Promoted Anodizing Reaction and Enhanced Coating Performance of Al–11Si Alloy: The Role of an Equal-Channel-Angular-Pressed Substrate
Abstract
1. Introduction
2. Experimental
2.1. Processing Route
2.2. Microstructure Characterizations
2.3. Corrosion Tests
3. Results
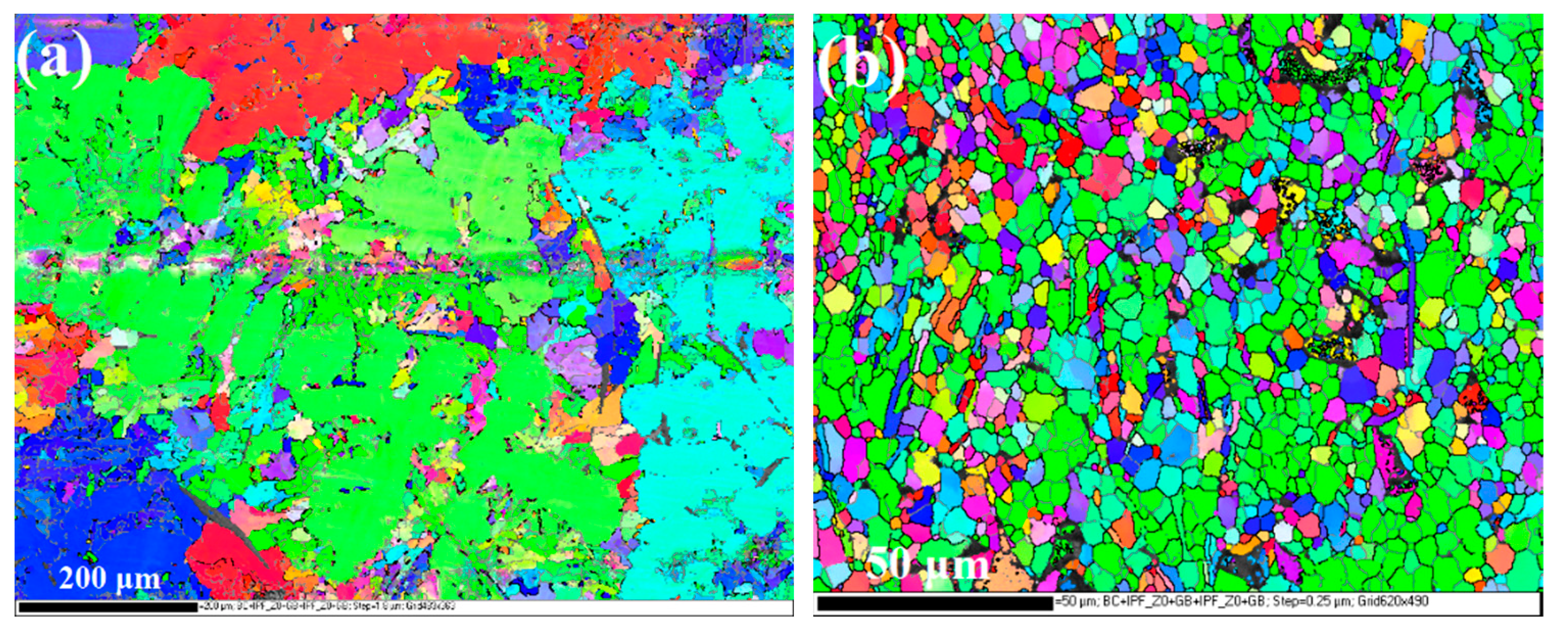
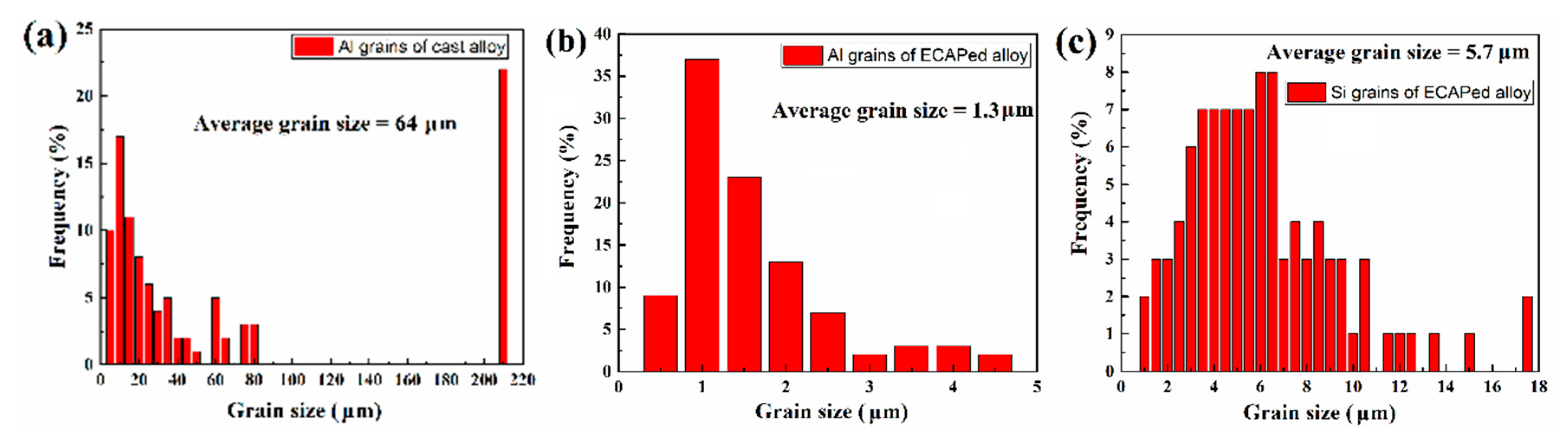
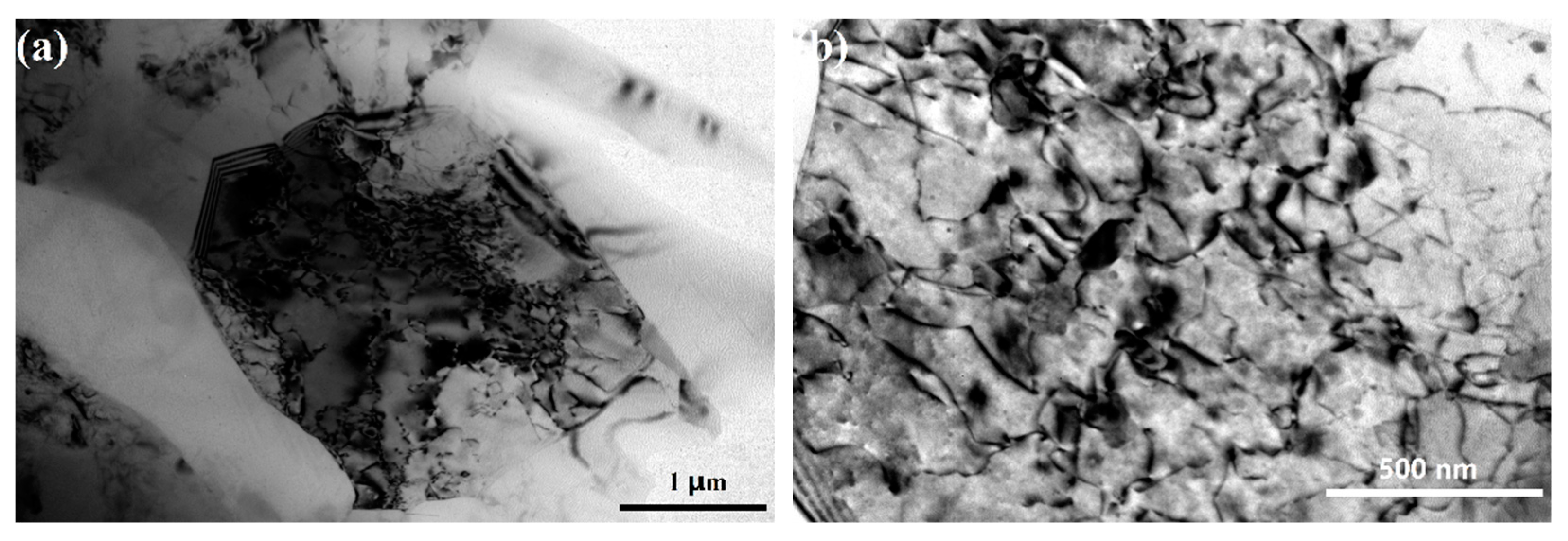
3.1. Microstructure Characteristics of the Substrate and the Anodizing Coating
