Reactive Insertion of PEDOT-PSS in SWCNT@Silica Composites and its Electrochemical Performance
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
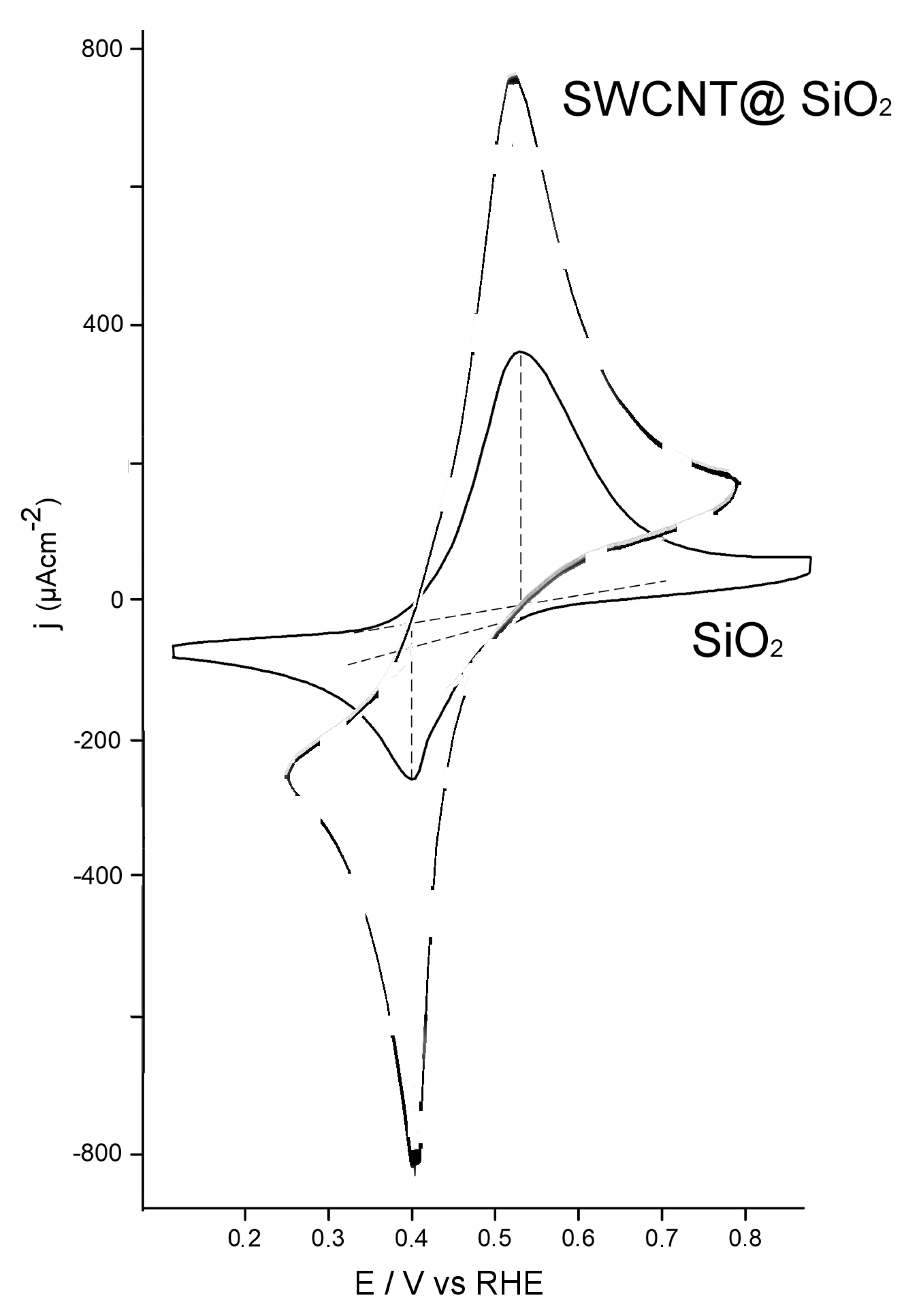
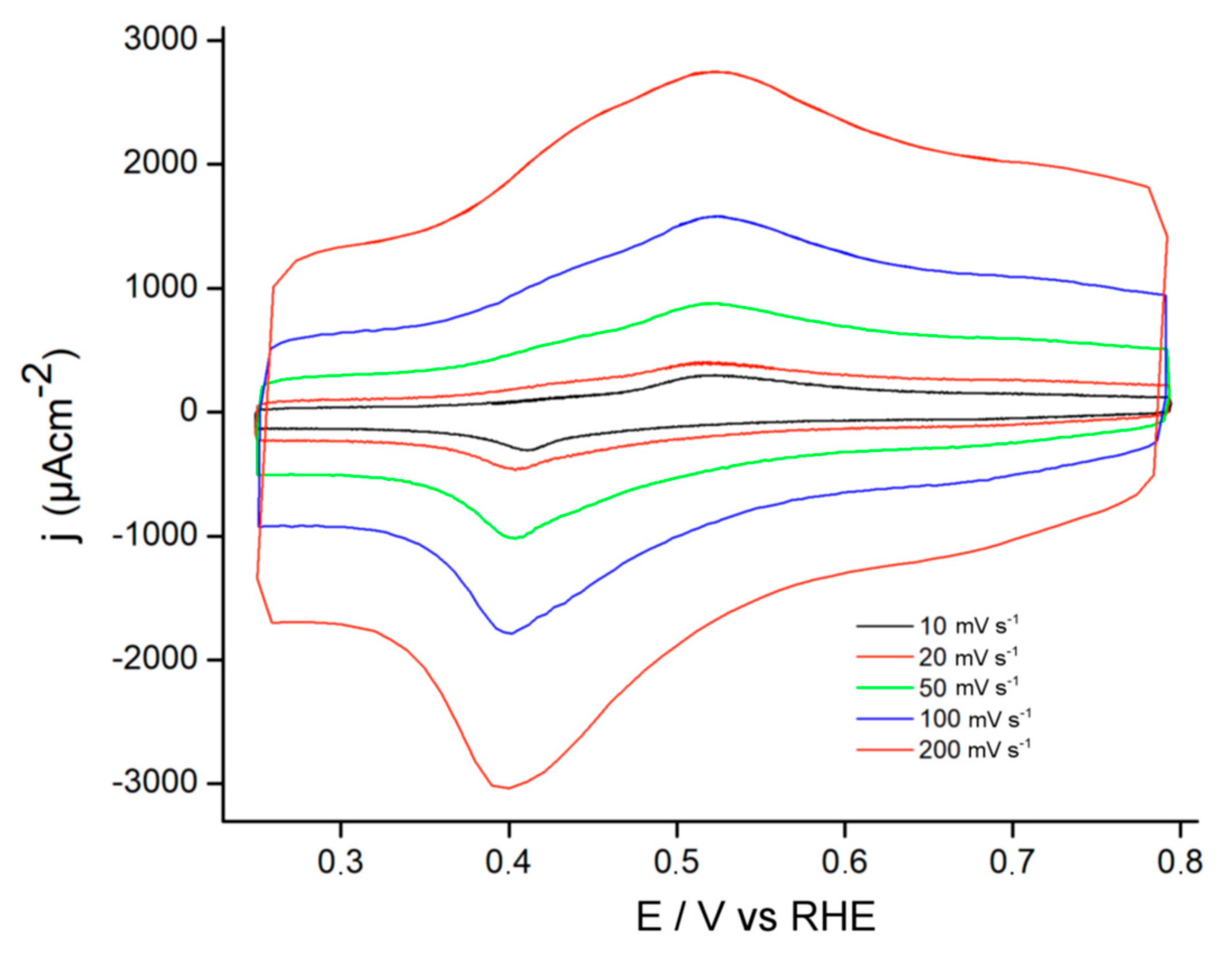
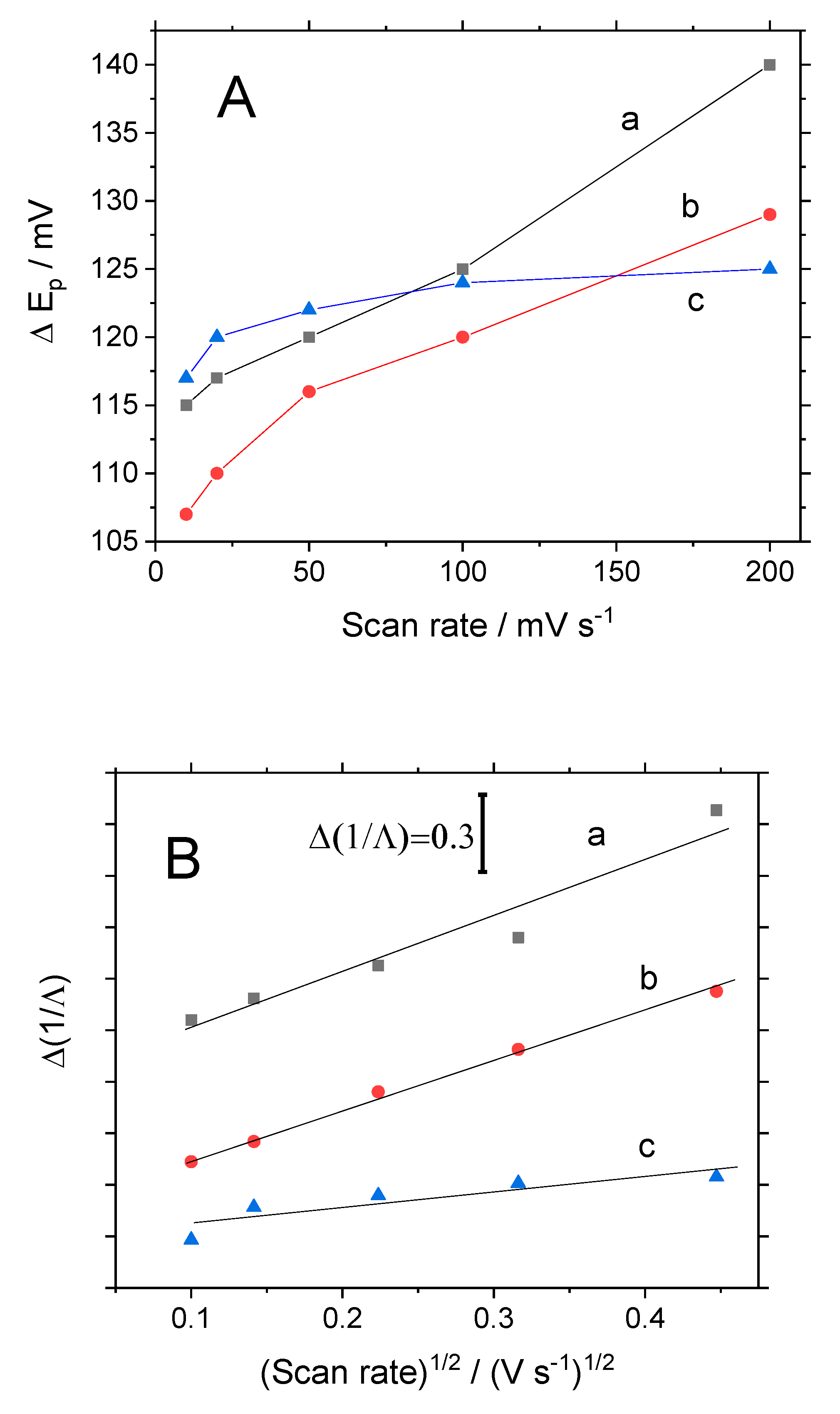
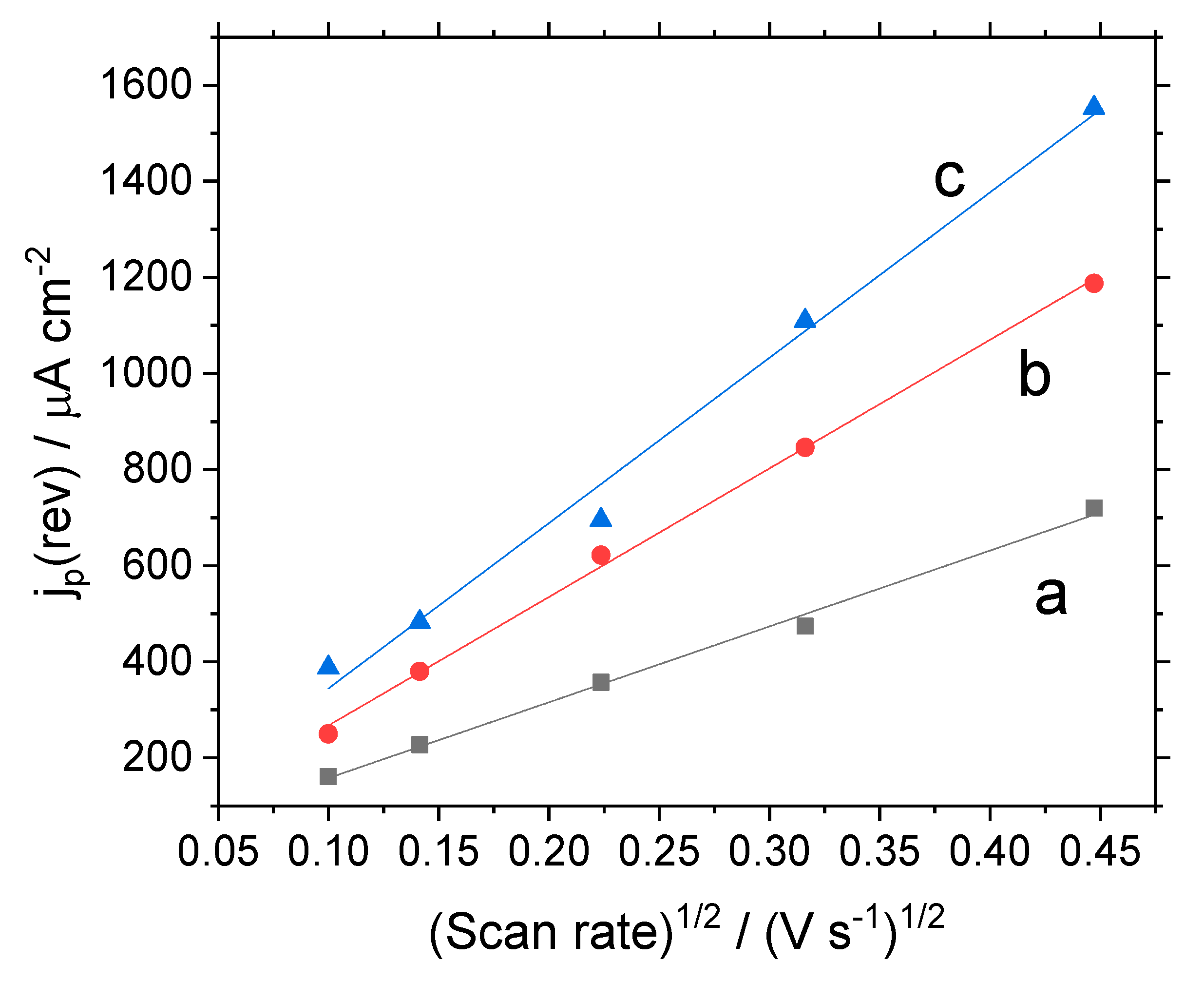
3.1. Electrochemical Behavior of Modified Electrodes
3.2. Surface Characterizations by Scanning Electron Microscopy
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sanghavi, B.J.; Wolfbeis, O.S.; Hirsch, T.; Swami, N.S. Nanomaterial-based electrochemical sensing of neurological drugs and neurotransmitters. Microchim. Acta 2015, 182, 1–41. [Google Scholar] [CrossRef]
- Walcarius, A.; Minteer, S.D.; Wang, J.; Lin, Y.; Merkoçi, A. Nanomaterials for bio-functionalized electrodes: Recent trends. J. Mater. Chem. B 2013, 1, 4878. [Google Scholar] [CrossRef]
- Wang, J. Nanomaterial-based electrochemical biosensors. Analyst 2005, 130, 421. [Google Scholar] [CrossRef] [PubMed]
- Britto, P.J.; Santhanam, K.S.V.; Ajayan, P.M. Carbon nanotube electrode for oxidation of dopamine. Bioelectrochem. Bioenerg. 1996, 41, 121–125. [Google Scholar] [CrossRef]
