Comparative Study of the Adsorption of Acid Blue 40 on Polyaniline, Magnetic Oxide and Their Composites: Synthesis, Characterization and Application
Abstract
1. Introduction
2. Experiment
2.1. Materials
2.2. Synthesis of PANI
2.3. Synthesis of Fe3O4
2.4. Synthesis of PANI/Fe3O4 Composites
2.5. Batch Adsorption Study for Removal of AB40 Dye
2.6. Characterization
3. Results and Discussion
3.1. Scanning Electron Microscopy (SEM)
3.1.1. Optical Studies
3.1.2. Energy Dispersive X-ray (EDX) Study
3.1.3. FTIR Study
3.1.4. Surface Area Study
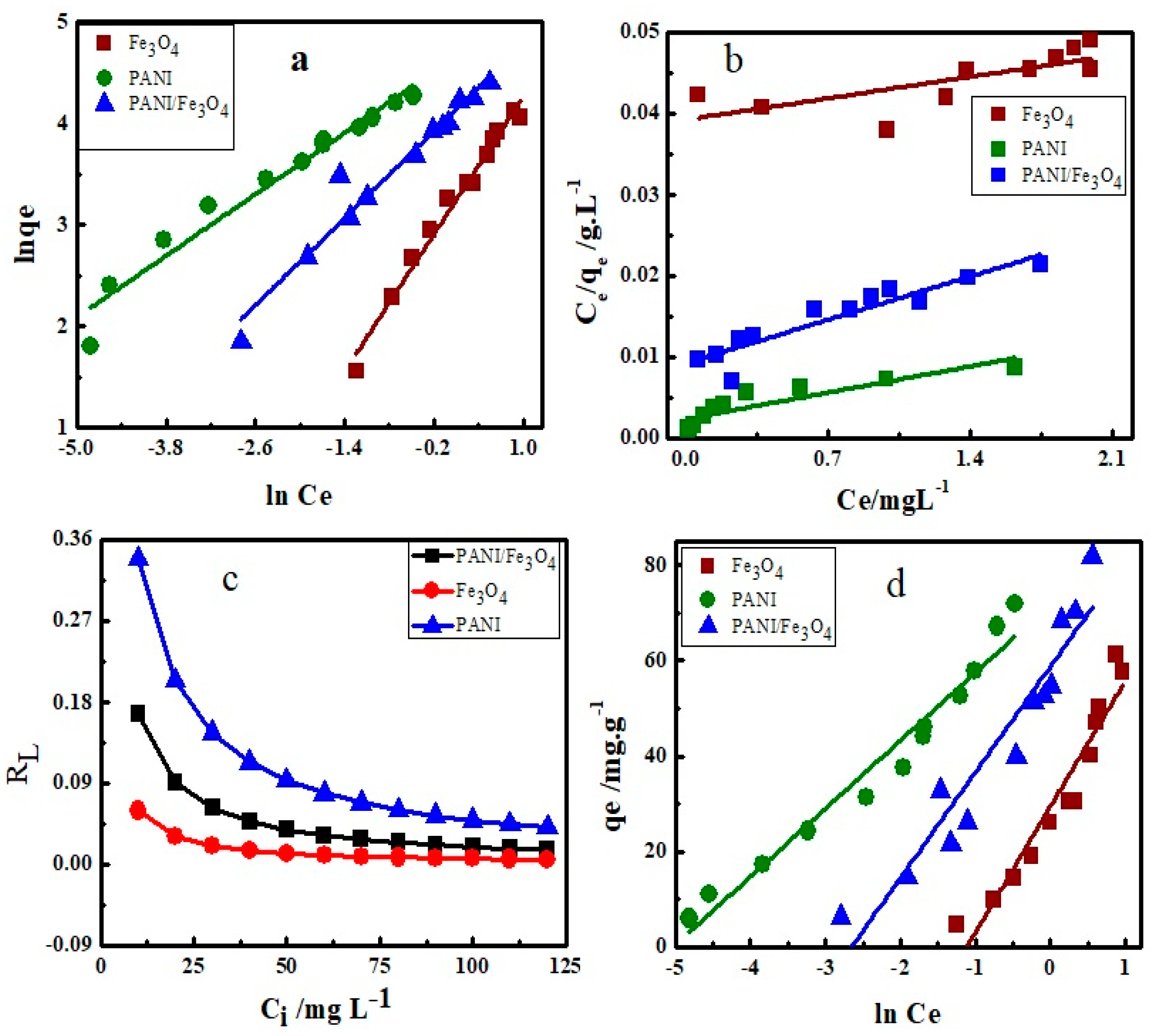
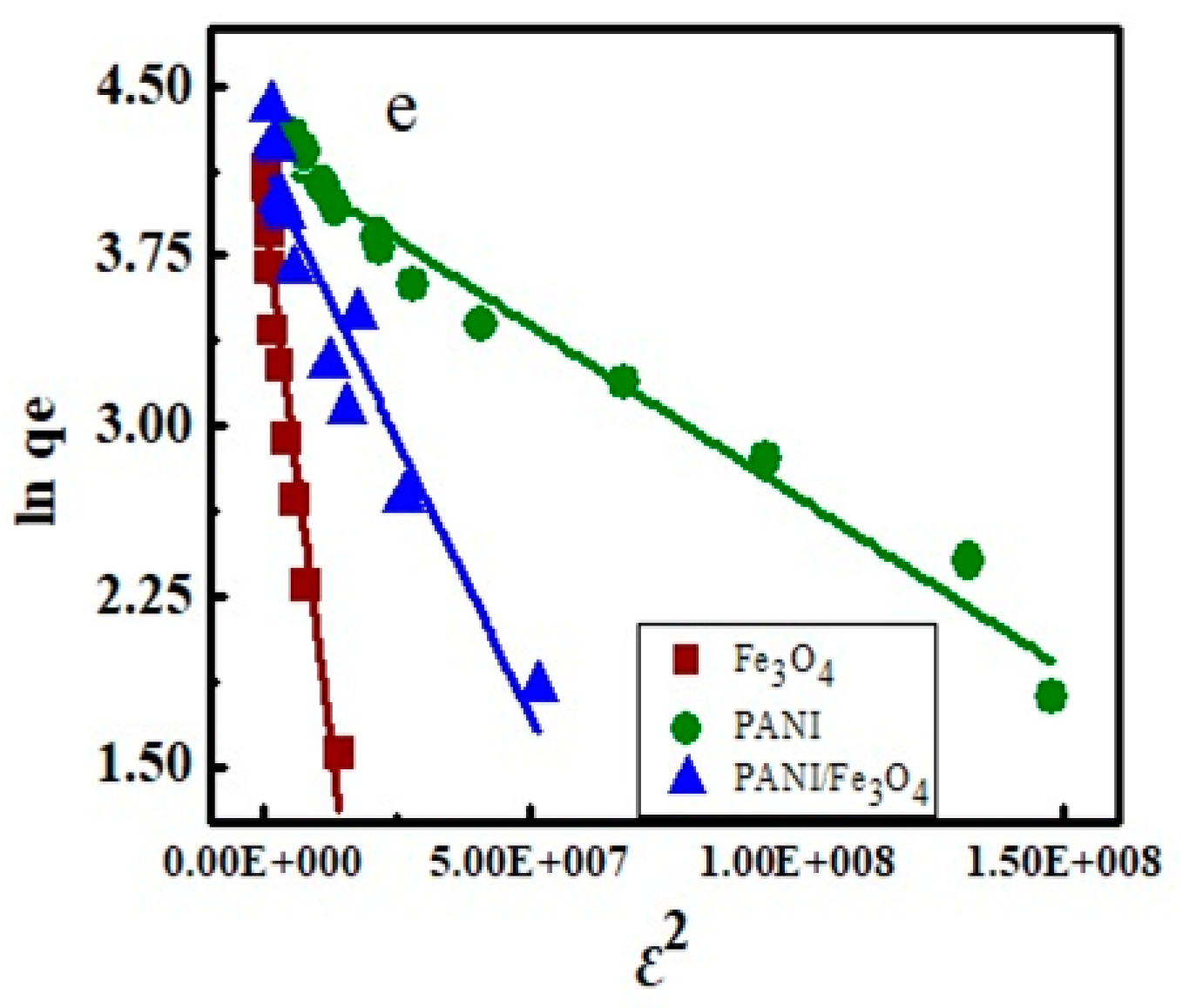
3.2. Isotherms Study
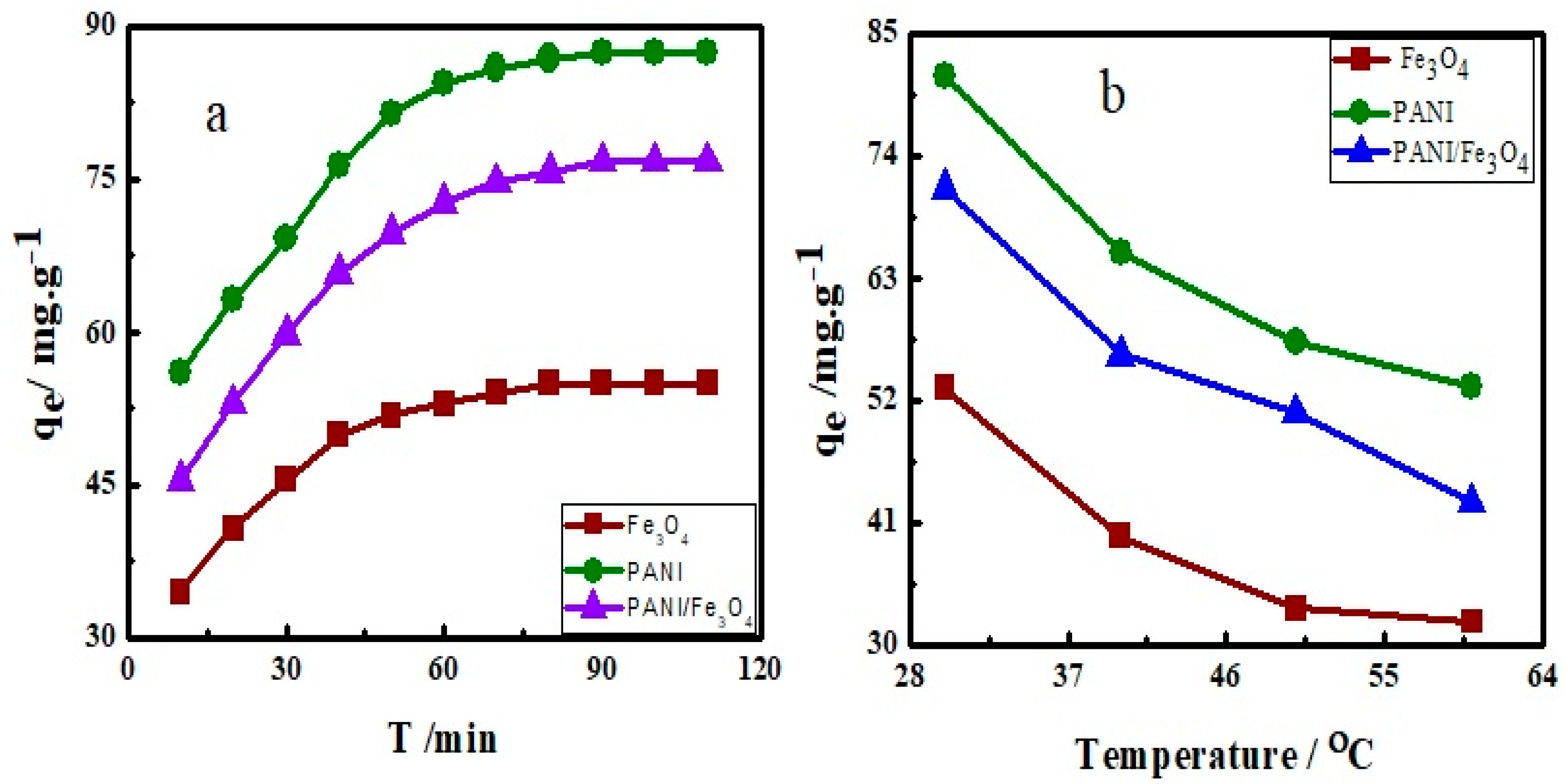
3.3. Effect of Contact Time and Temperature on Adsorption
3.4. Effect of pH on Adsorption
3.5. Effect of Ionic Strength on Adsorption
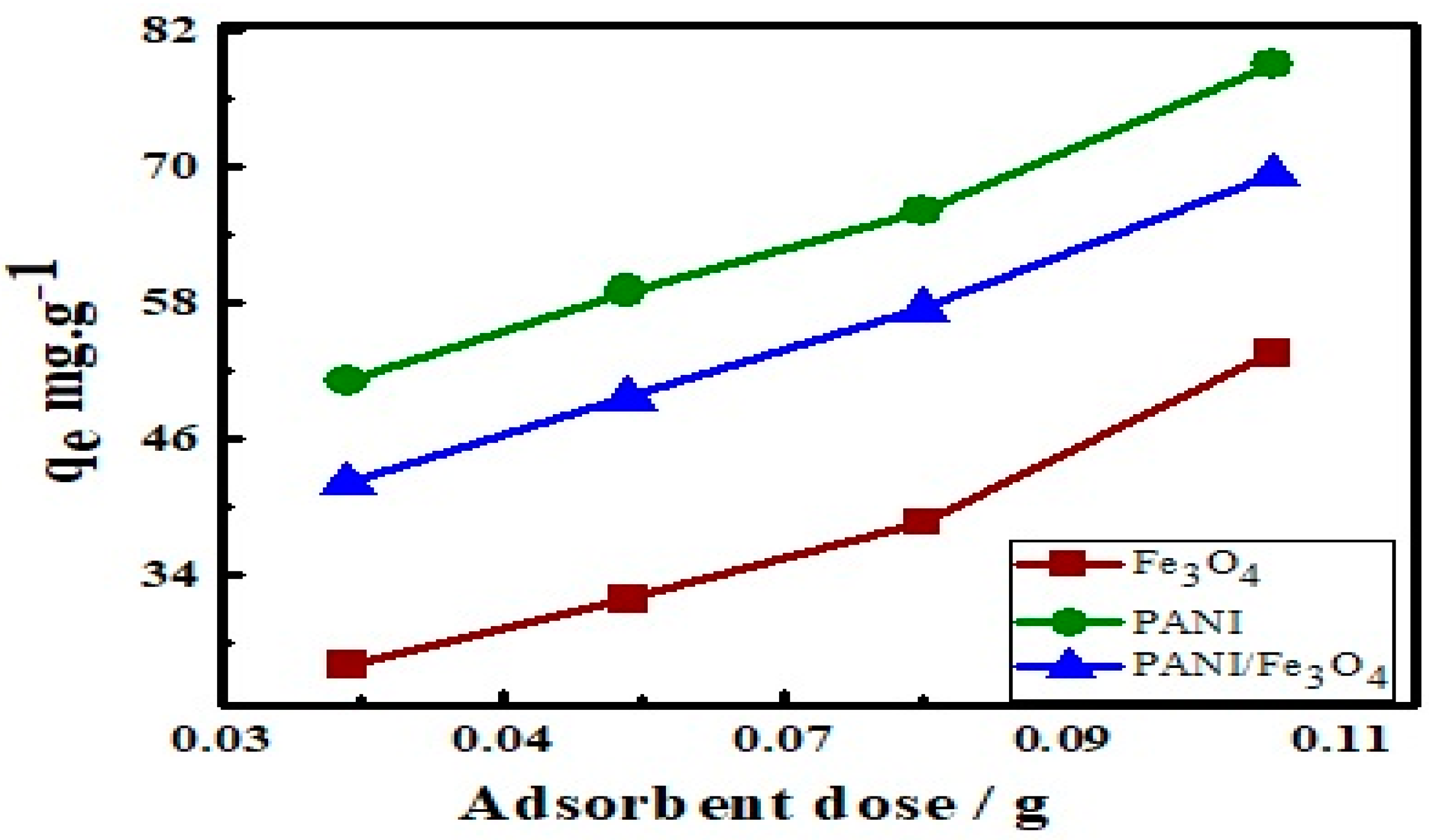
3.6. Effect of Adsorbent Dosage on Adsorption
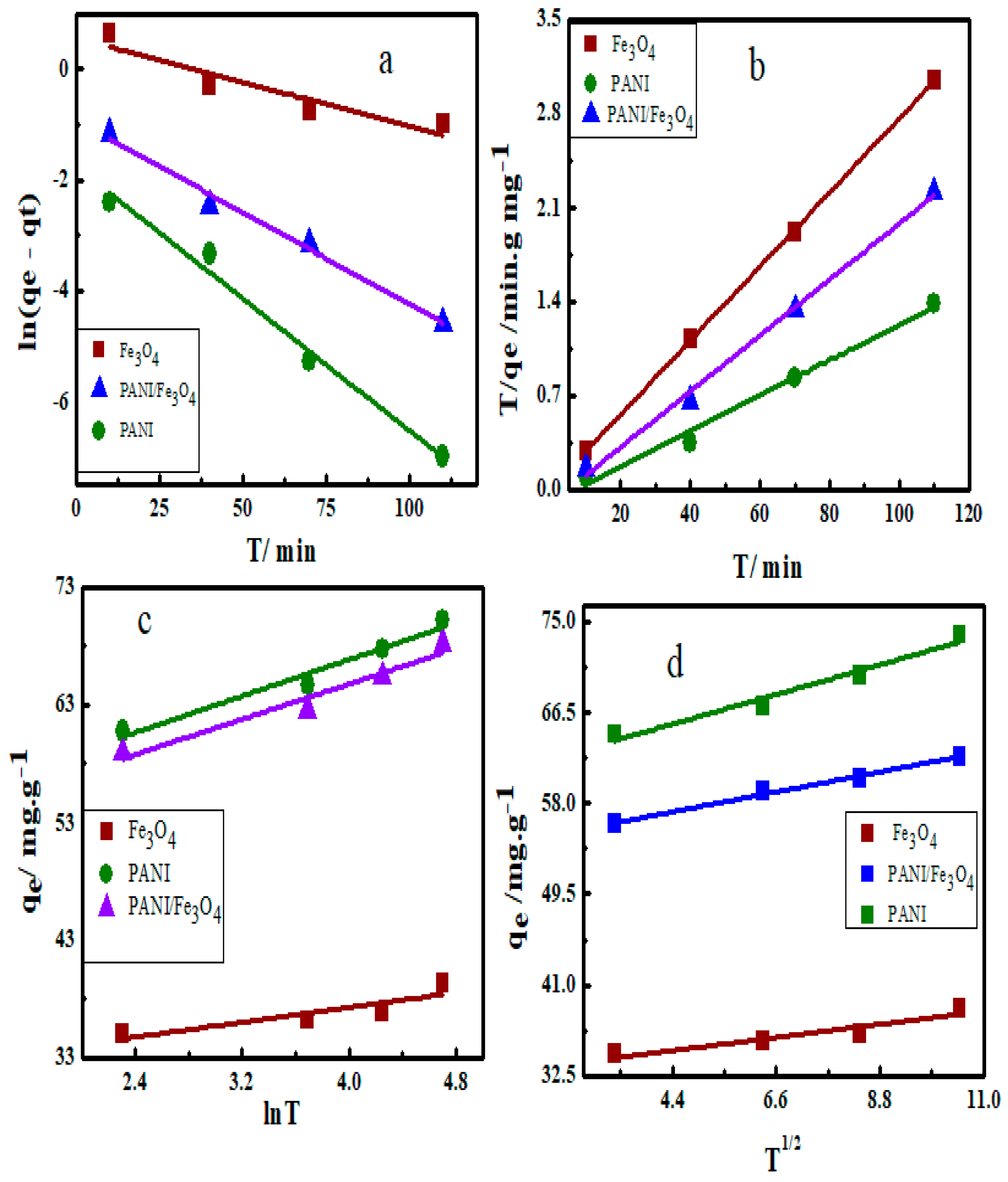
3.7. Adsorption Kinetics
3.8. Adsorption Mechanism
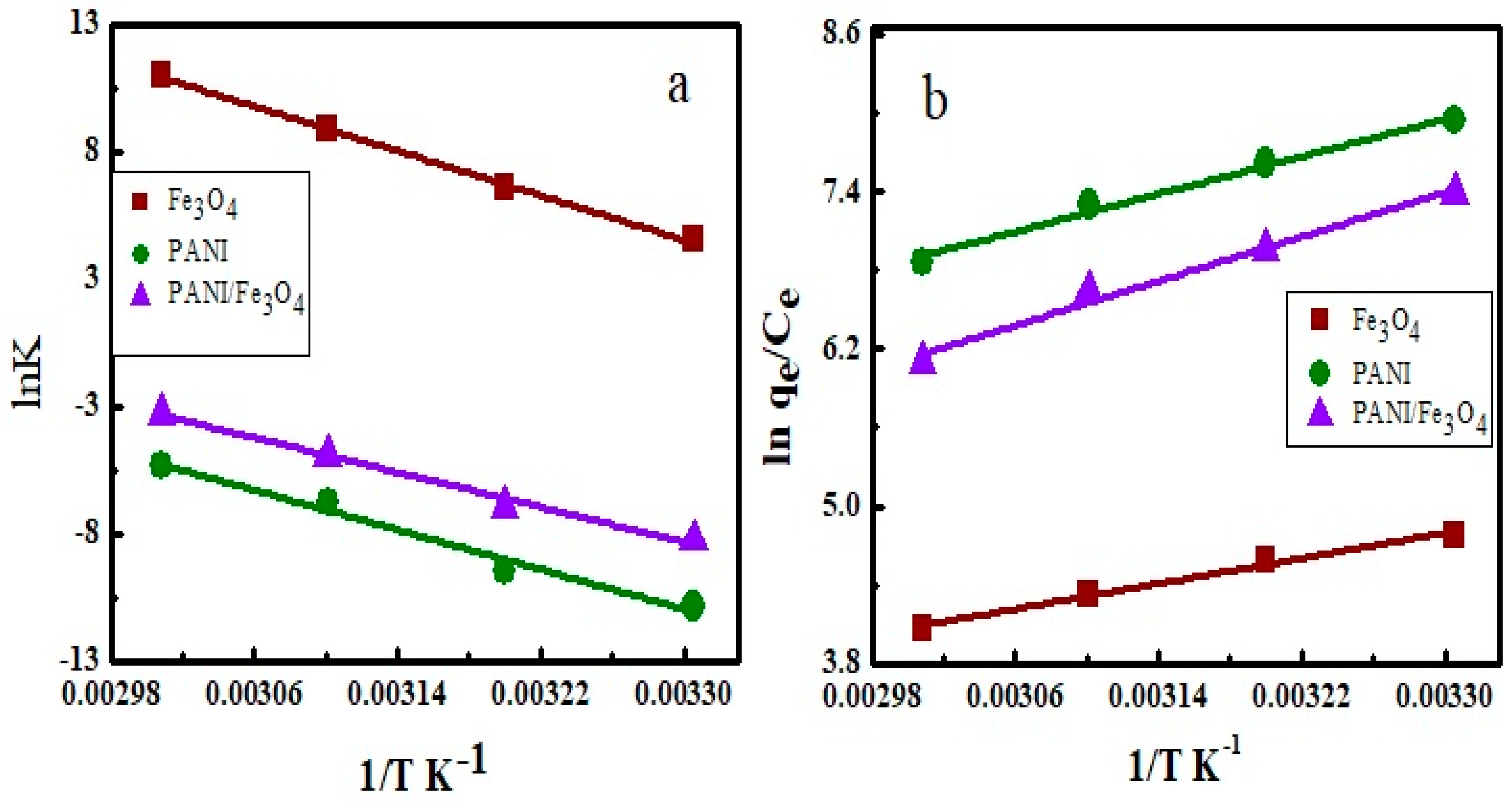
3.9. Thermodynamics of Adsorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bhadraa, S.; Khastgir, D.; Singhaa, N.K.; Leeb, J.H. Progress in preparation, processing and applications of polyaniline. Prog. Polym. Sci. 2009, 34, 783–810. [Google Scholar] [CrossRef]
- Kiskan, B.; Yagci, Y. Synthesis and characterization of thermally curable polyacetylenes by polymerization of propargyl benzoxazine using rhodium catalyst. Polymer 2008, 49, 2455–2460. [Google Scholar] [CrossRef]
- Shah, A.-U.-H.A.; Kamran, M.; Bilal, S.; Ullah, R. Cost Effective Chemical Oxidative Synthesis of Soluble and Electroactive Polyaniline Salt and Its Application as Anticorrosive Agent for Steel. Materials 2019, 12, 1527. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, M.A.; Diaz, F.R.; Armijo, F.; Soto, P. Electro-synthesis and characterization of polythiophene nano-wires/platinum nano-particles composite electrodes. Study of formic acid electro-catalytic oxidation. Electrochim. Acta 2012, 71, 277–282. [Google Scholar] [CrossRef]
- Shen, C.; Sun, Y.; Yao, W.; Lu, Y. Facile synthesis of polypyrrole nanospheres and their carbonized products for potential application in high-performance supercapacitors. Polymer 2014, 55, 2817–2824. [Google Scholar] [CrossRef]
- Fahim, M.; Shah, A.H.A.; Bilal, S. Highly stable and efficient performance of binder free symmetric supercapacitor fabricated with electroactive polymer synthesized via interfacial polymerization. Materials 2019, 12, 1626. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, R.; Shanmugam, S.; Gedanken, A. Pulsed sonoelectrochemical synthesis of polyaniline nanoparticles and their capacitance properties. Synth. Met. 2008, 158, 848–853. [Google Scholar] [CrossRef]
- Kulkarni, S.B.; Joshi, S.S.; Lokhande, C.D. Facile and efficient route for preparation of nanostructured polyaniline thin films: Schematic model for simplest oxidative chemical polymerization. Chem. Eng. J. 2011, 166, 1179–1185. [Google Scholar] [CrossRef]
