Effect of Curing Condition on Resistance to Chloride Ingress in Concrete Using Ground Granulated Blast Furnace Slag
Abstract
:1. Introduction
2. Materials and Specimens
2.1. Materials
2.2. Concrete Mix Proportion
2.3. Specimens
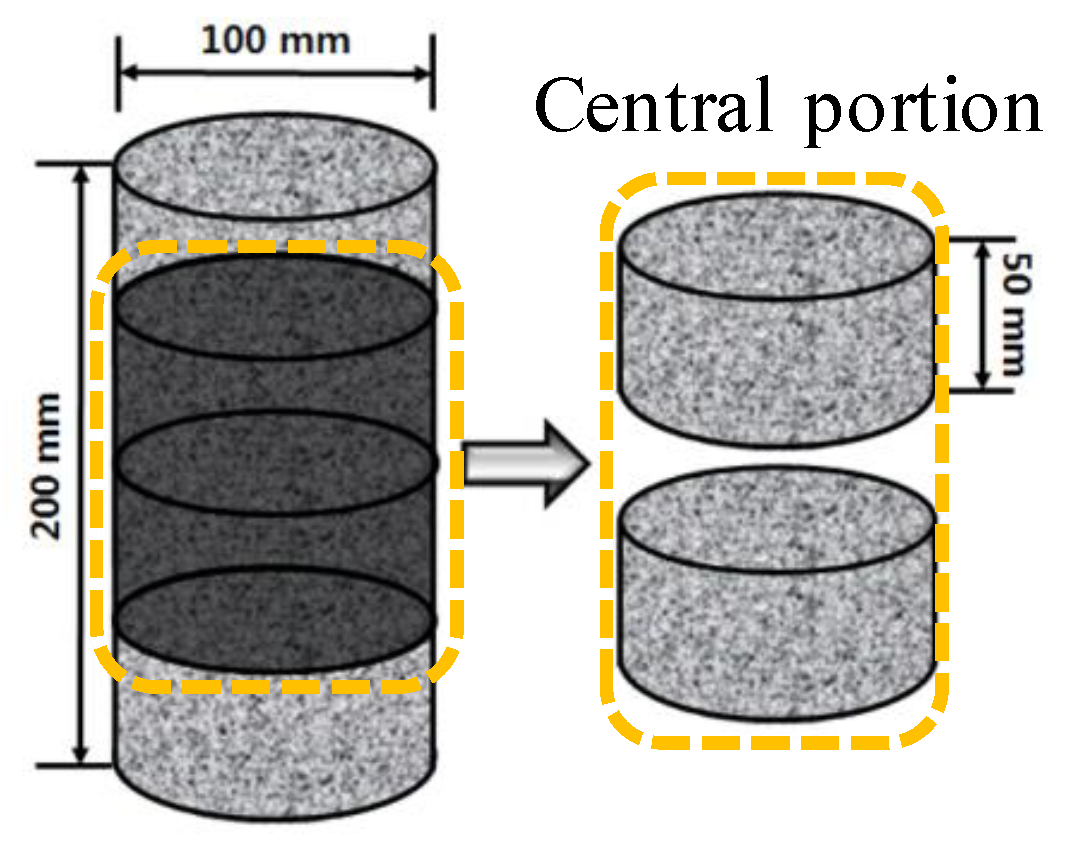
2.3.1. Specimen for Chloride Ion Migration Coefficient of Concrete
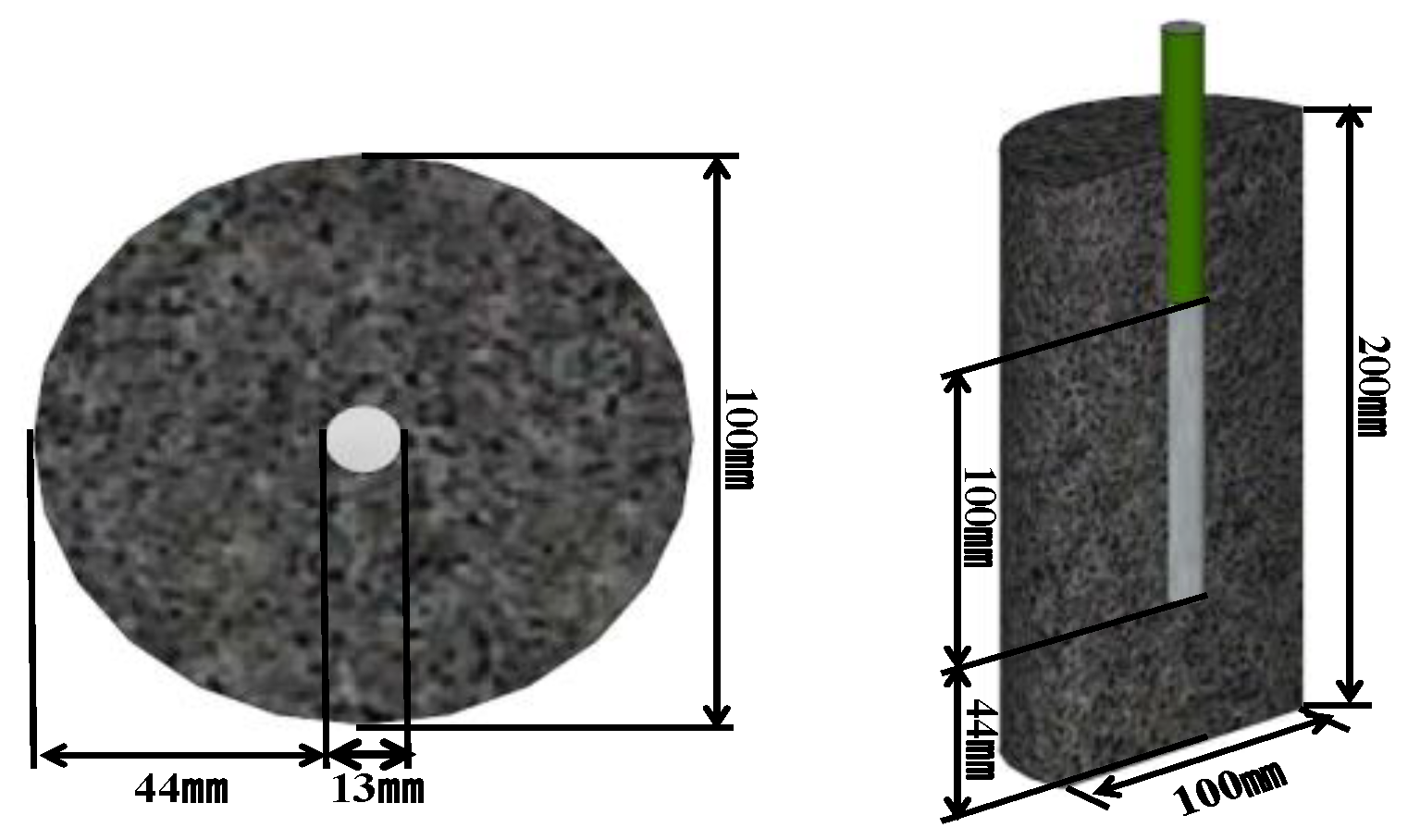
2.3.2. Specimen for Electrochemical Measurements