- Sieben, J.M.; Anson-Casaos, A.; Montilla, F.; Martinez, M.T.; Morallon, E. Electrochemical behaviour of different redox probes on single wall carbon nanotube buckypaper-modified electrodes. Electrochim. Acta 2014, 135, 404–411. [Google Scholar] [CrossRef]
- Wang, J. Carbon-nanotube based electrochemical biosensors: A review. Electroanalysis 2005, 17, 7–14. [Google Scholar] [CrossRef]
- Salinas-Torres, D.; Huerta, F.; Montilla, F.; Morallón, E. Study on electroactive and electrocatalytic surfaces of single walled carbon nanotube-modified electrodes. Electrochim. Acta 2011, 56, 2464–2470. [Google Scholar] [CrossRef]
- Wang, Z.J.; Etienne, M.; Poller, S.; Schuhmann, W.; Kohring, G.W.; Mamane, V.; Walcarius, A. Dehydrogenase-Based Reagentless Biosensors: Electrochemically Assisted Deposition of Sol-Gel Thin Films on Functionalized Carbon Nanotubes. Electroanalysis 2012, 24, 376–385. [Google Scholar] [CrossRef]
- Katz, E.; Willner, I. Biomolecule-functionalized carbon nanotubes: Applications in nanobioelectronics. ChemPhysChem 2004, 5, 1084–1104. [Google Scholar] [CrossRef]
- Jacobs, C.B.; Peairs, M.J.; Venton, B.J. Carbon nanotube based electrochemical sensors for biomolecules. Anal. Chim. Acta 2010, 662, 105–127. [Google Scholar] [CrossRef]
