Anisotropic Cryostructured Collagen Scaffolds for Efficient Delivery of RhBMP–2 and Enhanced Bone Regeneration
Abstract
1. Introduction
2. Materials and Methods
2.1. Production of Cryostructured Bone Scaffolds
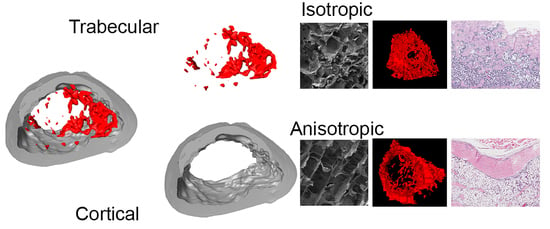
2.2. Scaffold Characterization by Scanning Electron Microscope (SEM) and Energy Dispersive X-Ray Spectroscopy (EDX).
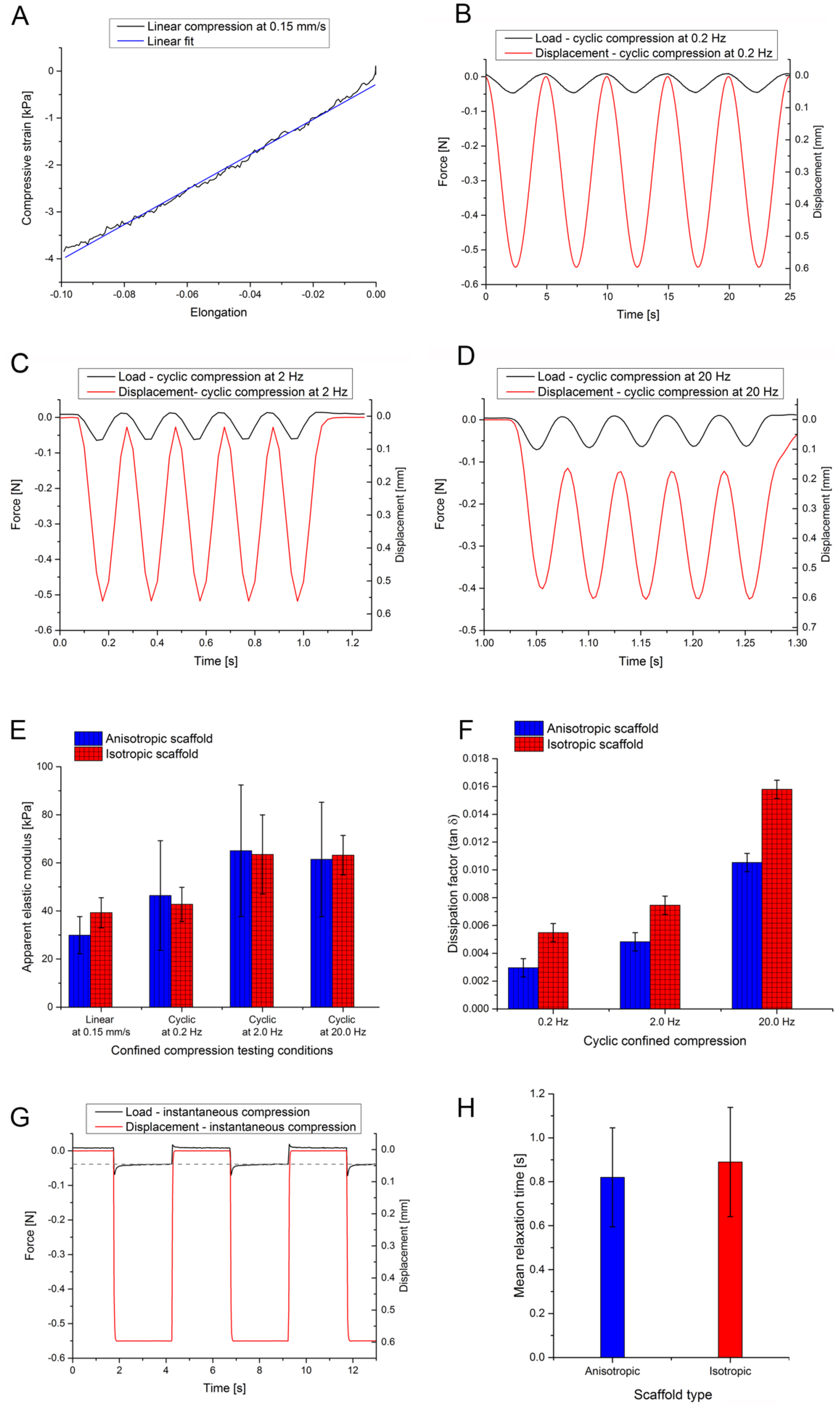
2.3. Scaffold Mechanical Testing
2.4. Bone Morphogenetic Protein 2 (BMP–2) Loading and Release
2.5. Nonunion In Vivo Model
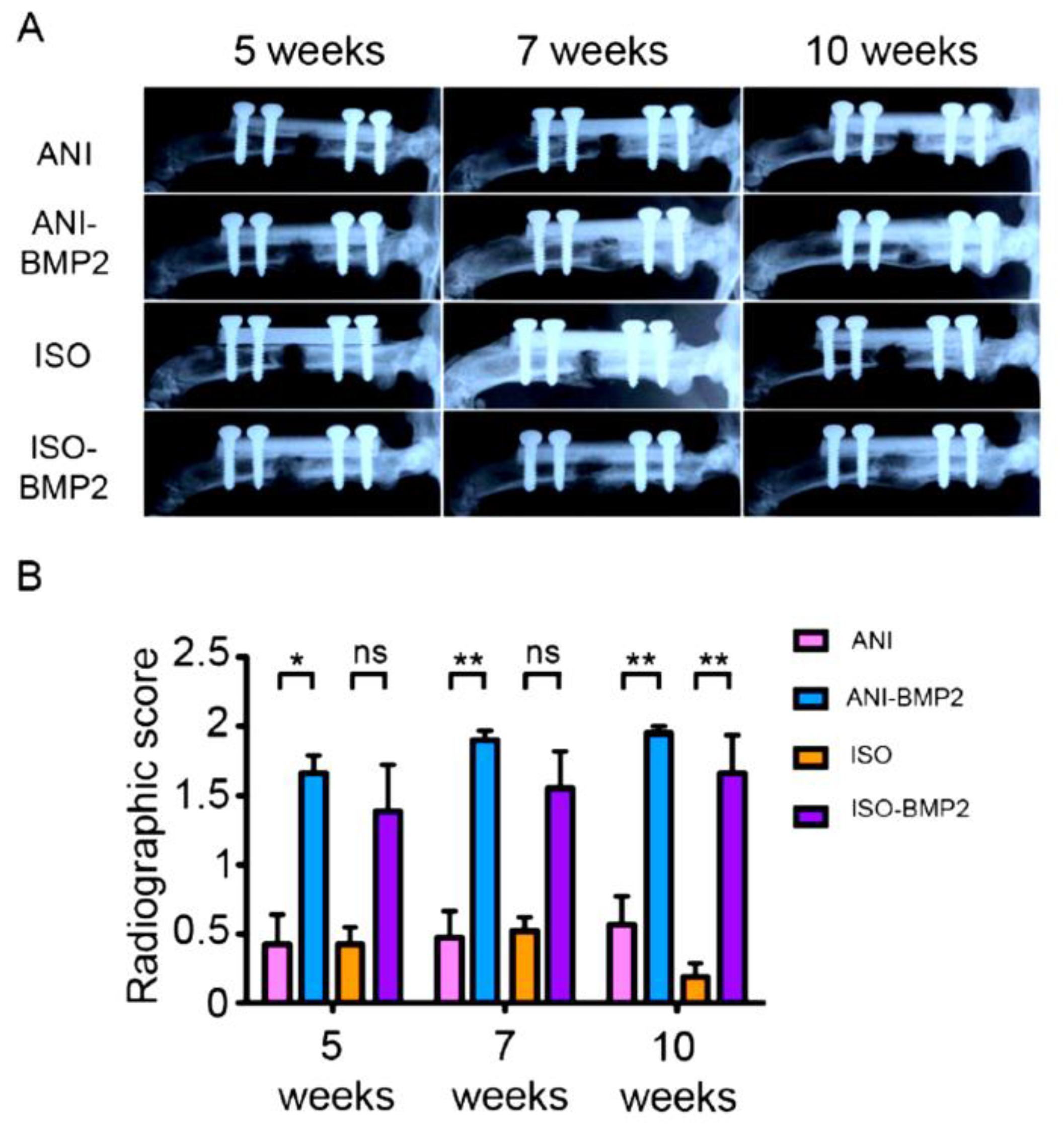
2.6. Radiography
2.7. Micro computed Tomography (µCT)
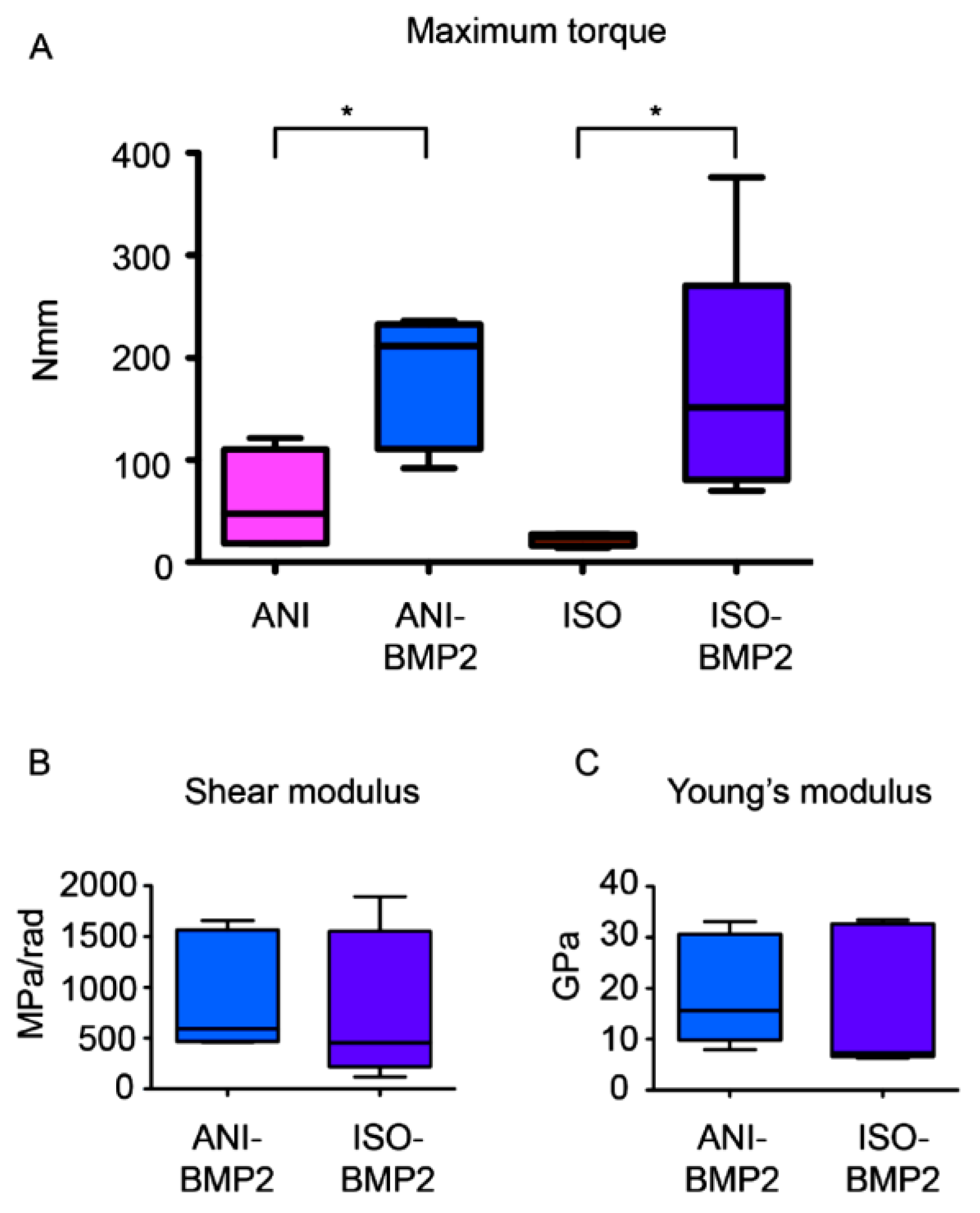
2.8. Torsional Biomechanical Testing
2.9. Histology
2.10. Statistical Analysis
3. Results
3.1. Cryostructured Scaffolds Characterization
3.2. In Vivo Assessment of Cryostructured Scaffolds
3.3. Biomechanical Testing
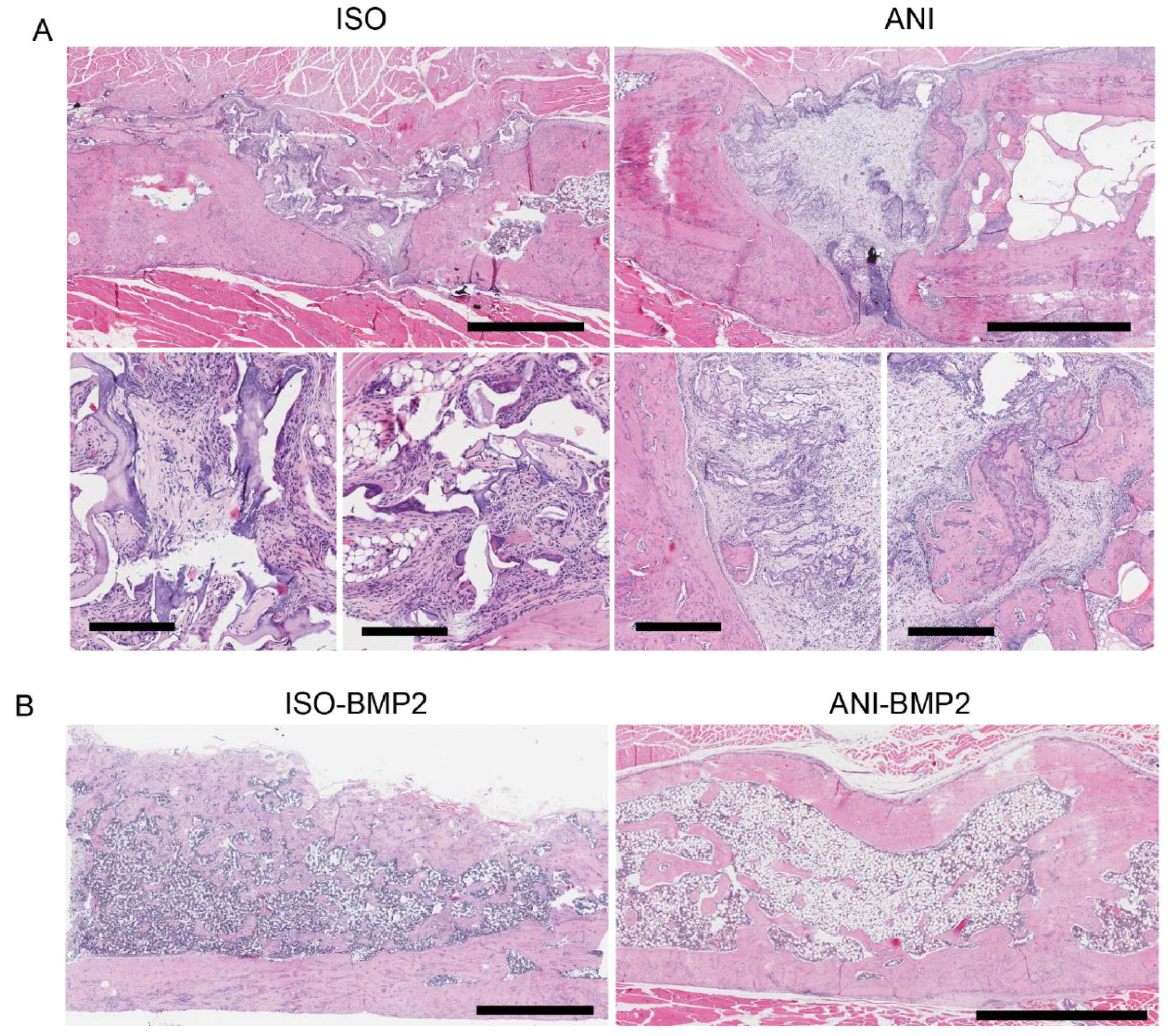
3.4. Histological Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jakob, F.; Ebert, R.; Ignatius, A.; Matsushita, T.; Watanabe, Y.; Groll, J.; Walles, H. Bone tissue engineering in osteoporosis. Maturitas 2013, 75, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Wegst, U.G.K.; Bai, H.; Saiz, E.; Tomsia, A.P.; Ritchie, R.O. Bioinspired structural materials. Nat. Mater. 2014, 14, 23. [Google Scholar] [CrossRef] [PubMed]
