Morphology Study on Inclusion Modifications Using Mg–Ca Treatment in Resulfurized Special Steel
Abstract
:1. Introduction
2. Laboratory Study
2.1. Sample Preparation
2.2. Analysis of Inclusions
2.3. Hot Forging Experiment
- (1)
- (2)
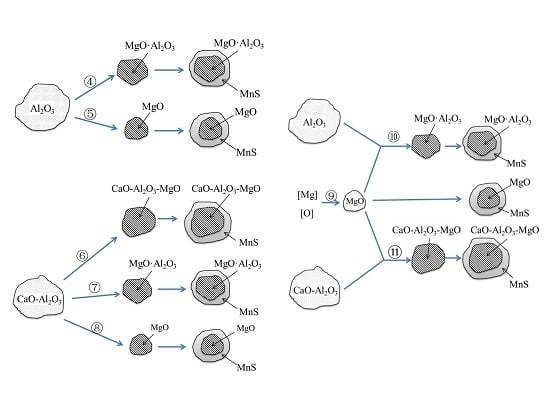
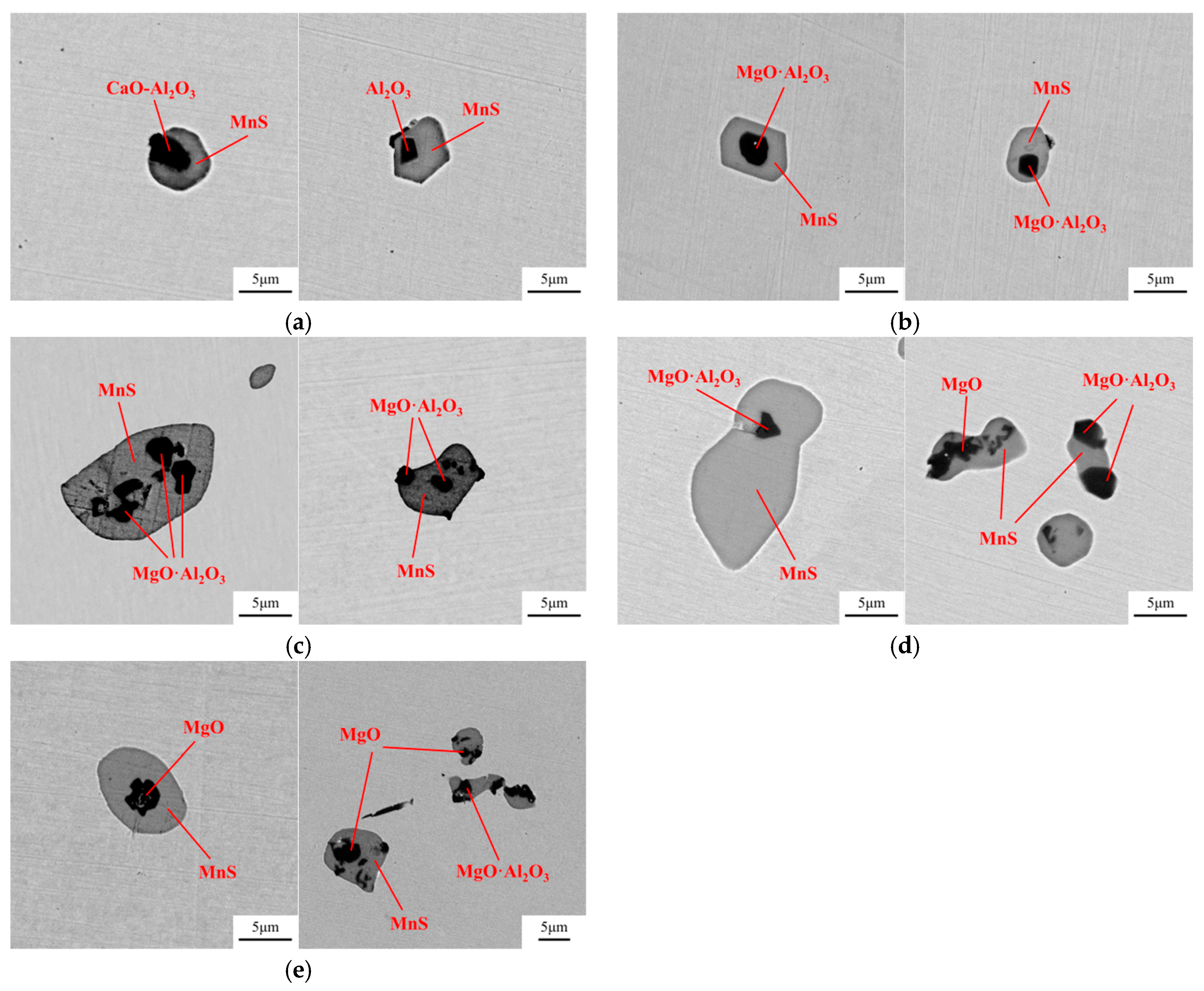
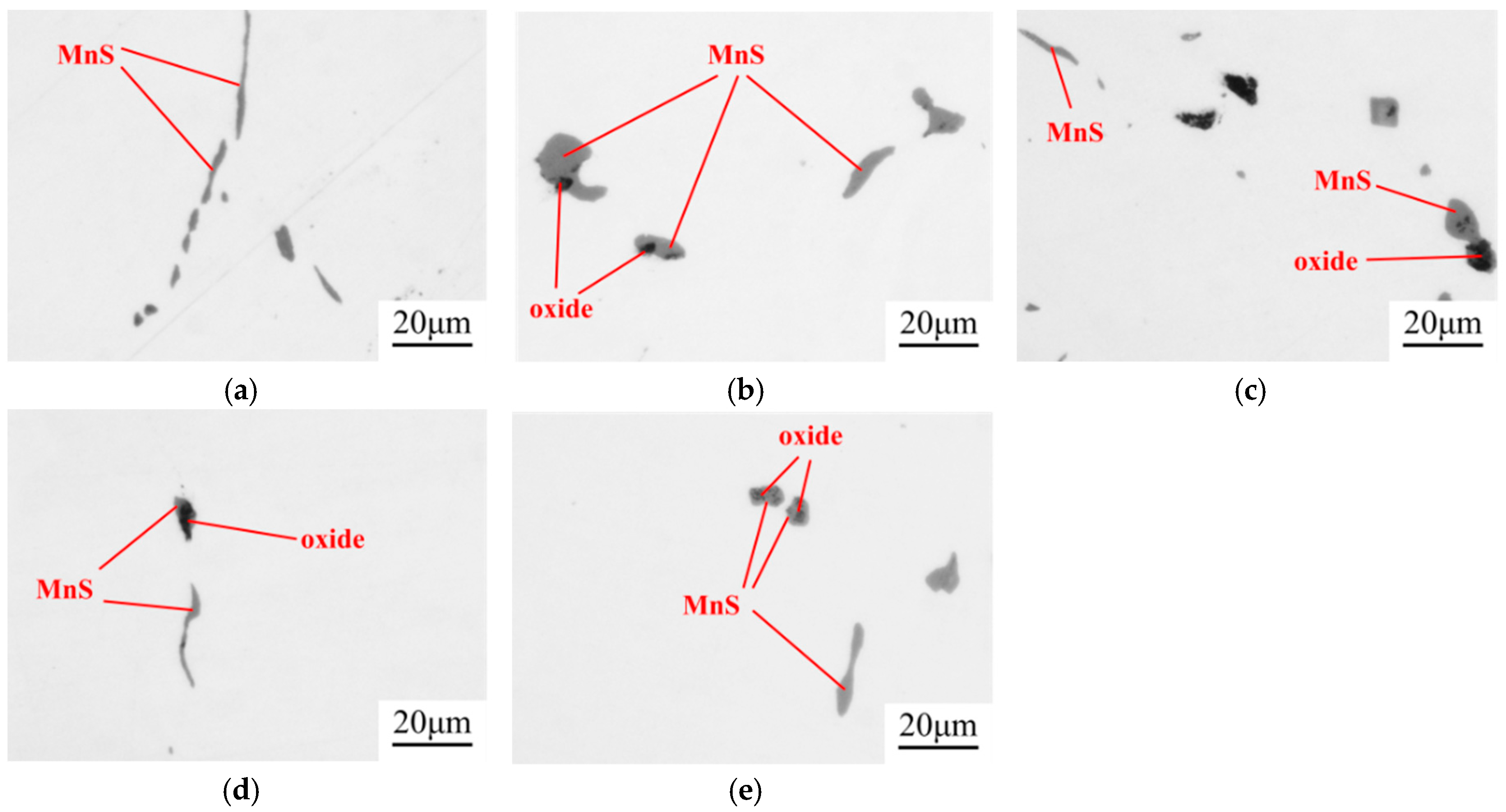
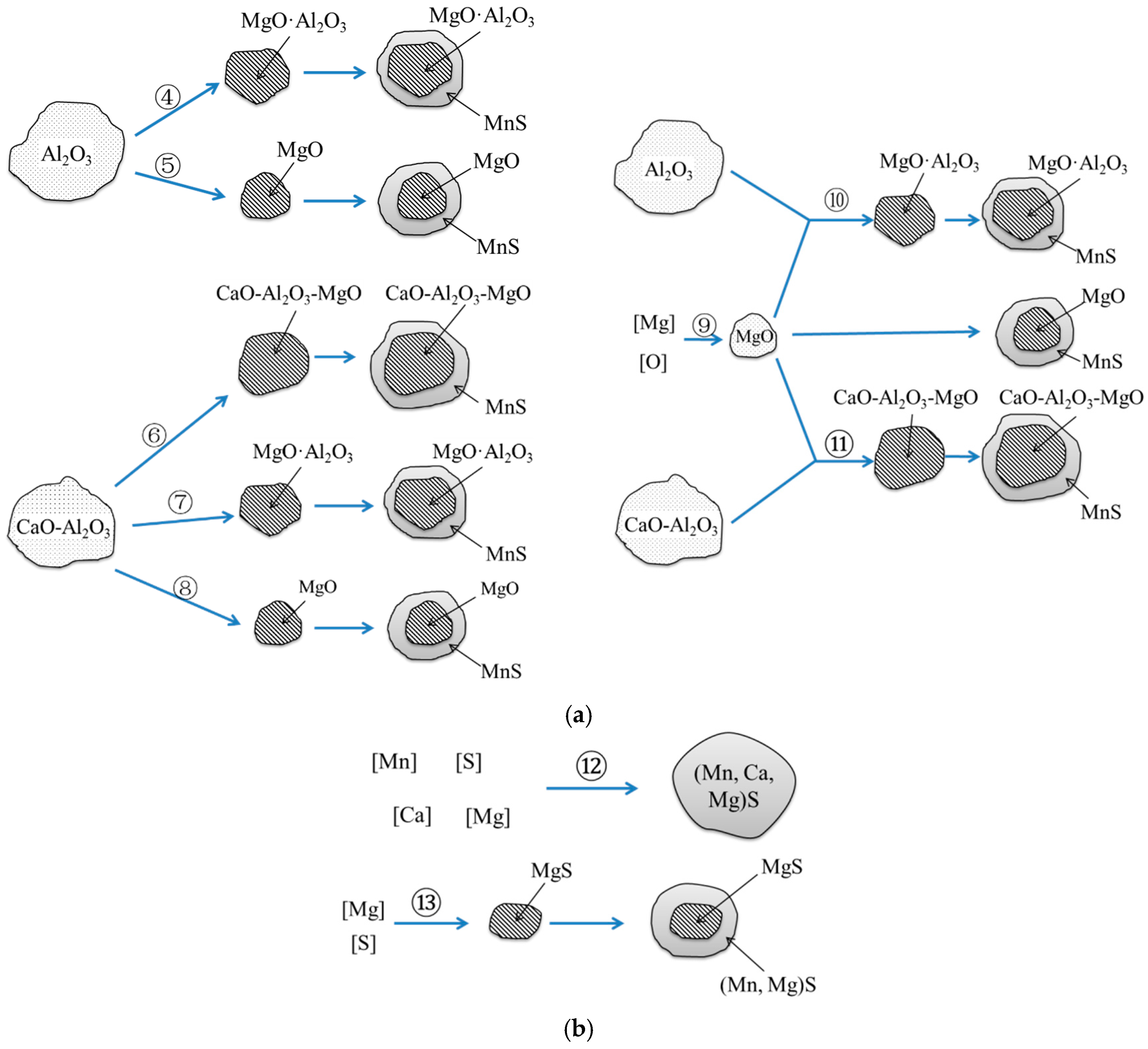
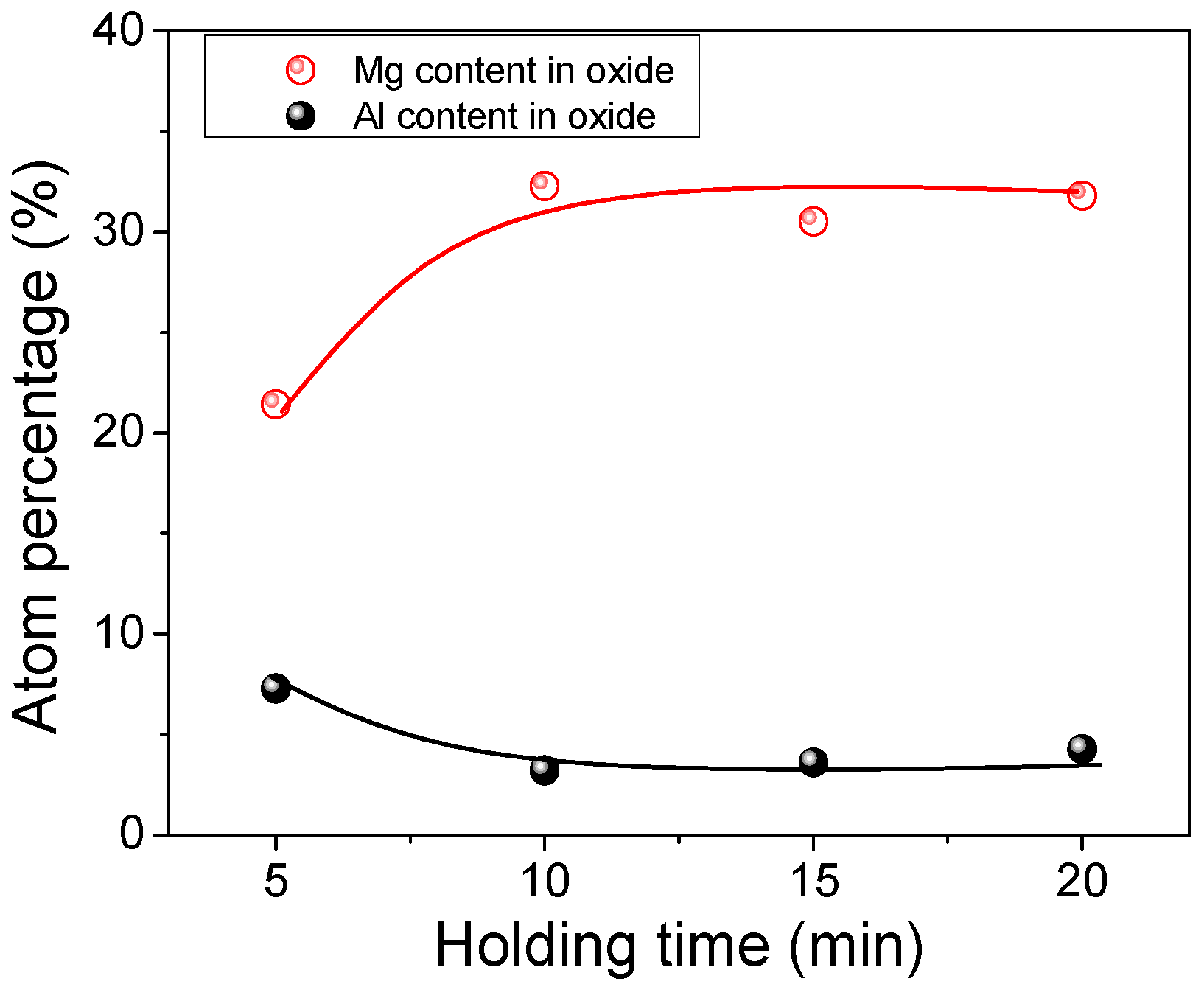