- Djelad, H.; Huerta, F.; Morallón, E.; Montilla, F. Modulation of the electrocatalytic performance of PEDOT-PSS by reactive insertion into a sol-gel silica matrix. Eur. Polym. J. 2018, 105, 323–330. [Google Scholar] [CrossRef]
- Walcarius, A. Electrochemical applications of silica-based organic-inorganic hybrid materials. Chem. Mater. 2001, 13, 3351–3372. [Google Scholar] [CrossRef]
- Shrivastava, S.; Jadon, N.; Jain, R. Next-generation polymer nanocomposite-based electrochemical sensors and biosensors: A review. TRAC Trends Anal. Chem. 2016, 82, 55–67. [Google Scholar] [CrossRef]
- Kaur, G.; Adhikari, R.; Cass, P.; Bown, M.; Gunatillake, P. Electrically conductive polymers and composites for biomedical applications. RSC Adv. 2015, 5, 37553–37567. [Google Scholar] [CrossRef]
- Wang, J.; Dai, J.; Yarlagadda, T. Carbon Nanotube−Conducting-Polymer Composite Nanowires. Langmuir 2005, 21, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Gamero-Quijano, A.; Huerta, F.; Salinas-Torres, D.; Morallón, E.; Montilla, F. Enhancement of the electrochemical performance of SWCNT dispersed in a silica sol-gel matrix by reactive insertion of a conducting polymer. Electrochim. Acta 2014, 135, 114–120. [Google Scholar] [CrossRef]