- Hak, D.J.; Fitzpatrick, D.; Bishop, J.A.; Marsh, J.L.; Tilp, S.; Schnettler, R.; Simpson, H.; Alt, V. Delayed union and nonunions: Epidemiology, clinical issues, and financial aspects. Injury 2014, 45, S3–S7. [Google Scholar] [CrossRef] [PubMed]
- Bauer, T.W.; Muschler, G.F. Bone graft materials. An overview of the basic science. Clin. Orthop. Relat. Res. 2000, 371, 10–27. [Google Scholar] [CrossRef]
- Salazar, V.S.; Gamer, L.W.; Rosen, V. BMP signalling in skeletal development, disease and repair. Nat. Rev. Endocrinol. 2016, 12, 203. [Google Scholar] [CrossRef] [PubMed]
- Muinos-López, E.; Ripalda-Cemboráin, P.; López-Martínez, T.; González-Gil, A.B.; Lamo-Espinosa, J.M.; Valentí, A.; Mortlock, D.P.; Valentí, J.R.; Prósper, F.; Granero-Moltó, F. Hypoxia and reactive oxygen species homeostasis in mesenchymal progenitor cells define a molecular mechanism for fracture nonunion. Stem Cells 2016, 34, 2342–2353. [Google Scholar] [CrossRef]
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone regeneration: current concepts and future directions. BMC Med. 2011, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Dimar, J.R.; Glassman, S.D.; Burkus, K.J.; Carreon, L.Y. Clinical outcomes and fusion success at 2 years of single-level instrumented posterolateral fusions with recombinant human bone morphogenetic protein-2/compression resistant matrix versus iliac crest bone graft. Spine (Phila. Pa. 1976). 2006, 31, 2534–2539. [Google Scholar] [CrossRef]