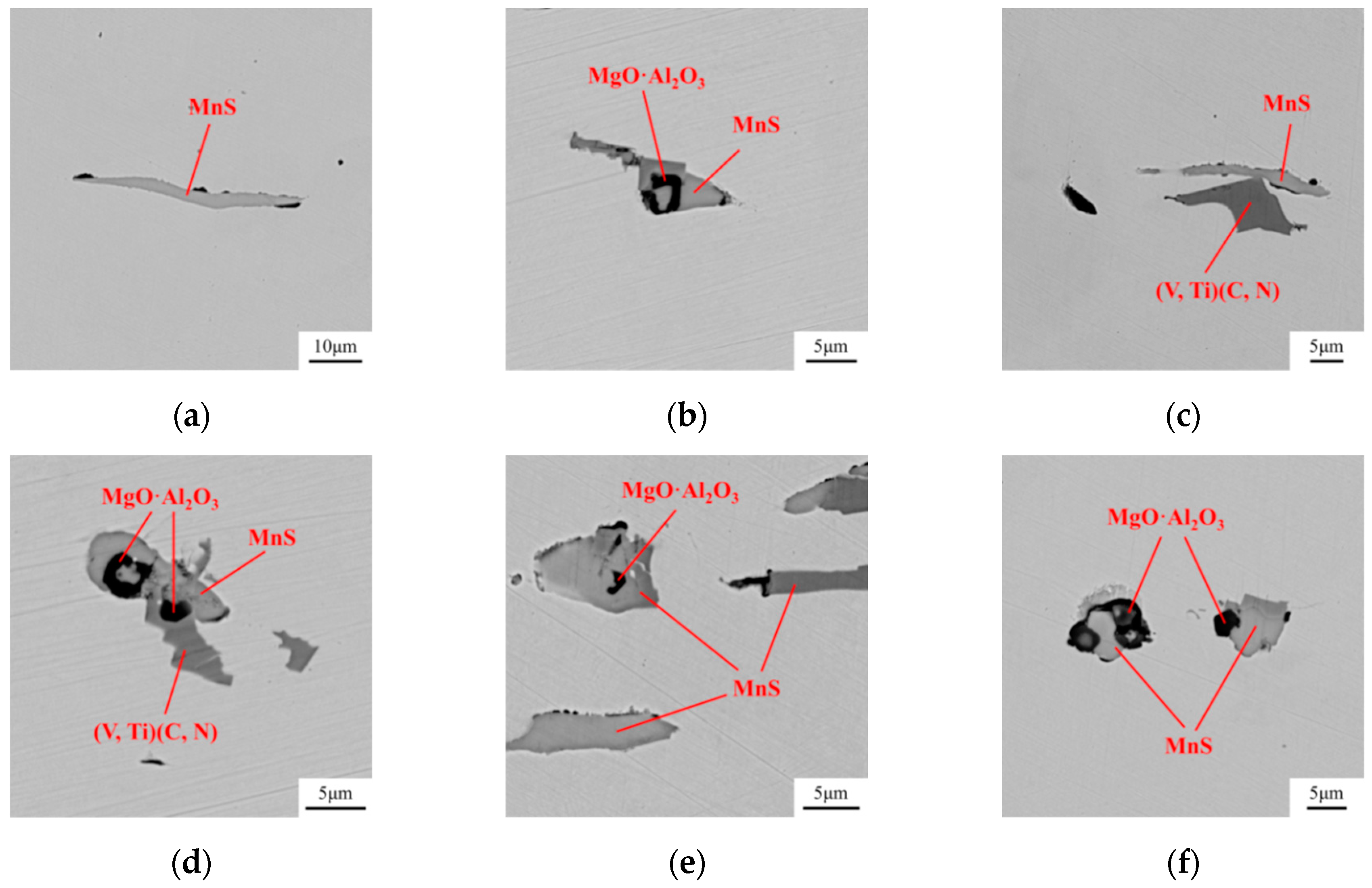
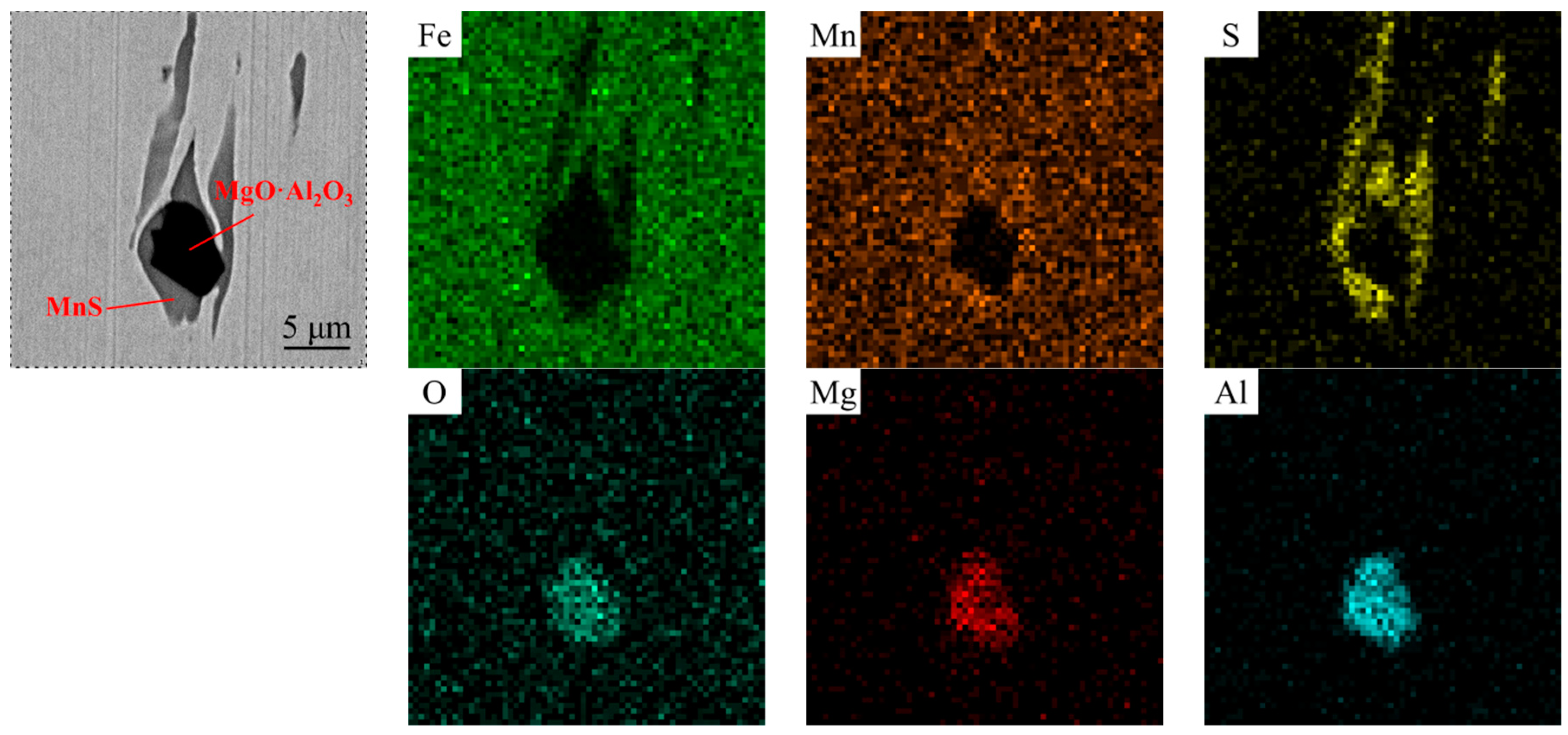
- Compared with the single MnS inclusion, the oxide inclusion had a higher strength and hardness [22]. The oxide restricted the deformation of MnS inclusion during the rolling process when it became the core of MnS. Magnesium modified the Al2O3 inclusions, forming large quantity and dispersive distributed MgO·Al2O3 spinel inclusion or MgO inclusion with a relatively small size [18,23]. It was reported that MgO·Al2O3 and MgO inclusion had a much weaker tendency to aggregate than Al2O3 inclusions [17,18,24]. During the solidification of steels, MnS took the tiny spinel inclusion or MgO inclusion as the heterogeneous nucleation point, forming the complex inclusion. The increase of Mg content caused the proportion increase of this kind of inclusion, which was beneficial to restrict more inclusions to deform into a long strip.