2.3.3. Curing Method
3. Experimental Method
3.1. Evaluation of Concrete Compressive Strength
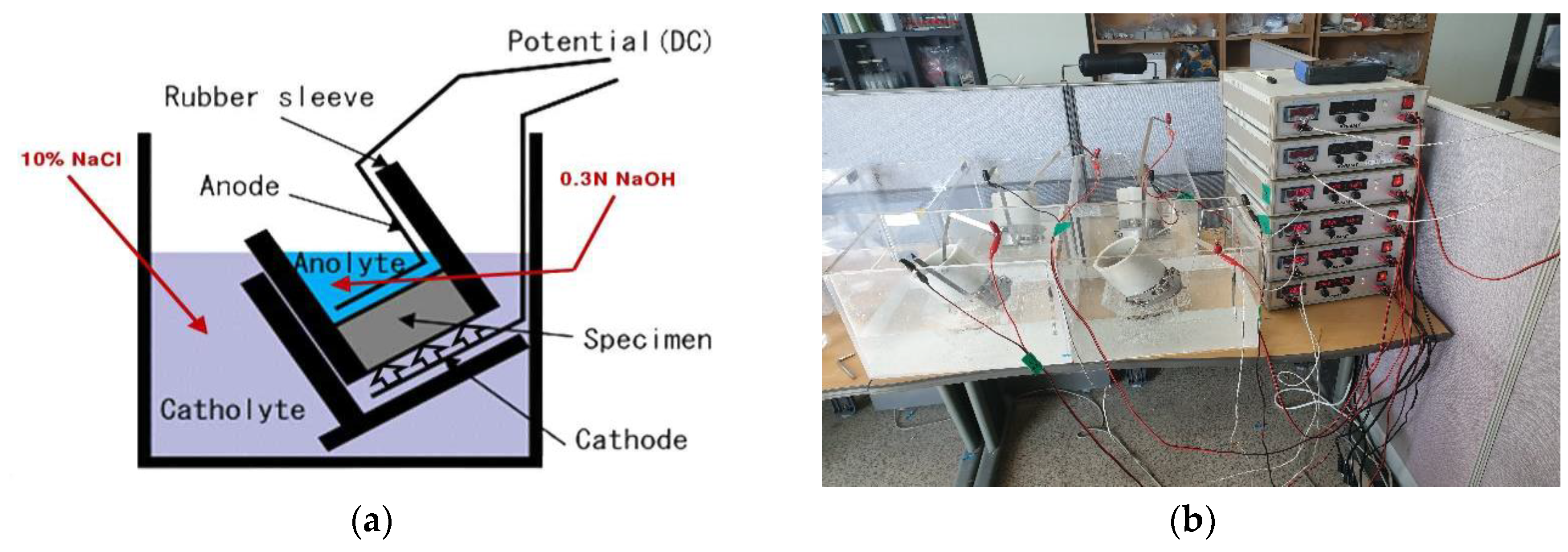
3.2. Evaluation of Resistance to Chloride Ion Penetration of Concrete
- Dnssm is a non-steady-state migration coefficient (×10−12 m2/s)
- U is the absolute value of the applied voltage (V)
- T is the average of the initial and final temperatures in the anolyte solution (°C)