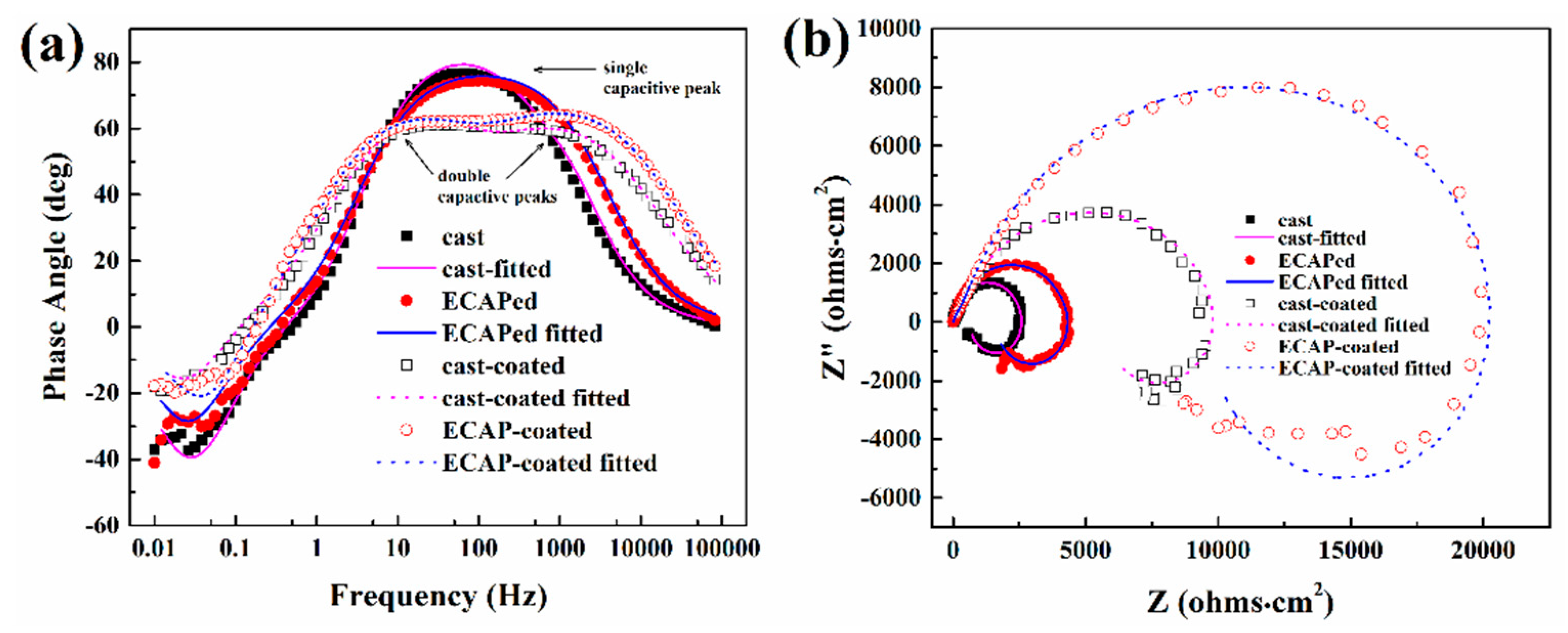
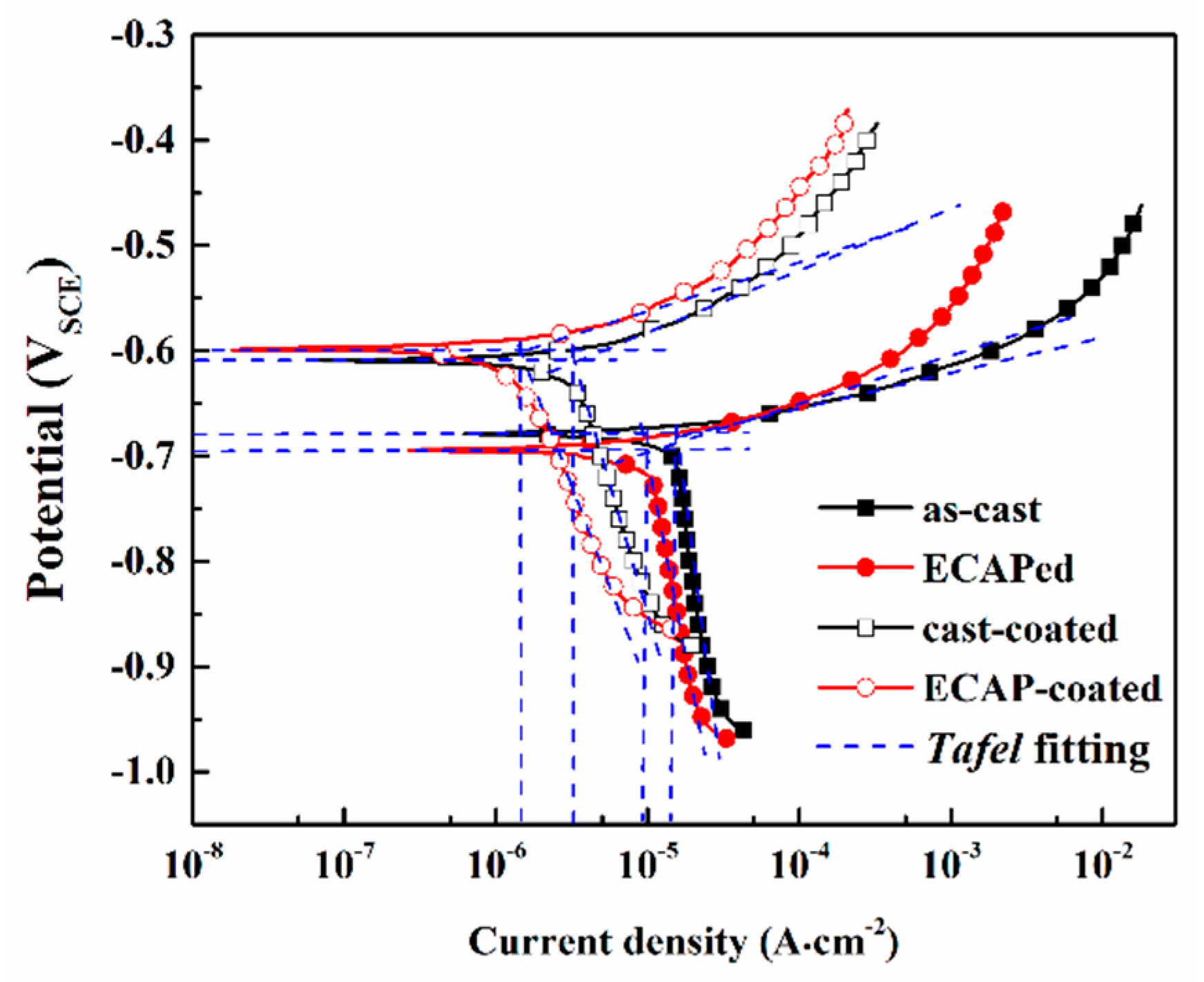
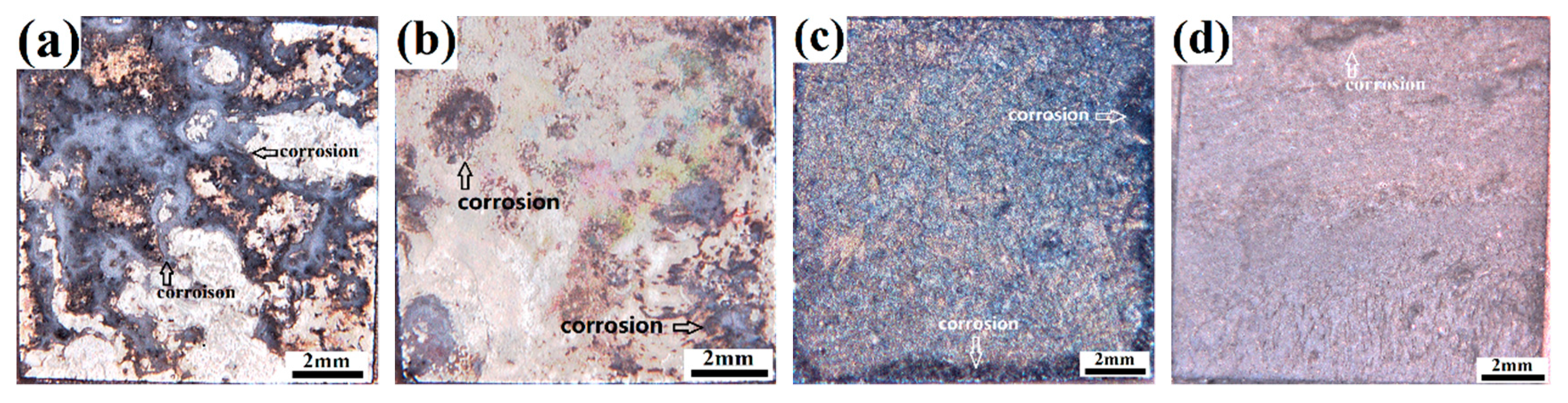
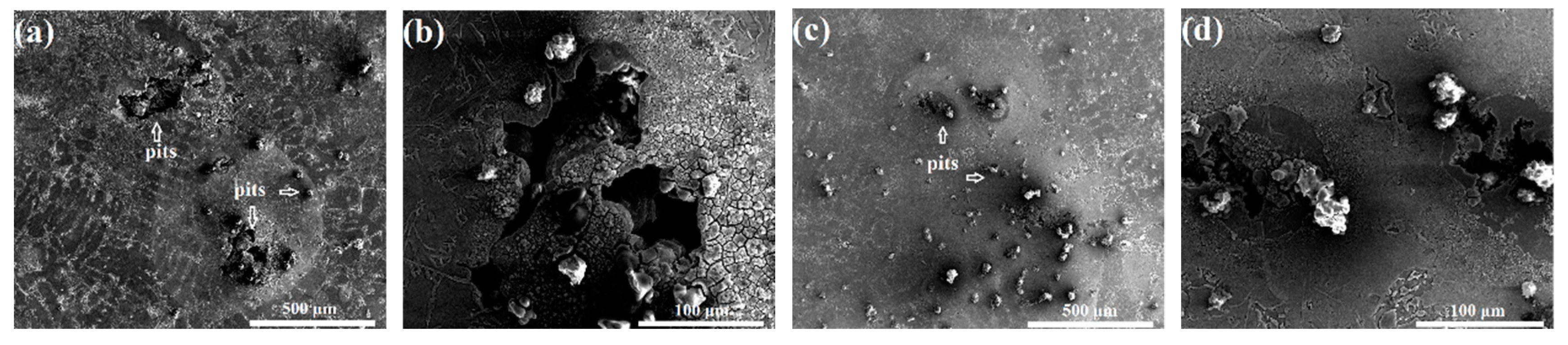
3.2. Corrosion Behavior in NaCl Medium
4. Discussion
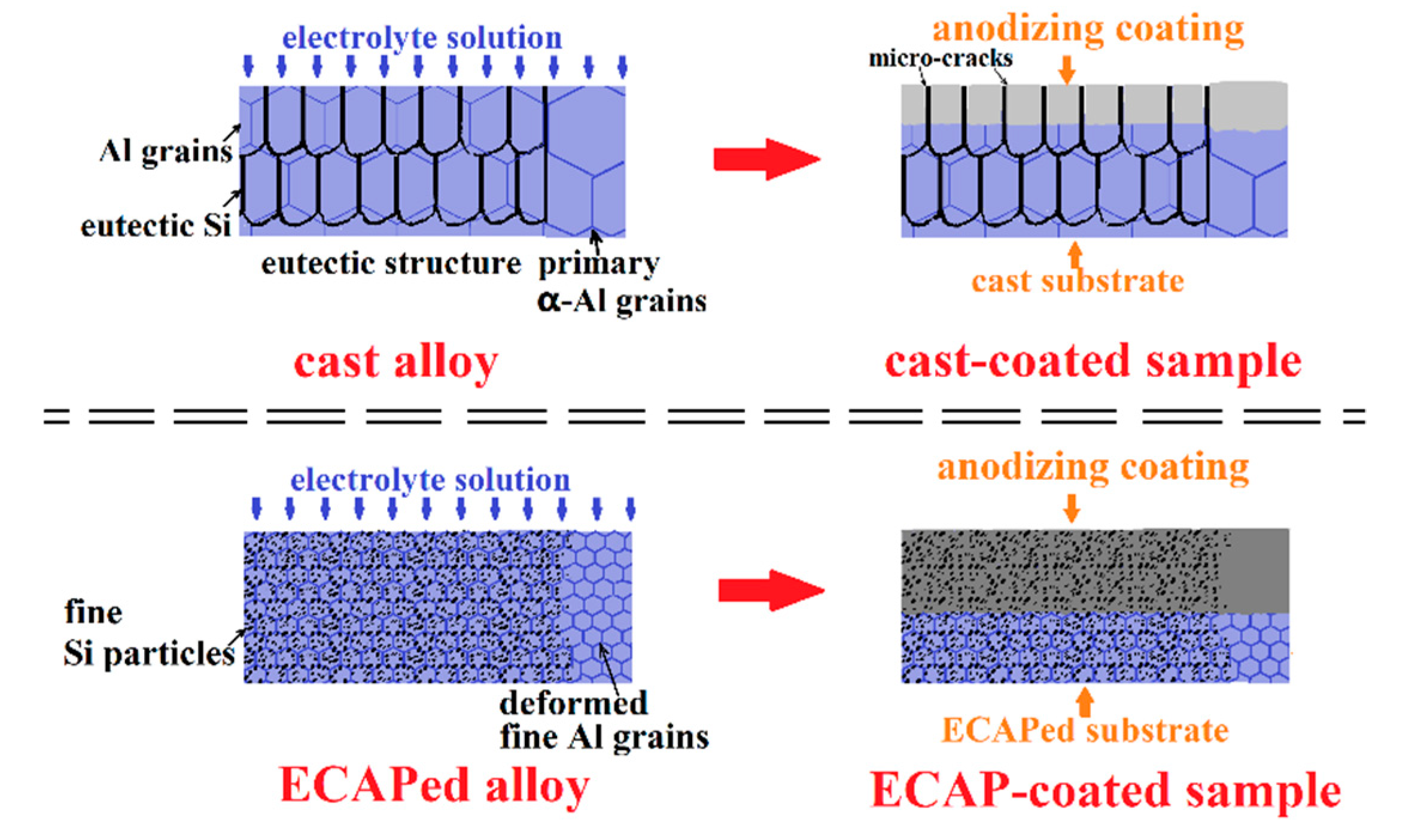
4.1. Effect of Fine Al Grains in the ECAPed Substrate on the Promoted Anodizing Reaction
4.2. Effect of Fragmentation of the Eutectic Si Phase on Enhancing Coating Compactness
4.3. Mechanism of the Significantly Improved Anticorrosion Performance of the ECAP-Coated Sample
5. Conclusions
- Optimum anodizing coating for both coated samples can be fabricated after anodizing reaction for 30 min. There is a competition between the cast and the ECAPed alloys in obtaining the quota of the anodizing current. During the whole anodizing duration, the anodizing current of the ECAP-coated alloy is always higher than that of the cast-coated alloy.