- Vivekanandan, J.; Ponnusamy, V.; Mahudeswaran, A.; Vijayanand, P.S. Synthesis, characterization and conductivity study of polyaniline prepared by chemical oxidative and electrochemical methods. Arch. Appl. Sci. Res. 2011, 3, 147–153. [Google Scholar]
- Xu, P.; Singh, A.; Kaplan, D.L. Enzymatic Catalysis in the Synthesis of Polyanilines and Derivatives of Polyanilines. Adv. Polym. Sci. 2006, 194, 69–94. [Google Scholar]
- Abdolahi, A.; Hamzah, E.; Ibrahim, Z.; Hashim, S. Synthesis of Uniform Polyaniline Nanofibers through Interfacial Polymerization. Materials 2012, 5, 1487–1494. [Google Scholar] [CrossRef]
- Guo, X.; Fei, G.T.; Su, H.; Zhang, L.D. Synthesis of polyaniline micro/nanospheres by a copper(II)-catalyzed self-assembly method with superior adsorption capacity of organic dye from aqueous solution. J. Mater. Chem. 2011, 21, 8618. [Google Scholar] [CrossRef]
- Lee, S.; Hong, J.Y.; Jang, J. Synthesis and electrical response of polyaniline/poly(styrene sulfonate)-coated silica spheres prepared by seed-coating method. J. Colloid. Inter. Sci. 2013, 398, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shao, Y.; Zhang, T.; Meng, G.; Wang, F. The effect of epoxy coating containing emeraldine base and hydrofluoric acid doped polyaniline on the corrosion protection of AZ91D magnesium alloy. Corros. Sci. 2011, 53, 3747–3755. [Google Scholar] [CrossRef]
- Xu, W.; Zhao, K.; Niu, C.; Zhang, L.; Cai, Z.; Han, C.; He, L.; Shen, T.; Yan, M.; Qu, L. Heterogeneous branched core–shell SnO2–PANI nanorod arrays with mechanical integrity and three dimensional electron transport for lithium batteries. Nano Energy 2014, 8, 196–204. [Google Scholar] [CrossRef]
- Farooq, S.; Tahir, A.; Krewer, U.; Shah, A.A.; Bilal, S. Efficient photocatalysis through conductive polymer coated FTO counter electrode in platinum free dye sensitized solar cells. Electrochim. Acta 2019, 320, 134544. [Google Scholar] [CrossRef]
- Mujawar, S.H.; Ambade, S.B.; Battumur, T.; Ambade, R.B.; Lee, S.H. Electropolymerization of polyaniline on titanium oxide nanotubes for supercapacitor application. Electrochim. Acta 2011, 56, 4462–4466. [Google Scholar] [CrossRef]
- Li, R.; Liu, L.; Yang, F. Preparation of polyaniline/reduced graphene oxide nanocomposite and its application in adsorption of aqueous Hg(II). Chem. Eng. J. 2013, 229, 460–468. [Google Scholar] [CrossRef]
- Shen, J.; Shahid, S.; Amura, I.; Sarihan, A.; Mi Tian, M.; Emanuelsson, E.A. Enhanced adsorption of cationic and anionic dyes from aqueous solutions by polyacid doped polyaniline. Synth. Met. 2018, 245, 151–159. [Google Scholar] [CrossRef]
- Huang, Y.; Li, J.; Chen, X.; Wang, X. Applications of conjugated polymer based composites in wastewater purification. RSC Adv. 2014, 4, 62160–62178. [Google Scholar] [CrossRef]
