Fabrication and Biocompatibility Evaluation of Nanodiamonds-Gelatin Electrospun Materials Designed for Prospective Tissue Regeneration Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Electrospun NDs-loaded FG Fibrous Scaffolds
2.2. Characterization of the Fibrous Scaffolds
2.3. Biocompatibility Evaluation of the Fibrous Scaffolds
3. Results
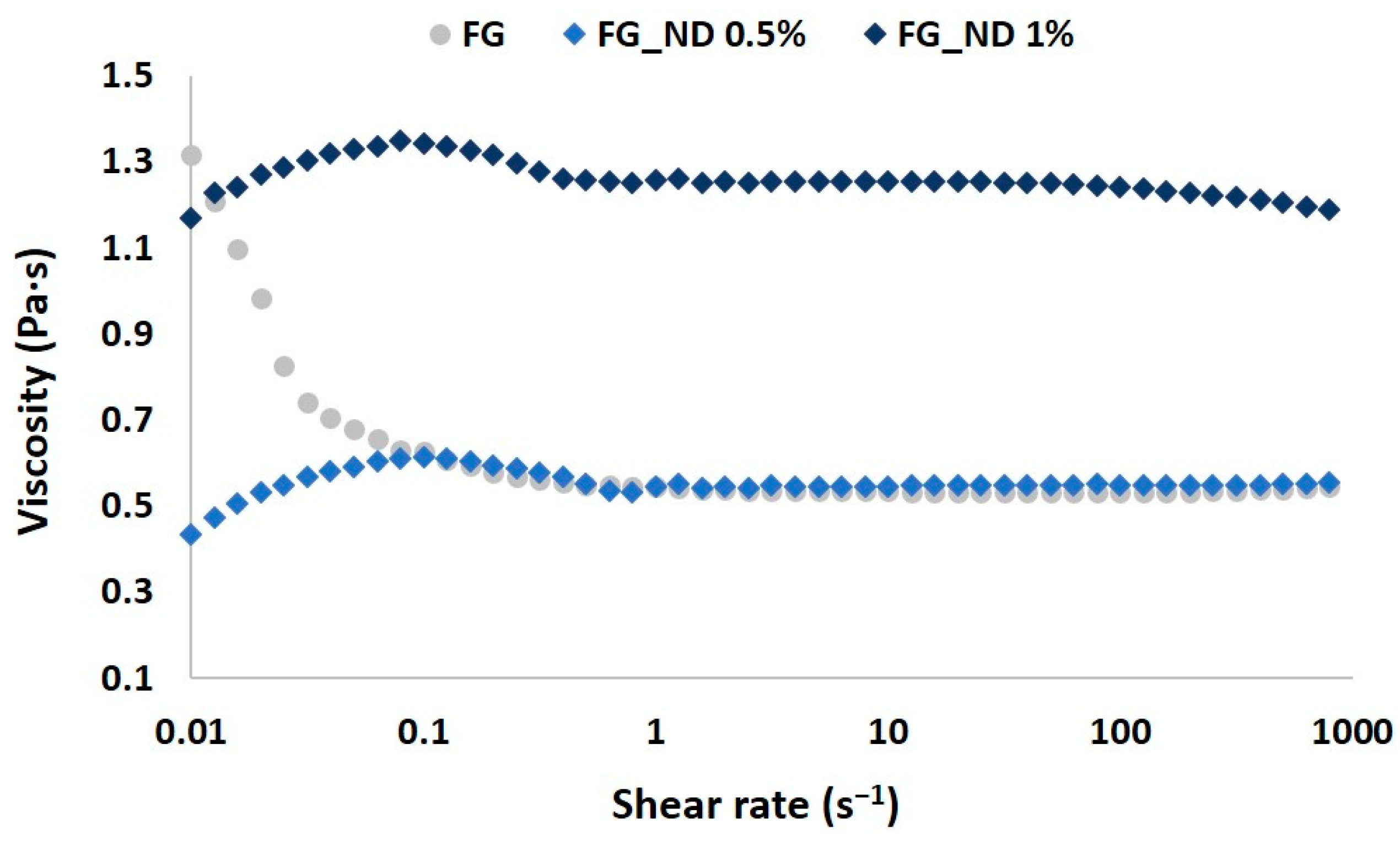
3.1. Rheological Evaluation of the Precursors
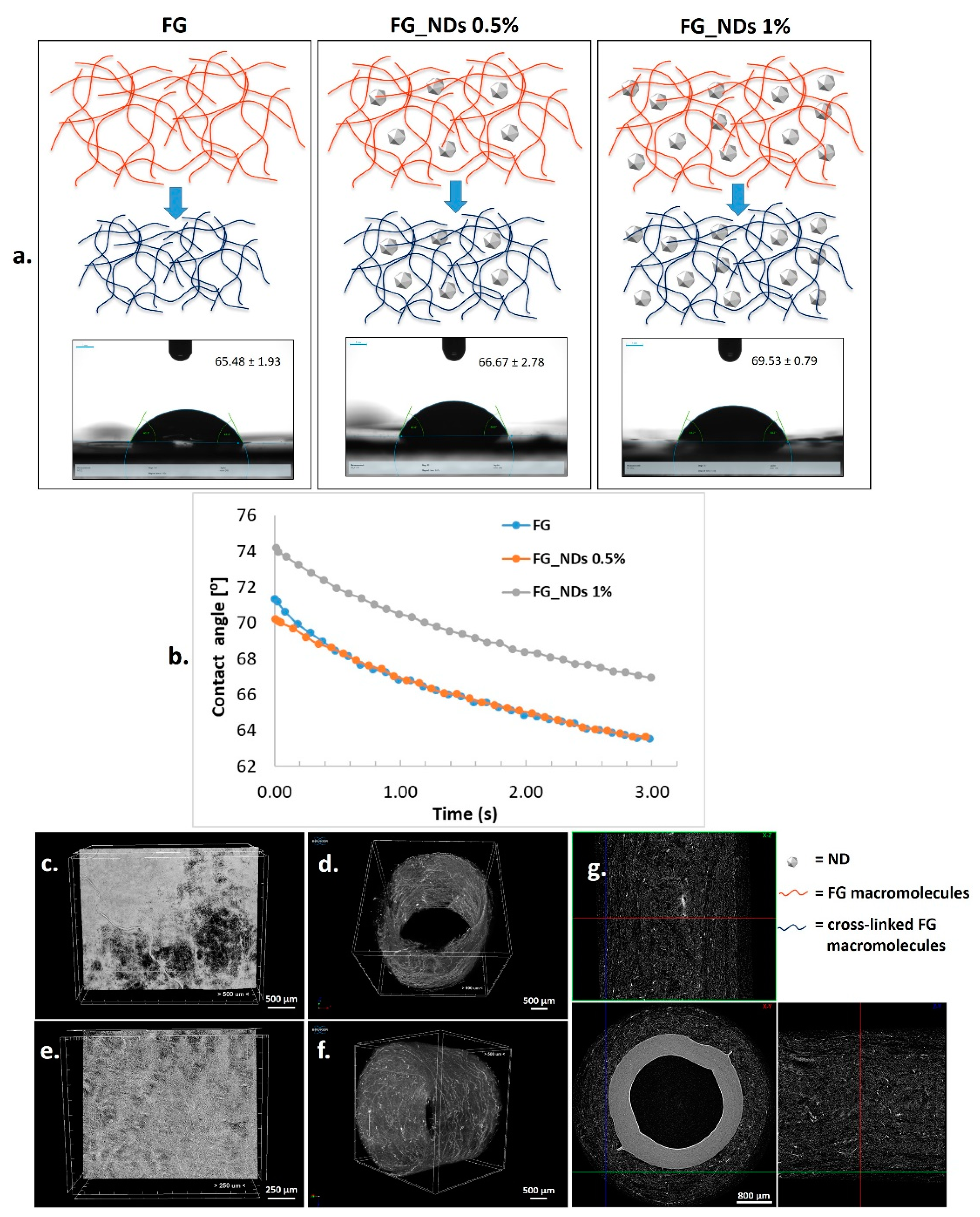
3.2. Wettability
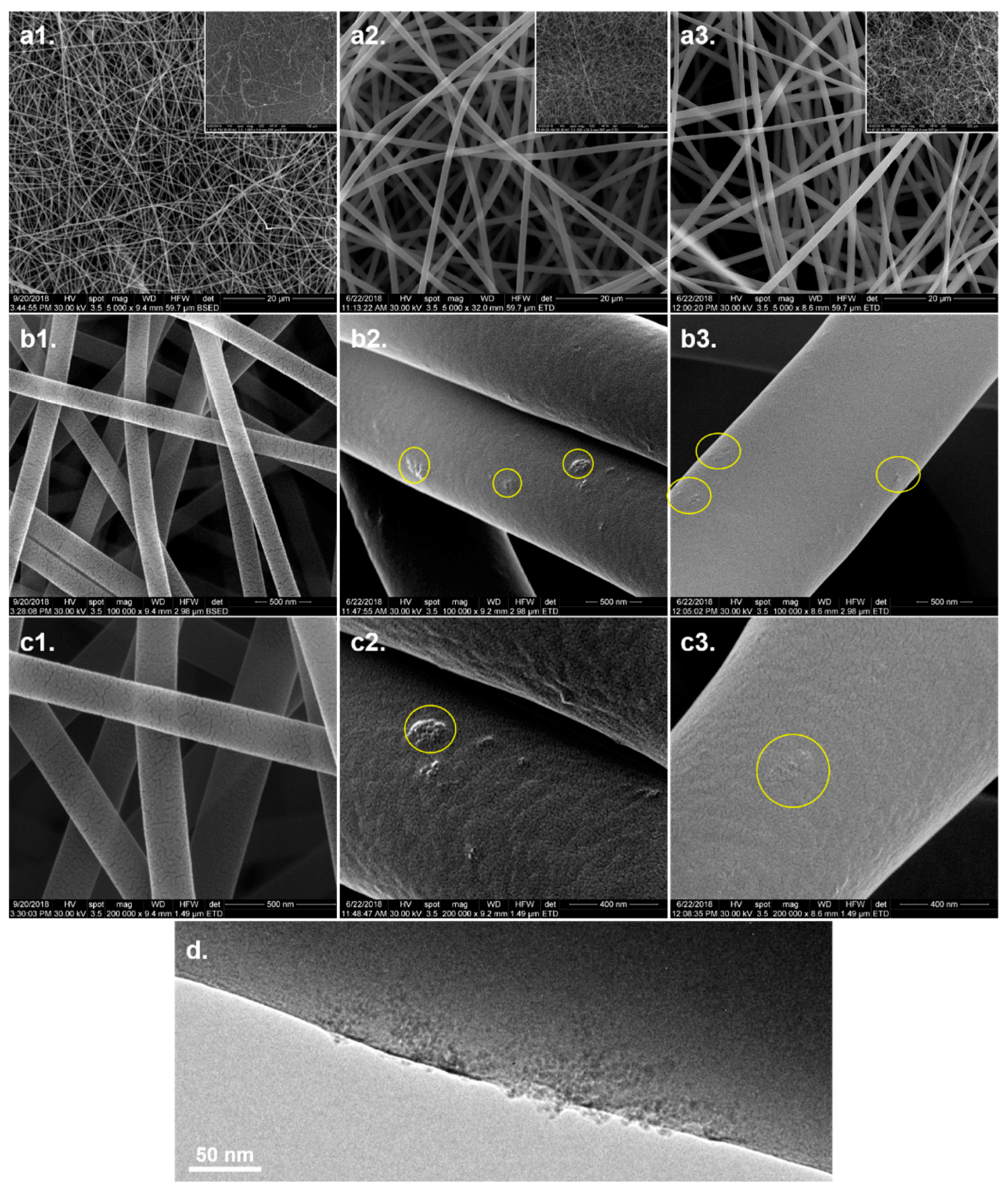
3.3. Microstructural Analysis
3.4. Nanomechanical Investigation
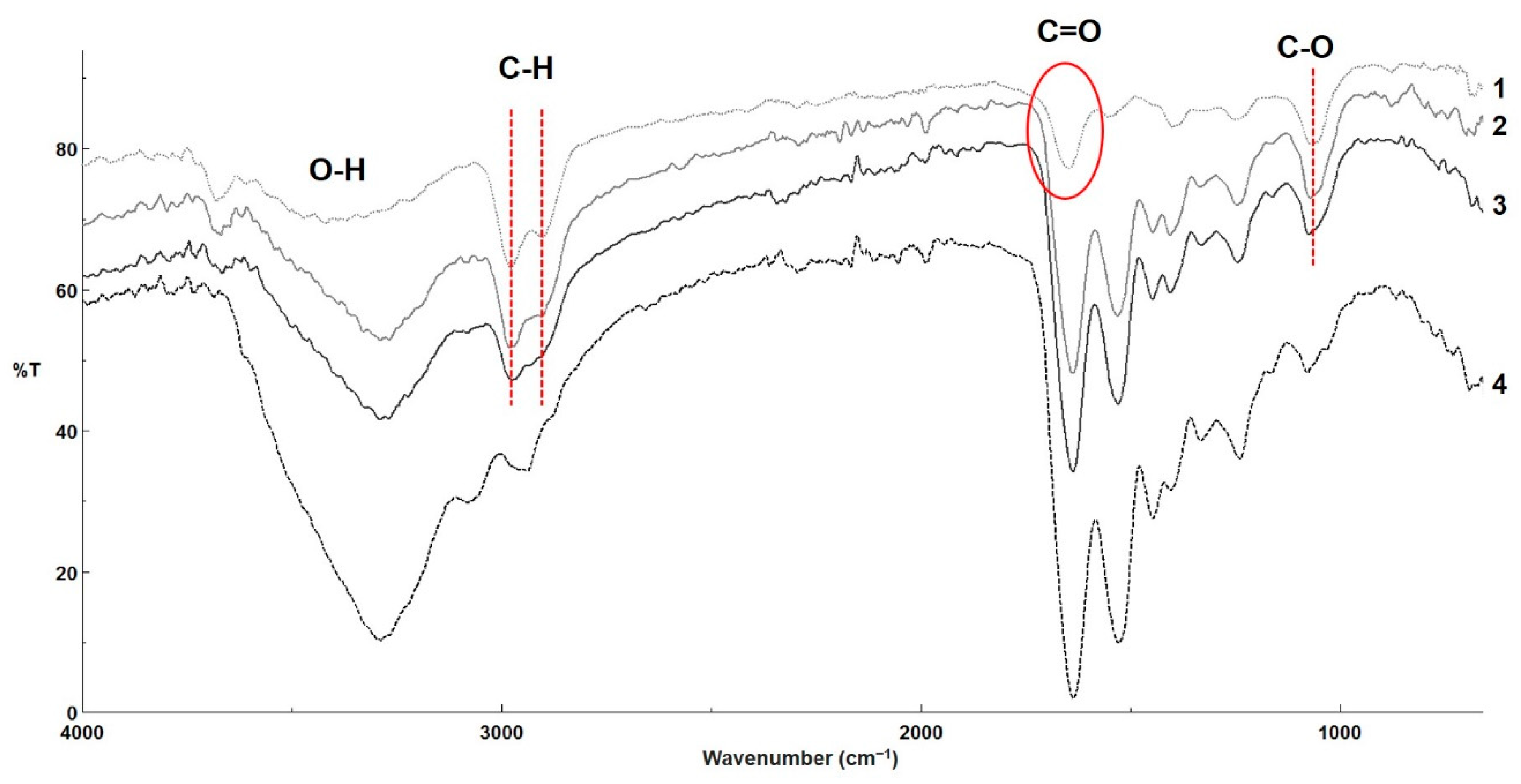
3.5. FT-IR Analysis
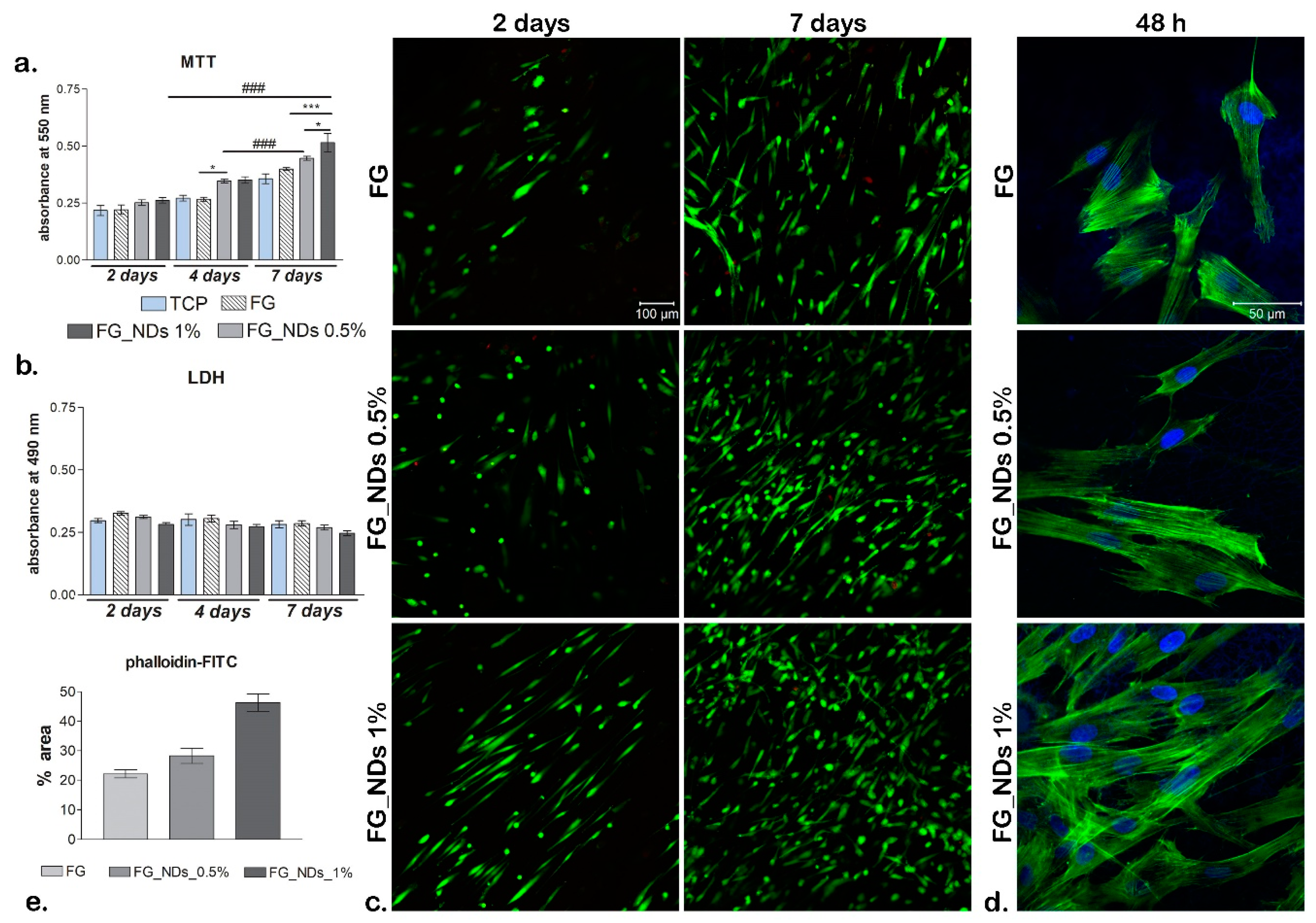
3.6. Biocompatibility Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dzobo, K.; Thomford, N.E.; Senthebane, D.A.; Shipanga, H.; Rowe, A.; Dandara, C.; Pillay, M.; Motaung, K.S.C.M. Advances in regenerative medicine and tissue engineering: Innovation and transformation of medicine. Stem Cells Int. 2018. [Google Scholar] [CrossRef] [PubMed]
- Salgado, A.J.; Oliveira, J.M.; Martins, A.; Teixeira, F.G.; Silva, N.A.; Neves, N.M.; Sousa, N.; Reis, R.L. Tissue engineering and regenerative medicine: Past, present, and future. In Title of International Review of Neurobiology; Stefano, G., Isabelle, P., Pierluigi, T., Bruno, B., Eds.; Academic Press: Boston, MA, USA, 2013; Volume 108, pp. 1–33. [Google Scholar]
- Sell, S.A.; Wolfe, P.S.; Garg, K.; McCool, J.M.; Rodriguez, I.A.; Bowlin, G.L. The use of natural polymers in tissue engineering: A focus on electrospun extracellular matrix analogues. Polymers 2010, 2, 522–553. [Google Scholar] [CrossRef]
- Karim, A.A.; Bhat, R. Fish gelatin: Properties, challenges, and prospects as an alternative to mammalian gelatins. Food Hydrocolloid 2009, 23, 563–576. [Google Scholar] [CrossRef]