- The ECAP-coated sample has a thicker, more compact, and more uniform anodizing coating compared to the cast-coated sample. Fine Al grains with profuse grain boundaries and dislocations promote the anodizing reaction from both thermodynamics and kinetics perspectives, which leads to a thicker coating. The fragmentation of the eutectic Si into uniformly distributed fine Si-particles in the ECAPed alloy effectively suppresses the development of microscale porosity and cracks in the coating, leading to enhanced coating compactness and uniformity.
- The ECAP-coated sample has the optimum anticorrosion performance, presenting the lowest icorr and largest Rp in PDP test, largest Rt and Rpore in EIS test, and the least corrosion damage in the salt spray test. The enhanced protection from the anodizing coating and the improved corrosion resistance of the substrate are the two factors contributing to the improved anticorrosion performance of the ECAP-coated sample.
Author Contributions
Funding
Conflicts of Interest
References
- Davis, J.R. (Ed.) Aluminum and Aluminum Alloys; ASM International: Cleveland, OH, USA, 1993; p. 627. [Google Scholar]
- Wang, Z.; Zhu, F.; Zheng, K.; Jia, J.; Wei, Y.; Li, H.; Huang, L.; Zheng, Z. Effect of the thickness reduction on intergranular corrosion in an under–aged Al–Mg–Si–Cu alloy during cold–rolling. Corros. Sci. 2018, 142, 201–212. [Google Scholar] [CrossRef]
- Williams, J.C.; Starke, E.A., Jr. Progress in structural materials for aerospace systems. Acta Mater. 2003, 51, 5775–5799. [Google Scholar] [CrossRef]
- Li, H.; Zhao, P.; Wang, Z.; Mao, Q.; Fang, B.; Song, R.; Zheng, Z. The intergranular corrosion susceptibility of a heavily overaged Al-Mg-Si-Cu alloy. Corros. Sci. 2016, 107, 113–122. [Google Scholar] [CrossRef]
- El-Menshawy, K.; El-Sayed, A.-W.A.; El-Bedawy, M.E.; Ahmed, H.A.; El-Raghy, S.M. Effect of aging time at low aging temperatures on the corrosion of aluminum alloy 6061. Corros. Sci. 2012, 54, 167–173. [Google Scholar] [CrossRef]
- Karunakaran, M.; Vadivu, M.P. Magnetic and micro-mechanical behavior of Cu-Ni-P-W-TiO2 hybrid composite electroplating on Al alloy substrate. J. Magn. Magn. Mater. 2019, 475, 359–367. [Google Scholar] [CrossRef]
- Zhou, L.; Friis, H.; Roefzaad, M.; Hansen, K.B.; Eisenhardt, S.; Andersen, A.G.; Tabrizian, N.; Zangenberg, N. Steam initiated hydrotalcite conversion coatings: Application to environmental Al alloy surface treatment. Surf. Coat. Tech. 2018, 340, 45–54. [Google Scholar] [CrossRef]
- Thirupathi, K.; Bárczy, P.; Vad, K.; Csík, A.; Somosvári, B.M. Effects of vacuum and ageing on Zr4/Cr3 based conversion coatings on aluminium alloys. Appl. Surf. Sci. 2018, 441, 1043–1047. [Google Scholar] [CrossRef]
- Kubisztal, J.; Kubisztal, M.; Stach, S.; Haneczok, G. Corrosion resistance of anodic coatings studied by scanning microscopy and electrochemical methods. Surf. Coat. Tech. 2018, 350, 419–427. [Google Scholar] [CrossRef]
- Chen, L.; Liu, Z.; Shen, Q. Enhancing tribological performance by anodizing micro-textured surfaces with nano-MoS2 coatings prepared on aluminum-silicon alloys. Tribol. Int. 2018, 122, 84–95. [Google Scholar] [CrossRef]
- Garcia-Vergara, S.; Skeldon, P.; Thompson, G.; Habazaki, H. A flow model of porous anodic film growth on aluminium. Electrochim. Acta 2006, 52, 681–687. [Google Scholar] [CrossRef]