- Gamero-Quijano, A.; Huerta, F.; Salinas-Torres, D.; Morallón, E.; Montilla, F. Electrocatalytic Performance of SiO2-SWCNT Nanocomposites Prepared by Electroassisted Deposition. Electrocatalysis 2013, 4, 259–266. [Google Scholar] [CrossRef]
- McCreery, R.L. Advanced carbon electrode materials for molecular electrochemistry. Chem. Rev. 2008, 108, 2646–2687. [Google Scholar] [CrossRef]
- Liu, G.; Liu, J.; Böcking, T.; Eggers, P.K.; Gooding, J.J. The modification of glassy carbon and gold electrodes with aryl diazonium salt: The impact of the electrode materials on the rate of heterogeneous electron transfer. Chem. Phys. 2005, 319, 136–146. [Google Scholar] [CrossRef]
- Smalley, J.F.; Finklea, H.O.; Chidsey, C.E.D.; Linford, M.R.; Creager, S.E.; Ferraris, J.P.; Chalfant, K.; Zawodzinsk, T.; Feldberg, S.W.; Newton, M.D. Heterogeneous electron-transfer kinetics for ruthenium and ferrocene redox moieties through alkanethiol monolayers on gold. J. Am. Chem. Soc. 2003, 125, 2004–2013. [Google Scholar] [CrossRef]
- Ronkainen, N.J.; Halsall, H.B.; Heineman, W.R. Electrochemical Biosensors. Chemical Society Reviews. 2010, 39, 1747–1763. [Google Scholar] [CrossRef] [PubMed]