- L is the thickness of the specimen (mm)
- is the average value of the penetration depths (mm)
- t is the test duration (hour)
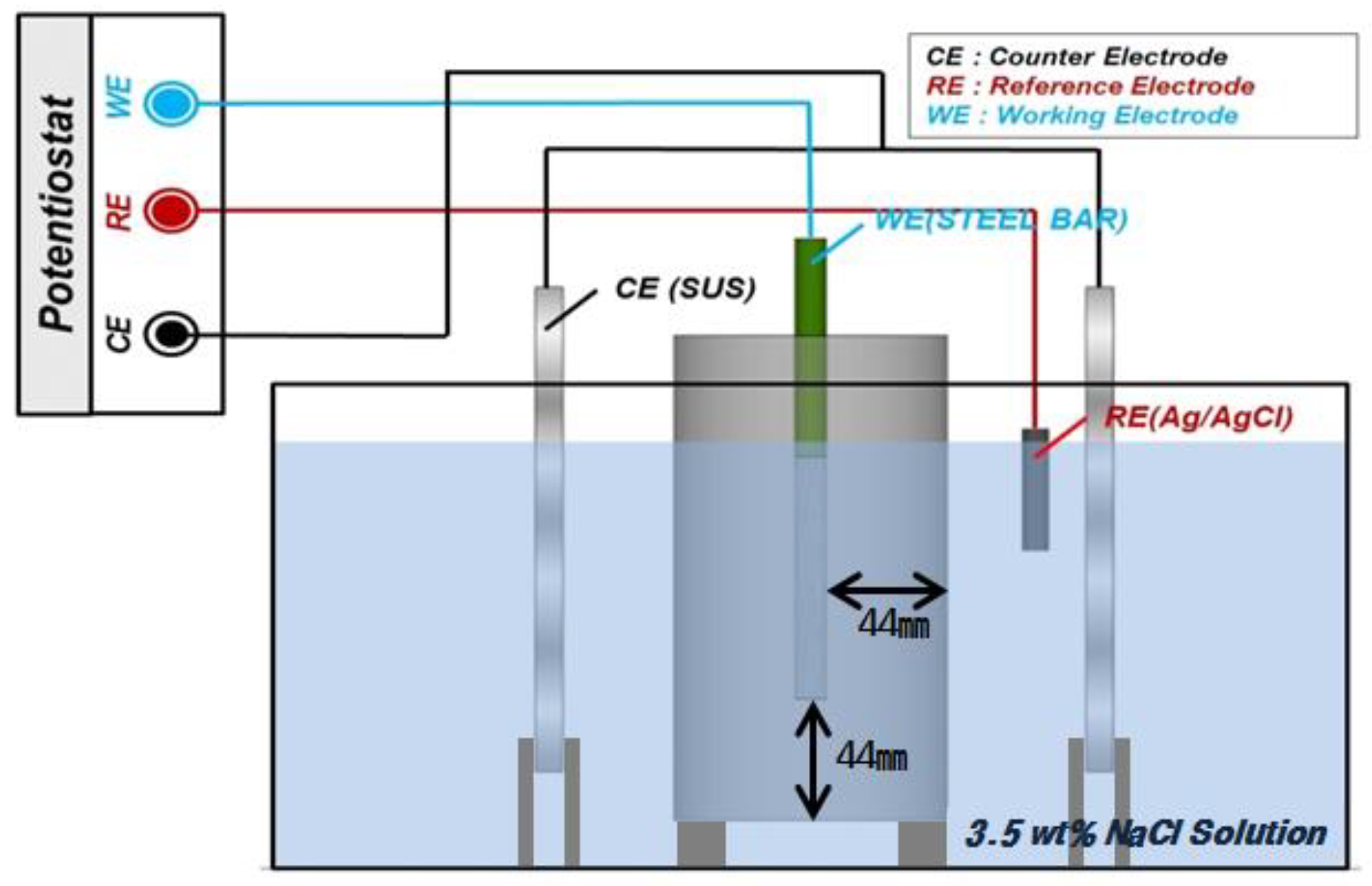
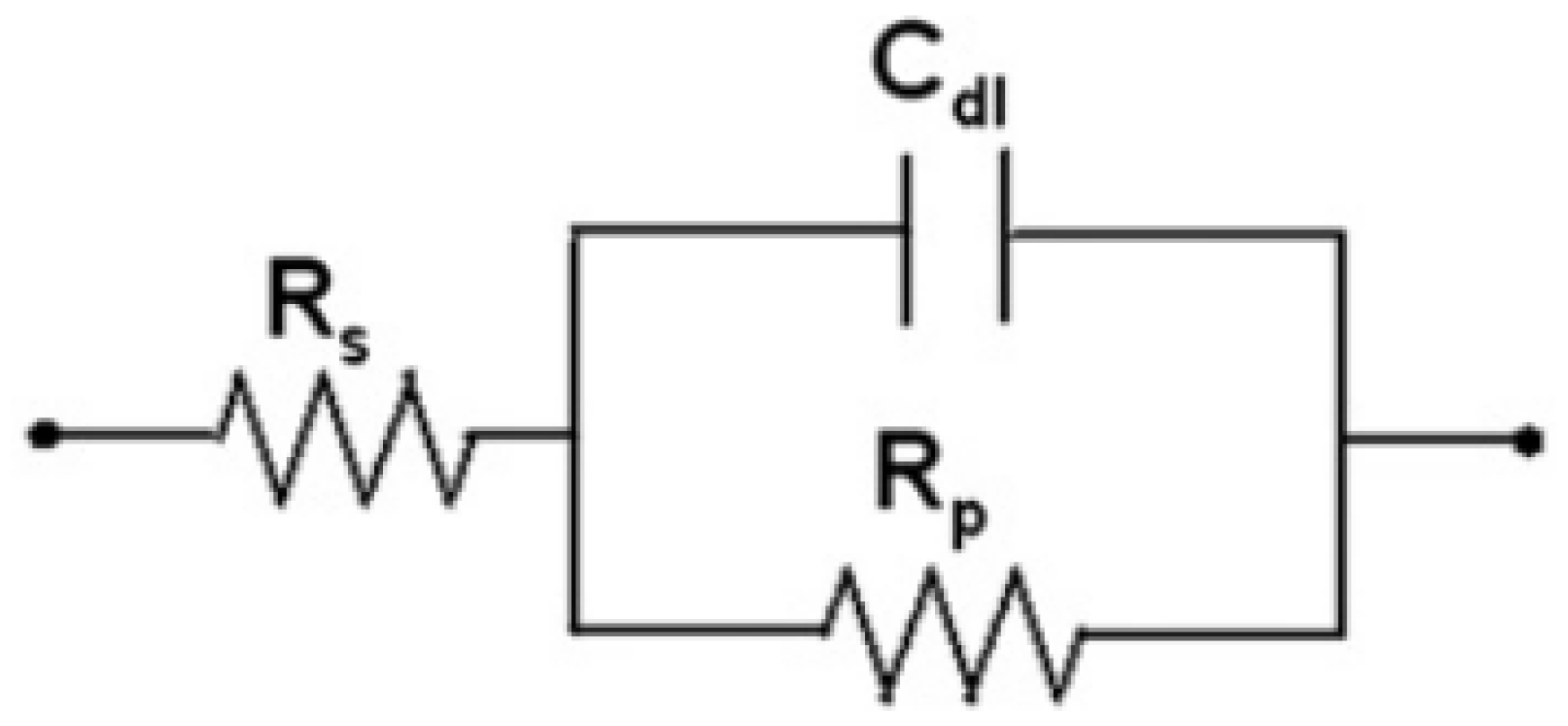
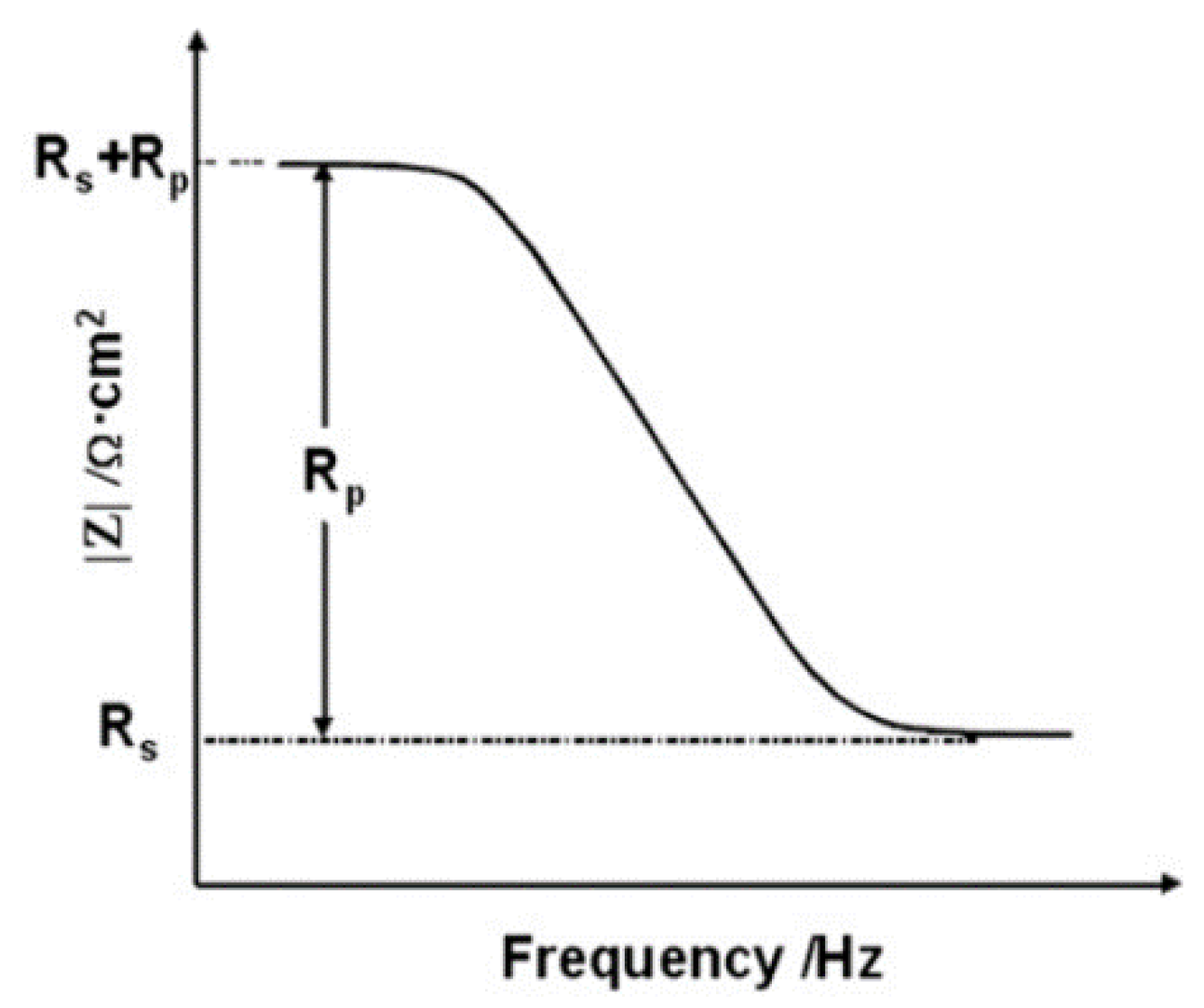
3.3. Electrochemical Measurement
4. Results and Discussion
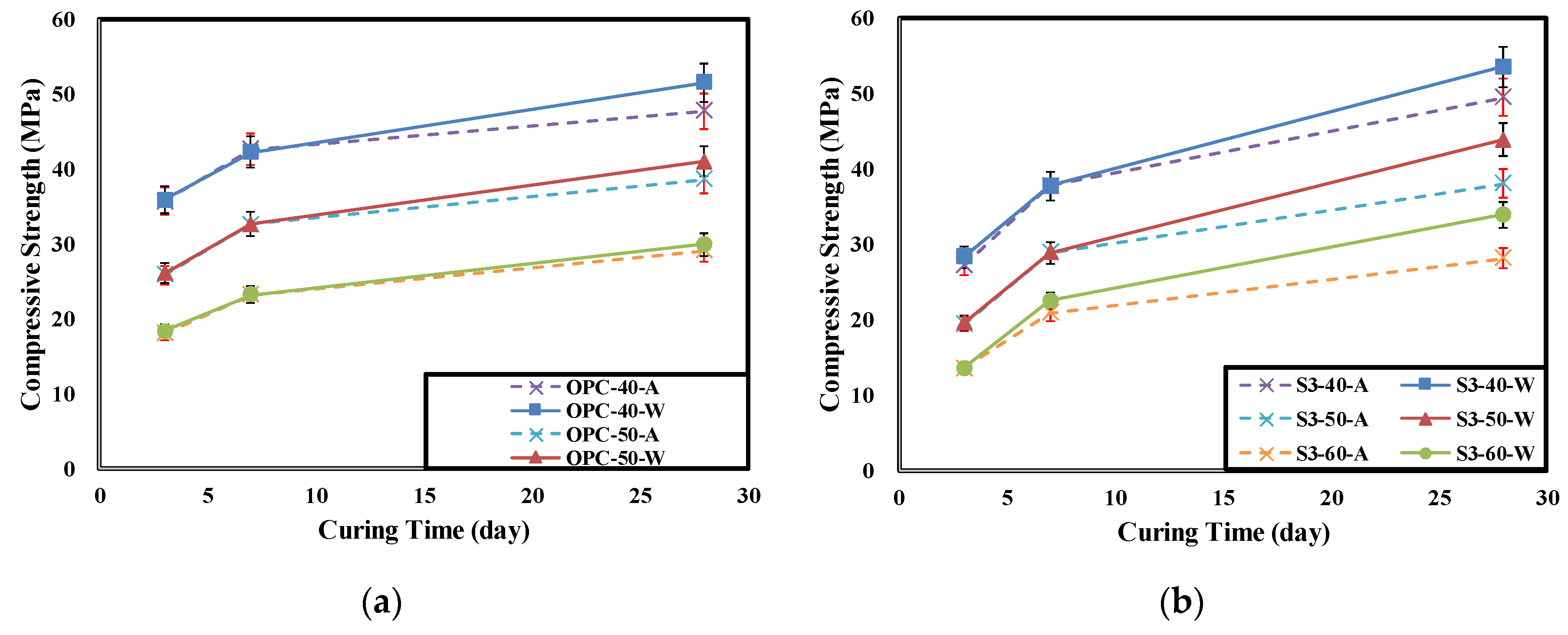
4.1. Result of Concrete Compressive Strength
