Preparation and in Vitro Evaluation of New Composite Mesh Functionalized with Cationic Antimicrobial Peptide
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
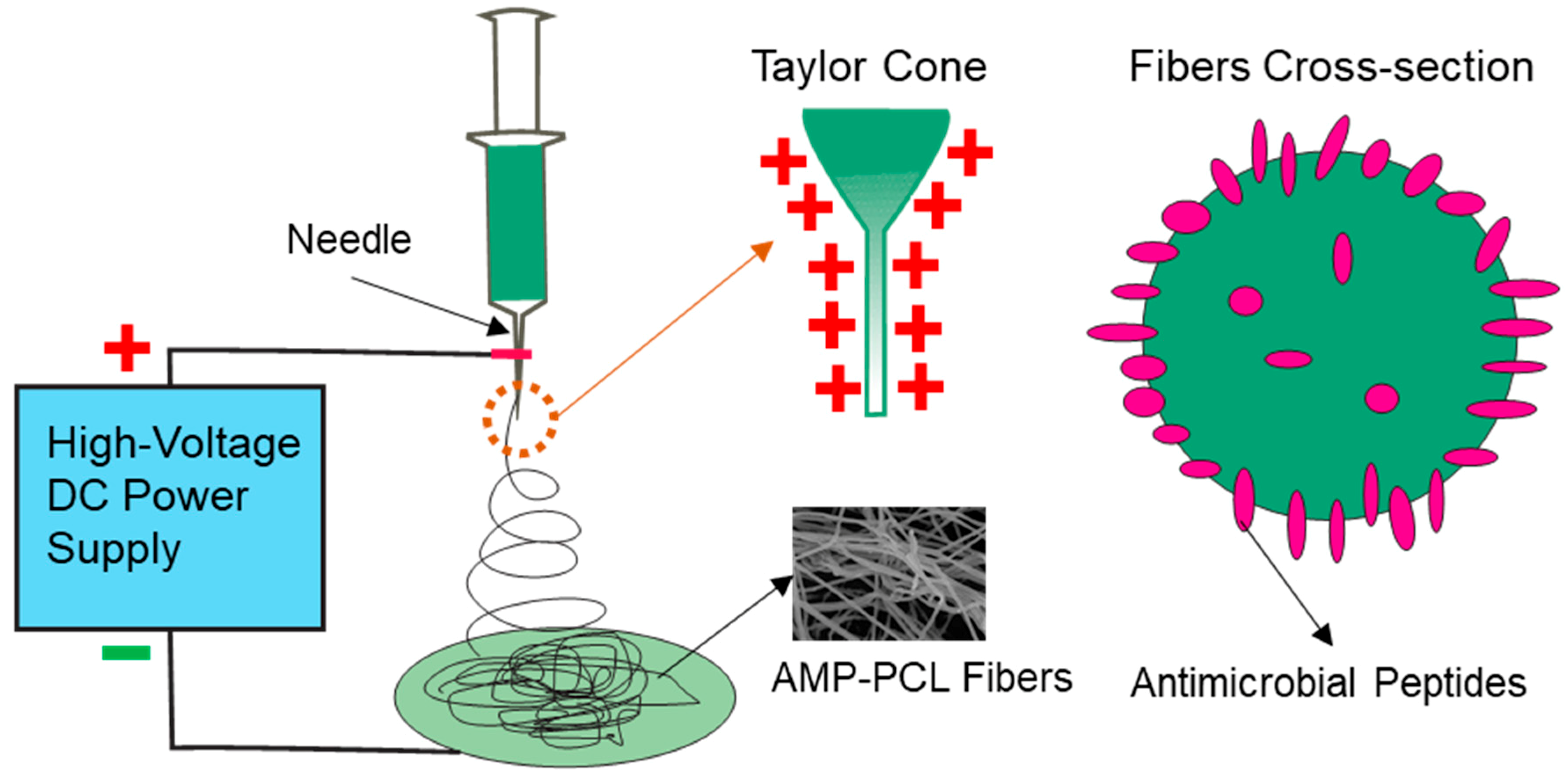
2.2. Preparation of Composite Mesh
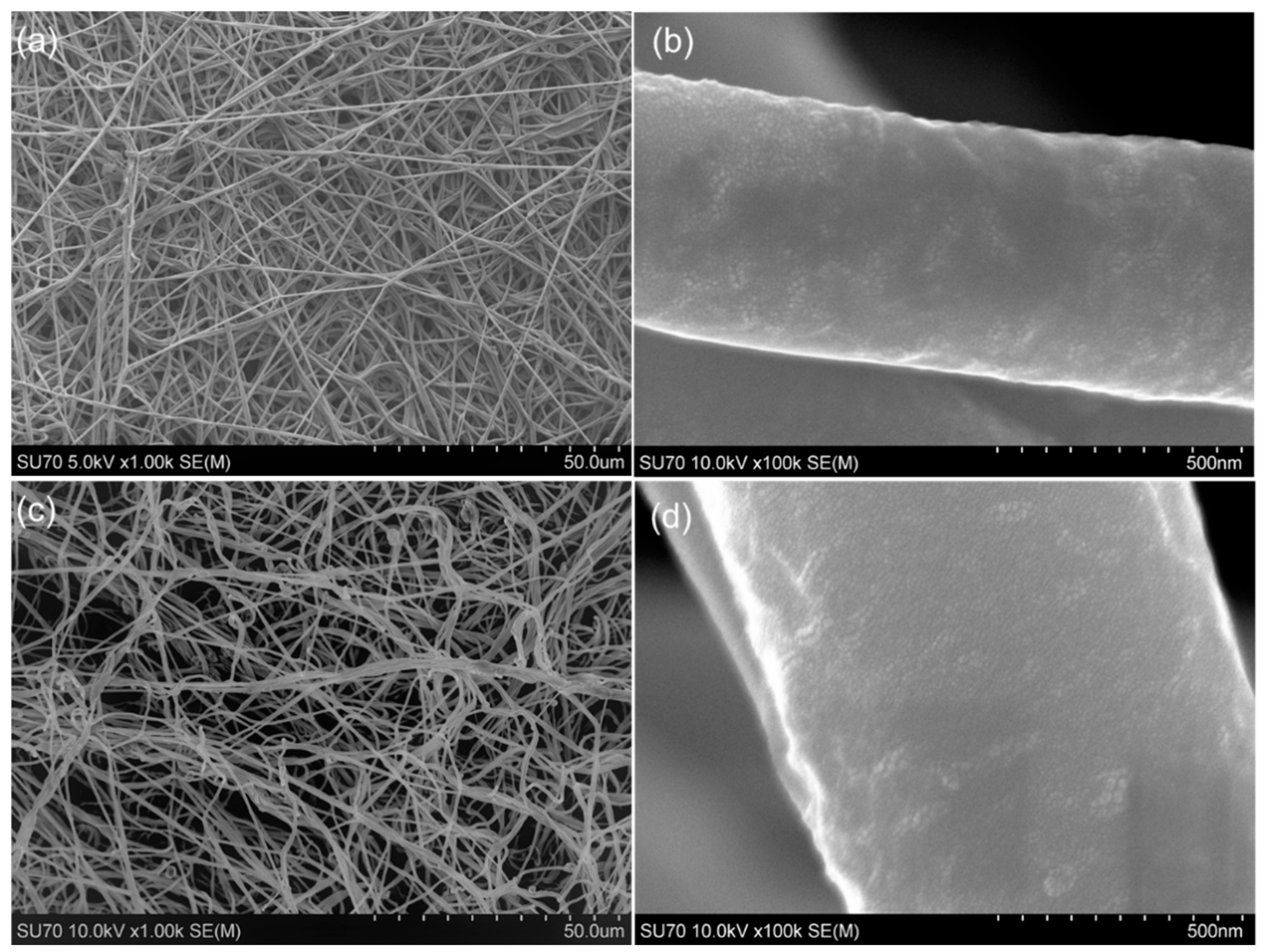
2.3. Field-Emission Scanning Electron Microscopy
2.4. FTIR Spectroscopy
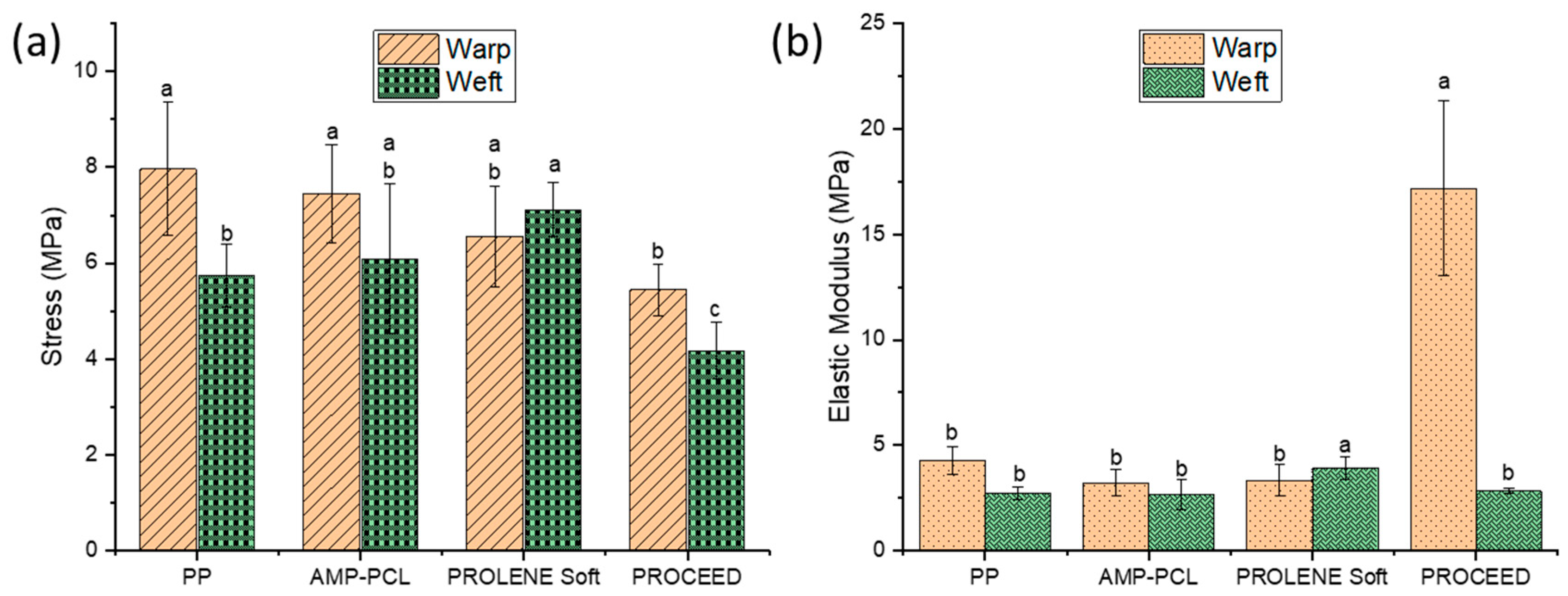
2.5. Tensile Strength Test
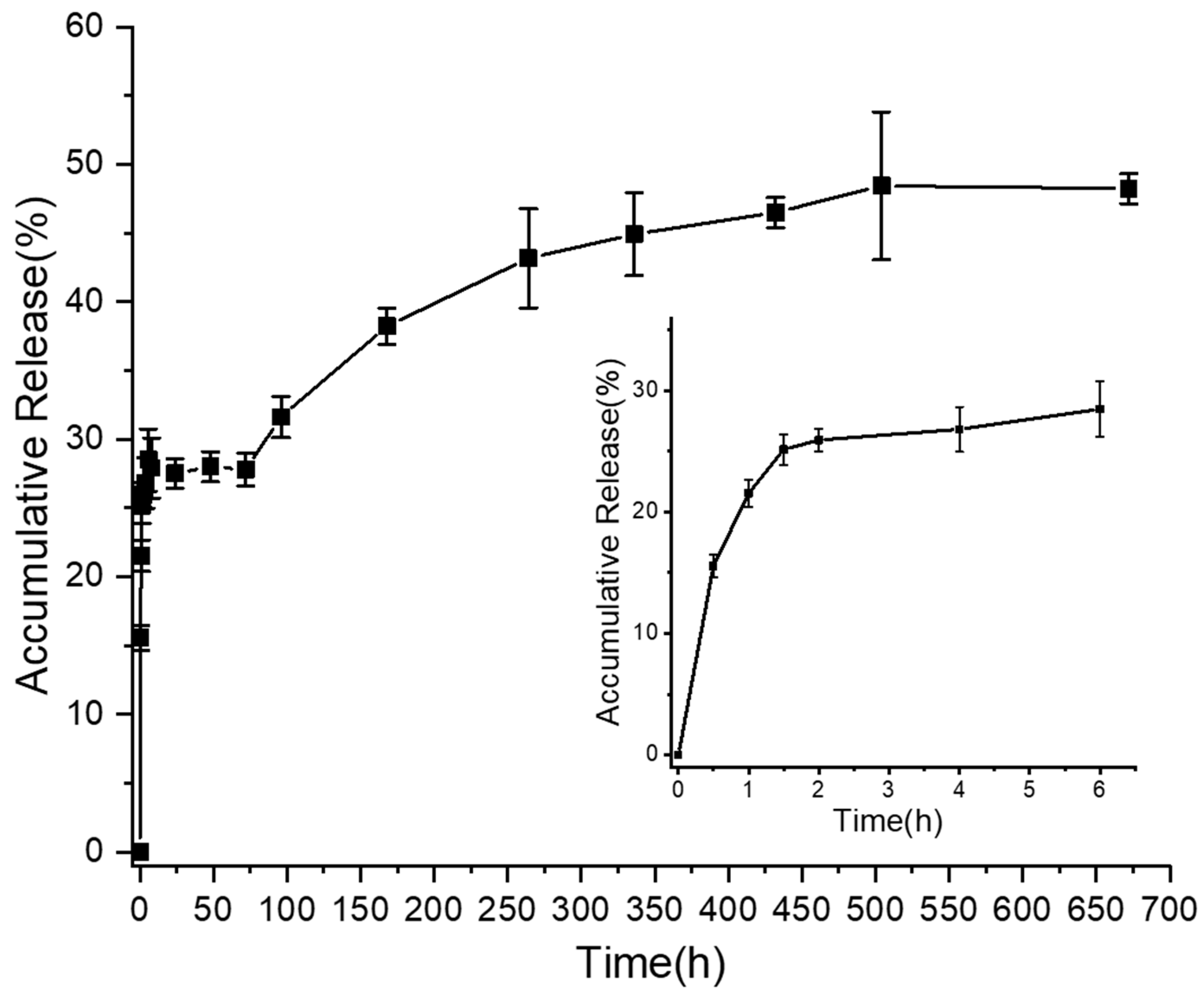
2.6. In Vitro Release
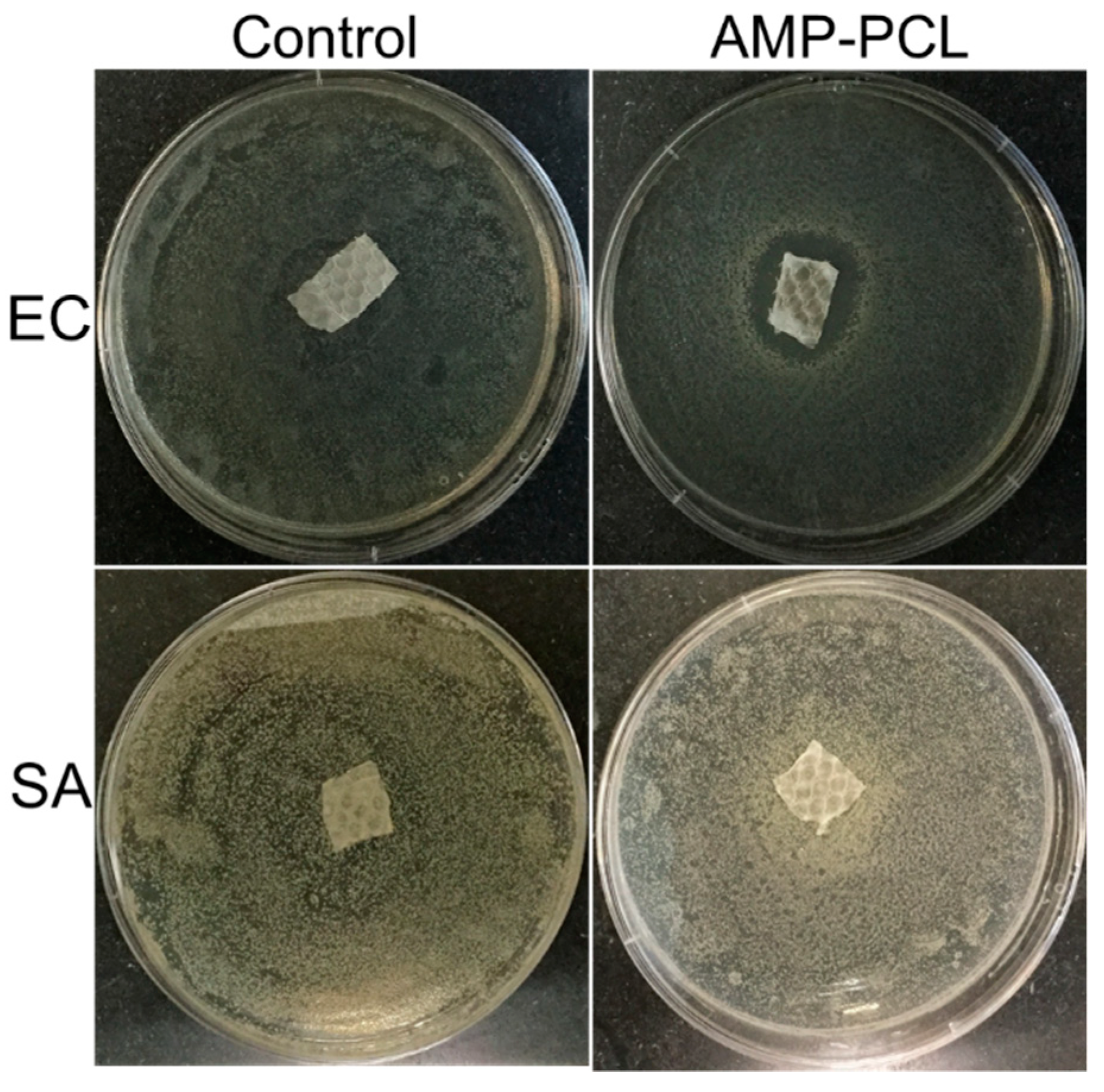
2.7. Antibacterial Activity
2.8. Cytotoxicity Assay
3. Results
3.1. Composite Mesh Surface Morphology
3.2. Fourier Transform Infrared Spectroscopy
3.3. Tensile strength test
3.4. In Vitro Release Activity
3.5. In Vitro Antibacterial Activity
3.6. In Vitro Cytotoxicity Test