3. Mechanism of the Modification of Inclusions
3.1. Modification of Oxide and MnS Inclusions
3.2. Transformation of Inclusions
4. Industrial Production
5. Conclusions
- (1)
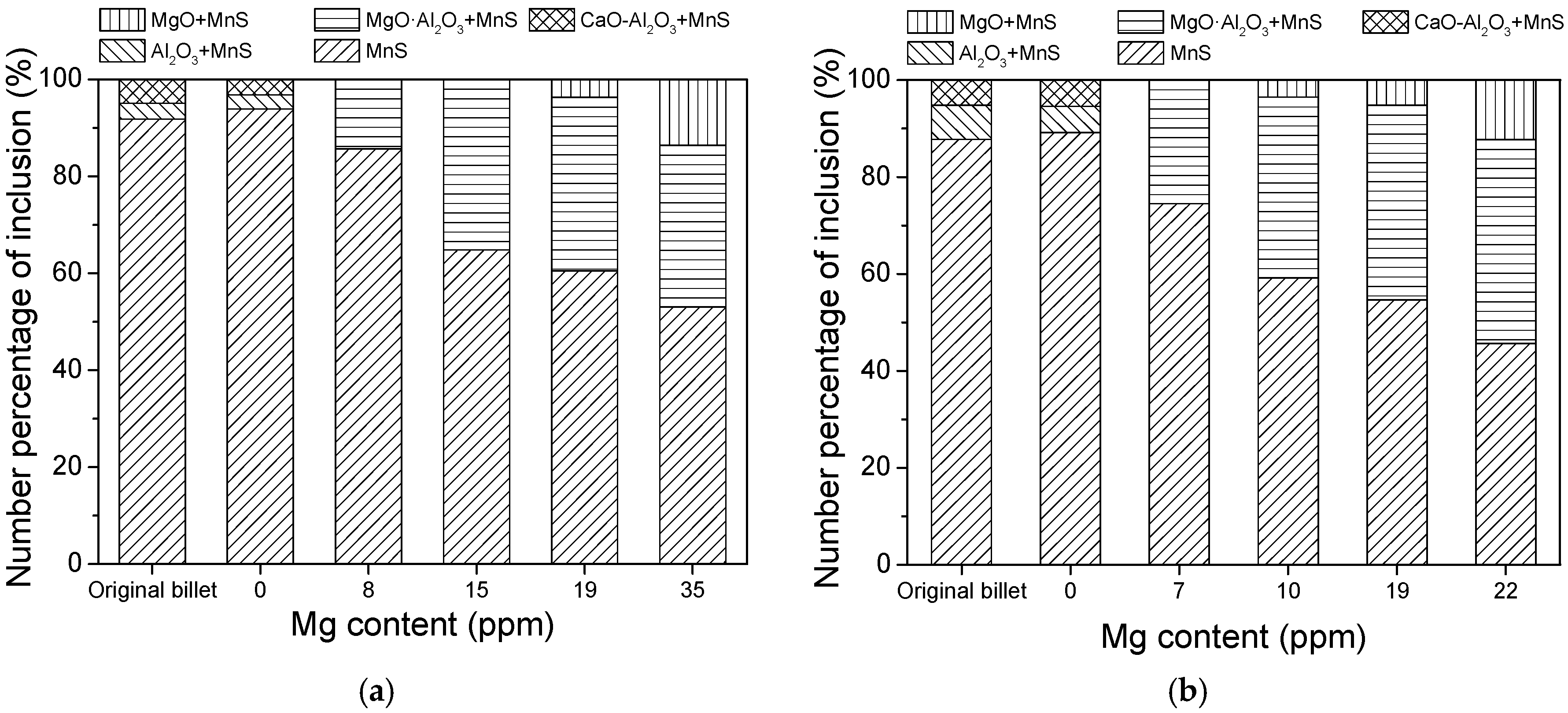
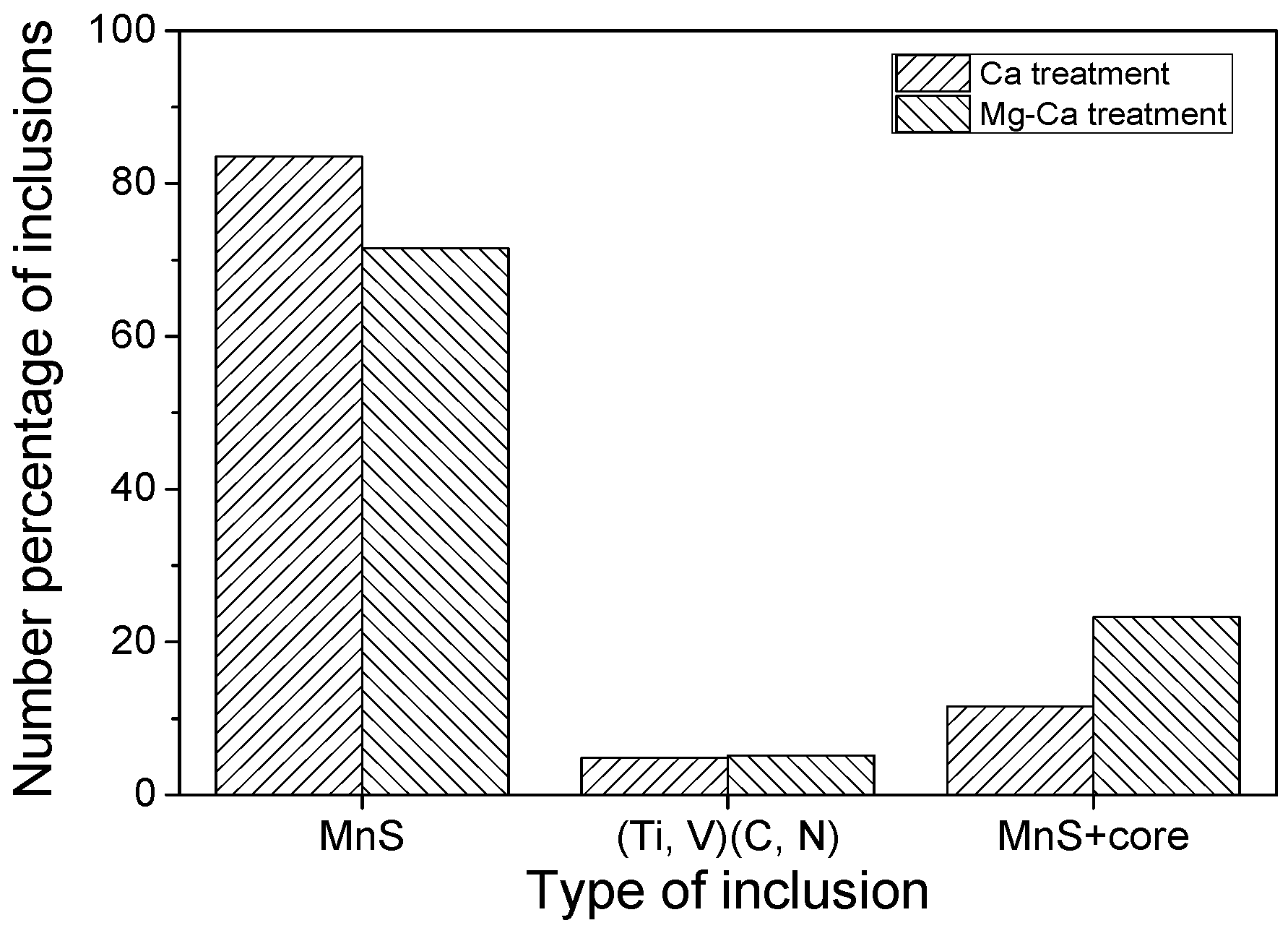
- In the laboratory study, Ni–Mg alloy was added into the Ca treated steel. With the increase of Mg content, the Al2O3 and CaO–Al2O3 gradually transformed to MgO·Al2O3 and MgO. Besides, part of Mg dissolved into MnS, and a solid solution was generated. The oxide inclusion was wrapped by sulfide in the solidification process, forming a complex inclusion with an oxide core and sulfide outer layer. With the increase of Mg content, the number percentage of complex inclusions increased, while the ratio of single MnS decreased. In addition, more inclusions transformed from type II inclusion to type III or type I inclusion.