- UW is the result of underwater curing condition
- IA is the result of air-dry curing condition
4.2. Results of Chloride Ion Diffusion Coefficient of Concrete
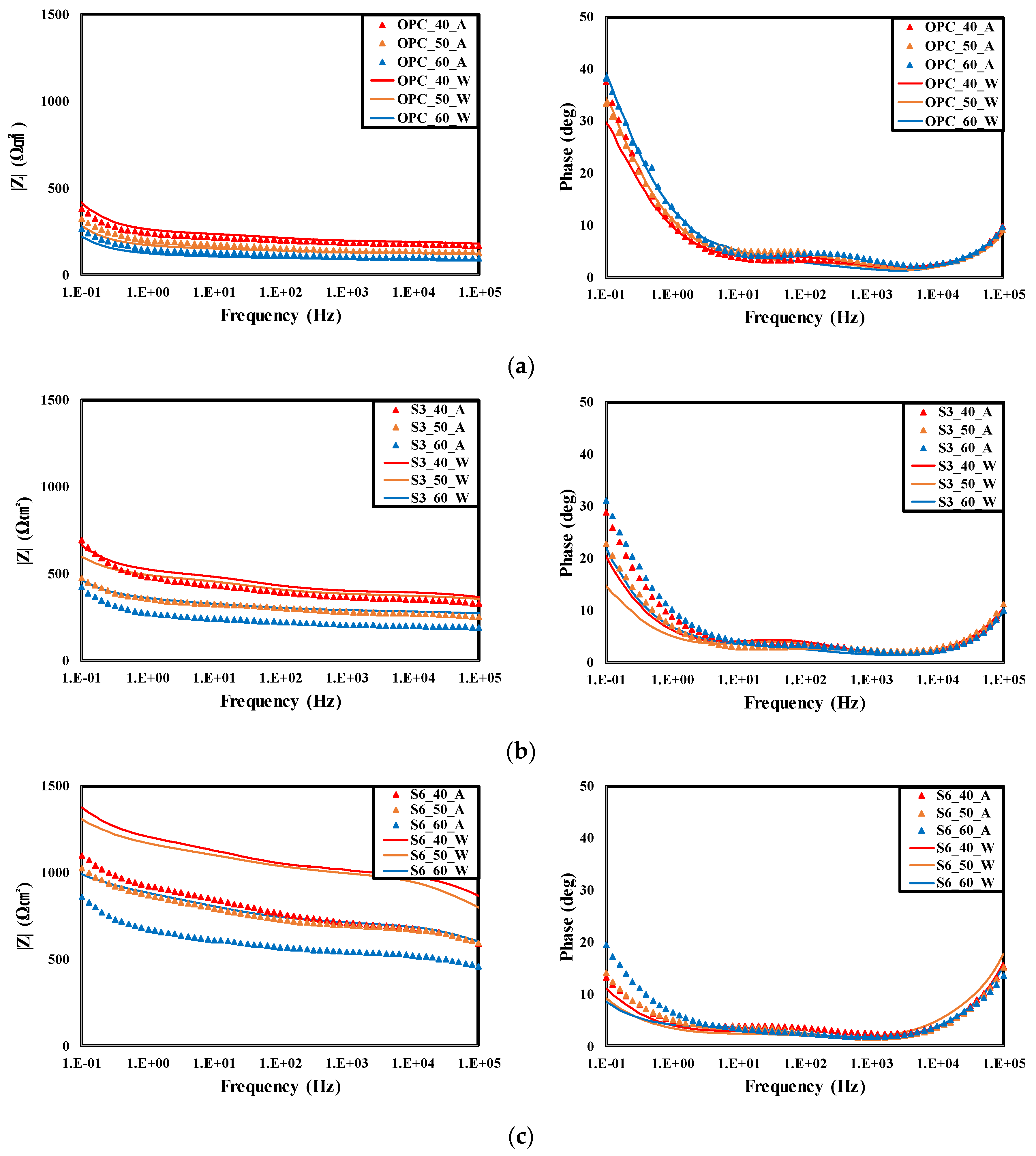
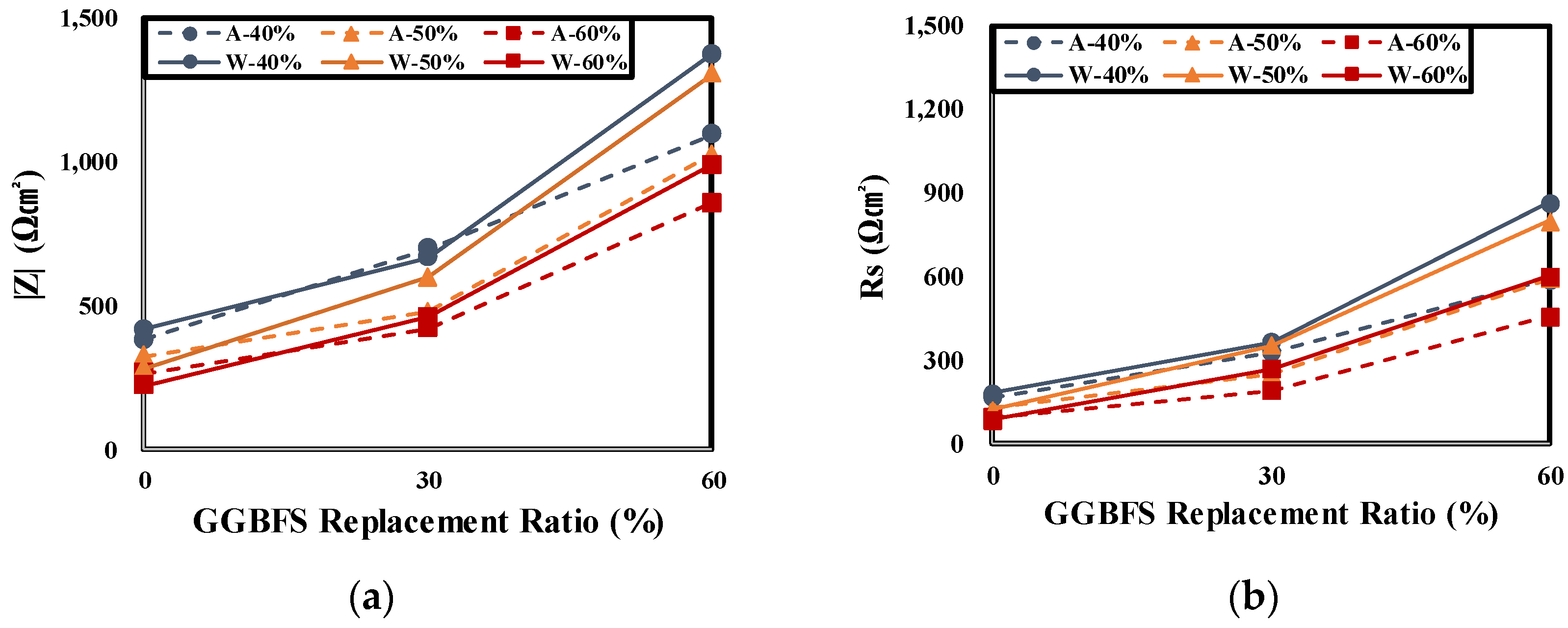
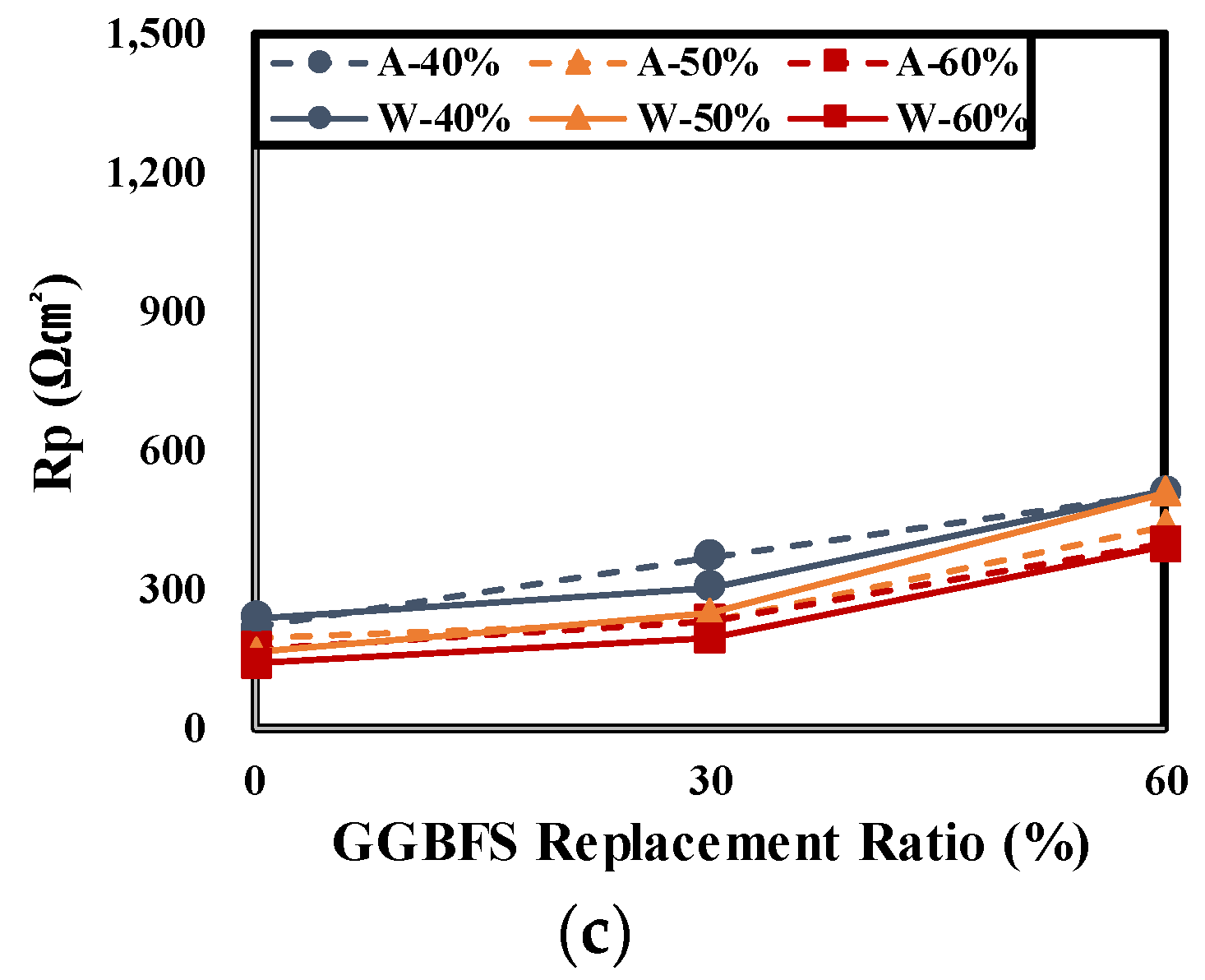
4.3. Result of Electrochemical Measurements
5. Conclusions
- The evaluation results of the concrete compressive strength showed that the compressive strength of the air cured specimen decreased compared to the underwater cured specimen. As the GGBFS replacement ratio increased, the compressive strength reduction rate increased. The reason for this seems to be that when air curing is performed, pore water is not enough for sufficient exhibition of latent hydraulic activity of the GGBFS and thus, it decreases the generation of hydration products.