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Usher, F.C.; Ochsner, J.; Tuttle, J.L. Use of marlex mesh in the repair of incisional hernias. Am. Surg. 1958, 24, 969–974. [Google Scholar]
- Luijendijk, R.W.; Hop, W.C.; Mp, V.D.T.; de Lange, D.C.; Braaksma, M.M.; Ijzermans, J.N.; Boelhouwer, R.U.; de Vries, B.C.; Salu, M.K.; Wereldsma, J.C. A comparison of suture repair with mesh repair for incisional hernia. N. Engl. J. Med. 2000, 343, 392. [Google Scholar] [CrossRef]
- Collaboration, E.H.T. Mesh compared with non-mesh methods of open groin hernia repair: Systematic review of randomized controlled trials. Br. J. Surg. 2000, 87, 854–859. [Google Scholar]
- Basoglu, M.; Yildirgan, M.I.; Yilmaz, I.; Balik, A.; Celebi, F.; Atamanalp, S.S.; Polat, K.Y.; Oren, D. Late complications of incisional hernias following prosthetic mesh repair. Acta Chir. Belg. 2004, 104, 425–448. [Google Scholar] [CrossRef]
- Berger, D.; Bientzle, M.; Müller, A. Postoperative complications after laparoscopic incisional hernia repair. Surg. Endosc. 2002, 16, 1720–1723. [Google Scholar] [CrossRef]
- Robinson, T.N.; Clarke, J.H.; Schoen, J.; Walsh, M.D. Major mesh-related complications following hernia repair. Surg. Endosc. 2005, 19, 1556–1560. [Google Scholar] [CrossRef]
- Mavros, M.N.; Athanasiou, S.; Alexiou, V.G.; Mitsikostas, P.K.; Peppas, G.; Falagas, M.E. Risk Factors for Mesh-related Infections After Hernia Repair Surgery: A Meta-analysis of Cohort Studies. World J. Surg. 2011, 35, 2389. [Google Scholar] [CrossRef]
- Arnold, M.R.; Coakley, K.M.; Fromke, E.J.; Groene, S.A.; Prasad, T.; Colavita, P.D.; Augenstein, V.A.; Kercher, K.W.; Heniford, B.T. Long-term assessment of surgical and quality-of-life outcomes between lightweight and standard (heavyweight) three-dimensional contoured mesh in laparoscopic inguinal hernia repair. Surgery 2019, 165, 820–824. [Google Scholar] [CrossRef]