- Zaraska, L.; Sulka, G.D.; Szeremeta, J.; Jaskula, M. Porous anodic alumina formed by anodization of aluminumalloy (AA1050) and high purity aluminum. Electrochim. Acta 2010, 55, 4377–4386. [Google Scholar] [CrossRef]
- Stępniowski, W.J.; Nowak-Stępniowska, A.; Bojar, Z. Quantitative arrangement analysis of anodic alumina formed by short anodizations in oxalic acid. Mater. Charact. 2013, 78, 79–86. [Google Scholar] [CrossRef]
- Caliari, D.; Timelli, G.; Zabala, B.; Igartua, A. Microstructural and tribological investigations of diecast and hard anodized AlSiCu alloys. Surf. Coat. Tech. 2018, 352, 462–473. [Google Scholar] [CrossRef]
- Wang, W.-L.; Tsai, Y.-C. Microstructural characterization and mechanical property of active soldering anodized 6061 Al alloy using Sn–3.5Ag–xTi active solders. Mater. Charact. 2012, 68, 42–48. [Google Scholar] [CrossRef]
- Mehdizade, M.; Soltanieh, M.; Eivani, A.R. Investigation of anodizing time and pulse voltage modes on the corrosion behavior of nanostructured anodic layer in commercial pure aluminum. Surf. Coat. Tech. 2019, 358, 741–752. [Google Scholar] [CrossRef]
- Valiev, R.Z.; Langdon, T.G. Principles of equal-channel angular pressing as a processing tool for grain refinement. Prog. Mater. Sci. 2006, 51, 881–981. [Google Scholar] [CrossRef]
- Ovid’Ko, I.; Valiev, R.; Zhu, Y. Review on superior strength and enhanced ductility of metallic nanomaterials. Prog. Mater. Sci. 2018, 94, 462–540. [Google Scholar] [CrossRef]
- Natori, K.; Utsunomiya, H.; Tanaka, T. Improvement in formability of semi-solid cast hypoeutectic Al-Si alloys by equal-channel angular pressing. J. Mater. Process. Technol. 2017, 240, 240–248. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, M.; Chen, X.; Cao, Y.; Roven, H.J.; Murashkin, M.; Valiev, R.Z.; Zhou, H. Effect of Mg on microstructure and mechanical properties of Al-Mg alloys produced by high pressure torsion. Scr. Mater. 2019, 159, 137–141. [Google Scholar] [CrossRef]
- Ma, A.; Nishida, Y.; Suzuki, K.; Shigematsu, I.; Saito, N. Characteristics of plastic deformation by rotary-die equal-channel angular pressing. Scr. Mater. 2005, 52, 433–437. [Google Scholar] [CrossRef]
- Dong, J.F.; Dong, Q.; Dai, Y.B.; Xing, H.; Han, Y.F.; Ma, J.B.; Zhang, J.; Wang, J.; Sun, B.D. Microstructure Evolution in High Purity Aluminum Single Crystal Processed by Equal Channel Angular Pressing (ECAP). Materials 2017, 10, 87. [Google Scholar] [CrossRef] [PubMed]
- Ma, A.; Suzuki, K.; Nishida, Y.; Saito, N.; Shigematsu, I.; Takagi, M.; Iwata, H.; Watazu, A.; Imura, T. Impact toughness of an ultrafine-grained Al–11mass%Si alloy processed by rotary-die equal-channel angular pressing. Acta Mater. 2005, 53, 211–220. [Google Scholar] [CrossRef]
- Hansen, N. Hall–Petch relation and boundary strengthening. Scr. Mater. 2004, 51, 801–806. [Google Scholar] [CrossRef]
- Jiang, J.-H.; Ma, A.-B.; Song, D.; Saito, N.; Yuan, Y.-C.; Nishida, Y. Corrosion behavior of hypereutectic Al-23%Si alloy (AC9A) processed by severe plastic deformation. Trans. Nonferrous Met. Soc. China 2010, 20, 195–200. [Google Scholar] [CrossRef]
- Jiang, J.H.; Ma, A.B.; Lu, F.M.; Saito, N.; Watazu, A.; Song, D.; Zhang, P.; Nishida, Y. Improving corrosion resistance of Al-11mass%Si alloy through a large number of ECAP passes. Mater. Corros. 2011, 62, 848–852. [Google Scholar] [CrossRef]