- The evaluation result of the concrete chloride ion diffusion coefficient showed that the chloride ion diffusion coefficient of the air cured specimen was higher than that of the underwater cured specimen. Furthermore, the higher the GGBFS replacement ratio, the lower is the chloride ion diffusion coefficient. The high fineness and latent hydraulic activity reaction of GGBFS decreased the concrete chloride ion diffusion coefficient compared to the OPC but when air curing was performed, the chloride ion diffusion coefficient increased relative to underwater curing.
- The impedance measurement results of rebars embedded in concrete showed that the |Z| of the air cured specimen decreased in every frequency range relative to the underwater cured one at all levels. As the GGBFS replacement ratio increased, |Z| increased in every frequency range. This showed the same tendency as the evaluation result of the concrete chloride ion diffusion coefficient. It is considered that the higher the chloride penetration resistance is, the higher is the |Z| value of the rebars embedded in concrete.
- As the GGBFS replacement rate increased, the performance of the underwater cured specimen was higher relative to the air cured specimen in every experiment. This suggests a high dependence on underwater curing. Therefore, when concrete is manufactured using GGBFS, special care should be devoted to the curing conditions.
Author Contributions
Funding
Conflicts of Interest
References
- Watson, R. Spaghetti Junction lifts off£ 400m repairs. New Civ. Eng. 1990, 28, 7. [Google Scholar]
- Wallbank, E.J. The Performance of Concrete in Bridges. A Survey of 200 Highway Bridges; Department of Transport, HMSO: London, UK, 1989; p. 96. [Google Scholar]
- Rosenberg, A.; Hanson, C.M.; Andrade, C. Mechanisms of corrosion of steel in concrete. In Material Science of Concrete; Skalny, J., Ed.; American Ceramic Society: Westerville, OH, USA, 1989; pp. 285–313. [Google Scholar]
- Kumar, V. Protection of steel reinforcement for concrete—A review. Corrosion Reviewer. 1998, 16, 317–358. [Google Scholar] [CrossRef]
- Page, C.L.; Havdahl, J. Electrochemical monitoring of corrosion of steel in microsilica cement pastes. Mater. Struct. 1985, 18, 41–47. [Google Scholar] [CrossRef]
- Lomborg, B. The Skeptical Environmentalist: Measuring the Real State of the World; Cambridge University Prdess: Cambridge, UK, 2003. [Google Scholar]
- Shi, X.; Xie, N.; Fortune, K.; Gong, J. Durability of steel reinforced concrete in chloride environments: An overview. Constr. Build. Mater. 2012, 30, 125–138. [Google Scholar] [CrossRef]
- Jang, S.Y.; Karthick, S.; Kwon, S.J. Investigation on durability performance in early aged high-performance concrete containing GGBFS and FA. Adv. Mater. Sci. Eng. 2017, 2017, 3214696. [Google Scholar] [CrossRef]
- Page, C.; Short, N.R.; Tarras, A. Diffusion of chloride ions in hardened cement pastes. Cem. Concr. Res. 1981, 11, 395–406. [Google Scholar] [CrossRef]
- Hussain, S.E. In Mechanisms of High Durability Performance Plane and Blended Cements. Ph.D. Thesis, Department Civil Engineering, King Fahd University of Petroleum and Minerals, Dhahran, Saudi Arabia, 1991; pp. 221–228. [Google Scholar]
- Al-Amoudi, O.S.B.; Maslehuddin, M.; Al-Mana, A.I. Prediction of long-term corrosion resistance of plain and blended cement concretes. Mater. J. 1993, 90, 564–570. [Google Scholar]
- Fulton, F.S. The Properties of Portland Cements Containing Milled Granulated Blast-Furnace Slag; Portland Cement Institute: Johannesburg, South Africa, 1974. [Google Scholar]
- Boğa, A.R.; Topçu, I.B. Influence of fly ash on corrosion resistance and chloride ion permeability of concrete. Constr. Build. Mater. 2012, 31, 258–264. [Google Scholar] [CrossRef]
- Thomas, M.D.A.; Hooton, R.; Scott, A.; Zibara, H. The effect of supplementary cementitious materials on chloride binding in hardened cement paste. Cem. Concr. Res. 2012, 42, 1–7. [Google Scholar] [CrossRef]
- Lee, H.S.; Ismail, M.; Hussin, M.W. Durability Probabilistic Evaluation of RC Structures Subjected to Chloride Ion. Renew. Energy Sustain. Dev. 2015, 1, 160–166. [Google Scholar]
- Malhotra, V.M. Role of supplementary cementing materials in reducing greenhouse gas emissions. In Concrete Technology for a Sustainable Development in the 21st Century; E&FN Spon: London, UK, 2000; pp. 226–235. [Google Scholar]
- Berke, N.S. Resistance of Microsilica Concrete to Steel Corrosion Erosion and Chemical Attack. Spec. Publ. 1989, 114, 861–886. [Google Scholar]
- Swamy, R.N. Blended Cements in Construction; Elsevier Applied Science: London, UK, 1991. [Google Scholar]
- Hussain, S.E. Rasheeduzzafar. Corrosion resistance performance of fly ash blended cement concrete. ACI Mater. J. 1994, 91, 264–272. [Google Scholar]
- Ozyildirim, C.; Halstead, W.J. Improved concrete quality with combinations of fly ash and silica fume. ACI Mater. J. 1994, 91, 587–594. [Google Scholar]
- Güneyisi, E.; Özturan, T.; Gesoğlu, M. Laboratory investigation of chloride permeability for high performance concrete containing fly-ash and silica fume. In Innovations and Developments in Concrete Materials and Construction: Proceedings of the International Conference Held at the University of Dundee, Scotland, UK, 9–11 September 2002; Thomas Telford Publishing: Dundee, UK, 2002; pp. 295–305. [Google Scholar]
- Luo, R.; Cai, Y.; Walng, C.; Huang, X. Study of chloride binding and diffusion in GGBS concrete. Cem. Concr. Res. 2003, 33, 1–7. [Google Scholar] [CrossRef]
- Cheng, A.; Huang, R.; Wu, J.-K.; Chen, C.-H. Influence of GGBS on durability and corrosion behavior of reinforced concrete. Mater. Chem. Phys. 2005, 93, 404–411. [Google Scholar] [CrossRef]