- (2)
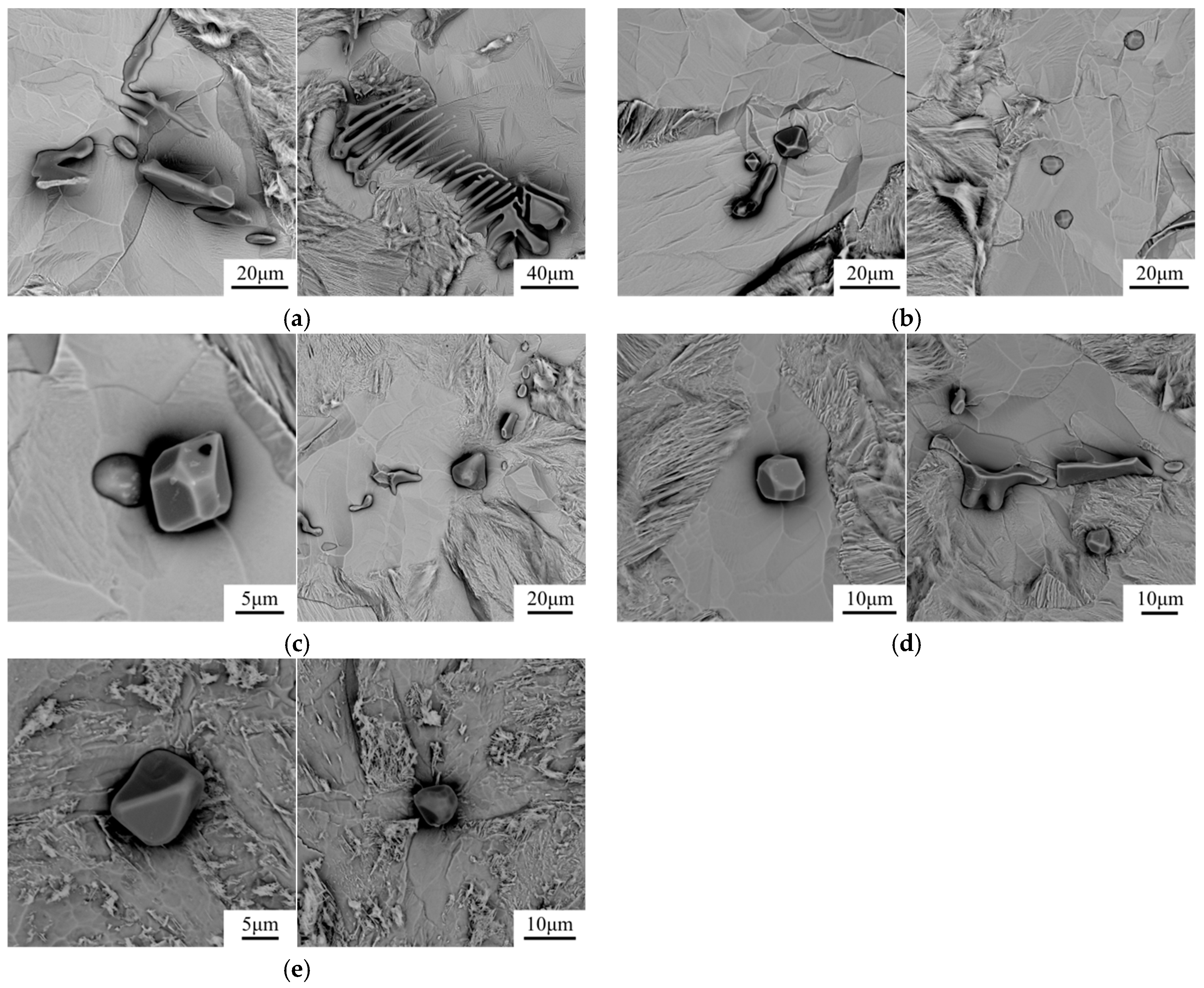
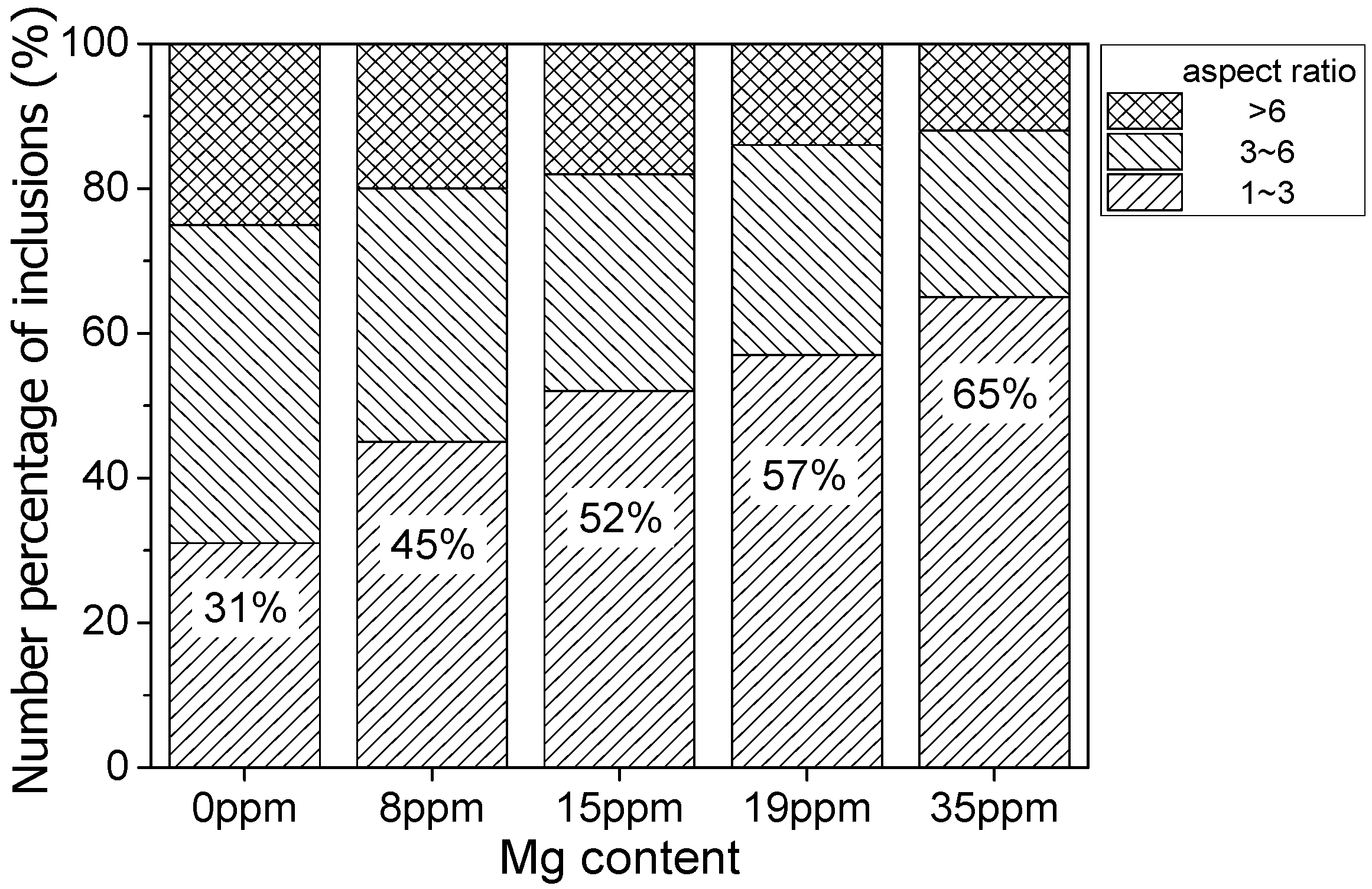
- In the hot forging experiment of 16MnCrS5 steel, the MnS inclusion without an oxide core was deformed into a long strip. While the complex inclusions showed less deformation and kept the morphology with low aspect ratio. With the increase of Mg content, the number percentage of inclusions with a small aspect ratio range increased. The addition of Mg had a significant influence on preventing the deformation of MnS inclusion.