- Jiang, J.; Ma, A.; Song, D.; Yang, D.; Shi, J.; Wang, K.; Zhang, L.; Chen, J. Anticorrosion behavior of ultrafine-grained Al-26 wt% Si alloy fabricated by ECAP. J. Mater. Sci. 2012, 47, 7744–7750. [Google Scholar] [CrossRef]
- Son, I.-J.; Nakano, H.; Oue, S.; Kobayashi, S.; Fukushima, H.; Horita, Z. Pitting Corrosion Resistance of Anodized Aluminum Alloy Processed by Severe Plastic Deformation. Mater. Trans. 2007, 48, 21–28. [Google Scholar] [CrossRef]
- Okayasu, M.; Ota, K.; Takeuchi, S.; Ohfuji, H.; Shiraishi, T. Influence of microstructural characteristics on mechanical properties of ADC12 aluminum alloy. Mater. Sci. Eng. A 2014, 592, 189–200. [Google Scholar] [CrossRef]
- Makhlouf, M.; Guthy, H. The aluminum–silicon eutectic reaction: mechanisms and crystallography. J. Light Met. 2001, 1, 199–218. [Google Scholar] [CrossRef]
- Heiberg, G.; Nogita, K.; Dahle, A.; Arnberg, L. Columnar to equiaxed transition of eutectic in hypoeutectic aluminium–silicon alloys. Acta Mater. 2002, 50, 2537–2546. [Google Scholar] [CrossRef]
- Ganiger, B.; Chandrashekharaiah, T.; Prasad, T.; Kabadi, V. Studies on Relationship between Wear Behaviour and Microstructure of a Hypereutectic Al-Si Alloy. Mater. Today Proc. 2018, 5, 25165–25173. [Google Scholar] [CrossRef]
- Ganiger, B.; Chandrashekharaiah, T.M.; Prasad, T.B.; Kabadi, V.R. Wear behavior and microstructure of hypereutectic Al–Si alloys prepared by selective laser melting. Appl. Surf. Sci. 2016, 378, 142–149. [Google Scholar]
- Ahn, S.S.; Pathan, S.; Koo, J.M.; Baeg, C.H.; Jeong, C.U.; Son, H.T.; Kim, Y.H.; Lee, K.H.; Hong, S.J. Enhancement of the Mechanical Properties in Al–Si–Cu–Fe–Mg Alloys with Various Processing Parameters. Materials 2018, 11, 2150. [Google Scholar] [CrossRef] [PubMed]
- Bugelnig, K.; Germann, H.; Steffens, T.; Sket, F.; Adrien, J.; Maire, E.; Boller, E.; Requena, G. Revealing the Effect of Local Connectivity of Rigid Phases during Deformation at High Temperature of Cast AlSi12Cu4Ni(2,3)Mg Alloys. Materials 2018, 11, 1300. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Ji, Z.; Hu, M.; Xu, H.; Xu, S. A novel modifier on eutectic Si and mechanical properties of Al-Si alloy. Mater. Lett. 2019, 239, 13–16. [Google Scholar] [CrossRef]
- Jeon, J.; Shin, J.; Bae, D. Si phase modification on the elevated temperature mechanical properties of Al-Si hypereutectic alloys. Mater. Sci. Eng. A 2019, 748, 367–370. [Google Scholar] [CrossRef]
- Jiang, W.Y.; Yu, W.Z.; Li, J.; You, Z.X.; Li, C.M.; Lv, X.W. Segregation and Morphological Evolution of Si Phase during Electromagnetic Directional Solidification of Hypereutectic Al–Si Alloys. Materials 2019, 12, 10. [Google Scholar] [CrossRef]
- Rakhmonov, J.; Timelli, G.; Bonollo, F. Characterization of the solidification path and microstructure of secondary Al-7Si-3Cu-0.3Mg alloy with Zr, V and Ni additions. Mater. Charact. 2017, 128, 100–108. [Google Scholar] [CrossRef]
- Wu, Y.; Liao, H.; Yang, J.; Zhou, K. Effect of Si Content on Dynamic Recrystallization of Al–Si–Mg Alloys During Hot Extrusion. J. Mater. Sci. Technol. 2014, 30, 1271–1277. [Google Scholar] [CrossRef]
- Wang, Y.; Liao, H.; Wu, Y.; Yang, J. Effect of Si content on microstructure and mechanical properties of Al–Si–Mg alloys. Mater. Des. 2014, 53, 634–638. [Google Scholar] [CrossRef]