- Zara, J.N.; Siu, R.K.; Zhang, X.; Shen, J.; Ngo, R.; Lee, M.; Li, W.; Chiang, M.; Chung, J.; Kwak, J.; et al. High Doses of Bone Morphogenetic protein 2 induce structurally abnormal bone and inflammation In Vivo. Tissue Eng. Part A 2011, 17, 1389–1399. [Google Scholar] [CrossRef]
- Geiger, M.; Li, R.; Friess, W. Collagen sponges for bone regeneration with RhBMP-2. Adv. Drug Deliv. Rev. 2003, 55, 1613–1629. [Google Scholar] [CrossRef]
- Bessa, P.C.; Casal, M.; Reis, R.L. Bone morphogenetic proteins in tissue engineering: The road from laboratory to clinic, Part II (BMP delivery). J. Tissue Eng. Regen. Med. 2008, 2, 81–96. [Google Scholar] [CrossRef] [PubMed]
- James, A.W.; LaChaud, G.; Shen, J.; Asatrian, G.; Nguyen, V.; Zhang, X.; Ting, K.; Soo, C. A review of the clinical side effects of bone morphogenetic protein-2. Tissue Eng. Part B. Rev. 2016, 22, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Carragee, E.J.; Hurwitz, E.L.; Weiner, B.K. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: Emerging safety concerns and lessons learned. Spine J. 2011, 11, 471–491. [Google Scholar] [CrossRef] [PubMed]
- Arosarena, O.; Collins, W. Comparison of BMP-2 and -4 for rat mandibular bone regeneration at various doses. Orthod. Craniofac. Res. 2005, 8, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Arosarena, O.A.; Collins, W.L. Bone regeneration in the rat mandible with bone morphogenetic protein-2: A comparison of two carriers. Otolaryngol. Neck Surg. 2005, 132, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Young, S.; Patel, Z.S.; Kretlow, J.D.; Murphy, M.B.; Mountziaris, P.M.; Baggett, L.S.; Ueda, H.; Tabata, Y.; Jansen, J.A.; Wong, M.; et al. Dose effect of dual delivery of vascular endothelial growth factor and bone morphogenetic protein-2 on bone regeneration in a rat critical-size defect model. Tissue Eng. Part A 2009, 15, 2347–2362. [Google Scholar] [CrossRef]
- Patel, Z.S.; Young, S.; Tabata, Y.; Jansen, J.A.; Wong, M.E.K.; Mikos, A.G. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone 2008, 43, 931–940. [Google Scholar] [CrossRef]
- Aryal, R.; Chen, X.; Fang, C.; Hu, Y. Bone morphogenetic protein-2 and vascular endothelial growth factor in bone tissue regeneration: New insight and perspectives. Orthop. Surg. 2014, 6, 171–178. [Google Scholar] [CrossRef]
- Stuckensen, K.; Schwab, A.; Knauer, M.; Muiños-López, E.; Ehlicke, F.; Reboredo, J.; Granero-Moltó, F.; Gbureck, U.; Prósper, F.; Walles, H.; et al. Tissue mimicry in morphology and composition promotes hierarchical matrix remodeling of invading stem cells in osteochondral and meniscus scaffolds. Adv. Mater. 2018, 30, e1706754. [Google Scholar] [CrossRef]
- Dodla, M.C.; Bellamkonda, R.V. Differences between the effect of anisotropic and isotropic laminin and nerve growth factor presenting scaffolds on nerve regeneration across long peripheral nerve gaps. Biomaterials 2008, 29, 33–46. [Google Scholar] [CrossRef]