- Lee, Y.M.; Nam, S.Y.; Ha, S.Y. Pervaporation of water/isopropanol mixtures through polyaniline membranes doped with poly(acrylic acid). J. Membr. Sci. 1999, 159, 41–46. [Google Scholar]
- Bober, P.; Stejskal, J.; Trchováa, M.; Prokes, J. In-situ prepared polyaniline–silver composites: Single-and two-step strategies. Electrochim. Acta 2014, 122, 259–266. [Google Scholar] [CrossRef]
- He, K.; Li, M.; Guo, L. Preparation and photocatalytic activity of PANI-CdS composites for hydrogen evolution. Int. J. Hydrogen Energy 2012, 37, 755–759. [Google Scholar] [CrossRef]
- Yilmaz, H.; Zengin, H.S.; Unal, H.I. Synthesis and electrorheological properties of polyaniline/silicon dioxide composites. J. Mater. Sci. 2012, 47, 5276–5286. [Google Scholar] [CrossRef]
- Wanga, J.G.; Yang, Y.; Huanga, Z.H.; Kang, F. Interfacial synthesis of mesoporous MnO2/polyaniline hollow spheres and their application in electrochemical capacitors. J. Power Sources 2012, 204, 236–243. [Google Scholar] [CrossRef]
- Chandraa, S.; Lang, H.; Bahadur, D. Polyaniline-iron oxide nanohybrid film as multi-functional label-free electrochemical and biomagnetic sensor for catechol. Anal. Chim. Acta 2013, 795, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Vellakkat, M.; Kamath, A.; Raghu, S.; Chapi, S.; Hundekal, D. Dielectric Constant and Transport Mechanism of Percolated Polyaniline Nanoclay Composites. Ind. Eng. Chem. Res. 2014, 53, 16873–16882. [Google Scholar] [CrossRef]
- Salem, M.A.; Salem, I.A.; Hanfy, M.G.; Ahmed, B.; Zak, A.B. Removal of titan yellow dye from aqueous solution by polyaniline/Fe3O4 nanocomposite. Eur. Chem. Bull. 2016, 5, 113–118. [Google Scholar]
- Das, S.; Chakraborty, P.; Ghosh, R.; Paul, S.; Mondal, S.; Panja, A.; Nand, A.K. Folic Acid-Polyaniline Hybrid Hydrogel for Adsorption/Reduction of Chromium(VI) and Selective Adsorption of Anionic Dye from Water. Chem. Eng. 2017, 5, 9325–9337. [Google Scholar] [CrossRef]
- Neuberger, T.; Schöpf, B.; Hofmann, H.; Hofmann, M.; Rechenberg, B. Superparamagnetic nanoparticles for biomedical applications: Possibilities and limitations of a new drug delivery system. J. Magn. Magn. Mater. 2005, 293, 483–496. [Google Scholar] [CrossRef]
- Ito, A.; Shinkai, M.; Honda, H.; Kobayashi, T. Medical application of functionalized magnetic nanoparticles. J. Biosci. Bioeng. 2005, 100, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pankhurst, Q.A.; Connolly, J.; Jones, S.K.; Dobson, J. Applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2003, 36, 167–181. [Google Scholar] [CrossRef]
- Khurshid, H.; Hadjipanayis, C.; Chen, H.W.; Li, H.; Mao, H.; Machaidze, R.; Tzitzios, V.; Hadjipanay, G.C. Core/shell structured iron/iron-oxide nanoparticles as excellent MRI contrast enhancement agents. J. Magn. Magn. Mater. 2013, 331, 17–20. [Google Scholar] [CrossRef]
- Wang, G.; Chang, Y.; Wang, L.; Wei, Z.; Kang, J.; Sang, L.; Dong, X.; Chen, G.; Wang, H.; Qi, H. Preparation and characterization of PVPI-coated Fe3O4 nanoparticles as an MRI contrast agent. J. Magn. Magn. Mater. 2013, 340, 57–60. [Google Scholar] [CrossRef]