- Song, H.W.; Saraswathy, V. Studies on the corrosion resistance of reinforced steel in concrete with ground granulated blast-furnace slag—An overview. J. Hazard. Mater. 2006, 138, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Dong, J.H.; Ke, W. Corrosion resistant performance of a chemical quenched rebar in concrete. Constr. Build. Mater. 2011, 25, 1243–1247. [Google Scholar] [CrossRef]
- Wang, Z.; Zeng, Q.; Wang, L.; Yao, Y.; Li, K. Corrosion of rebar in concrete under cyclic freeze–thaw and Chloride salt action. Constr. Build. Mater. 2014, 53, 40–47. [Google Scholar] [CrossRef]
- Topçu, İ.B.; Boğa, A.R. Effect of ground granulate blast-furnace slag on corrosion performance of steel embedded in concrete. Mater. Des. 2010, 31, 3358–3365. [Google Scholar] [CrossRef]
- Moreno, M.; Morris, W.; Alvarez, M.G.; Duffó, G.S. Corrosion of reinforcing steel in simulated concrete pore solutions: Effect of carbonation and chloride content. Corros. Sci. 2004, 46, 2681–2699. [Google Scholar] [CrossRef]
- Kim, E.K.; Kim, J.-K.; Lete, D.-H.; Kim, Y.-U.; Kim, Y.-C. A Study on Corrosion Resistance of the Reinforement in Concrete Using Blast-Furnace Slag Powder. J. Korea Concr. Inst. 2004, 16, 1–9. [Google Scholar] [CrossRef]
- Wang, J.; Liu, E. The relationship between steady-state chloride diffusion and migration coefficients in cementitious materials. Mag. Concr. Res. 2019, 223, 595–604. [Google Scholar] [CrossRef]
- Ramezanianpour, A.A.; Malhotra, V.M. Effect of curing on the compressive strength, resistance to chloride-ion penetration and porosity of concretes incorporating slag, fly ash or silica fume. Cem. Concr. Compos. 1995, 17, 125–133. [Google Scholar] [CrossRef]
- Aldea, C.M.; Young, F.; Walng, K.; Shah, S.P. Effects of curing conditions on properties of concrete using slag replacement. Cem. Concr. Res. 2000, 30, 465–472. [Google Scholar] [CrossRef]
- Papadakis, V.G.; Vayenas, C.G.; Fardis, M.N. Fundamental modeling and experimental investigation of concrete carbonation. Mater. J. 1991, 88, 363–373. [Google Scholar]
- Ngala, V.T.; Page, C.L. Effects of carbonation on pore structure and diffusional properties of hydrated cement pastes. Cem. Concr. Res. 1997, 27, 995–1007. [Google Scholar] [CrossRef]
- Thiéry, M.; Faure, P.; Morandeau, A.; Platret, G.; Bouteloup, J.F.; Dangla, P.; Baroghel-Bouny, V. Effect of carbonation on the microstructure and moisture properties of cement-based materials. In XII DBMC (12th International Conference on Building Materials and Components); Rilem: Porto, Portugal, 2011; Volume 3, pp. 1–8. [Google Scholar]
- Yokozuka, K.; Li, C.; Nakarai, K. Influence of carbonation at early ages on depth distribution of oxygen diffusion coefficient of hardened cement paste. Cem. Sci. Concr. Technol. 2010, 64, 370–376. [Google Scholar] [CrossRef]
- Bilim, C.; Karahan, O.; Atis, C.D.; Ilkentapar, S. Influence of admixtures on the properties of alkali-activated slag mortars subjected to different curing conditions. Mater. Des. 2013, 44, 540–547. [Google Scholar] [CrossRef]
- Mehta, P.K. Studies on blended Portland cements containing Santorin earth. Cem. Concr. Res. 1981, 11, 507–518. [Google Scholar] [CrossRef]
- Güneyisi, E.; Mermerdaş, K. Comparative study on strength, sorptivity, and chloride ingress characteristics of air-cured and water-cured concretes modified with metakaolin. Mater. Struct. 2007, 40, 1161–1171. [Google Scholar] [CrossRef]
- Mehta, P.K.; Monteiro, P.J. Concrete: Microstructure, Properties, and Materials; McGraw-Hill: New York, NY, USA, 2005. [Google Scholar]
- Goto, S.; Roy, D.M. The effect of w/c ratio and curing temperature on the permeability of hardened cement paste. Cem. Concr. Res. 1981, 11, 575–579. [Google Scholar] [CrossRef]
- Astm, C. 150, Standard specification for Portland cement. In Annual Book of ASTM Standards; ASTM International: West Conshohocken, PA, USA, 2001; Volume 4, p. 1. [Google Scholar]
- Astm, C. 989, Standard specification for ground granulated blast furnace slag for use in concrete and mortars. In Annual Book of ASTM Standards; ASTM International: West Conshohocken, PA, USA, 1999. [Google Scholar]
- Kim, J.-H.; Yoon, S.-C.; Jee, N.-Y. The effect of combined aggregates on fluidity of the high fluid concrete containing GGBFS. J. Korea Inst. Build. Constr. 2003, 3, 79–86. [Google Scholar]
- Kang, M.-J.; Lee, P.-K.; Youm, S.-J. Assessment of Environmental Contamination caused by the Stone-dust using Leaching Tests. J. Soil Groundw. Environ. 2010, 15, 52–60. [Google Scholar]
- Build, N.T. 492. Concrete, mortar and cement-based repair materials: Chloride migration coefficient from non-steady-state migration experiments. Nord. Method Finland 1999. [Google Scholar]
- Astm, A. C39. Standard Test Method for Compressive Strength of Concrete; ASTM International: West Conshohocken, PA, USA, 2012. [Google Scholar]
- Cao, C.N.; Zhang, J.Q. Introduction of electrochemical impedance spectra. Beijing Sci. 2002, 21, 37–44. [Google Scholar]
- Mansfeld, F. Electrochemical Impedance Spectroscopy (EIS) as a new tool for investigating methods of corrosion protection. Electrochim Acta 1990, 35, 1533–1544. [Google Scholar] [CrossRef]
- Lay, P.; Lawrence, P.F.; Wilkins, N.J.M.; Williams, D.E. An ac impedance study of steel in concrete. J. Appl. Electrochem. 1985, 15, 755–766. [Google Scholar] [CrossRef]
- Glass, G.K. An assessment of the coulostatic method applied to the corrosion of steel in concrete. Corros. Sci. 1995, 37, 597–605. [Google Scholar] [CrossRef]
- Wang, W.; Chen, H.; Li, X.; Zhu, Z. Corrosion behavior of steel bars immersed in simulated pore solutions of alkali-activated slag mortar. Constr. Build. Mater. 2017, 143, 289–297. [Google Scholar] [CrossRef]
- Ismail, M.; Ohtsu, M. Corrosion rate of ordinary and high-performance concrete subjected to chloride attack by AC impedance spectroscopy. Constr. Build. Mater. 2006, 20, 458–469. [Google Scholar] [CrossRef]