- Kirsch, T.; Nickel, J.; Sebald, W. Isolation of recombinant BMP receptor IA ectodomain and Its 2:1 complex with BMP-2. FEBS Lett. 2000, 468, 215–219. [Google Scholar] [CrossRef]
- Jarcho, M.; Kay, J.F.; Gumaer, K.I.; Doremus, R.H.; Drobeck, H.P. Tissue, cellular and subcellular events at a bone-ceramic hydroxylapatite interface. J. Bioeng. 1977, 1, 79. [Google Scholar] [PubMed]
- Bishop, G.B.; Einhorn, T.A. Current and future clinical applications of bone morphogenetic proteins in orthopaedic trauma surgery. Int. Orthop. 2007, 31, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.; Gross, G. Tendon and ligament engineering in the adult organism: Mesenchymal stem cells and gene-therapeutic approaches. Int. Orthop. 2007, 31, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Chubinskaya, S.; Hurtig, M.; Rueger, D.C. OP-1/BMP-7 in cartilage repair. Int. Orthop. 2007, 31, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Abbah, S.A.; Hu, T.; Toh, S.Y.; Lam, R.W.M.; Goh, J.C.-H.; Wong, H.-K. Minimizing the severity of RhBMP-2–Induced inflammation and heterotopic ossification with a polyelectrolyte carrier incorporating heparin on microbead templates. Spine (Phila. Pa. 1976). 2013, 38, 1452–1458. [Google Scholar] [CrossRef]
- Krishnan, L.; Priddy, L.B.; Esancy, C.; Klosterhoff, B.S.; Stevens, H.Y.; Tran, L.; Guldberg, R.E. Delivery vehicle effects on bone regeneration and heterotopic ossification induced by high dose BMP-2. Acta Biomater. 2017, 49, 101–112. [Google Scholar] [CrossRef]