- Hyun Do, S.I.; Hoon Jo, Y.; Park, J.Y.; Hong, S.H. As3+removal by Ca–Mn–Fe3O4with and without H2O2: Effects ofcalcium oxide in Ca–Mn–Fe3O4. J. Hazard. Mater. 2014, 280, 322–330. [Google Scholar]
- Hu, J.; Irene, M.C.; Chen, G. Fast Removal and Recovery of Cr(VI) Using Surface-Modified Jacobsite (MnFe2O4) Nanoparticles. Langmuir 2005, 21, 11173–11179. [Google Scholar] [CrossRef]
- Aphesteguy, J.C.; Kurlyandskaya, G.V.; de Celis, J.P.; Safronov, A.P.; Schegoleva, N.N. Magnetite nanoparticles prepared by co-precipitation method in different conditions. Mater. Chem. Phys. 2015, 161, 243–249. [Google Scholar] [CrossRef]
- Amer, M.A.; Meaz, T.M.; Attalah, S.S.; Ghoneim, A.I. Structural and magnetic characterization of the Mg0.2_xSrxMn0.8Fe2O4 nanoparticles. J. Magn. Magn. Mater. 2014, 363, 60–65. [Google Scholar] [CrossRef]
- Lam, U.T.; Mammucari, R.; Suzuki, K.; Foster, N.R. Processing of iron oxide nanoparticles by supercritical fluids. Ind. Eng. Chem. Res. 2008, 47, 599–614. [Google Scholar] [CrossRef]
- Tavakoli, A.; Sohrabi, M.; Kargari, A. A review of methods for synthesis of nano structured metals with emphasis on iron compounds. Chem. Pap. 2007, 61, 151–170. [Google Scholar] [CrossRef]
- Teja, A.S.; Koh, P. Y Synthesis, properties, and applications of magnetic iron oxide nanoparticles. Prog. Cryst. Growth Charact. Mater. 2009, 55, 22–45. [Google Scholar] [CrossRef]
- Majewski, P.; Thierry, B. Functionalized magnetite nanoparticles synthesis, properties, and bio-applications. Solid State Mater. Sci. 2007, 32, 203–215. [Google Scholar] [CrossRef]
- Jia, Z.; Yujun, W.; Yangcheng, L.; Jingyu, M.; Guangsheng, L. In situ preparation of magnetic chitosan/Fe3O4 composite nanoparticles in tiny pools of water-in-oil microemulsion. React. Funct. Polym. 2006, 66, 1552–1558. [Google Scholar]
- Khan, A.; Aldwayyan, A.S.; Alhoshan, M.; Alsalhi, M. Synthesis by in situ chemical oxidative polymerization and characterization of polyaniline/iron oxide nanoparticle composite. Polym. Int. 2010, 59, 1690–1694. [Google Scholar] [CrossRef]
- Rasha, M.K. Synthesis, characterization, magnetic and electrical properties of the novel conductive and magnetic Polyaniline/MgFe2O4 nanocomposite having the core–shell structure. J. Alloys Compounds 2011, 509, 9849–9857. [Google Scholar]
- Bhaumik, M.; Choi, H.J.; McCrindle, R.I.; Maity, A. Composite nanofibers prepared from metallic iron nanoparticles and polyaniline: High performance for water treatment applications. J. Colloid. Interface Sci. 2014, 425, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.S. The role of polyaniline salts in the removal of direct blue 78 from aqueous solution: A kinetic study. React. Funct. Polym. 2010, 70, 707–714. [Google Scholar]
- Cui, H.; Qian, Y.; Li, Q.; Zhang, Q.; Zhai, J. Adsorption of aqueous Hg(II) by a polyaniline/attapulgite composite. Chem. Eng. J. 2012, 211, 216–223. [Google Scholar]