- Shakila, R.J.; Jeevithan, E.; Varatharajakumar, A.; Jeyasekaran, G.; Sukumar, D. Comparison of the properties of multi-composite fish gelatin films with that of mammalian gelatin films. Food Chem. 2012, 135, 2260–2267. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.J.; Shin, S.R.; Cha, J.M.; Lee, S.H.; Kim, J.H.; Do, J.T.; Song, H.; Bae, H. Cold water fish gelatin methacryloyl hydrogel for tissue engineering application. PloS ONE 2016, 11, e0163902. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Hydrogels for tissue engineering. Chem. Rev. 2001, 101, 1869–1880. [Google Scholar] [CrossRef] [PubMed]
- Afewerki, S.; Sheikhi, A.; Kannan, S.; Ahadian, S.; Khademhosseini, A. Gelatin-polysaccharide composite scaffolds for 3D cell culture and tissue engineering: Towards natural therapeutics. Bioeng. Transl. Med. 2019, 4, 96–115. [Google Scholar] [CrossRef]
- Manikandan, A.; Thirupathi Kumara Raja, S.; Thiruselvi, T.; Gnanamani, A. Engineered fish scale gelatin: An alternative and suitable biomaterial for tissue engineering. J. Bioact. Compat. Polym. 2018, 33, 332–346. [Google Scholar] [CrossRef]
- Fu, C.; Bai, H.; Hu, Q.; Gao, T.; Bai, Y. Enhanced proliferation and osteogenic differentiation of MC3T3-E1 pre-osteoblasts on graphene oxide-impregnated PLGA–gelatin nanocomposite fibrous membranes. Rsc Adv. 2017, 7, 8886–8897. [Google Scholar] [CrossRef]
- Lai, J.Y.; Li, Y.T.; Cho, C.H.; Yu, T.C. Nanoscale modification of porous gelatin scaffolds with chondroitin sulfate for corneal stromal tissue engineering. Int. J. Naonomed. 2012, 7, 1101. [Google Scholar] [CrossRef]
- Huber, B.; Borchers, K.; Tovar, G.E.; Kluger, P.J. Methacrylated gelatin and mature adipocytes are promising components for adipose tissue engineering. J. Biomater. Appl. 2016, 30, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi-Mobarakeh, L.; Prabhakaran, M.P.; Morshed, M.; Nasr-Esfahani, M.H.; Ramakrishna, S. Electrospun poly (ε-caprolactone)/gelatin nanofibrous scaffolds for nerve tissue engineering. Biomaterials 2008, 29, 4532–4539. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Tabata, Y.; Ikada, Y. Growth factor release from gelatin hydrogel for tissue engineering. J. Bioact. Compat. Polym. 1999, 14, 474–489. [Google Scholar] [CrossRef]