- Sawyer, R.G.; Swenson, B.R.; Camp, T.R.; Mulloy, D.P. Antimicrobial-impregnated surgical incise drapes in the prevention of mesh infection after ventral hernia repair. Surg. Infect. 2008, 9, 23. [Google Scholar]
- Jose, B.L.; Yurena, S.Q.; Inmaculada, G.I.G.; Javier, V.U.; Fernando, C.T.; Santiago, B.D.; Providencia, G.P.; Ricardo, B.V.; José, M.P. Prosthetic infection after hernioplasty. Five years experience. Cirugía Espaola 2009, 85, 158–164. [Google Scholar]
- Deligiannidis, N.; Papavasiliou, I.; Sapalidis, K.; Kesisoglou, I.; Papavramidis, S.; Gamvros, O. The use of three different mesh materials in the treatment of abdominal wall defects. Hernia 2002, 6, 51–55. [Google Scholar] [CrossRef]
- Cobb, W.S.; Harris, J.B.; Lokey, J.S.; Mcgill, E.S.; Klove, K.L. Incisional herniorrhaphy with intraperitoneal composite mesh: A report of 95 cases. Am. Surg. 2003, 69, 784–787. [Google Scholar]
- Kathju, S.; Nistico, L.; Melton-Kreft, R.; Lasko, L.A.; Stoodley, P. Direct Demonstration of Bacterial Biofilms on Prosthetic Mesh after Ventral Herniorrhaphy. Surg. Infect. 2015, 16, 45–53. [Google Scholar] [CrossRef]
- Stremitzer, S.; Bachleitner-Hofmann, T.; Gradl, B.; Gruenbeck, M.; Bachleitner-Hofmann, B.; Mittlboeck, M.; Bergmann, M. Mesh Graft Infection Following Abdominal Hernia Repair: Risk Factor Evaluation and Strategies of Mesh Graft Preservation. A Retrospective Analysis of 476 Operations. World J. Surg. 2010, 34, 1702–1709. [Google Scholar] [CrossRef]
- Chung, L.; Tse, G.H.; O’Dwyer, P.J. Outcome of patients with chronic mesh infection following abdominal wall hernia repair. Hernia 2014, 18, 701–704. [Google Scholar] [CrossRef]
- Labay, C.; Canal, J.M.; Modic, M.; Cvelbar, U.; Quiles, M.; Armengol, M.; Arbos, M.A.; Gil, F.J.; Canal, C. Antibiotic-loaded polypropylene surgical meshes with suitable biological behaviour by plasma functionalization and polymerization. Biomaterials 2015, 71, 132–144. [Google Scholar] [CrossRef]
- Guillaume, O.; Garric, X.; Lavigne, J.-P.; Van Den Berghe, H.; Coudane, J. Multilayer, degradable coating as a carrier for the sustained release of antibiotics: Preparation and antimicrobial efficacy in vitro. J. Control. Release 2012, 162, 492–501. [Google Scholar] [CrossRef]
- Lancet, T. Antibiotic resistance: A final warning. Lancet 2013, 382, 1072. [Google Scholar] [CrossRef]
- Stewart, P.S.; Costerton, J.W. Antibiotic resistance of bacteria in biofilms. Lancet 2001, 358, 135–138. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2013; 2013. Available online: https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf (accessed on 1 April 2019).