- Ortega, J.M.; Sánchez, I.; Climent, M.A. Impedance spectroscopy study of the effect of environmental conditions in the microstructure development of OPC and slag cement mortars. Arch. Civ. Mech. Eng. 2015, 15, 569–583. [Google Scholar] [CrossRef]
- Sánchez, I.; López, M.P.; Ortega, J.M.; Climent, M.A. Impedance spectroscopy: An efficient tool to determine the non-steady-state chloride diffusion coefficient in building materials. Mater. Corros. 2011, 62, 139–145. [Google Scholar] [CrossRef]
- Vedalakshmi, R.R.; Devi, R.R.; Emmanuel, B.; Palaniswamy, N. Determination of diffusion coefficient of chloride in concrete: An electrochemical impedance spectroscopic approach. Mater. Struct. 2008, 41, 1315–1326. [Google Scholar] [CrossRef]



| Name | Chemical Compositions (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | TiO2 | Fe2O3 | CaO | MgO | SO3 | K2O | etc. | L.O.I | |
| OPC | 19.74 | 5.33 | 0.30 | 2.93 | 61.74 | 3.78 | 2.47 | 0.89 | 2.82 | 2.3 |
| GGBFS | 33.35 | 13.36 | 0.59 | 0.33 | 44.62 | 4.12 | 2.69 | 0.41 | 0.53 | 0.1 |
| Name | W/B (%) | Unit Weight (kg/m3) | Unit Weight (g/m3) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| W | C | GG BFS | S1 1 | S2 2 | G 3 | S.P. | A.E. | ||
| OPC-40 | 40 | 180 | 450 | - | 553 | 240 | 867 | 3600 | 150 |
| OPC-50 | 50 | 180 | 360 | - | 607 | 260 | 867 | 3240 | 117 |
| OPC-60 | 60 | 180 | 300 | - | 640 | 273 | 867 | 3007 | 93 |
| S3-40 | 40 | 180 | 315 | 135 | 547 | 240 | 867 | 3150 | 157 |
| S3-50 | 50 | 180 | 252 | 108 | 600 | 260 | 867 | 2880 | 130 |
| S3-60 | 60 | 180 | 210 | 90 | 640 | 273 | 867 | 2400 | 120 |
| S6-40 | 40 | 180 | 180 | 270 | 540 | 233 | 867 | 2700 | 430 |
| S6-50 | 50 | 180 | 144 | 216 | 600 | 253 | 867 | 2160 | 300 |
| S6-60 | 60 | 180 | 120 | 180 | 633 | 267 | 867 | 2100 | 250 |
| Initial Current I30V (with 30V) (mA) | Applied Voltage U (after Adjustment) (V) | Possible New Initial Current I0 (mA) | Test Duration (Hour) |
|---|---|---|---|
| I0 < 5 | 60 | I0 < 10 | 96 |
| 5 ≤ I0 < 10 | 60 | 10 ≤ I0 < 20 | 48 |
| 10 ≤ I0 < 15 | 60 | 20 ≤ I0 < 30 | 24 |
| 15 ≤ I0 < 20 | 50 | 25 ≤ I0 < 35 | 24 |
| 20 ≤ I0 < 30 | 40 | 25 ≤ I0 < 40 | 24 |
| 30 ≤ I0 < 40 | 35 | 35 ≤ I0 < 50 | 24 |
| 40 ≤ I0 < 60 | 30 | 40 ≤ I0 < 60 | 24 |
| 60 ≤ I0 < 90 | 25 | 50 ≤ I0 < 75 | 24 |
| 90 ≤ I0 < 120 | 20 | 60 ≤ I0 < 80 | 24 |
| 120 ≤ I0 < 180 | 15 | 60 ≤ I0 < 90 | 24 |
| 180 ≤ I0 < 360 | 10 | 60 ≤ I0 < 120 | 24 |
| I0 ≥ 360 | 10 | I0 ≥ 120 | 6 |
| Frequency Range | 105 ~ 10−2 Hz |
|---|---|
| Specimen Size | Ø100 × 200 mm2 |
| Cover concrete | 44 mm |
| WE | Ø13 mm rebar (SD400) |
| RE | Ag/AgCl |
| CE | STS 304 |
| Name | Compressive Strength (MPa) | Rate of Change 28d (%) | |||
|---|---|---|---|---|---|
| 3d | 7d | 28d | |||
| OPC-40 | W * | 35.9 | 42.3 | 51.7 | −7.43 |
| A ** | 35.8 | 42.7 | 47.8 | ||
| OPC-50 | W | 26.1 | 32.7 | 41.1 | −5.79 |
| A | 25.9 | 32.7 | 38.7 | ||
| OPC-60 | W | 18.4 | 23.2 | 30.0 | −2.66 |
| A | 18.0 | 23.2 | 29.2 | ||
| S3-40 | W | 28.3 | 37.8 | 53.6 | −7.58 |
| A | 27.2 | 37.8 | 49.5 | ||
| S3-50 | W | 19.6 | 28.9 | 43.9 | −13.28 |
| A | 19.4 | 28.9 | 38.1 | ||
| S3-60 | W | 13.6 | 22.6 | 34.0 | −17.06 |
| A | 13.6 | 20.9 | 28.2 | ||
| S6-40 | W | 25.2 | 40.8 | 50.7 | −9.61 |
| A | 24.7 | 37.1 | 45.8 | ||
| S6-50 | W | 17.3 | 30.1 | 42.7 | −15.85 |
| A | 16.6 | 28.8 | 35.9 | ||
| S6-60 | W | 11.8 | 22.4 | 33.4 | −17.98 |
| A | 11.8 | 20.9 | 27.4 | ||
| Name | Chloride Diffusion Coefficient (28d) (×10−12 m2/s) | Rate of Change (%) | |
|---|---|---|---|
| OPC-40 | W | 9.92 | 28.53 |
| A | 12.75 | ||
| OPC-50 | W | 16.56 | 15.70 |
| A | 19.16 | ||
| OPC-60 | W | 22.05 | 17.94 |
| A | 37.03 | ||
| S3-40 | W | 4.47 | −0.45 |
| A | 4.45 | ||
| S3-50 | W | 5.41 | 33.83 |
| A | 7.24 | ||
| S3-60 | W | 7.42 | 111.19 |
| A | 15.67 | ||
| S6-40 | W | 2.51 | 52.99 |
| A | 3.84 | ||
| S6-50 | W | 3.24 | 87.96 |
| A | 6.09 | ||
| S6-60 | W | 4.12 | 96.60 |
| A | 8.10 | ||
| Name | |Z|max (Ωcm2) | |Z|min (Rs) (Ωcm2) | |Z|max − |Z|min (Rp) (Ωcm2) | |
|---|---|---|---|---|
| OPC-40 | W | 418.463 | 181.009 | 237.454 |
| A | 382.322 | 166.431 | 215.891 | |
| OPC-50 | W | 281.896 | 121.460 | 160.436 |
| A | 323.150 | 127.378 | 195.772 | |
| OPC-60 | W | 223.554 | 87.651 | 135.903 |
| A | 263.696 | 94.007 | 169.689 | |
| S3-40 | W | 668.187 | 365.856 | 302.331 |
| A | 697.065 | 329.151 | 367.914 | |
| S3-50 | W | 598.828 | 353.875 | 244.953 |
| A | 478.571 | 252.401 | 226.170 | |
| S3-60 | W | 460.889 | 270.274 | 190.615 |
| A | 421.707 | 191.777 | 229.930 | |
| S6-40 | W | 1374.260 | 864.382 | 509.878 |
| A | 1095.780 | 588.412 | 507.368 | |
| S6-50 | W | 1306.230 | 800.663 | 505.567 |
| A | 1027.270 | 593.435 | 433.835 | |
| S6-60 | W | 990.183 | 601.283 | 388.900 |
| A | 857.888 | 458.342 | 399.546 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Park, C.; Joh, S.; Lee, H. Effect of Curing Condition on Resistance to Chloride Ingress in Concrete Using Ground Granulated Blast Furnace Slag. Materials 2019, 12, 3233. https://doi.org/10.3390/ma12193233
Park J, Park C, Joh S, Lee H. Effect of Curing Condition on Resistance to Chloride Ingress in Concrete Using Ground Granulated Blast Furnace Slag. Materials. 2019; 12(19):3233. https://doi.org/10.3390/ma12193233
Chicago/Turabian StylePark, JangHyun, Cheol Park, SungHyung Joh, and HanSeung Lee. 2019. "Effect of Curing Condition on Resistance to Chloride Ingress in Concrete Using Ground Granulated Blast Furnace Slag" Materials 12, no. 19: 3233. https://doi.org/10.3390/ma12193233
APA StylePark, J., Park, C., Joh, S., & Lee, H. (2019). Effect of Curing Condition on Resistance to Chloride Ingress in Concrete Using Ground Granulated Blast Furnace Slag. Materials, 12(19), 3233. https://doi.org/10.3390/ma12193233