- Houshyar, S.; Kumar, S.; Rifai, A.; Tran, N.; Nayak, R.; Shanks, R.A.; Padhye, R.; Fox, K.; Bhattacharyya, A. Nanodiamond/poly-ϵ-caprolactone nanofibrous scaffold for wound management. Mat. Sci. Eng. C-Mater. 2019, 100, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Chen, J. Nanobiomechanics of living cells: A review. Interface Focus 2014, 4, 20130055. [Google Scholar] [CrossRef] [PubMed]
- Bacakova, L.; Broz, A.; Liskova, J.; Stankova, L.; Potocky, S.; Kromka, A. The application of nanodiamond in biotechnology and tissue engineering. In Title of Diamond and Carbon Composites and Nanocomposites; Mahmood, A., Ed.; IntechOpen: London, UK, 2016; pp. 59–88. [Google Scholar]
- Bacakova, L.; Grausova, L.; Vandrovcova, M.; Vacik, J.; Frazcek, A.; Blazewicz, S.; Kromka, A.; Rezek, B.; Vanecek, M.; Nesladek, M.; et al. Carbon nanoparticles as substrates for cell adhesion and growth. In Title of Nanopartiles: New Research; Simone, L.L., Ed.; Nova Science Publishes, Inc.: New York, NY, USA, 2008; pp. 39–107. [Google Scholar]
- Serafim, A.; Cecoltan, S.; Lungu, A.; Vasile, E.; Iovu, H.; Stancu, I.C. Electrospun fish gelatin fibrous scaffolds with improved biointeractions due to carboxylated nanodiamond loading. Rsc Adv. 2015, 5, 95467–95477. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Maleki, A.; de la Guardia, M.; Bani, M.S.; Chenab, K.K.; Pashazadeh-Panahi, P.; Baradaran, B.; Mokhtarzadeh, A.; Hamblin, M.R. Carbon based nanomaterials for tissue engineering of bone: Building new bone on small black scaffolds: A review. J. Adv. Res. 2019, 18, 185–201. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.; Pedersen, T.O.; Wu, X.; Xue, Y.; Sun, Y.; Finne-Wistrand, A.; Kloss, F.R.; Waag, R.; Krueger, A.; Steinmuller-Nethl, D.; et al. Biological effects of functionalizing copolymer scaffolds with nanodiamond particles. Tissue Eng. Part. A 2013, 19, 1783–1791. [Google Scholar] [CrossRef]
