Facile Preparation of Micrometer KClO4/Zr Energetic Composite Particles with Enhanced Light Radiation
Abstract
:1. Introduction
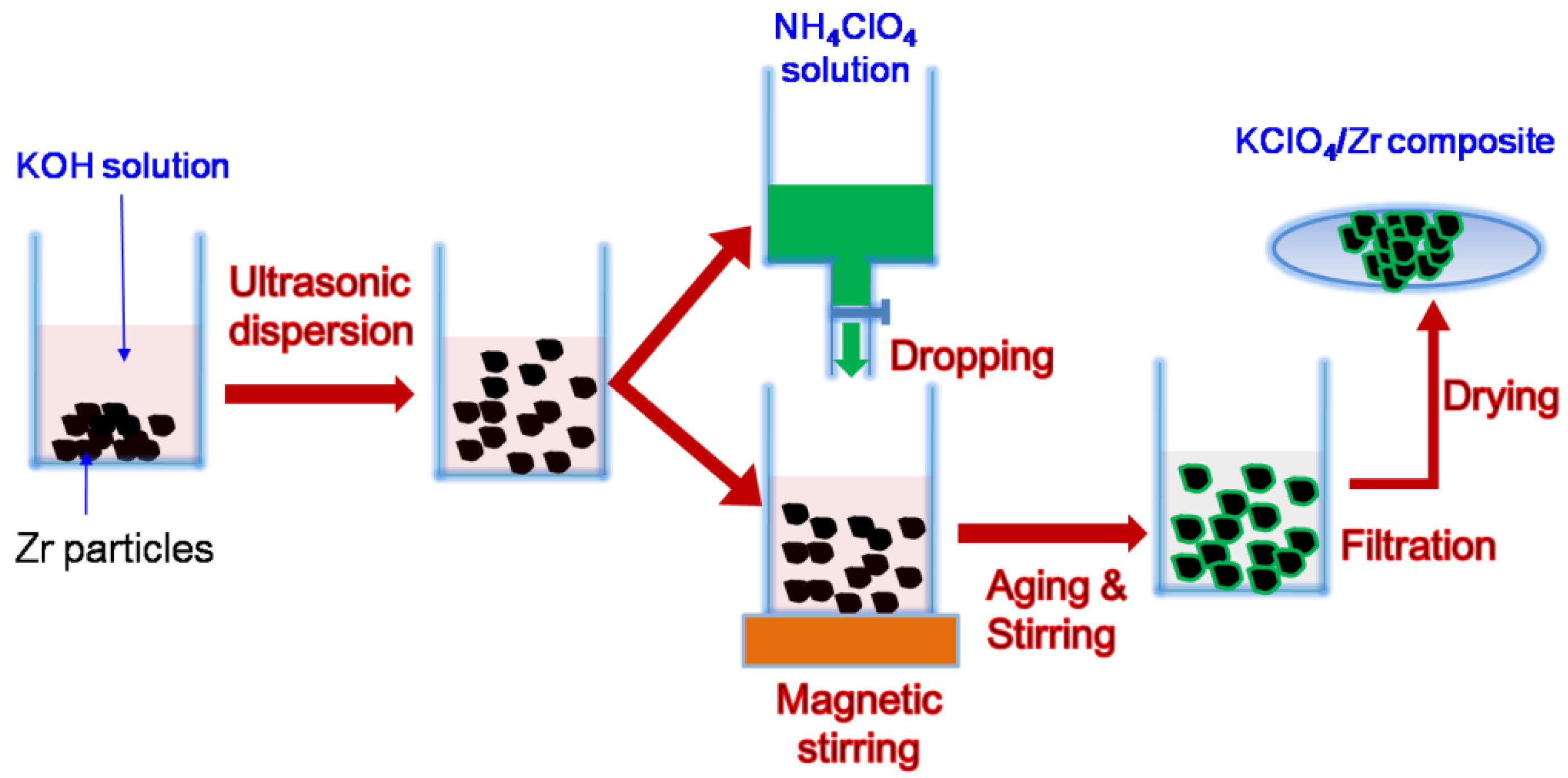
2. Materials and Methods
3. Results
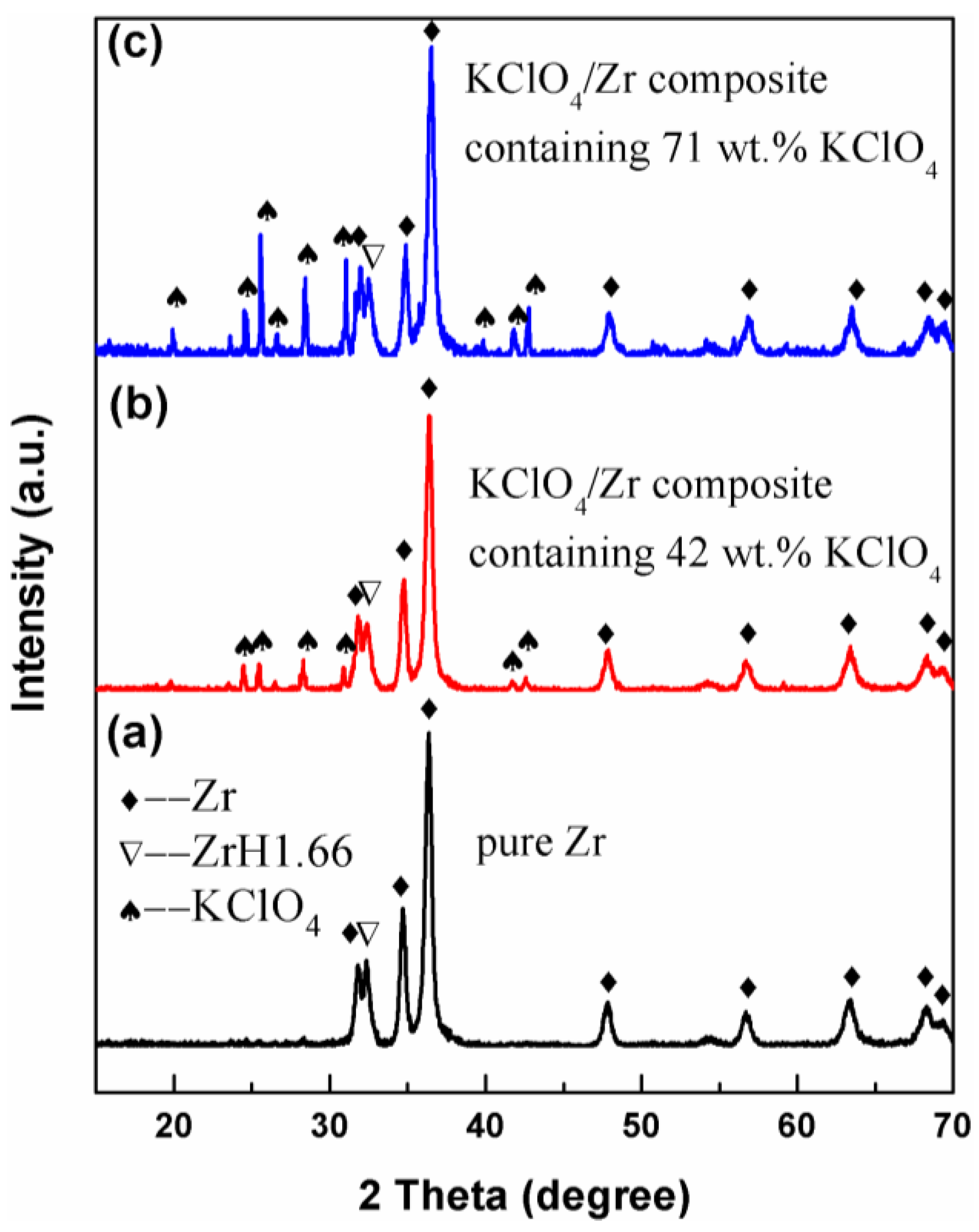
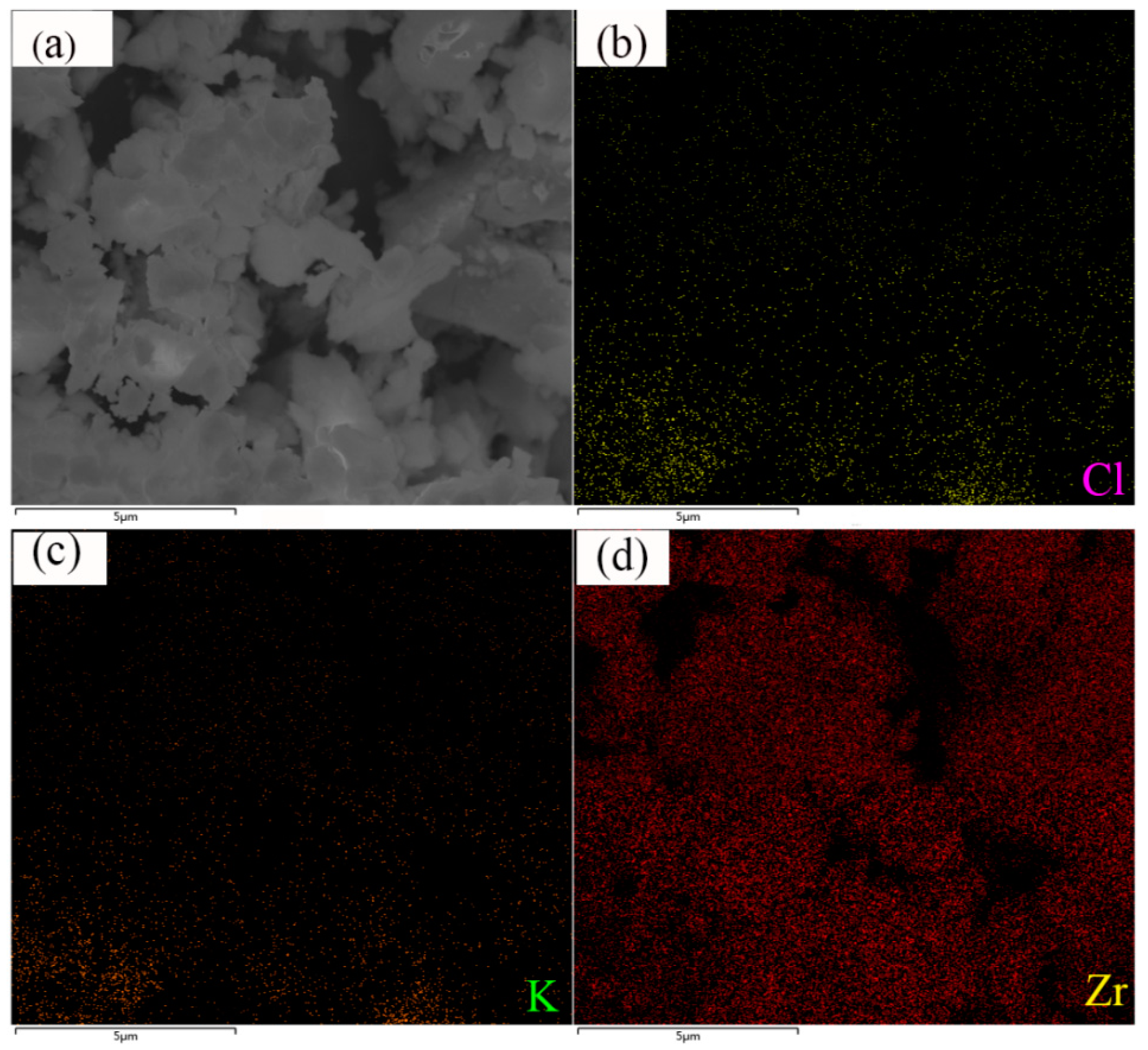
3.1. Sample Characterization
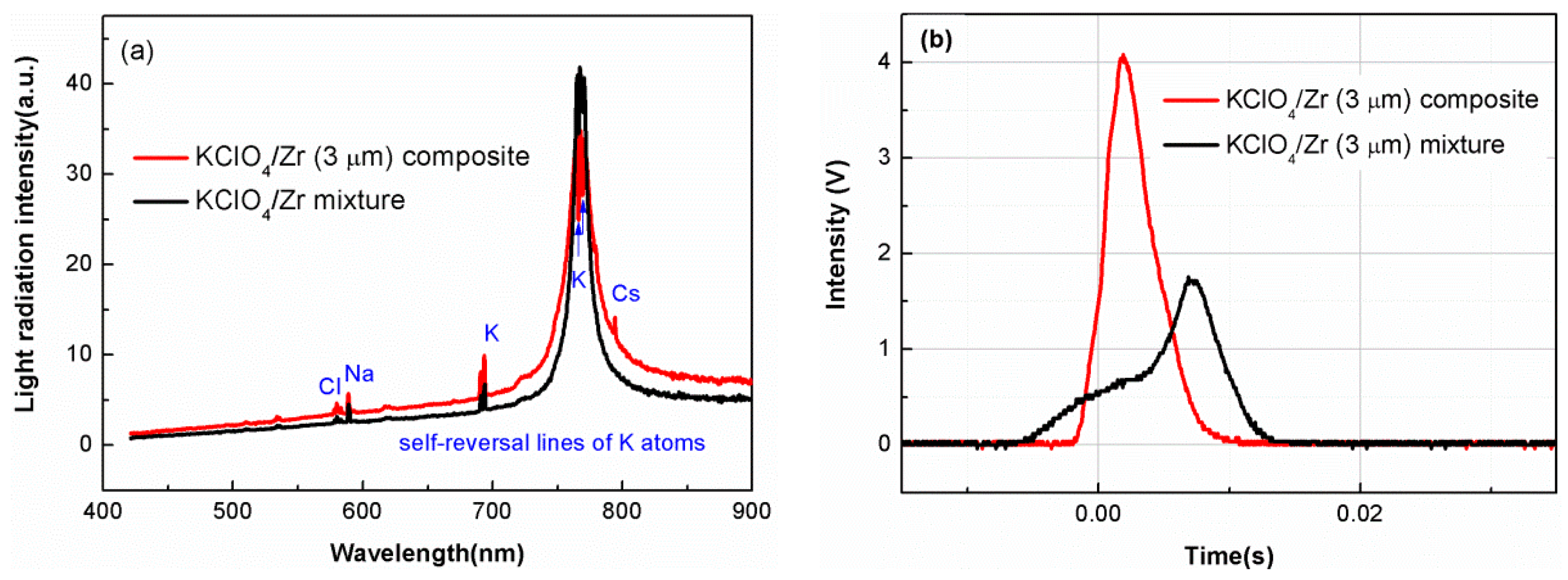
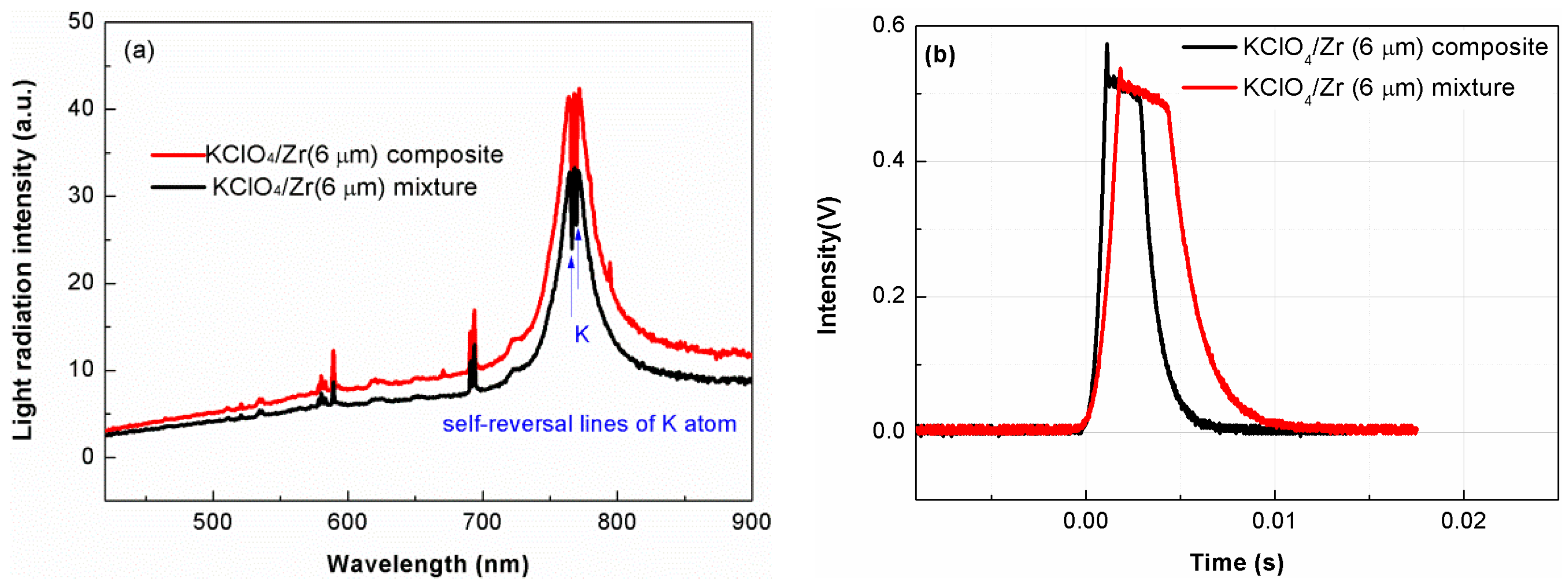
3.2. Light-Radiation Property
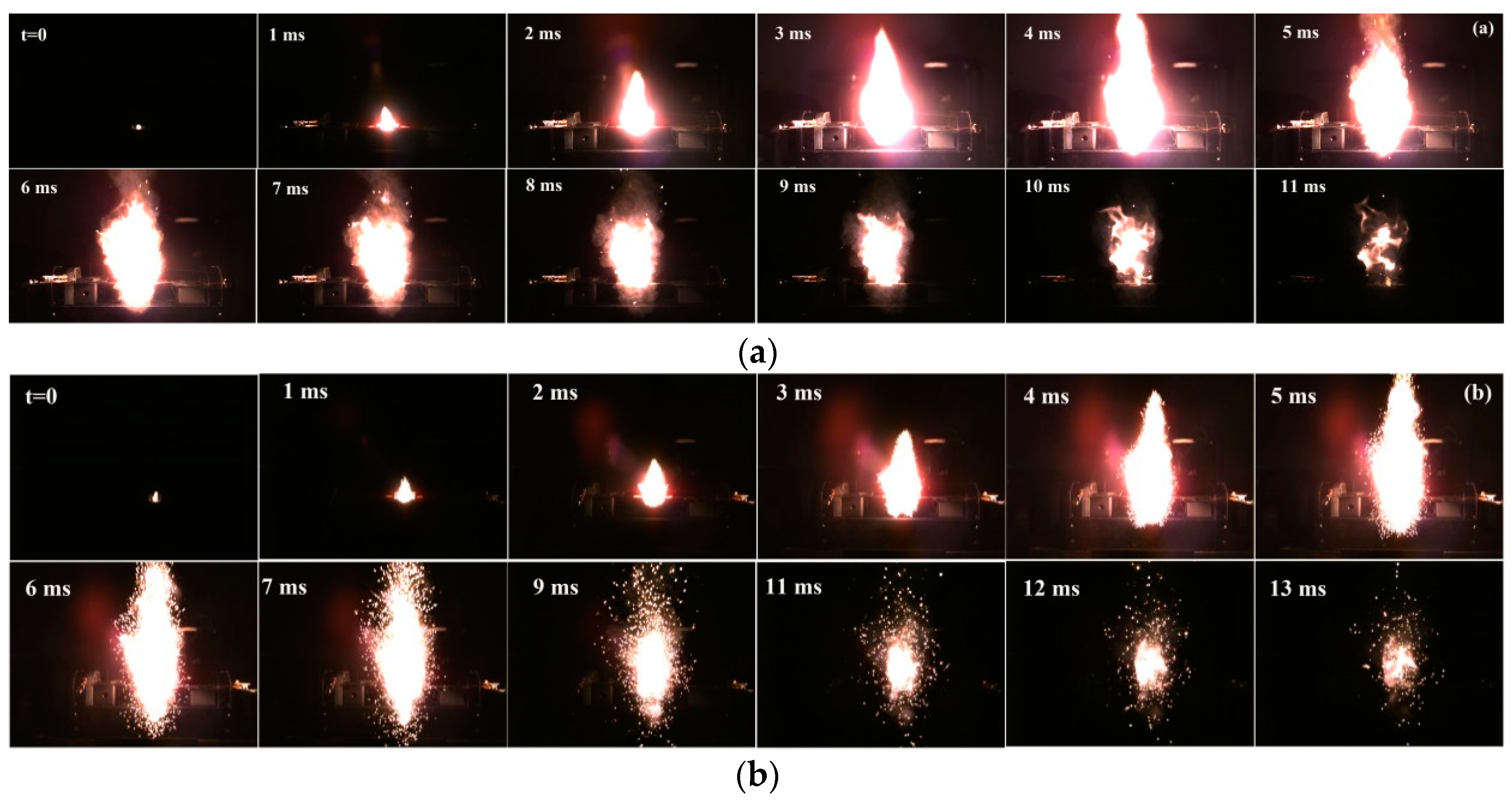
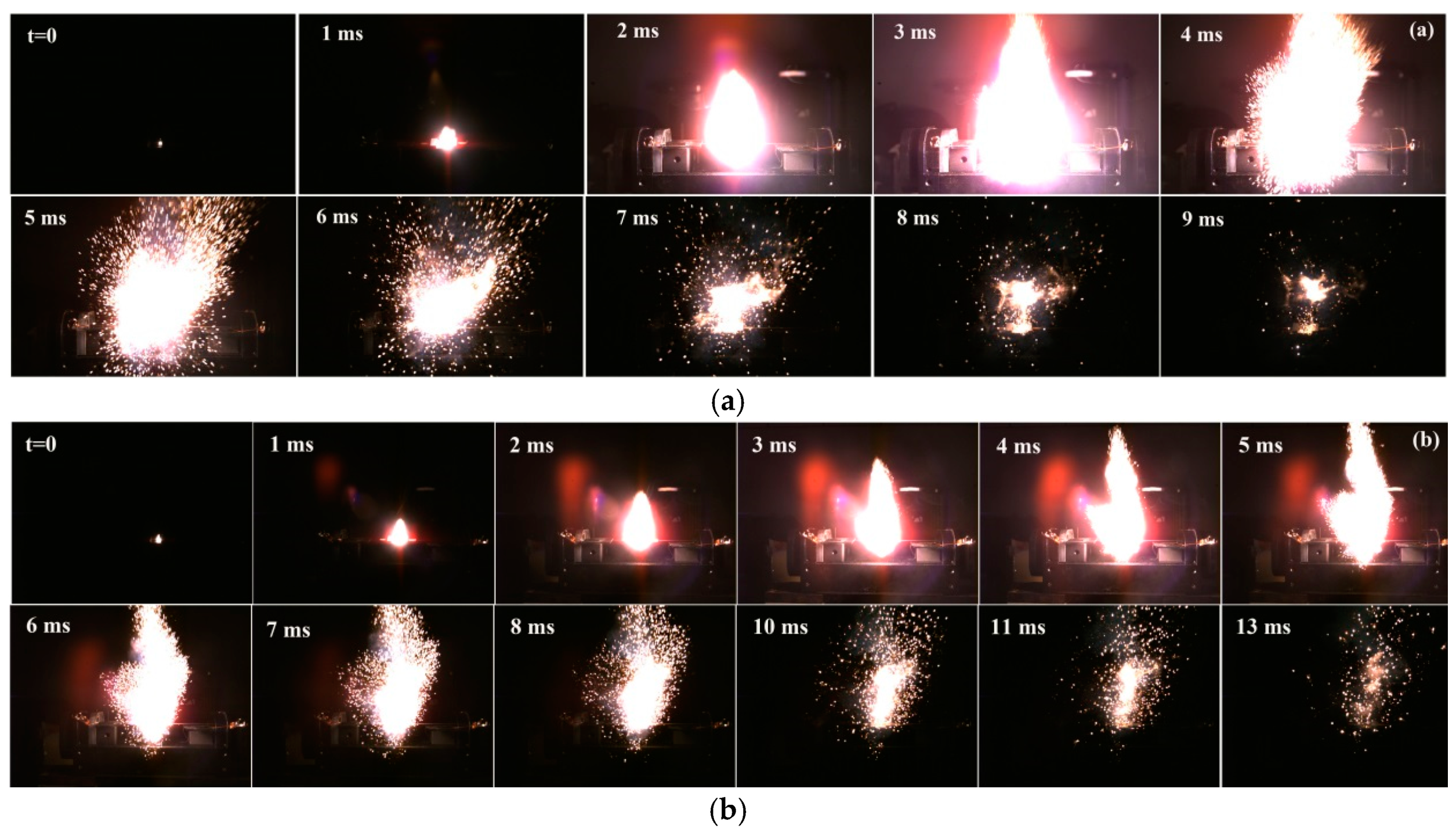
3.3. Flame Photographs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Carrico, C.M.; Gomez, S.L.; Dubey, M.K.; Aikenb, A.C. Low hygroscopicity of ambient fresh carbonaceous aerosols from pyrotechnics smoke. Atmos. Environ. 2018, 178, 101–108. [Google Scholar] [CrossRef]
- Yin, J.W.; Zhao, L.S.; Du, Z.M.; Xing, Q.F.; Zhao, Z.H. Study on combustion heat of pyrotechnics. Procedia Eng. 2014, 84, 849–853. [Google Scholar]