- Yoo, E.-H.; Lee, S.-Y. Glucose Biosensors: An Overview of Use in Clinical Practice. Sensors 2010, 10, 4558–4576. [Google Scholar] [CrossRef] [PubMed]
- Setti, L.; Fraleoni-Morgera, A.; Ballarin, B.; Filippini, A.; Frascaro, D.; Piana, C. An amperometric glucose biosensor prototype fabricated by thermal inkjet printing. Biosens. Bioelectron. 2005, 20, 2019–2026. [Google Scholar] [CrossRef] [PubMed]
- López-Bernabeu, S.; Huerta, F.; Morallón, E.; Montilla, F. Direct Electron Transfer to Cytochrome c Induced by a Conducting Polymer. J. Phys. Chem. C 2017, 121, 15870–15879. [Google Scholar] [CrossRef]
- Park, J.; Kim, H.K.; Son, Y. Glucose biosensor constructed from capped conducting microtubules of PEDOT. Sens. Actuators B Chem. 2008, 133, 244–250. [Google Scholar] [CrossRef]
- Santhosh, P.; Manesh, K.M.; Uthayakumar, S.; Komathi, S.; Gopalan, A.I.; Lee, K.-P. Fabrication of enzymatic glucose biosensor based on palladium nanoparticles dispersed onto poly(3,4-ethylenedioxythiophene) nanofibers. Bioelectrochemistry 2009, 75, 61–66. [Google Scholar] [CrossRef]
- Porcel-Valenzuela, M.; Salinas-Castillo, A.; Morallón, E.; Montilla, F. Molecularly Imprinted Silica Films Prepared by Electroassisted Deposition for the Selective Detection of Dopamine. Sens. Actuators B Chem. 2015, 222, 63–70. [Google Scholar] [CrossRef]