- Mahdavi, M.; Mahmoudi, N.; Anaran, F.R.; Simchi, A. Electrospinning of Nanodiamond-Modified Polysaccharide Nanofibers with Physico-Mechanical Properties Close to Natural Skins. Mar. Drugs 2016, 14, 128. [Google Scholar] [CrossRef]
- Thalhammer, A.; Edgington, R.J.; Cingolani, L.A.; Schoepfer, R.; Jackman, R.B. The use of nanodiamond monolayer coatings to promote the formation of functional neuronal networks. Biomaterials 2010, 31, 2097–2104. [Google Scholar] [CrossRef]
- Yu, G.; Floyd, Z.E.; Wu, X.; Hebert, T.; Halvorsen, Y.D.C.; Buehrer, B.M.; Gimble, J.M. Adipogenic Differentiation of Adipose-Derived Stem Cells. In Title of Adipose-Derived Stem Cells. Methods in Molecular Biology (Methods and Protocols); Gimble, J., Bunnell, B., Eds.; Humana Press: Totowa, NJ, USA, 2011; Volume 702, pp. 193–200. [Google Scholar]
- Grottkau, B.E.; Lin, Y. Osteogenesis of adipose-derived stem cells. Bone Res. 2013; 2, 133–145. [Google Scholar]
- Oh, S.J.; Park, H.Y.; Choi, K.U.; Choi, S.W.; Kim, S.D.; Kong, S.K.; Cho, K.S. Auricular Cartilage Regeneration with Adipose-Derived Stem Cells in Rabbits. Mediat. Inflamm. 2018, 2018, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ferroni, L.; Gardin, C.; Tussardi, I.T. Potential for Neural Differentiation of Mesenchymal Stem Cells. Adv. Biochem. Eng. Biotechnol. 2012, 129, 89–115. [Google Scholar]
- Jahan-Abad, A.J.; Morteza-Zadeh, P.; Negah, S.S.; Gorji, A. Curcumin attenuates harmful effects of arsenic on neural stem/progenitor cells. Avicenna J. Phytomed. 2017, 7, 376. [Google Scholar]
- Krishna, L.; Dhamodaran, K.; Jayadev, C.; Chatterjee, K.; Shetty, R.; Khora, S.S.; Das, D. Nanostructured scaffold as a determinant of stem cell fate. Stem Cell Res. 2016, 7, 188. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ruan, J.; Cui, D. Advances and prospect of nanotechnology in stem cells. Nanoscale Res. Lett. 2009, 4, 593. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; He, D.; Kleiner, G.; Kuluz, J. Neuron-like Differentiation of Adipose-Derived Stem Cells From Infant Piglets in Vitro. J. Spinal Cord Med. 2007, 30, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Darling, E.M.; Topel, M.; Zauscher, S.; Vail, T.P.; Guilak, F. Viscoelastic properties of human mesenchymally-derived stem cells and primary osteoblasts, chondrocytes, and adipocytes. J. Biomech. 2008, 41, 454–464. [Google Scholar] [CrossRef]