- Lyons, G.W.; Raspet, R. Chemical kinetics theory of pyrotechnic whistles. J. Acoust. Soc. Am. 2015, 137, 2200. [Google Scholar] [CrossRef]
- Poret, J.; Sabatini, J. Comparison of barium and amorphous boron pyrotechnics for green light emission. J. Energy Mater. 2013, 31, 27–34. [Google Scholar] [CrossRef]
- Berger, B. Parameters influencing the pyrotechnic reaction. Propell. Explos. Pyrot. 2005, 30, 27–35. [Google Scholar] [CrossRef]
- Calais, T.; Bancaud, A.; Estève, A.; Rossi, C. Correlation between DNA self-assembly kinetics, microstructure, and thermal properties of tunable highly energetic Al-CuO nanocomposites for micropyrotechnic applications. ACS Appl. Nano Mater. 2018, 1, 4716–4725. [Google Scholar] [CrossRef]
- Wang, H.; DeLisio, J.B.; Jian, G.; Zhou, W.; Zachariah, M.R. Electrospray formation and combustion characteristics of iodine-containing Al/CuO nanothermite microparticles. Combust. Flame 2018, 162, 2823–2829. [Google Scholar] [CrossRef]
- Petrantoni, M.; Rossi, C.; Salvagnac, L.; Conédéra, V.; Estève, A.; Tenailleau, C.; Alphonse, P.; Chabal, Y.J. Multilayered Al/CuO thermite formation by reactive magnetron sputtering: Nano versus micro. J. Appl. Phys. 2010, 108, 832. [Google Scholar] [CrossRef]
- Kuntz, J.D.; Cervantes, O.G.; Gash, A.E.; Munirb, Z.A. Tantalum-tungsten oxide thermite composites prepared by sol-gel synthesis and spark plasma sintering. Combust. Flame 2010, 157, 1566–1571. [Google Scholar] [CrossRef]
- Gao, K.; Li, G.; Luo, Y.; Wang, L.; Shen, L.H.; Wang, G. Preparation and characterization of the AP/Al/Fe2O3 ternary nano-thermites. J. Therm. Anal. Calorim. 2014, 118, 43–49. [Google Scholar] [CrossRef]
- Qin, L.; Gong, T.; Hao, H.; Wang, K.Y.; Feng, H. Core-shell-structured nanothermites synthesized by atomic layer deposition. J. Nanopart. Res. 2013, 15, 1–15. [Google Scholar] [CrossRef]