- Findlay, B.; Zhanel, G.G.; Schweizer, F. Cationic Amphiphiles, a New Generation of Antimicrobials Inspired by the Natural Antimicrobial Peptide Scaffold. Antimicrob. Agents Chemother. 2010, 54, 4049–4058. [Google Scholar] [CrossRef]
- Kumar, P.; Kizhakkedathu, J.N.; Straus, S.K. Antimicrobial Peptides: Diversity, Mechanism of Action and Strategies to Improve the Activity and Biocompatibility In Vivo. Biomolecules 2018, 8, 4. [Google Scholar] [CrossRef]
- Zhang, E.; Bai, P.Y.; Cui, D.Y.; Chu, W.C.; Hua, Y.G.; Liu, Q.; Yin, H.Y.; Zhang, Y.J.; Qin, S.; Liu, H.M. Synthesis and bioactivities study of new antibacterial peptide mimics: The dialkyl cationic amphiphiles. Eur. J. Med. Chem. 2018, 143, 1489–1509. [Google Scholar] [CrossRef]
- Park, S.C.; Kim, M.H.; Hossain, M.A.; Shin, S.Y.; Kim, Y.; Stella, L.; Wade, J.D.; Park, Y.; Hahm, K.S. Amphipathic alpha-helical peptide, HP (2–20), and its analogues derived from Helicobacter pylori: Pore formation mechanism in various lipid compositions. Biochim. Biophys. Acta 2008, 1778, 229–241. [Google Scholar] [CrossRef]
- Onate-Garzon, J.; Manrique-Moreno, M.; Trier, S.; Leidy, C.; Torres, R.; Patino, E. Antimicrobial activity and interactions of cationic peptides derived from Galleria mellonella cecropin D-like peptide with model membranes. J. Antibiot. 2017, 70, 238–245. [Google Scholar] [CrossRef]
- Hancock, R.E.W. Cationic peptides: Effectors in innate immunity and novel antimicrobials. Lancet Infect. Dis. 2001, 1, 156–164. [Google Scholar] [CrossRef]
- Wang, G.; Li, X.; Wang, Z. APD3: The antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2015, 44, D1087–D1093. [Google Scholar] [CrossRef]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef]
- Bechinger, B.; Gorr, S.U. Antimicrobial Peptides: Mechanisms of Action and Resistance. J. Dent. Res. 2017, 96, 254–260. [Google Scholar] [CrossRef]
- Wenzel, M.; Chiriac, A.I.; Otto, A.; Zweytick, D.; May, C.; Schumacher, C.; Gust, R.; Albada, H.B.; Penkova, M.; Kramer, U.; et al. Small cationic antimicrobial peptides delocalize peripheral membrane proteins. Proc. Natl. Acad. Sci. USA 2014, 111, E1409–1418. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Haney, E.F.; Vogel, H.J. The expanding scope of antimicrobial peptide structures and their modes of action. Trends Biotechnol. 2011, 29, 464–472. [Google Scholar] [CrossRef]
- Michael, Z. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389–395. [Google Scholar]
- Yeaman, M.R.; Yount, N.Y. Mechanisms of antimicrobial peptide action and resistance. Pharmacol. Rev. 2003, 55, 27–55. [Google Scholar] [CrossRef]
- Pfalzgraff, A.; Brandenburg, K.; Weindl, G. Antimicrobial Peptides and Their Therapeutic Potential for Bacterial Skin Infections and Wounds. Front. Pharmacol. 2018, 9, 281. [Google Scholar] [CrossRef]
- Hancock, R.; Patrzykat, A. Clinical development of cationic antimicrobial peptides: From natural to novel antibiotics. Curr. Drug Targets Infect. Disord. 2002, 2, 79–83. [Google Scholar] [CrossRef]
- Hancock, R.E.; Sahl, H.-G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006, 24, 1551. [Google Scholar] [CrossRef]
- Liu, P.; Shao, H.; Chen, N.; Jiang, J. Physico-Mechanical Performance Evaluation of Large Pore Synthetic Meshes with Different Textile Structures for Hernia Repair Applications. Fibres Text. East. Eur. 2018, 26, 79–86. [Google Scholar] [CrossRef]
- Santajit, S.; Indrawattana, N. Mechanisms of Antimicrobial Resistance in ESKAPE Pathogens. BioMed Res. Int. 2016, 2016, 8. [Google Scholar] [CrossRef]
- Hall Barrientos, I.J.; Paladino, E.; Brozio, S.; Passarelli, M.K.; Moug, S.; Black, R.A.; Wilson, C.G.; Lamprou, D.A. Fabrication and characterisation of drug-loaded electrospun polymeric nanofibers for controlled release in hernia repair. Int. J. Pharm. 2017, 517, 329–337. [Google Scholar] [CrossRef]