- Seeherman, H.; Wozney, J.M. Delivery of bone morphogenetic proteins for orthopedic tissue regeneration. Cytokine Growth Factor Rev. 2005, 16, 329–345. [Google Scholar] [CrossRef]
- Nguyen, P.D.; Lin, C.D.; Allori, A.C.; Schachar, J.S.; Ricci, J.L.; Saadeh, P.B.; Warren, S.M. Scaffold-based rhBMP-2 therapy in a rat alveolar defect model: implications forhuman gingivoperiosteoplasty. Plast. Reconstr. Surg. 2009, 124, 1829–1839. [Google Scholar] [CrossRef]
- Ruhé, P.Q.; Boerman, O.C.; Russel, F.G.M.; Spauwen, P.H.M.; Mikos, A.G.; Jansen, J.A. Controlled release of rhBMP-2 loaded Poly(Dl-Lactic-Co-Glycolic Acid)/calcium phosphate cement composites in vivo. J. Control. Release 2005, 106, 162–171. [Google Scholar] [CrossRef]
- Boerckel, J.D.; Kolambkar, Y.M.; Dupont, K.M.; Uhrig, B.A.; Phelps, E.A.; Stevens, H.Y.; García, A.J.; Guldberg, R.E. Effects of protein dose and delivery system on BMP-mediated bone regeneration. Biomaterials 2011, 32, 5241–5251. [Google Scholar] [CrossRef] [PubMed]
- de Guzman, R.C.; Saul, J.M.; Ellenburg, M.D.; Merrill, M.R.; Coan, H.B.; Smith, T.L.; Van Dyke, M.E. Bone regeneration with BMP-2 delivered from keratose scaffolds. Biomaterials 2013, 34, 1644–1656. [Google Scholar] [CrossRef] [PubMed]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stuckensen, K.; Lamo-Espinosa, J.M.; Muiños-López, E.; Ripalda-Cemboráin, P.; López-Martínez, T.; Iglesias, E.; Abizanda, G.; Andreu, I.; Flandes-Iparraguirre, M.; Pons-Villanueva, J.; et al. Anisotropic Cryostructured Collagen Scaffolds for Efficient Delivery of RhBMP–2 and Enhanced Bone Regeneration. Materials 2019, 12, 3105. https://doi.org/10.3390/ma12193105