- Galateanu, B.; Dinescu, S.; Cimpean, A.; Dinischiotu, A.; Costache, M. Modulation of Adipogenic Conditions for Prospective Use of hADSCs in Adipose Tissue Engineering. Int. J. Mol. Sci. 2012, 13, 15881–15900. [Google Scholar] [CrossRef]
- Dinescu, S.; Galateanu, B.; Albu, M.; Cimpean, A.; Dinischiotu, A.; Costache, M. Sericin enhances the bioperformance of collagen-based matrices preseeded with hADSCs. Int. J. Mol. Sci. 2013, 14, 1870–1889. [Google Scholar] [CrossRef]
- Zhang, Y.; Hua, Q.; Zhang, J.M.; Zhao, Y.; Yin, H.; Dai, Z.; Zheng, L.; Tang, J. Enhanced thermal and mechanical properties by cost-effective carboxylated nanodiamonds in poly (vinyl alcohol). Nanocomposites 2018, 4, 58–67. [Google Scholar] [CrossRef]
- Cecoltan, S.; Serafim, A.; Dragusin, D.-M.; Lungu, A.; Lagazzo, A.; Barberis, F.; Stancu, I.-C. The potential of NDPs-loaded fish gelatin fibers as reinforcing agent for fish gelatin hydrogels. Key Eng. Mater. 2016, 695, 278–283. [Google Scholar] [CrossRef]
- Woo, D.J.; Sneed, B.; Peerally, F.; Heer, F.C.; Brewer, L.N.; Hooper, J.P.; Osswald, S. Synthesis of nanodiamond-reinforced aluminum metal composite powders and coatings using high-energy ball milling and cold spray. Carbon 2013, 63, 404–415. [Google Scholar] [CrossRef]
- Hardiman, M.; Vaughan, T.J.; McCarthy, C.T. Fibrous composite matrix characterisation using nanoindentation: The effect of fibre constraint and the evolution from bulk to in-situ matrix properties. Compos. Part. A Appl. Sci. Manuf. 2015, 68, 296–303. [Google Scholar] [CrossRef]