- (3)
- The Mg–Ca modification process employed in the manufacture of non-quenched and tempered steel 49MnVS3 indicated that the extra Mg treatment resulted in the improvement of the assessment of sulfide inclusions compared with that with only Ca treatment. More inclusions had a small size and less deformation of inclusions occurred after hot rolling. The composition and ratio of different kinds of inclusions were in agreement with that in the laboratory study.
Author Contributions
Funding
Conflicts of Interest
References
- Chang, K.; Wang, P.; Liu, W. Development status and application prospect of non-quenched tempered steel. Heat Treat. Met. 2011, 36, 80–85. [Google Scholar] [CrossRef]
- Wu, S.; Liu, Z.; Wu, Y.; Wang, X. Production and application of gear steel in China. Spec. Steel 2003, 24, 30–33. [Google Scholar] [CrossRef]
- Knyazeva, M.; Rozo Vasquez, J.; Gondecki, L.; Weibring, M.; Pöhl, F.; Kipp, M.; Tenberge, P.; Theisen, W.; Walther, F.; Biermann, D. Micro-magnetic and microstructural characterization of wear progress on case-hardened 16MnCr5 gear wheels. Materials 2018, 11, 2290. [Google Scholar] [CrossRef] [PubMed]
- Pessard, E.; Morel, F.; Morel, A.; Bellett, D. Modelling the role of non-metallic inclusions on the anisotropic fatigue behaviour of forged steel. Int. J. Fatigue 2011, 33, 568–577. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Wang, F.; Hui, R.; Cao, W. Effects of sulfur addition methods and Ca-Si treatment on the microstructure and properties of 30MnVS. Int. J. Miner. Metall. Mater. 2009, 16, 650–653. [Google Scholar] [CrossRef]
- Shao, X.; Wang, X.; Jiang, M.; Wang, W.; Huang, F. Effect of heat treatment conditions on shape control of large-sized elongated MnS inclusions in resulfurized free-cutting steels. ISIJ Int. 2011, 51, 1995–2001. [Google Scholar] [CrossRef]
- Tsunekage, N.; Tsubakino, H. Effects of sulfur content and sulfide-forming elements addition on impact properties of ferrite-pearlitic microalloyd steels. ISIJ Int. 2001, 41, 498–505. [Google Scholar] [CrossRef]
- Cerullo, M. Sub-surface fatigue crack growth at alumina inclusions in AISI 52100 roller bearings. Procedia Eng. 2014, 74, 333–338. [Google Scholar] [CrossRef]
- Yang, J.; Wang, X.; Jiang, M.; Wang, W. Effect of calcium treatment on non-metallic inclusions in ultra-low oxygen steel refined by high basicity high Al2O3 slag. J. Iron. Steel Res. Int. 2011, 18, 8–14. [Google Scholar] [CrossRef]
- Deng, Z.; Zhu, M. A new double calcium treatment method for clean steel refining. Steel Res. Int. 2013, 84, 519–525. [Google Scholar] [CrossRef]
- Lind, M.; Holappa, L. Transformation of alumina inclusions by calcium treatment. Metall. Mater. Trans. B 2010, 41, 359–366. [Google Scholar] [CrossRef]
- Yan, G. Study of Technology Theory and Quality Control for Free-Cutting Machinability of Medium-Carbon-Steel; University of Science and Technology Beijing: Beijing, China, 2006. [Google Scholar]
- Qiao, M.R.; Guo, S.Q.; Su, X.; Zheng, H.Y.; Qin, L.B. Thermodynamics on the modification of inclusions by Ca and Mg treatment in GCr18Mo bearing steel. Defect Diffus. Forum 2018, 382, 73–79. [Google Scholar] [CrossRef]
- Blais, C.; L’Espérance, G.; Lehuy, H.; Forget, C. Development of an integrated method for fully characterizing multiphase inclusions and its application to calcium-treated steels. Mater. Charact. 1997, 38, 25–37. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, Y.; Zhang, L. A kinetic model for Ca treatment of Al-killed steels using FactSage macro processing. Ironmak. Steelmak. 2016, 44, 497–504. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhuang, Y.; Li, Y.; Li, S. Effect of modification treatment on inclusions in 430 stainless steel by Mg-Al alloys. J. Iron. Steel Res. Int. 2013, 20, 6–10. [Google Scholar] [CrossRef]