- Muhammad, A.; Shah, A.H.A.; Bilal, S.; Rahman, G. Basic Blue Dye Adsorption from Water using Polyaniline/Magnetite(Fe3O4) Composites: Kinetic and Thermodynamic Aspects. Materials 2019, 12, e1764. [Google Scholar] [CrossRef]
- Keyhanian, F.; Shariati, S.; Faraji, M.; Hesabi, M. Magnetite nanoparticles with surface modification for removal of methyl violet from aqueous solutions. Arab. J. Chem. 2016, 9, 348–354. [Google Scholar] [CrossRef]
- Patra, B.N.; Majhi, D. Removal of Anionic Dyes from Water by Potash Alum Doped Polyaniline: Investigation of Kinetics and Thermodynamic Parameters of Adsorption. J. Phys. Chem. B 2015, 119, 8154–8164. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.H.; Asadnia, A. Polyaniline/Fe3O4 coated on MnFe2O4 nanocomposite: Preparation, characterization, and applications in microwave absorption. Int. J. Phys. Sci. 2013, 8, 1209–1217. [Google Scholar]
- Tung, L.M.; Cong, N.X.; Huy, L.T.; Lan, N.T.; Phan, V.N.; Hoa, N.Q.; Vinh, L.K.; Thinh, N.V.; Tai, L.T.; Ngo, D.T.; et al. Synthesis, Characterizations of Superparamagnetic Fe3O4–Ag Hybrid Nanoparticles and Their Application for Highly Eective Bacteria Inactivation. J. Nanosci. Nanotechnol. 2016, 16, 5902–5912. [Google Scholar] [CrossRef]
- Bachan, N.; Asha, A.; Jeyarani, W.J.; Kumar, D.A.; Shyla, J.M. A Comparative Investigation on the Structural, Optical and Electrical Properties of SiO2–Fe3O4 Core–Shell Nanostructures with Their Single Components. Acta Metall. Sin. Engl. Lett. 2015, 28, 1317–1325. [Google Scholar] [CrossRef]
- Bilal, S.; Gul, S.; Holze, R.; Shah, A.A. An impressive emulsion polymerization route for the synthesis of highly soluble and conducting polyaniline salt. Synth. Met. 2015, 206, 131–144. [Google Scholar] [CrossRef]
- Hatamzadeh, M.; Ahar, M.J.; Jaymand, M. In Situ Chemical Oxidative Graft Polymerization of Aniline from Fe3O4 Nanoparticles. Int. J. Nanosci. Nanotechnol. 2012, 8, 51–60. [Google Scholar]
- Akar, T.; Ozcan, A.S.; Tunali, S.; Ozcan, A. Biosorption of a textile dye (Acid Blue 40) by cone biomass of Thuja orientalis: Estimation of equilibrium, thermodynamic and kinetic parameters. Bioresour. Technol. 2008, 99, 3057–3065. [Google Scholar] [CrossRef] [PubMed]
- Khoshsang, H.; Ghaffarinejad, A.; Kazemi, H.; Jabarian, S. Synthesis of Mesoporous Fe3O4 and Fe3O4/C Nanocomposite for Removal of Hazardous Dye from Aqueous Media. J. Water Environ. Nanotechnol. 2018, 3, 191–206. [Google Scholar]
- Ballav, N.; Debnath, S.; Pillay, K.; Maity, A. Efficient removal of Reactive Black from aqueous solution usingpolyaniline coated ligno-cellulose composite as a potential adsorbent. J. Mol. Liq. 2015, 209, 387–396. [Google Scholar] [CrossRef]
- Konicki, W.; Pełech, I.; Mijowska, E.; Jasinska, I. Adsorption of anionic dye Direct Red 23 onto magnetic multi-walled carbonnanotubes-Fe3C nanocomposite: Kinetics, equilibrium and thermodynamics. Chem. Eng. J. 2012, 210, 87–95. [Google Scholar] [CrossRef]