- Yan, N.; Qin, L.J.; Hao, H.X.; Hui, L.F.; Zhao, F.Q.; Feng, H. Iron oxide/aluminum/graphene energetic nanocomposites synthesized by atomic layer deposition: Enhanced energy release and reduced electrostatic ignition hazard. Appl. Surf. Sci. 2017, 408, 51–59. [Google Scholar] [CrossRef]
- Wang, H.; Zachariah, M.R.; Xie, L.; Rao, G.N. Ignition and combustion characterization of nano-Al-AP and nano-Al-CuO-AP micro-sized composites produced by electrospray technique. Energy Procedia 2015, 66, 109–112. [Google Scholar] [CrossRef]
- Sullivan, K.T.; Worsley, M.A.; Kuntz, J.D.; Gash, A.E. Electrophoretic deposition of binary energetic composites. Combust. Flame 2012, 159, 2210–2218. [Google Scholar] [CrossRef]
- Li, R.; Xu, H.M.; Hu, H.L.; Yang, G.C.; Wang, J.; Shen, J.P. Microstructured Al/Fe2O3/nitrocellulose energetic fibers realized by electrospinning. J. Energy Mater. 2014, 32, 50–59. [Google Scholar] [CrossRef]
- Shim, H.M.; Kim, J.K.; Kim, H.S.; Koo, K.K. Production of the spherical nano-Al/AP composites by drowning-out/agglomeration and their solid-reaction kinetics. Ind. Eng. Chem. Res. 2016, 55, 10227–10234. [Google Scholar] [CrossRef]
- Lan, Y.; Jin, M.; Luo, Y. Preparation and characterization of graphene aerogel/Fe2O3/ammonium perchlorate nanostructured energetic composite. J. Sol-Gel Sci. Technol. 2015, 74, 161–167. [Google Scholar] [CrossRef]
- Yang, F.; Kang, X.L.; Luo, J.S.; Yi, Z.; Tang, Y.J. Preparation of core-shell structure KClO4@Al/CuO Nanoenergetic material and enhancement of thermal behavior. Sci. Rep. 2017, 7, 3730. [Google Scholar] [CrossRef]
- Kaminskii, A.A.; Bagayev, S.N.; Ueda, K.; Takaichi, K.; Yagi, H.; Yanagitani, T. 5.5 J pyrotechnically pumped Nd3+:Y3Al5O12 ceramic laser. Laser Phys. Lett. 2010, 3, 124–128. [Google Scholar] [CrossRef]
- Kang, X.L.; Zhang, Q.; Luo, J.S.; Tang, Y.J. Selective emissions during combustion of KClO4/Zr pyrotechnics for laser pump application. Combust. Sci. Technol. 2011, 183, 1401–1411. [Google Scholar] [CrossRef]