- Matsuda, H.; Ayabe, Y. Zur Theorie der Randles-Sevcikschen Kathodenstrahl-Polarographie. Z. Elektrochem. 1955, 59, 494–503. [Google Scholar]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djelad, H.; Benyoucef, A.; Morallón, E.; Montilla, F. Reactive Insertion of PEDOT-PSS in SWCNT@Silica Composites and its Electrochemical Performance. Materials 2020, 13, 1200. https://doi.org/10.3390/ma13051200
Djelad H, Benyoucef A, Morallón E, Montilla F. Reactive Insertion of PEDOT-PSS in SWCNT@Silica Composites and its Electrochemical Performance. Materials. 2020; 13(5):1200. https://doi.org/10.3390/ma13051200
Chicago/Turabian StyleDjelad, Halima, Abdelghani Benyoucef, Emilia Morallón, and Francisco Montilla. 2020. "Reactive Insertion of PEDOT-PSS in SWCNT@Silica Composites and its Electrochemical Performance" Materials 13, no. 5: 1200. https://doi.org/10.3390/ma13051200
APA StyleDjelad, H., Benyoucef, A., Morallón, E., & Montilla, F. (2020). Reactive Insertion of PEDOT-PSS in SWCNT@Silica Composites and its Electrochemical Performance. Materials, 13(5), 1200. https://doi.org/10.3390/ma13051200