- Sun, M.; Zhu, A.; Zhang, Q.; Liu, Q. A facile strategyto synthesize mono disperse super paramagnetic OA-modified Fe3O4 nanoparticles with PEG assistant. J. Magn. Magn. Mater. 2014, 369, 49–54. [Google Scholar] [CrossRef]
- Asgari, S.; Fakhari, Z.; Berijanic, S. Synthesis and Characterization of Fe3O4 Magnetic Nanoparticles Coated with Carboxymethyl Chitosan Grafted Sodium Methacrylate. J. Nanostruct. 2014, 4, 55–63. [Google Scholar]
- Ömeroglu Ay, C.; Özcan, A.S.; Erdogan, Y.; Özcan, A. Characterization of Punica granatum L. peels and quantitatively determination of its biosorption behavior towards lead(II) ions and Acid Blue 40. Colloids Surf. B 2012, 100, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Gul, H.; Shah, A.A.; Bilal, S. Fabrication of Eco-Friendly Solid-State Symmetric Ultracapacitor Device Based on Co-Doped PANI/GO Composite. Polymers 2019, 11, 1315. [Google Scholar] [CrossRef] [PubMed]
- Kellenberger, A.; Dmitrieva, E.; Dunsch, L. Structure Dependence of Charged States in Linear Polyaniline as Studied by In Situ ATR-FTIR Spectroelectrochemistry. J. Phys. Chem. B 2012, 116, 4377–4385. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Mao, H.; Zhang, W. Fabrication of DBSA-Doped Polyaniline Nanorods by Interfacial Polymerization. J. Appl. Polym. Sci. 2008, 109, 2842–2847. [Google Scholar] [CrossRef]
- Umare, S.S.; Shambharkar, B.H.; Ningthoujam, R.S. Synthesis and characterization of polyaniline–Fe3O4 nanocomposite: Electrical conductivity, magnetic, electrochemical studies. Synth. Met. 2010, 160, 1815–1821. [Google Scholar] [CrossRef]
- Oppong, S.O.B.; Anku, W.; Shukla, S.K.; Govender, P. Lanthanum doped–TiO2 decorated on graphene oxide nanocomposite: A photocatalyst for enhanced degradation of acid blue 40 under simulated solar light. Adv. Mater. Lett. 2017, 8, 295–302. [Google Scholar] [CrossRef]
- Ayad, M.; Zaghlol, S. Nanostructured crosslinked polyaniline with high surface area: Synthesis, characterization and adsorption for organic dye. Chem. Eng. J. 2012, 204, 79–86. [Google Scholar] [CrossRef]
- Germain, J.; Frechet, J.M.; Svec, F. Hypercrosslinked polyanilines with nanoporous structure and high surface area: Potential adsorbents for hydrogen storage. J. Mater. Chem. 2007, 17, 4989–4997. [Google Scholar] [CrossRef]
- Kegl, T.; Ban, I.; Lobnik, A.; Košak, A. Synthesis and characterization of novel γ-Fe2O3-NH4OH@SiO2(APTMS) nanoparticles for dysprosium adsorption. J. Hazard. Mater. 2019, 378, 120764. [Google Scholar] [CrossRef] [PubMed]
- Javadian, H.; Angaji, M.T.; Naushad, M. Synthesis and characterization of polyaniline/g-alumina nanocomposite: A comparative study for the adsorption of three different anionic dyes. J. Ind. Eng. Chem. 2014, 20, 3890–3900. [Google Scholar] [CrossRef]
- Crini, G. Kinetic and equilibrium studies on the removal of cationic dyes from aqueous solution by adsorption onto a cyclodextrin polymer. Dyes Pigm. 2008, 77, 415–426. [Google Scholar] [CrossRef]