- Lee, J.S.; Li, L.K.; Lin, C.H.; Chen, P.J.; Huang, C.W.; Chang, S.S. A study of zirconium/potassium perchlorate primer mixtures. Thermochim. Acta 1990, 173, 211–218. [Google Scholar] [CrossRef]
- Lee, J.S.; Hsu, C.K. The effect of different zirconium on thermal behaviors for Zr/KClO4 priming composition. Thermochim. Acta 2001, 367–368, 375–379. [Google Scholar] [CrossRef]
- Lee, J.S.; Huang, C.W. Thermal behavior and firing characteristics of Zr/KClO4 primer mixture containing CuO. J. Therm. Anal. 1993, 40, 357–361. [Google Scholar] [CrossRef]
- Pourmortazavi, S.M.; Hosseini, S.G.; Hajimirsadeghi, S.S.; Alamdari, R.F. Investigation on thermal analysis of binary zirconium/oxidant pyrotechnic Systems. Combust. Sci. Technol. 2008, 180, 2093–2102. [Google Scholar] [CrossRef]
- Ji, X.B.; Liu, Y.G.; Li, Z.F.; Yu, Q.; Gao, Y.; Zhang, H.B.; Wang, L. Thermal behavior of Al/Zr/KClO4 pyrotechnic compositions at high temperature. Thermochim. Acta 2018, 659, 55–58. [Google Scholar] [CrossRef]
- Shin, D.J.; Kim, W.D.; Lee, S.; Lee, D.C. Nanothermite of Al nanoparticles and three-dimensionally ordered macroporous CuO: Mechanistic insight into oxidation during thermite reaction. Combust. Flame 2018, 189, 87–91. [Google Scholar] [CrossRef]
- Bockmon, B.S.; Pantoya, M.L.; Son, S.F.; Asay, B.W.; Mang, J.T. Combustion velocities and propagation mechanisms of metastable interstitial composites. J. Appl. Phys. 2005, 98, 064903. [Google Scholar] [CrossRef]
- Malchi, J.Y.; Foley, T.J.; Son, S.F.; Yetter, R.A. The effect of added Al2O3 on the propagation behavior of an Al/CuO nanoscale thermite. Combust. Sci. Technol. 2008, 180, 1278–1294. [Google Scholar] [CrossRef]
- Malchi, J.Y.; Yetter, R.A.; Foley, T.J.; Foley, T.J. Dependence of flame propagation on pressure and pressurizing gas for an Al/CuO nanoscale thermite. Proc. Combust. Inst. 2009, 32, 1895–1903. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, X.; Li, C.; Zheng, Z.; Cui, X. Facile Preparation of Micrometer KClO4/Zr Energetic Composite Particles with Enhanced Light Radiation. Materials 2019, 12, 199. https://doi.org/10.3390/ma12020199
Kang X, Li C, Zheng Z, Cui X. Facile Preparation of Micrometer KClO4/Zr Energetic Composite Particles with Enhanced Light Radiation. Materials. 2019; 12(2):199. https://doi.org/10.3390/ma12020199
Chicago/Turabian StyleKang, Xiaoli, Chunhong Li, Zhou Zheng, and Xudong Cui. 2019. "Facile Preparation of Micrometer KClO4/Zr Energetic Composite Particles with Enhanced Light Radiation" Materials 12, no. 2: 199. https://doi.org/10.3390/ma12020199
APA StyleKang, X., Li, C., Zheng, Z., & Cui, X. (2019). Facile Preparation of Micrometer KClO4/Zr Energetic Composite Particles with Enhanced Light Radiation. Materials, 12(2), 199. https://doi.org/10.3390/ma12020199