- Sha, X.C.; Chen, X.F.; Ning, H.Y.; Xiao, L.R.; Yin, D.D.; Jiang, L.M.; Zhou, H. Modification of Eutectic Si in Al–Si-(Ba) Alloy by Inducing a Novel 9R Structure in Twins. Materials 2018, 11, 1151. [Google Scholar] [CrossRef]
- Alkahtani, S.A.; Elgallad, E.M.; Tash, M.M.; Samuel, A.M.; Samuel, F.H. Effect of Rare Earth Metals on the Microstructure of Al-Si Based Alloys. Materials 2016, 9, 45. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.; Gu, X.; Dai, N.; Qin, P.; Zhang, L.-C. Distinction of corrosion resistance of selective laser melted Al-12Si alloy on different planes. J. Alloy. Compd. 2018, 747, 648–658. [Google Scholar] [CrossRef]
- Fratila-Apachitei, L.; Apachitei, I.; Duszczyk, J. Characterization of cast AlSi(Cu) alloys by scanning Kelvin probe force microscopy. Electrochim. Acta 2006, 51, 5892–5896. [Google Scholar] [CrossRef]
- Li, X.; Nie, X.; Wang, L.; Northwood, D.O. Corrosion protection properties of anodic oxide coatings on an Al–Si alloy. Surf. Coat. Tech. 2005, 200, 1994–2000. [Google Scholar] [CrossRef]
- Cancellieri, C.; Evangelisti, F.; Geldmacher, T.; Araullo-Peters, V.; Ott, N.; Chiodi, M.; Döbeli, M.; Schmutz, P. The role of Si incorporation on the anodic growth of barrier-type Al oxide. Mater. Sci. Eng. B 2017, 226, 120–131. [Google Scholar] [CrossRef]
- Gavrilov, N.; Kamenetskikh, A.; Trernikov, P.; Emlin, D.; Chukin, A.; Surkov, Y. Al2O3 thin films deposition by reactive evaporation of Al in anodic arc with high levels of metal ionization. Surf. Coat. Tech. 2019, 359, 117–124. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, W.; Wang, Y.; Liu, Q.; Yang, J.; Zhang, L.; He, F. Fabrication of Al2O3 by anodic oxidation and hydrothermal synthesis of strong-bonding hydroxyapatite coatings on its surface. Appl. Surf. Sci. 2019, 470, 959–969. [Google Scholar] [CrossRef]
- Yao, C.; Lv, H.; Zhu, T.; Zheng, W.; Yuan, X.; Gao, W. Effect of Mg content on microstructure and corrosion behavior of hot dipped Zn–Al–Mg coatings. J. Alloy. Compd. 2016, 670, 239–248. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.; Ke, W. Study on influence of native oxide and corrosion products on atmospheric corrosion of pure Al. Corros. Sci. 2014, 80, 169–176. [Google Scholar] [CrossRef]
- Mosiałek, M.; Mordarski, G.; Nowak, P.; Simka, W.; Nawrat, G.; Hanke, M.; Socha, R.; Michalska, J. Phosphate–permanganate conversion coatings on the AZ81 magnesium alloy: SEM, EIS and XPS studies. Surf. Coat. Tech. 2011, 206, 51–62. [Google Scholar] [CrossRef]
- Razavi, M.; Fathi, M.; Savabi, O.; Vashaee, D.; Tayebi, L. In vitro study of nanostructured diopside coating on Mg alloy orthopedic implants. Mater. Sci. Eng. C 2014, 41, 168–177. [Google Scholar] [CrossRef]
- King, A.; Birbilis, N.; Scully, J. Accurate Electrochemical Measurement of Magnesium Corrosion Rates; a Combined Impedance, Mass-Loss and Hydrogen Collection Study. Electrochim. Acta 2014, 121, 394–406. [Google Scholar] [CrossRef]
- Curioni, M.; Scenini, F.; Monetta, T.; Bellucci, F. Correlation between electrochemical impedance measurements and corrosion rate of magnesium in M. Curioni estigated by real-time hydrogen measurement and optical imaging. Electrochim. Acta 2015, 166, 372–384. [Google Scholar] [CrossRef]