- Kimura, S.; Nakajima, K.; Mizoguchi, S. Behavior of alumina-magnesia complex inclusions and magnesia inclusions on the surface of molten low-carbon steels. Metall. Mater. Trans. B 2001, 32, 79–85. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, D.; Liu, C.; Jiang, M.; Lv, M.; Wang, B.; Zhang, S. Modification of inclusions in liquid iron by Mg treatment. J. Iron. Steel Res. Int. 2014, 21, 99–103. [Google Scholar] [CrossRef]
- Fu, J.; Yu, Y.; Wang, A.; Chen, B. Inclusion modification with Mg treatment for 35CrNi3MoV steel. J. Mater. Sci. Technol. 1998, 14, 53–56. [Google Scholar] [CrossRef]
- Oikawa, K.; Ohtani, H.; Ishida, K.; Nishizawa, T. The control of the morphology of MnS inclusions in steel during solidification. ISIJ Int. 2007, 35, 402–408. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Yang, W.; Dong, Y. Characterization of MnS particles in heavy rail steels using different methods. Steel Res. Int. 2017, 88, 1600080. [Google Scholar] [CrossRef]
- Ånmark, N.; Karasev, A.; Jönsson, P.G. The effect of different non-metallic inclusions on the machinability of steels. Materials 2015, 8, 751–783. [Google Scholar] [CrossRef]
- Yang, J.; Yamasaki, T.; Kuwabara, M. Behavior of inclusions in deoxidation process of molten steel with in situ produced Mg vapor. ISIJ Int. 2007, 47, 699–708. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, D.; Zhang, Y.; Jiang, M. Dynamic evolution of inclusions in Al-Mg deoxidation melts. J. Northeast. Univ. 2014, 35, 1270–1274. [Google Scholar] [CrossRef]
- Shen, P.; Yang, Q.; Zhang, D.; Yang, S.; Fu, J. The effect of tellurium on the formation of MnTe-MnS composite inclusions in non-quenched and tempered steel. Metals 2018, 8, 639. [Google Scholar] [CrossRef]
- Ye, G.; Jonsson, P.; Lund, T. Thermodynamics and kinetics of the modification of Al2O3 inclusions. ISIJ Int. 1996, 36, S105–S108. [Google Scholar] [CrossRef]
- Duan, Y.; Chen, X.; Wang, Y. Causes analysis and improvements of tundish nozzle clogging for aluminized steel. Iron Steel Vanadium Titanium 2015, 36, 123–127. [Google Scholar] [CrossRef]
- Fujii, K.; Nagasaka, T.; Hino, M. Activities of the constituents in spinel splid solution and free energies of formation of MgO, MgO·Al2O3. Trans. Inst. Iron Steel Inst. Jpn. 2000, 40, 1059–1066. [Google Scholar] [CrossRef]
- Holappa, L.; HäMäLäinen, M.; Liukkonen, M.; Lind, M. Thermodynamic examination of inclusion modification and precipitation from calcium treatment to solidified steel. Ironmak. Steelmak. 2002, 30, 111–115. [Google Scholar] [CrossRef]
- Xu, J.; Huang, F.; Wang, X. Formation mechanism of CaS-Al2O3 inclusions in low sulfur Al-killed steel after calcium treatment. Metall. Mater. Trans. B 2016, 47, 1217–1227. [Google Scholar] [CrossRef]
- Verma, N.; Pistorius, P.C.; Fruehan, R.J.; Potter, M.; Lind, M.; Story, S. Transient inclusion evolution during modification of alumina inclusions by calcium in liquid steel: Part I. Background, experimental techniques and analysis methods. Metall. Mater. Trans. B 2011, 42, 711–719. [Google Scholar] [CrossRef]
- Yang, S.; Wang, Q.; Zhang, L.; Li, J.; Peaslee, K. Formation and modification of MgO·Al2O3-based inclusions in alloy steels. Metall. Mater. Trans. B 2012, 43, 731–750. [Google Scholar] [CrossRef]