- Behler, K.D.; Stravato, A.; Mochalin, V.; Korneva, G.; Yushin, G. Nanodiamond-Polymer Composite Fibers and Coatings. Asc Nano 2009, 3, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Teramoto, Y.; Wang, S.; Pharr, G.M.; Rials, T.G. Nanoindentation of Biodegradable Cellulose Diacetate- graft -Poly (L -lactide) Copolymers: Effect of Molecular Composition and Thermal Aging on Mechanical Properties. J. Polym. Sci. Polym. Phys. 2006, 45, 1114–1121. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, D.; Kim, S.Y.; Kang, E.; Kim, C.K. Nanocomposite Synthesis of Nanodiamond and Molybdenum Disulfide. Nanomaterials 2019, 9, 927. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.A.; Kao, C.W.; Liu, K.K.; Huang, H.S.; Chiang, M.H.; Soo, C.R.; Chang, H.C.; Chiu, T.W.; Chao, J.I.; Hwang, E. The effect of fluorescent nanodiamonds on neuronal survival and morphogenesis. Sci. Rep. 2014, 4, 6919–6928. [Google Scholar] [CrossRef]
- Lee, G.Y.H.; Lim, C.T. Biomechanics approaches to studying human diseases. Trends Biotechnol. 2007, 25, 111–118. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, Q.; Wang, H. Synthesis and Characterization of Nanodiamond Reinforced Chitosan for Bone Tissue Engineering. J. Funct. Biomater. 2016, 7, 27–42. [Google Scholar]
- Ma, M.; Mao, Y.; Gupta, M.; Gleason, K.K.; Rutledge, G.C. Superhydrophobic fabrics produced by electrospinning and chemical vapor deposition. Macromolecules 2005, 38, 9742–9748. [Google Scholar] [CrossRef]
- Wang, X.; Yu, J.; Sun, G.; Ding, B. Electrospun nanofibrous materials: A versatile medium for effective oil/water separation. Mater. Today 2015, 19, 403–414. [Google Scholar] [CrossRef]
- Li, X.; Bian, F.; Lin, J.; Zeng, Y. Effect of electric field on the morphology and mechanical properties of electrospun fibers. Rsc Adv. 2016, 6, 50666–50672. [Google Scholar] [CrossRef]
- Pacelli, S.; Acosta, F.; Chakravarti, A.R.; Samanta, S.G.; Whitlow, J.; Modaresi, S.; Ahmed, R.P.H.; Rajasingh, J.; Paul, A. Nanodiamond-based injectable hydrogel for sustained growth factor release: Preparation, characterization and in vitro analysis. Acta Biomater. 2017, 58, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.; Zhang, R.; Luan, J.; Lin, F. Alginate and alginate/gelatin microspheres for human adipose-derived stem cell encapsulation and differentiation. Biofabrication 2012, 4, 025007. [Google Scholar] [CrossRef]