- Song, D.; Li, C.; Liang, N.; Yang, F.; Jiang, J.; Sun, J.; Wu, G.; Ma, A.; Ma, X. Simultaneously improving corrosion resistance and mechanical properties of a magnesium alloy via equal-channel angular pressing and post water annealing. Mater. Des. 2019, 166, 107621. [Google Scholar] [CrossRef]
- Afifi, M.A.; Wang, Y.C.; Pereira, P.H.R.; Huang, Y.; Wang, Y.; Cheng, X.; Li, S.; Langdon, T.G. Mechanical properties of an Al-Zn-Mg alloy processed by ECAP and heat treatments. J. Alloy. Compd. 2018, 769, 631–639. [Google Scholar] [CrossRef]
- Jia, H.; Bjørge, R.; Cao, L.; Song, H.; Marthinsen, K.; Li, Y. Quantifying the grain boundary segregation strengthening induced by post-ECAP aging in an Al-5Cu alloy. Acta Mater. 2018, 155, 199–213. [Google Scholar] [CrossRef]
- Zhu, B.; Seifeddine, S.; Persson, P.O.; Jarfors, A.E.; Leisner, P.; Zanella, C. A study of formation and growth of the anodised surface layer on cast Al-Si alloys based on different analytical techniques. Mater. Des. 2016, 101, 254–262. [Google Scholar] [CrossRef]
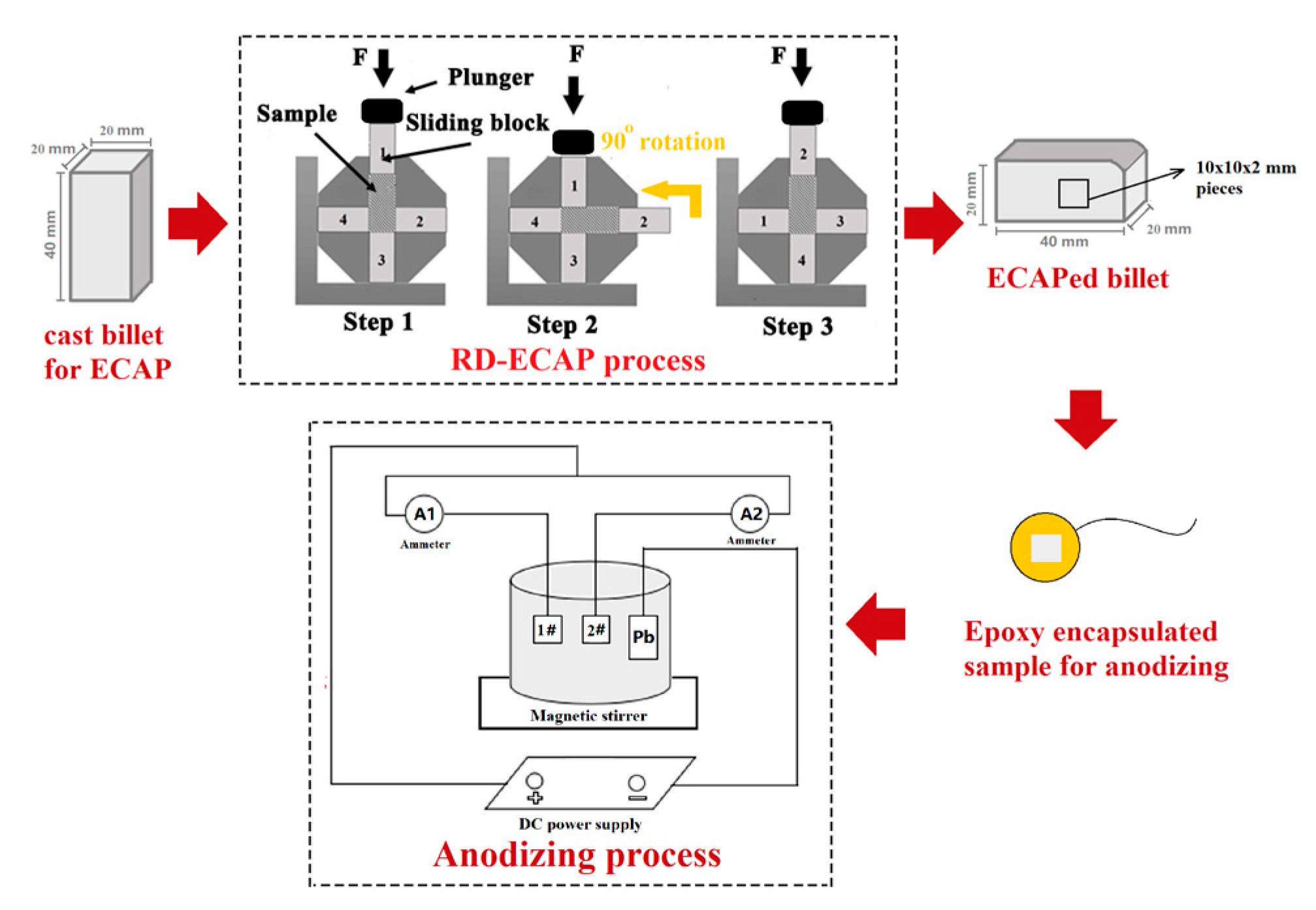
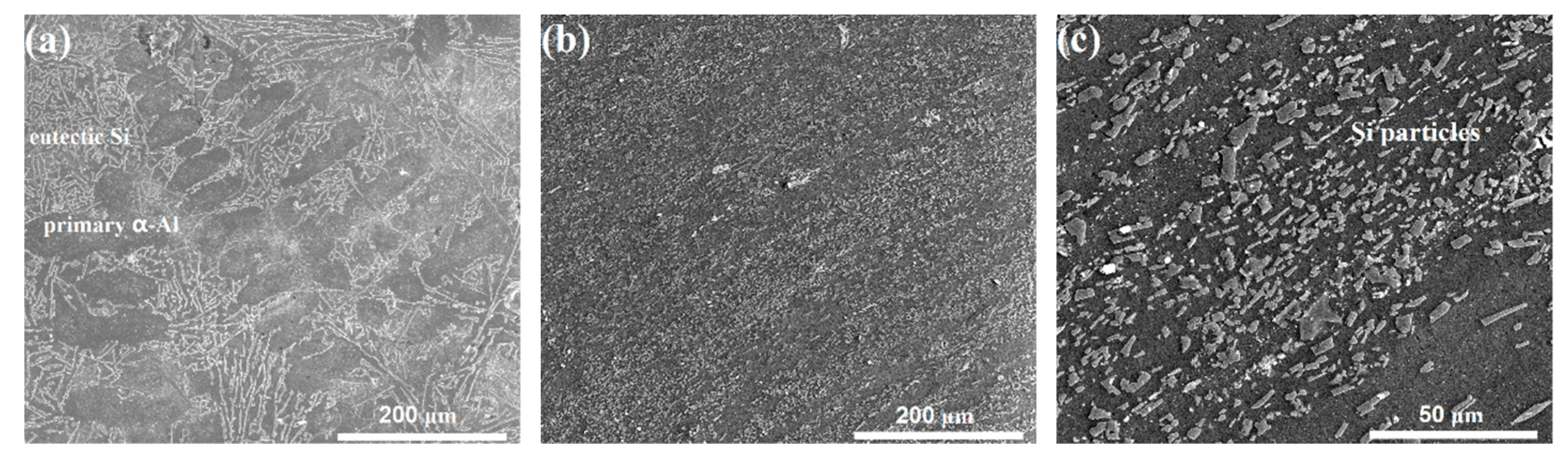
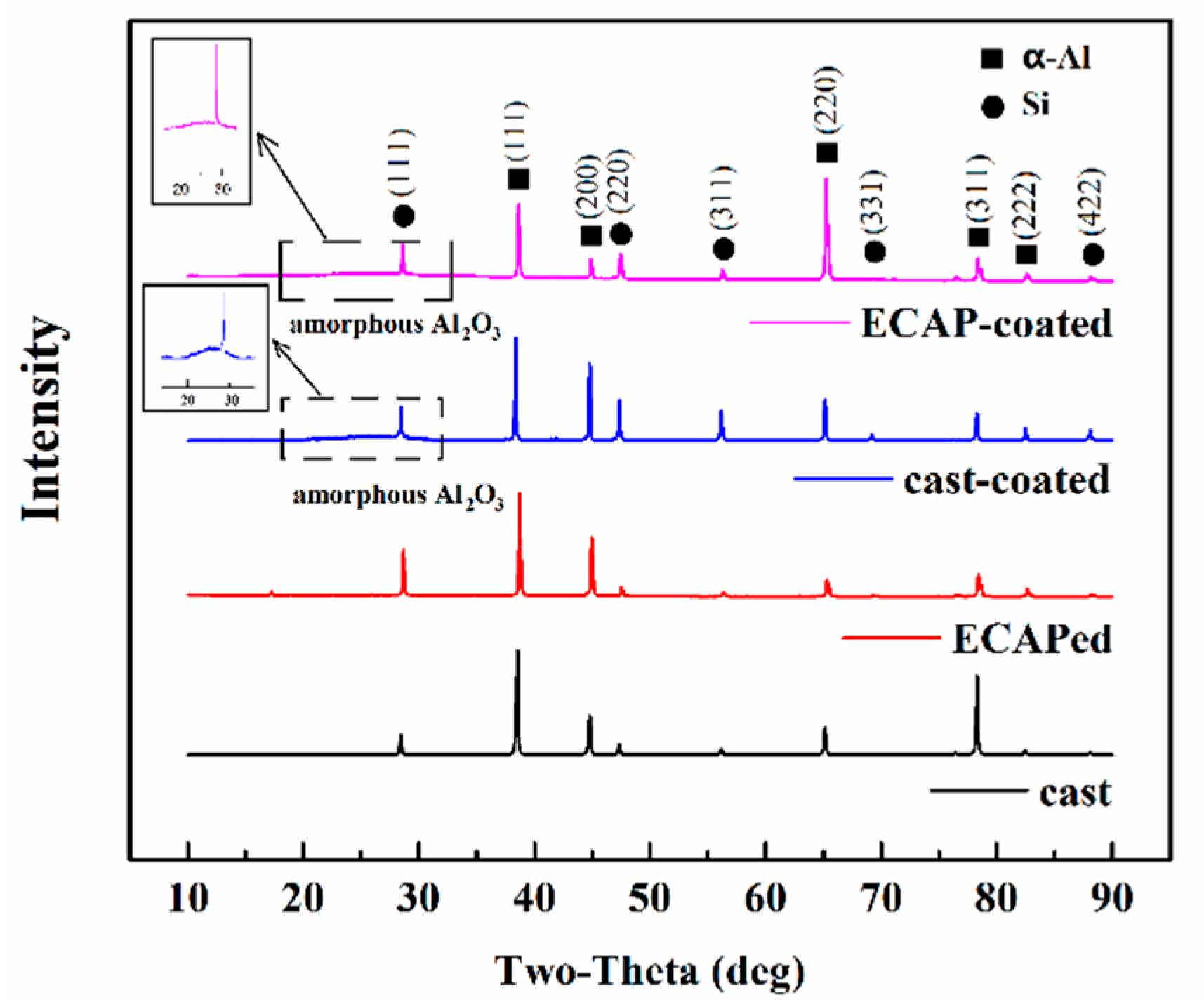


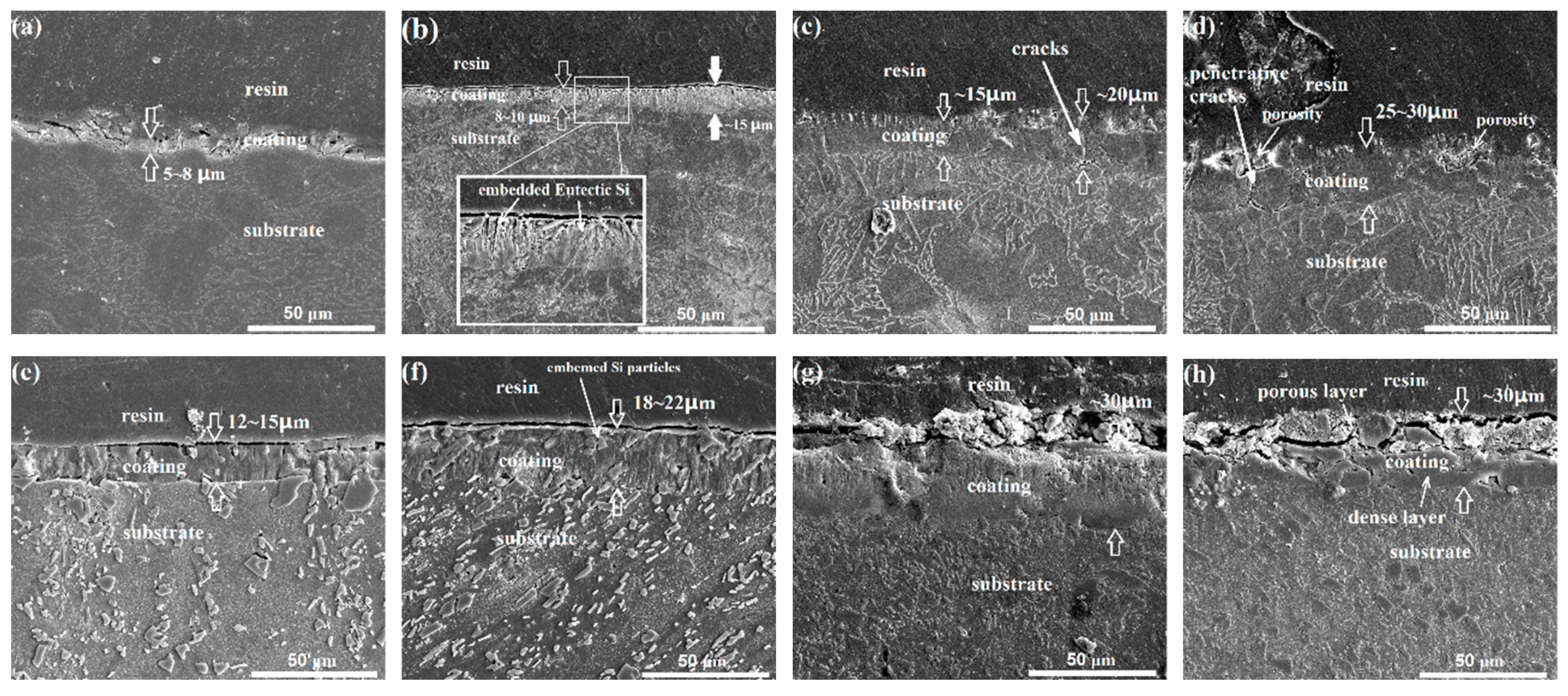



| Si | Cu | Fe | Mn | Mg | Zn | Al |
|---|---|---|---|---|---|---|
| 11.10 | 0.95 | 0.86 | 0.22 | 0.24 | 0.32 | Balance |
| Samples | Rs (Ω∙cm2) | Cf (μF∙cm−2) | Rpore (Ω∙cm2) | Cdl (μF∙cm−2) | Rt (Ω∙cm2) | RL (Ω∙cm2) | L (H∙cm2) |
|---|---|---|---|---|---|---|---|
| Cast | 9.5 | / | / | 18 | 646 | 2000 | 5355 |
| ECAPed | 8.3 | / | / | 15 | 1600 | 2814 | 10,200 |
| Cast-coated | 9.3 | 3.37 | 9376 | 14.2 | 1020 | 9206 | 89,180 |
| ECAP-coated | 8.9 | 3.55 | 19,450 | 7.2 | 1774 | 11,900 | 78,420 |
| Samples | Ecorr (V) | icorr (μA∙cm−2) | βa (mV∙dec−1) | βc (mV∙dec−1) | Rp (Ω∙cm2) |
|---|---|---|---|---|---|
| Cast | −0.69 | 15.2 | 32 | 1130 | 890 |
| ECAPed | −0.67 | 10.1 | 43 | 820 | 1760 |
| Cast-coated | −0.61 | 3.2 | 64 | 460 | 7630 |
| ECAP-coated | −0.60 | 1.3 | 67 | 340 | 18,700 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Z.; Song, D.; Liang, N.; Jiang, H.; Gao, B.; Wu, Y.; Ma, A.; Qiao, Y.; Sun, J.; Jiang, J.; et al. Promoted Anodizing Reaction and Enhanced Coating Performance of Al–11Si Alloy: The Role of an Equal-Channel-Angular-Pressed Substrate. Materials 2019, 12, 3255. https://doi.org/10.3390/ma12193255
Zhou Z, Song D, Liang N, Jiang H, Gao B, Wu Y, Ma A, Qiao Y, Sun J, Jiang J, et al. Promoted Anodizing Reaction and Enhanced Coating Performance of Al–11Si Alloy: The Role of an Equal-Channel-Angular-Pressed Substrate. Materials. 2019; 12(19):3255. https://doi.org/10.3390/ma12193255
Chicago/Turabian StyleZhou, Zhikai, Dan Song, Ningning Liang, Haiyang Jiang, Bo Gao, Yuna Wu, Aibin Ma, Yanxin Qiao, Jiapeng Sun, Jinghua Jiang, and et al. 2019. "Promoted Anodizing Reaction and Enhanced Coating Performance of Al–11Si Alloy: The Role of an Equal-Channel-Angular-Pressed Substrate" Materials 12, no. 19: 3255. https://doi.org/10.3390/ma12193255
APA StyleZhou, Z., Song, D., Liang, N., Jiang, H., Gao, B., Wu, Y., Ma, A., Qiao, Y., Sun, J., Jiang, J., & Ma, X. (2019). Promoted Anodizing Reaction and Enhanced Coating Performance of Al–11Si Alloy: The Role of an Equal-Channel-Angular-Pressed Substrate. Materials, 12(19), 3255. https://doi.org/10.3390/ma12193255