- Okuyama, G.; Yamaguchi, K.; Takeuchi, S.; Ken-Ichi, S. Effect of slag composition on the kinetics of formation of Al2O3-MgO inclusions in aluminum killed ferritic stainless steel. ISIJ Int. 2000, 40, 121–128. [Google Scholar] [CrossRef]
- Itoh, H.; Hino, M.; Ban-Ya, S. Thermodynamics on the formation of spinel nonmetallic inclusion in liquid steel. Metall. Mater. Trans. B 1997, 28, 953–956. [Google Scholar] [CrossRef]
- Jo, S.K.; Kim, S.H.; Bo, S. Thermodynamics on the formation of spinel (MgO·Al2O3) inclusion in liquid iron containing chromium. Metall. Mater. Trans. B 2002, 33, 703–709. [Google Scholar] [CrossRef]




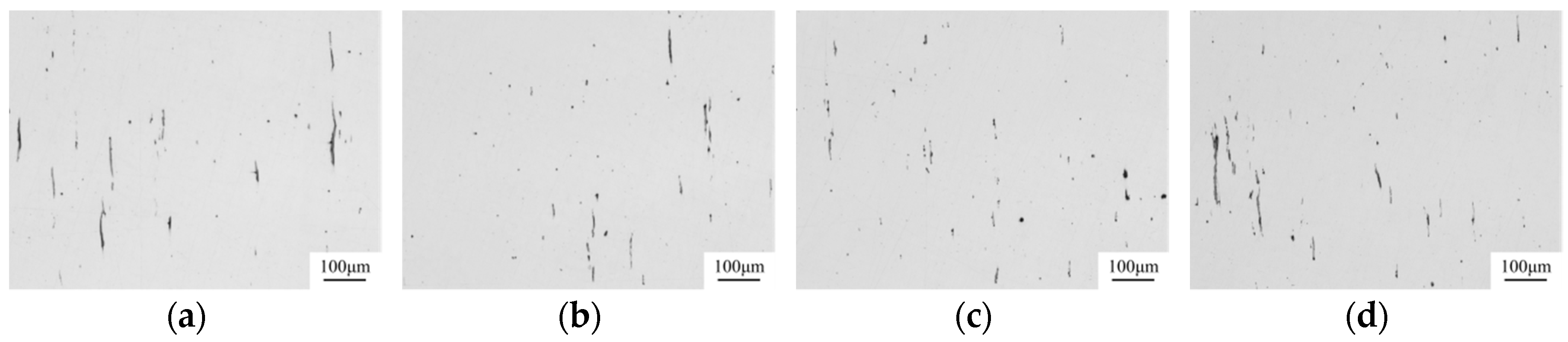
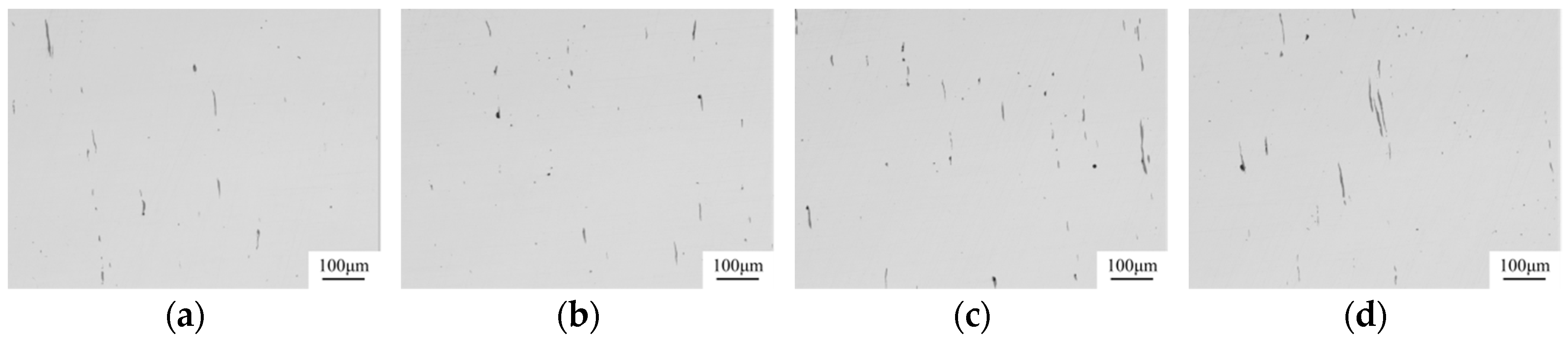
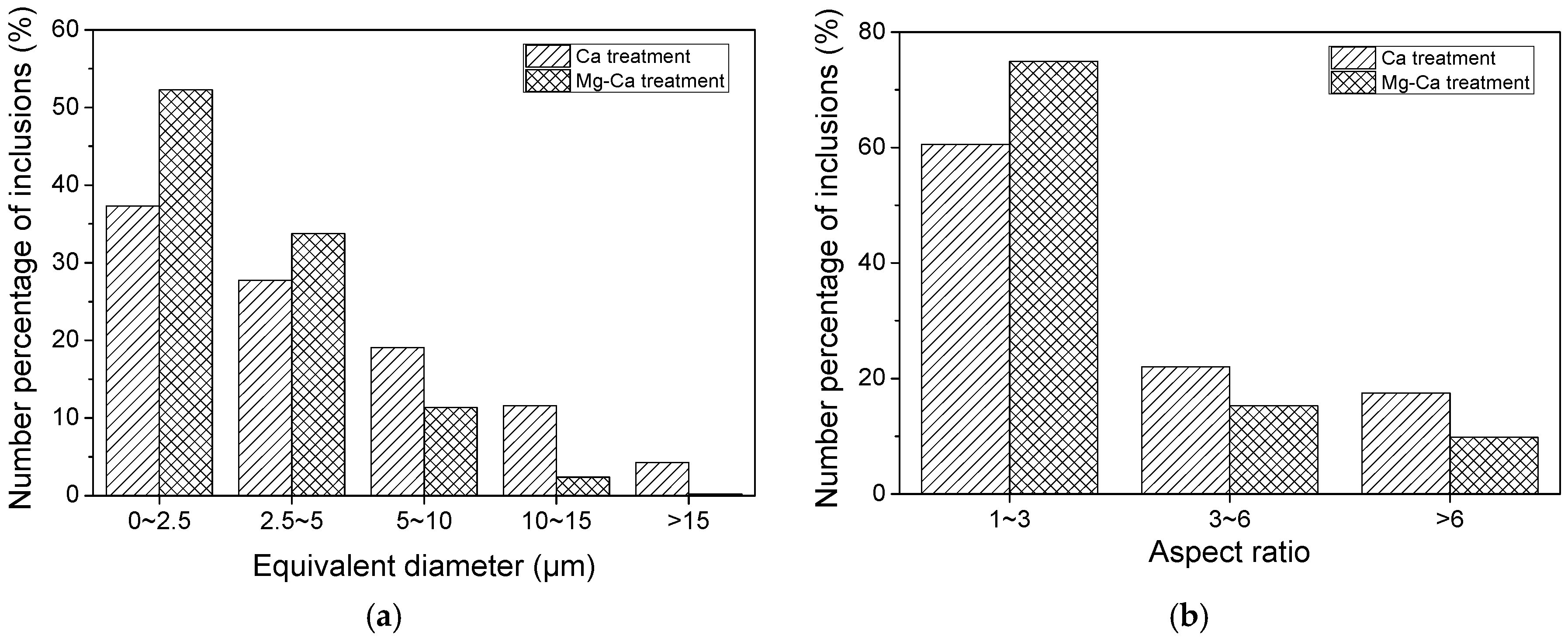
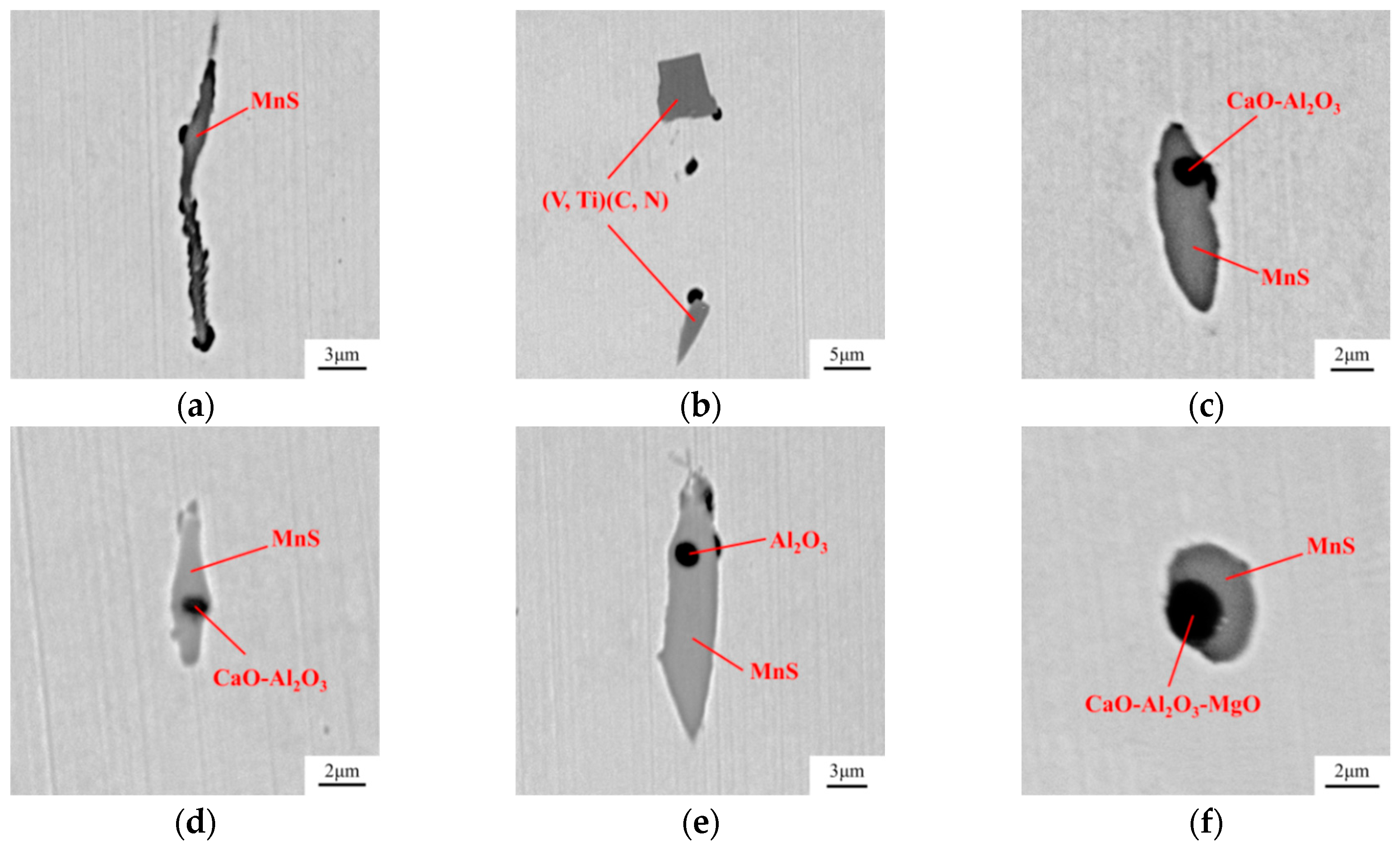
| Composition | Fe | C | Si | Mn | P | S | Alt | Cr | V | Ti | Ni | Mg | Ca | O |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 16MnCrS5 | balance | 0.16 | 0.16 | 1.18 | 0.0065 | 0.0278 | 0.031 | 1.023 | - | - | - | <0.0005 | 0.0012 | 0.0019 |
| 49MnVS3 | balance | 0.47 | 0.35 | 0.92 | 0.013 | 0.047 | 0.012 | 0.2 | 0.1 | 0.025 | - | <0.0005 | 0.0006 | 0.0015 |
| Ni–Mg alloy | 0.85 | 0.56 | 0.11 | - | - | - | - | - | - | - | 74.19 | 24.29 | - | - |
| Sample No. | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 16MnCrS5 | <5 | 8 | 15 | 19 | 35 |
| 49MnVS3 | <5 | 7 | 10 | 19 | 22 |
| No. | Reaction | Reference |
|---|---|---|
| 1 | Al2O3 + [Ca] → CaO–Al2O3 + [Al] | [29] |
| 2 | [Mn] + [S] + [Ca] → (Mn,Ca)S | - |
| 3 | [S] + [Ca] = CaS | [9,29,30,31] |
| 4 | 4Al2O3 + 3 [Mg] = 3MgO·Al2O3 + 2 [Al] | [9,32] |
| 5 | Al2O3 + 3 [Mg] = 3MgO + 2 [Al] | [32,33] |
| 6 | CaO–Al2O3 + [Mg] → CaO–Al2O3–MgO + [Ca] | - |
| 7 | CaO–Al2O3 + [Mg] → MgO·Al2O3 + [Ca] | [29] |
| 8 | CaO–Al2O3 + [Mg] → MgO + [Ca] + [Al] | - |
| 9 | [Mg] + [O] = MgO | [19,32] |
| 10 | Al2O3 + MgO = MgO·Al2O3 | [28,32,34,35] |
| 11 | CaO–Al2O3 + MgO → CaO–Al2O3–MgO | - |
| 12 | [Mn] + [S] + [Ca] + [Mg] → (Mn,Ca,Mg)S | - |
| 13 | [Mg] + [S] = MgS | [18] |
| Composition | C | Si | Mn | P | S | Cr | V | Ni | Ti | Al | N |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lower limit | 0.46 | 0.25 | 0.85 | -- | 0.04 | 0.15 | 0.09 | -- | 0.015 | -- | 0.008 |
| Upper limit | 0.49 | 0.4 | 0.95 | 0.025 | 0.06 | 0.3 | 0.12 | 0.2 | 0.03 | 0.02 | 0.02 |
| Target | 0.47 | 0.35 | 0.9 | ≤0.020 | 0.05 | 0.2 | 0.1 | ≤0.20 | 0.025 | 0.01 | 0.015 |
| Process | Average | Worst | ||
|---|---|---|---|---|
| Fine Series | Thick Series | Fine Series | Thick Series | |
| Ca treatment | 2.0 | 1.5 | 3.0 | 2.5 |
| Mg–Ca treatment | 1.5 | 1.0 | 2.0 | 1.5 |
| Process | Diameter (μm) | Number Density (mm−2) | Area Fraction (%) | Aspect Ratio |
|---|---|---|---|---|
| Ca treatment | 5.17 | 179 | 0.52 | 3.63 |
| Mg–Ca treatment | 3.19 | 263 | 0.25 | 2.73 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, P.; Fu, J. Morphology Study on Inclusion Modifications Using Mg–Ca Treatment in Resulfurized Special Steel. Materials 2019, 12, 197. https://doi.org/10.3390/ma12020197
Shen P, Fu J. Morphology Study on Inclusion Modifications Using Mg–Ca Treatment in Resulfurized Special Steel. Materials. 2019; 12(2):197. https://doi.org/10.3390/ma12020197
Chicago/Turabian StyleShen, Ping, and Jianxun Fu. 2019. "Morphology Study on Inclusion Modifications Using Mg–Ca Treatment in Resulfurized Special Steel" Materials 12, no. 2: 197. https://doi.org/10.3390/ma12020197
APA StyleShen, P., & Fu, J. (2019). Morphology Study on Inclusion Modifications Using Mg–Ca Treatment in Resulfurized Special Steel. Materials, 12(2), 197. https://doi.org/10.3390/ma12020197