- Tian, R.; Qiu, X.; Yuan, P.; Lei, K.; Wang, L.; Bai, Y.; Liu, S.; Chen, X. Fabrication of Self-Healing Hydrogels with On-Demand Antimicrobial Activity and Sustained Biomolecule Release for Infected Skin Regeneration. ACS Appl. Mater. Interfaces 2018, 10, 17018–17027. [Google Scholar] [CrossRef]
- Heunis, T.D.; Smith, C.; Dicks, L.M. Evaluation of a nisin-eluting nanofiber scaffold to treat Staphylococcus aureus-induced skin infections in mice. Antimicrob. Agents Chemother. 2013, 57, 3928–3935. [Google Scholar] [CrossRef]
- Machado, R.; da Costa, A.; Silva, D.M.; Gomes, A.C.; Casal, M.; Sencadas, V. Antibacterial and Antifungal Activity of Poly(Lactic Acid)-Bovine Lactoferrin Nanofiber Membranes. Macromol. Biosci. 2018. [Google Scholar] [CrossRef]
- Jang, C.H.; Cho, Y.B.; Jang, Y.S.; Kim, M.S.; Kim, G.H. Antibacterial effect of electrospun polycaprolactone/polyethylene oxide/vancomycin nanofiber mat for prevention of periprosthetic infection and biofilm formation. Int. J. Pediatr. Otorhinolaryngol. 2015, 79, 1299–1305. [Google Scholar] [CrossRef]
- ISO. Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. 10993-5. 2009. [Google Scholar]
- Bucio, E.; Concheiro, A.; Burillo, G. Cyclodextrin-functionalized polyethylene and polypropylene as biocompatible materials for diclofenac delivery. Int. J. Pharm. 2009, 382, 183–191. [Google Scholar]
- Balakrishnan, P.; Gardella, L.; Forouharshad, M.; Pellegrino, T.; Monticelli, O. Star poly(ε-caprolactone)-based electrospun fibers as biocompatible scaffold for doxorubicin with prolonged drug release activity. Colloids Surf. B 2017, 161, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Bandekar, J. Amide modes and protein conformation. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 1992, 1120, 123–143. [Google Scholar] [CrossRef]
- Lin, K.; Chua, K.N.; Christopherson, G.T.; Lim, S.; Mao, H.Q. Reducing electrospun nanofiber diameter and variability using cationic amphiphiles. Polymer 2007, 48, 6384–6394. [Google Scholar] [CrossRef]
- Bernardo, K.; Pakulat, N.; Fleer, S.; Schnaith, A.; Utermöhlen, O.; Krut, O.; Müller, S.; Krönke, M. Subinhibitory concentrations of linezolid reduce Staphylococcus aureus virulence factor expression. Antimicrob. Agents Chemother. 2004, 48, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Cooper, B.; Islam, N.; Xu, Y.; Beard, H.S.; Garrett, W.M.; Gu, G.; Nou, X. Quantitative Proteomic Analysis of Staphylococcus aureus Treated with Punicalagin, a Natural Antibiotic from Pomegranate that Disrupts Iron Homeostasis and Induces SOS. Proteomics 2018, 18, 1700461. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, P.; Chen, N.; Jiang, J.; Wen, X. Preparation and in Vitro Evaluation of New Composite Mesh Functionalized with Cationic Antimicrobial Peptide. Materials 2019, 12, 1676. https://doi.org/10.3390/ma12101676
Liu P, Chen N, Jiang J, Wen X. Preparation and in Vitro Evaluation of New Composite Mesh Functionalized with Cationic Antimicrobial Peptide. Materials. 2019; 12(10):1676. https://doi.org/10.3390/ma12101676
Chicago/Turabian StyleLiu, Pengbi, Nanliang Chen, Jinhua Jiang, and Xuejun Wen. 2019. "Preparation and in Vitro Evaluation of New Composite Mesh Functionalized with Cationic Antimicrobial Peptide" Materials 12, no. 10: 1676. https://doi.org/10.3390/ma12101676
APA StyleLiu, P., Chen, N., Jiang, J., & Wen, X. (2019). Preparation and in Vitro Evaluation of New Composite Mesh Functionalized with Cationic Antimicrobial Peptide. Materials, 12(10), 1676. https://doi.org/10.3390/ma12101676