- Chiou, B.S.; Avena-Bustillos, R.J.; Shey, J.; Yee, E.; Bechtel, P.J.; Imam, S.H.; Glenn, G.M.; Orts, W.J. Rheological and mechanical properties of cross-linked fish gelatins. Polymer 2006, 47, 6379–6386. [Google Scholar] [CrossRef]
- Liu, K.K.; Cheng, C.L.; Chang, C.C.; Chao, J.I. Biocompatible and detectable carboxylated nanodiamond on human cell. Nanotechnology 2007, 18, 325102. [Google Scholar] [CrossRef]
- Pacelli, S.; Maloney, R.; Chakravarti, A.R.; Whitlow, J.; Basu, S.; Modaresi, S.; Paul, A. Controlling adult stem cell behavior using nanodiamond-reinforced hydrogel: Implication in bone regeneration therapy. Sci. Rep. 2018, 7, 6577. [Google Scholar] [CrossRef]
- Guarino, V.; Alvarez-Perez, M.; Cirillo, V.; Ambrosio, L. hMSC interaction with PCL and PCL/gelatin platforms: A comparative study on films and electrospun membranes. J. Bioact. Compat. Polym. 2011, 26, 144–160. [Google Scholar] [CrossRef]
- Pereira, F.A.S.; Salles, G.N.; Rodrigues, B.V.M.; Marciano, F.R.; Pacheco-Soares, C.; Lobo, A.O. Diamond nanoparticles into poly (lactic acid) electrospun fibers: Cytocompatible and bioactive scaffolds with enhanced wettability and cell adhesion. Mater. Lett. 2016, 183, 420–424. [Google Scholar] [CrossRef]
- Hopper, A.P.; Dugan, J.M.; Gill, A.A.; Fox, O.J.L.; May, P.W.; Haycock, J.W.; Claeyssens, F. Amine functionalized nanodiamond promotes cellular adhesion, proliferation and neurite outgrowth. Biomed. Mater. 2014, 9, 045009. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.Y.; Lin, C.L.; Cheng, N.C.; Yu, J. Effects of nano-grooved gelatin films on neural induction of human adipose-derived stem cells. Rsc Adv. 2017, 7, 53537–53544. [Google Scholar] [CrossRef]
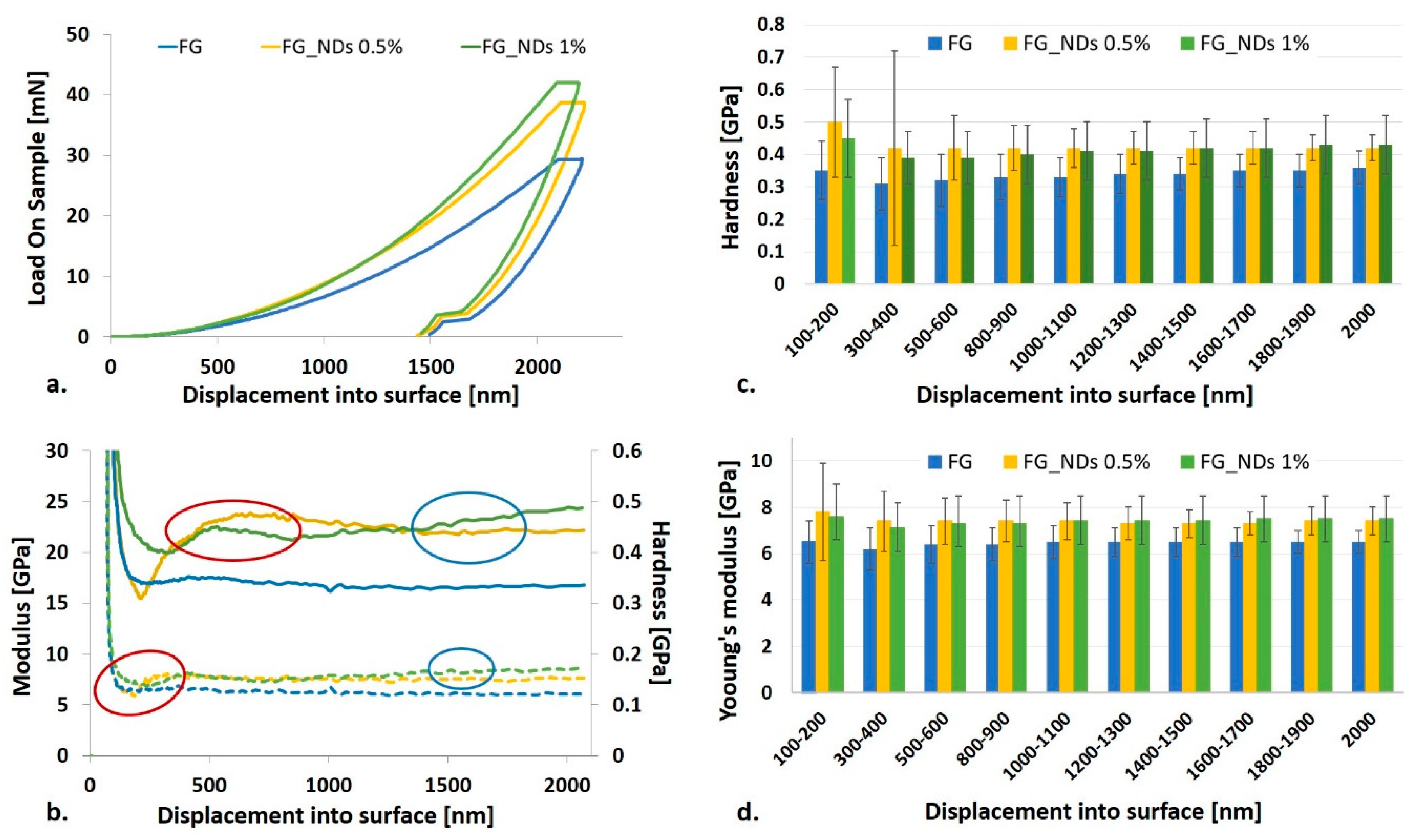
| Indentation Depth [nm] | Young’s Modulus (%) | Hardness (%) | ||
|---|---|---|---|---|