- Sharma, P.; Das, M.R. Removal of a Cationic Dye from Aqueous Solution Using Graphene Oxide Nanosheets: Investigation of Adsorption Parameters. J. Chem. Eng. Data 2013, 58, 151–158. [Google Scholar] [CrossRef]
- Bhatt, A.S.; Sakaria, P.L.; Vasudevan, M.; Radheshyam, R.; Sudheesh, P.N.; Bajaj, H.C.; Mody, H.M. Adsorption of an anionic dye from aqueous medium by organoclays: Equilibrium modeling, kinetic and thermodynamic exploration. RSC Adv. 2012, 2, 8663–8671. [Google Scholar] [CrossRef]
- Song, W.; Gao, B.; Xu, X.; Xing, L.; Han, S.; Duan, P.; Song, W.; Jia, R. Adsorption–desorption behavior of magnetic amine/Fe3O4functionalized biopolymer resin towards anionic dyes from wastewater. Bioresour. Technol. 2016, 210, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Mittal, J.; Malviya, A.; Gupta, V.K. Adsorptive removal of hazardous anionic dye “Congo red” from wastewaterusing waste materials and recovery by desorption. J. Colloid Interface Sci. 2009, 340, 16–26. [Google Scholar] [CrossRef]
- Patil, M.R.; Khairnar, S.D.; Shrivastava, V.S. Synthesis, characterisation of polyaniline–Fe3O4 magnetic nanocomposite and its application for removal of an acid violet 19 dye. Appl. Nanosci. 2016, 6, 495–502. [Google Scholar] [CrossRef]
- Abramian, L.; El-Rassy, H. Adsorption kinetics and thermodynamics of azo-dye Orange II onto highly porous titania aerogel. Chem. Eng. J. 2009, 150, 403–410. [Google Scholar] [CrossRef]
- German-Heins, J.; Flury, M. Sorption of Brilliant Blue FCF in soils as affected by pH and ionic strength. Geoderma 2000, 97, 87–101. [Google Scholar] [CrossRef]
- Alberghina, G.; Bianchini, R.; Fichera, M.; Fisichella, S. Dimerization of Cibacron Blue F3GA and other dyes: Influence of salts and temperature. Dyes. Pigm. 2000, 46, 129–137. [Google Scholar] [CrossRef]
- Mahanta, D.; Madras, G.; Radhakrishnan, S.; Patil, S. Adsorption and Desorption Kinetics of Anionic Dyes on Doped Polyaniline. J. Phys. Chem. B 2009, 113, 2293–2299. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.S.; Lin, J.X.; Fang, F.; Zhang, M.T.; Hu, Z.R. A new absorbent by modifying walnut shell for the removal of anionic dye: Kinetic and thermodynamic studies. Bioresour. Technol. 2014, 163, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.H.; Lin, Y.T.; Tzeng, T.W. Removal of Methylene Blue from Aqueous Solution by Adsorption onto Pineapple Leaf Powder. J. Hazard. Mater. 2009, 170, 417–424. [Google Scholar] [CrossRef]
- Özcan, S.; Erdem, B.; Özcan, A. Adsorption of Acid Blue 193 from Aqueous Solutions Onto Na-Bentonite and DTMA-Bentonite. J. Colloid Interface Sci. 2004, 280, 44–54. [Google Scholar] [CrossRef] [PubMed]
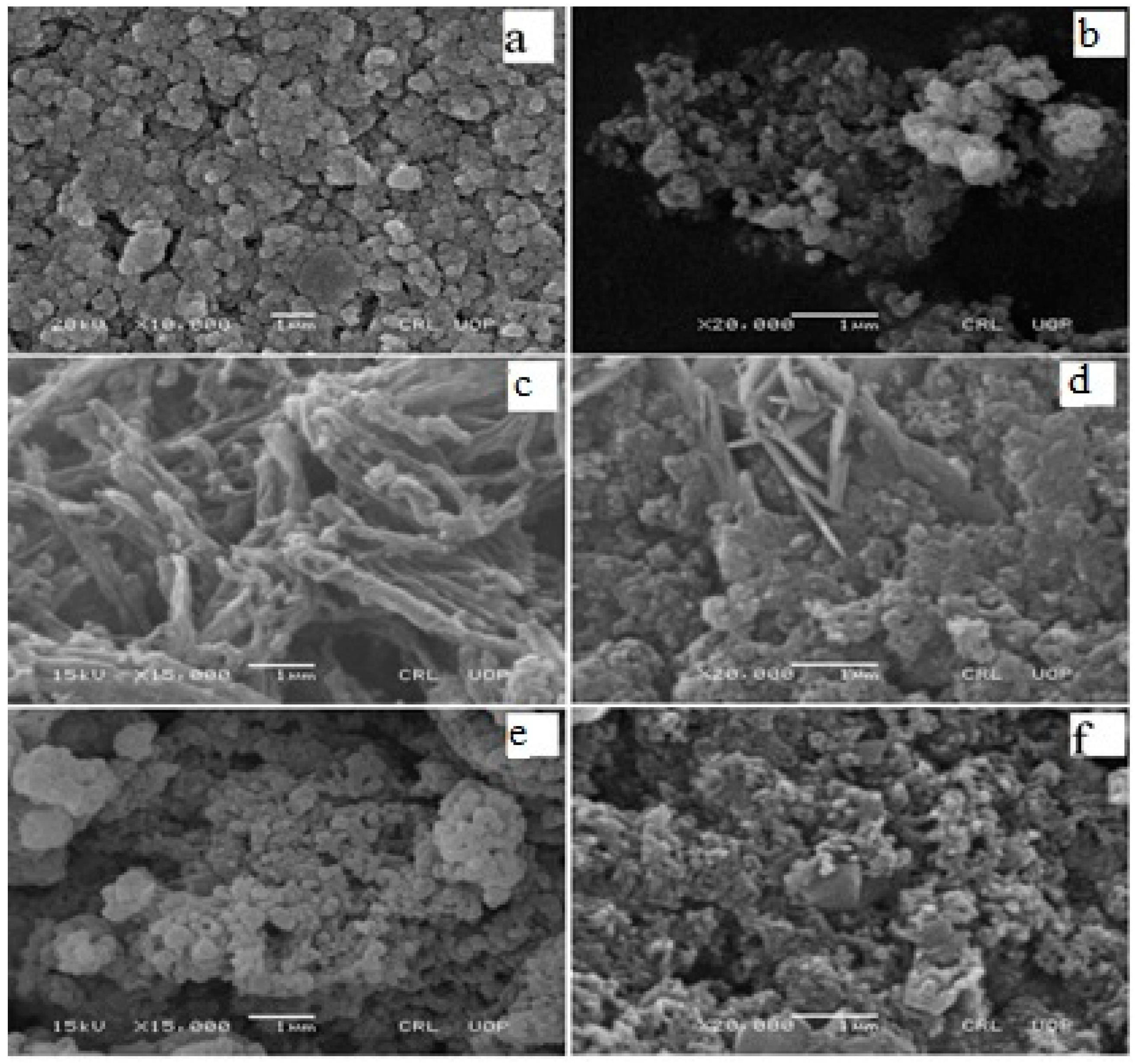
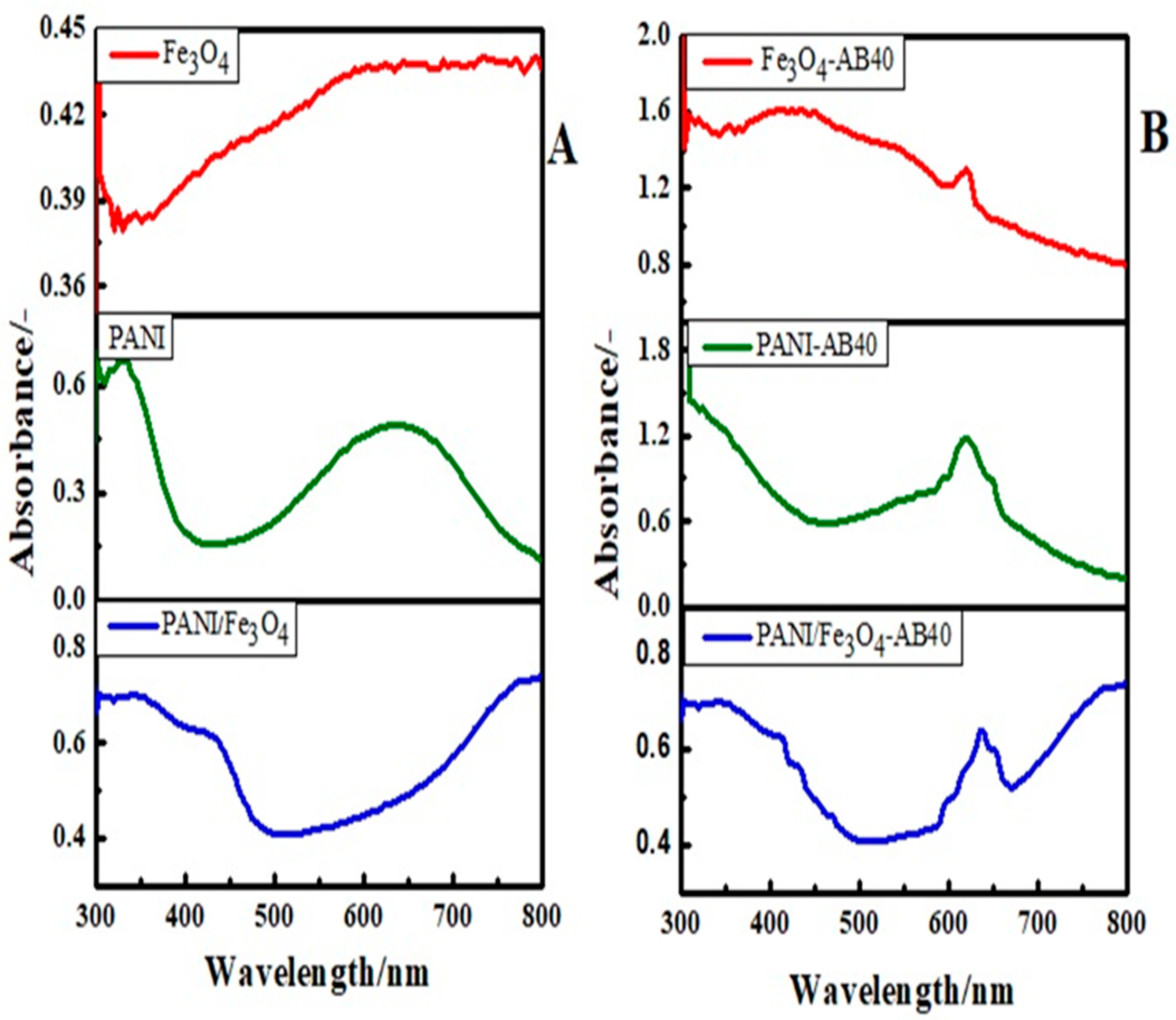
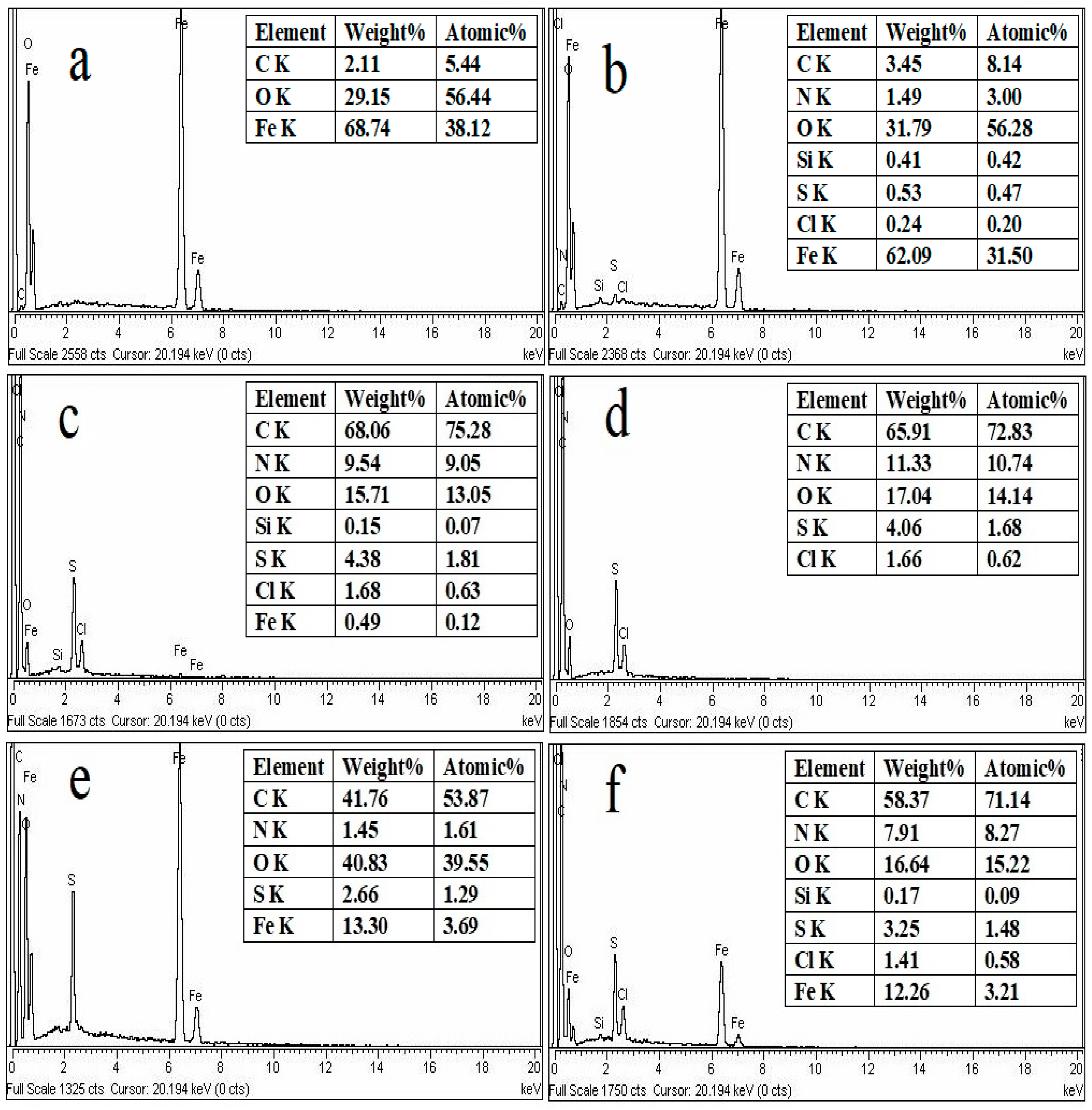
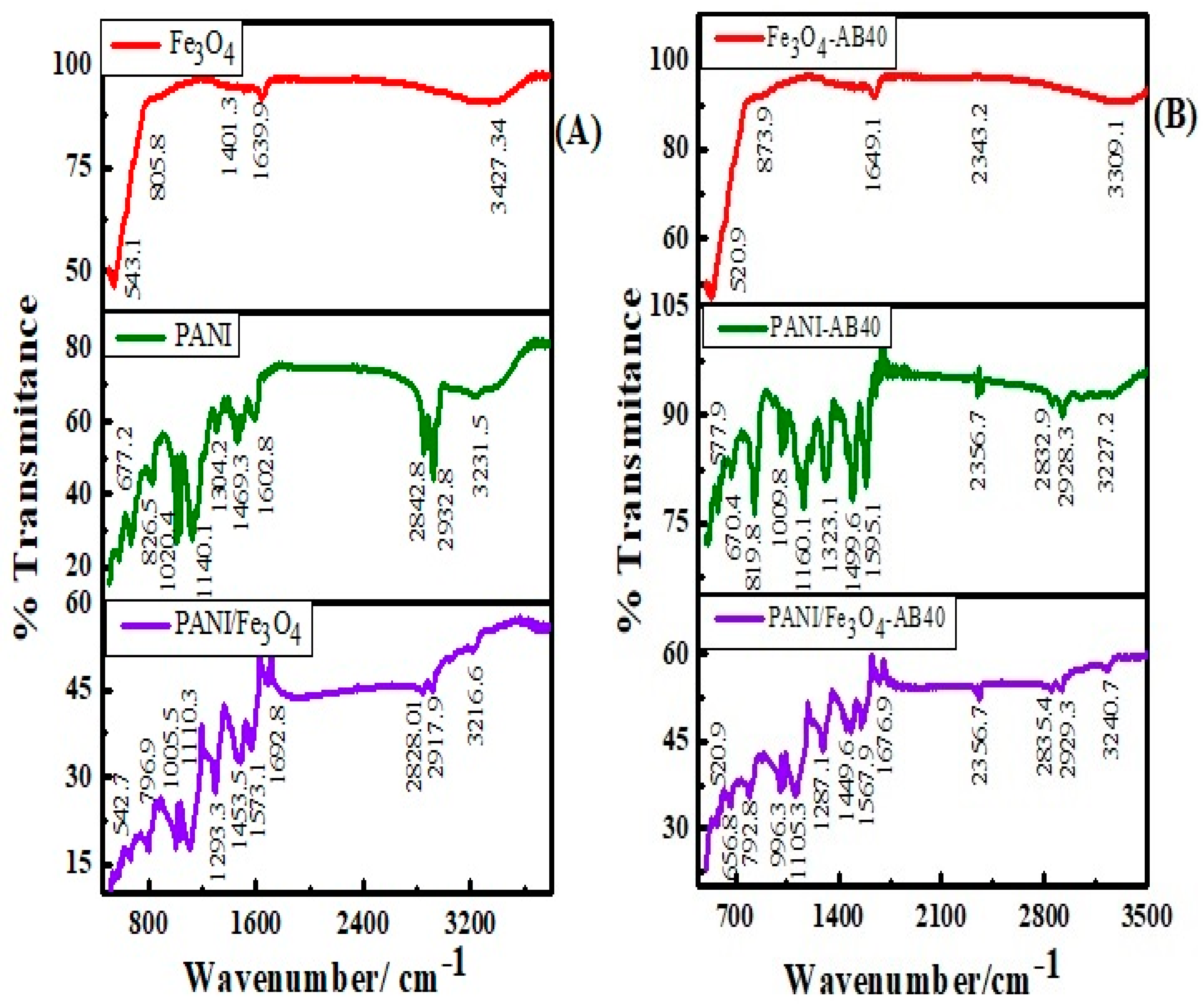
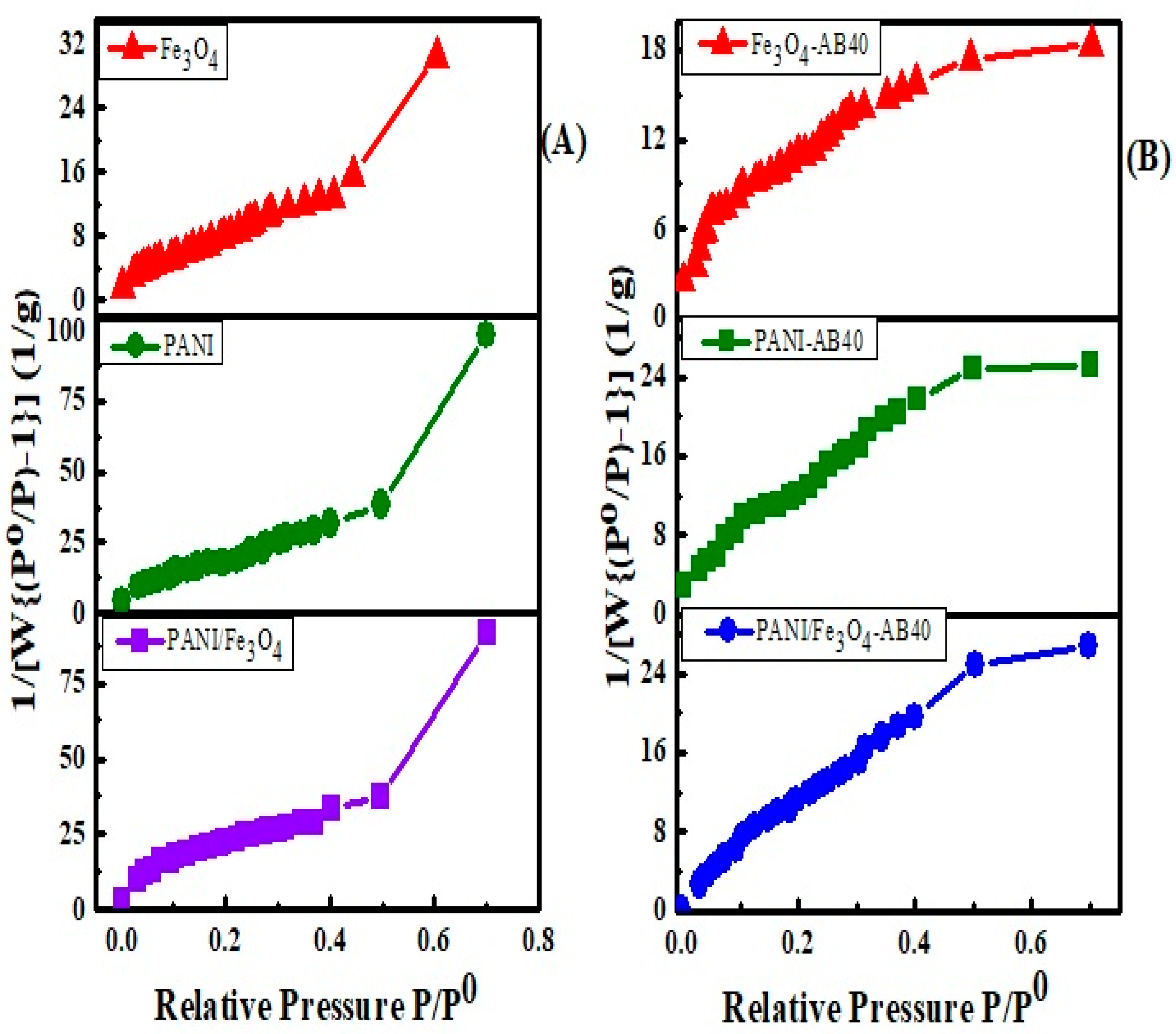


| Status | Materials | Surface Area (m2/g) | BJH Average Pore Radius (A0) | BJH Pore Volume (cc/g) |
