Effects of Iron, Zinc, and Copper on Dictyostelium discoideum Cell Growth and Resistance to Legionella pneumophila. Front. Cell. Infect. Microbiol. 2018, 7, 536. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.R. Critical Role of Zinc as Either an Antioxidant or a Prooxidant in Cellular Systems. Oxid. Med. Cell. Longev. 2018, 2018, 9156285. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bottagisio, M.; Lovati, A.B.; Galbusera, F.; Drago, L.; Banfi, G. A Precautionary Approach to Guide the Use of Transition Metal-Based Nanotechnology to Prevent Orthopedic Infections. Materials 2019, 12, 314. https://doi.org/10.3390/ma12020314
Bottagisio M, Lovati AB, Galbusera F, Drago L, Banfi G. A Precautionary Approach to Guide the Use of Transition Metal-Based Nanotechnology to Prevent Orthopedic Infections. Materials. 2019; 12(2):314. https://doi.org/10.3390/ma12020314
Chicago/Turabian StyleBottagisio, Marta, Arianna B. Lovati, Fabio Galbusera, Lorenzo Drago, and Giuseppe Banfi. 2019. "A Precautionary Approach to Guide the Use of Transition Metal-Based Nanotechnology to Prevent Orthopedic Infections" Materials 12, no. 2: 314. https://doi.org/10.3390/ma12020314
APA StyleBottagisio, M., Lovati, A. B., Galbusera, F., Drago, L., & Banfi, G. (2019). A Precautionary Approach to Guide the Use of Transition Metal-Based Nanotechnology to Prevent Orthopedic Infections. Materials, 12(2), 314. https://doi.org/10.3390/ma12020314