| FG_NDs 0.5% | FG_NDs 1% | FG_NDs 0.5% | FG_NDs 1% | |
| 100–200 | 20.00 | 16.92 | 42.86 | 28.57 |
| 300–400 | 19.35 | 14.52 | 35.48 | 25.81 |
| 500–600 | 15.63 | 14.06 | 31.25 | 21.88 |
| 800–900 | 15.63 | 14.06 | 27.27 | 21.21 |
| 1000–1100 | 13.85 | 13.85 | 27.27 | 24.24 |
| 1200–1300 | 12.31 | 13.85 | 23.53 | 20.59 |
| 1400–1500 | 12.31 | 13.85 | 23.53 | 23.53 |
| 1600–1700 | 12.31 | 15.38 | 20.00 | 20.00 |
| 1800–1900 | 13.85 | 15.38 | 20.00 | 22.86 |
| 2000 | 13.85 | 15.38 | 16.67 | 19.44 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Şelaru, A.; Drăgușin, D.-M.; Olăreț, E.; Serafim, A.; Steinmüller-Nethl, D.; Vasile, E.; Iovu, H.; Stancu, I.-C.; Costache, M.; Dinescu, S. Fabrication and Biocompatibility Evaluation of Nanodiamonds-Gelatin Electrospun Materials Designed for Prospective Tissue Regeneration Applications. Materials 2019, 12, 2933. https://doi.org/10.3390/ma12182933
Şelaru A, Drăgușin D-M, Olăreț E, Serafim A, Steinmüller-Nethl D, Vasile E, Iovu H, Stancu I-C, Costache M, Dinescu S. Fabrication and Biocompatibility Evaluation of Nanodiamonds-Gelatin Electrospun Materials Designed for Prospective Tissue Regeneration Applications. Materials. 2019; 12(18):2933. https://doi.org/10.3390/ma12182933
Chicago/Turabian StyleŞelaru, Aida, Diana-Maria Drăgușin, Elena Olăreț, Andrada Serafim, Doris Steinmüller-Nethl, Eugeniu Vasile, Horia Iovu, Izabela-Cristina Stancu, Marieta Costache, and Sorina Dinescu. 2019. "Fabrication and Biocompatibility Evaluation of Nanodiamonds-Gelatin Electrospun Materials Designed for Prospective Tissue Regeneration Applications" Materials 12, no. 18: 2933. https://doi.org/10.3390/ma12182933
APA StyleŞelaru, A., Drăgușin, D.-M., Olăreț, E., Serafim, A., Steinmüller-Nethl, D., Vasile, E., Iovu, H., Stancu, I.-C., Costache, M., & Dinescu, S. (2019). Fabrication and Biocompatibility Evaluation of Nanodiamonds-Gelatin Electrospun Materials Designed for Prospective Tissue Regeneration Applications. Materials, 12(18), 2933. https://doi.org/10.3390/ma12182933