|---|---|---|---|---|
| Before adsorption | Fe3O4 | 71.314 | 15.749 | 0.053 |
| PANI | 95.423 | 16.565 | 0.049 | |
| PANI/Fe3O4 | 98.184 | 15.501 | 0.069 | |
| After adsorption | Fe3O4-AB40 | 53.707 | 13.334 | 0.033 |
| PANI-AB40 | 43.938 | 11.743 | 0.043 | |
| PANI/Fe3O4-AB40 | 65.269 | 12.804 | 0.036 |
| Adsorbents | Adsorption Isotherms | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Freundlich | Langmuir | Tempkin | D-R | ||||||||||
| 1/n | Kf | R2 | qmax | KL | RL | R2 | ßT | KT | R2 | qS | Eads | R2 | |
| Fe3O4 | 0.126 | 22.88 | 0.933 | 130.5 | 0.195 | 0.059 | 0.499 | 26.38 | 3.050 | 0.917 | 98.83 | 3.199 | 0.933 |
| PANI | 0.504 | 98.21 | 0.971 | 264.9 | 1.579 | 0.339 | 0.773 | 14.24 | 153.6 | 0.957 | 166.7 | 23.63 | 0.971 |
| PANI/Fe3O4 | 0.723 | 58.99 | 0.946 | 216.9 | 0.499 | 0.167 | 0.859 | 22.15 | 14.17 | 0.902 | 134.5 | 11.29 | 0.909 |
| Pseudo 1st Order | Pseudo 2nd Order | |||||
|---|---|---|---|---|---|---|
| Adsorbents | K1 (min−1) | qe (mg g−1) | R2 | K2 (g mg−1 min−1) | qe (mg g−1) | R2 |
| Fe3O4 | -0.015 | 1.765 | 0.812 | 0.0665 | 126.3 | 0.999 |
| PANI | -0.033 | 4.823 | 0.885 | 0.0028 | 258.8 | 0.983 |
| PANI/ Fe3O4 | -0.047 | 2.495 | 0.881 | 0.0213 | 207.3 | 0.994 |
| Elovich Model | Intra Particle Diffusion Model | |||||
|---|---|---|---|---|---|---|
| Adsorbents | α (mg g−1min−1) | β (g mg−1) | R2 | kd (g mg−1min−1/2) | C (mg g−1) | R2 |
| Fe3O4 | 131.9 | 0.258 | 0.911 | 0.556 | 32.45 | 0.864 |
| PANI | 439.8 | 0.637 | 0.929 | 1.257 | 61.89 | 0.916 |
| PANI/ Fe3O4 | 378.7 | 0.267 | 0.707 | 0.847 | 53.47 | 0.917 |
| Adsorbents | ∆H (kJ mol−1) | ∆S (kJ mol−1) | ∆G (kJ mol−1) | Ea (kJ mol−1) |
|---|---|---|---|---|
| Fe3O4 | −6.077 | −0.026 | −11.93 | 30.12 |
| PANI | −8.993 | −0.032 | −19.87 | 22.09 |
| PANI/Fe3O4 | −10.62 | −0.054 | −19.75 | 26.13 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muhammad, A.; Shah, A.u.H.A.; Bilal, S. Comparative Study of the Adsorption of Acid Blue 40 on Polyaniline, Magnetic Oxide and Their Composites: Synthesis, Characterization and Application. Materials 2019, 12, 2854. https://doi.org/10.3390/ma12182854
Muhammad A, Shah AuHA, Bilal S. Comparative Study of the Adsorption of Acid Blue 40 on Polyaniline, Magnetic Oxide and Their Composites: Synthesis, Characterization and Application. Materials. 2019; 12(18):2854. https://doi.org/10.3390/ma12182854
Chicago/Turabian StyleMuhammad, Amir, Anwar ul Haq Ali Shah, and Salma Bilal. 2019. "Comparative Study of the Adsorption of Acid Blue 40 on Polyaniline, Magnetic Oxide and Their Composites: Synthesis, Characterization and Application" Materials 12, no. 18: 2854. https://doi.org/10.3390/ma12182854
APA StyleMuhammad, A., Shah, A. u. H. A., & Bilal, S. (2019). Comparative Study of the Adsorption of Acid Blue 40 on Polyaniline, Magnetic Oxide and Their Composites: Synthesis, Characterization and Application. Materials, 12(18), 2854. https://doi.org/10.3390/ma12182854