Stuckensen K, Lamo-Espinosa JM, Muiños-López E, Ripalda-Cemboráin P, López-Martínez T, Iglesias E, Abizanda G, Andreu I, Flandes-Iparraguirre M, Pons-Villanueva J, et al. Anisotropic Cryostructured Collagen Scaffolds for Efficient Delivery of RhBMP–2 and Enhanced Bone Regeneration. Materials. 2019; 12(19):3105. https://doi.org/10.3390/ma12193105
Chicago/Turabian StyleStuckensen, Kai, José M. Lamo-Espinosa, Emma Muiños-López, Purificación Ripalda-Cemboráin, Tania López-Martínez, Elena Iglesias, Gloria Abizanda, Ion Andreu, María Flandes-Iparraguirre, Juan Pons-Villanueva, and et al. 2019. "Anisotropic Cryostructured Collagen Scaffolds for Efficient Delivery of RhBMP–2 and Enhanced Bone Regeneration" Materials 12, no. 19: 3105. https://doi.org/10.3390/ma12193105
APA StyleStuckensen, K., Lamo-Espinosa, J. M., Muiños-López, E., Ripalda-Cemboráin, P., López-Martínez, T., Iglesias, E., Abizanda, G., Andreu, I., Flandes-Iparraguirre, M., Pons-Villanueva, J., Elizalde, R., Nickel, J., Ewald, A., Gbureck, U., Prósper, F., Groll, J., & Granero-Moltó, F. (2019). Anisotropic Cryostructured Collagen Scaffolds for Efficient Delivery of RhBMP–2 and Enhanced Bone Regeneration. Materials, 12(19), 3105. https://doi.org/10.3390/ma12193105