Comparative Analysis of the Corrosion Resistance of Titanium Alloys Intended to Come into Direct or Prolonged Contact with Live Tissues
Abstract
:1. Introduction
2. Materials and Methods
2.1. MaterialsTi-Based Alloys
2.2. Procedures to Analyse the Corrosion Resistance of Ti-Based Alloys
2.2.1. The First Procedure—Corrosion Resistance Evaluation
- pitting and crevice corrosion according to the ASTM F746-04 [25] standard;
- fatigue corrosion tests—taking into consideration that the titanium alloys considered are for orthopedic use.
- physiological solution—9 g/L NaCl;
- artificial plasma bone with the following composition: 6.8245 g/L NaCl, 0.2609 g/L CaCl2.2H2O, 0.4020 g/L KCl, 20.5066 g/L MgSO4.7H2O, 2.1993 g/L NaHCO3, 0.1674 g/L K2HPO4.3H2O, 0.0301 g/L NaH2PO4. H2O [19].
- Open circuit potential (Eoc), recorded during 15 h of immersion in the electrolyte to establish the pseudo-stationery conditions.
- Linear polarization resistance (Rp) in the domain of Mansfeld, determined from a polarization scan between ± 20 mV vs. SCE from the Eoc.
- Tafel plots, traced between ±150 mV/SCE close to the Eoc with a scan rate of 0.1 mV/s to determine the Tafel slopes (ba, bc) and the corrosion current density, icorr.
- Potentiodynamic polarizations plots, recorded between −1000 mV and 1000 mV/SCE with a scan rate of 0.5 mV/s to establish the breakdown potential, Ebreak, and to observe the existence of a passivation domain.
- Coulometric analysis, applied to determine the quantity of the electrical charge consumed on the anodic polarization curves. The anodic domain was divided in two different subdomains: first zone: E (I = 0) to 300 mV;second zone: 300 to 600 mV.
2.2.2. The Second Procedure—Cations Release Investigations
3. Results and Discussion
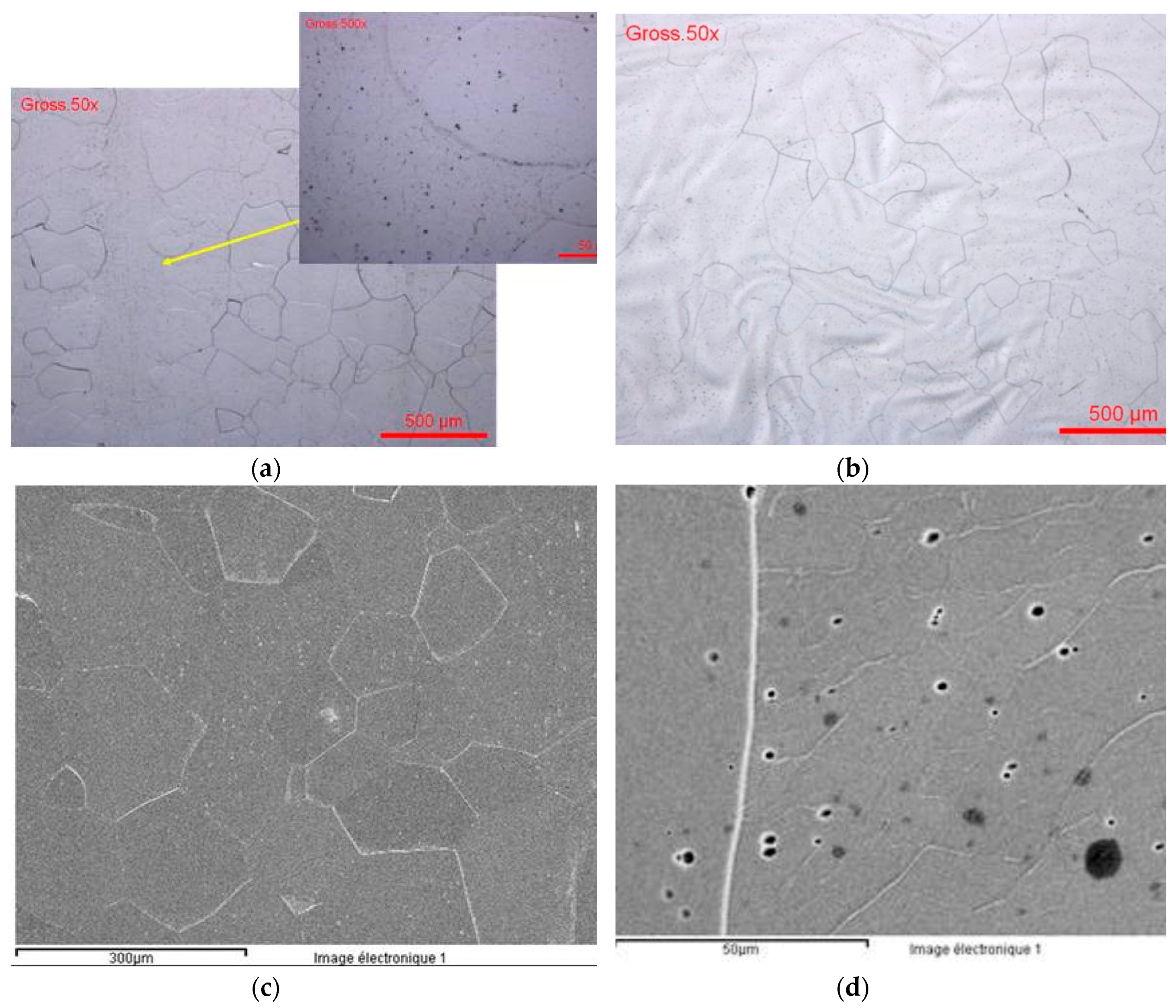
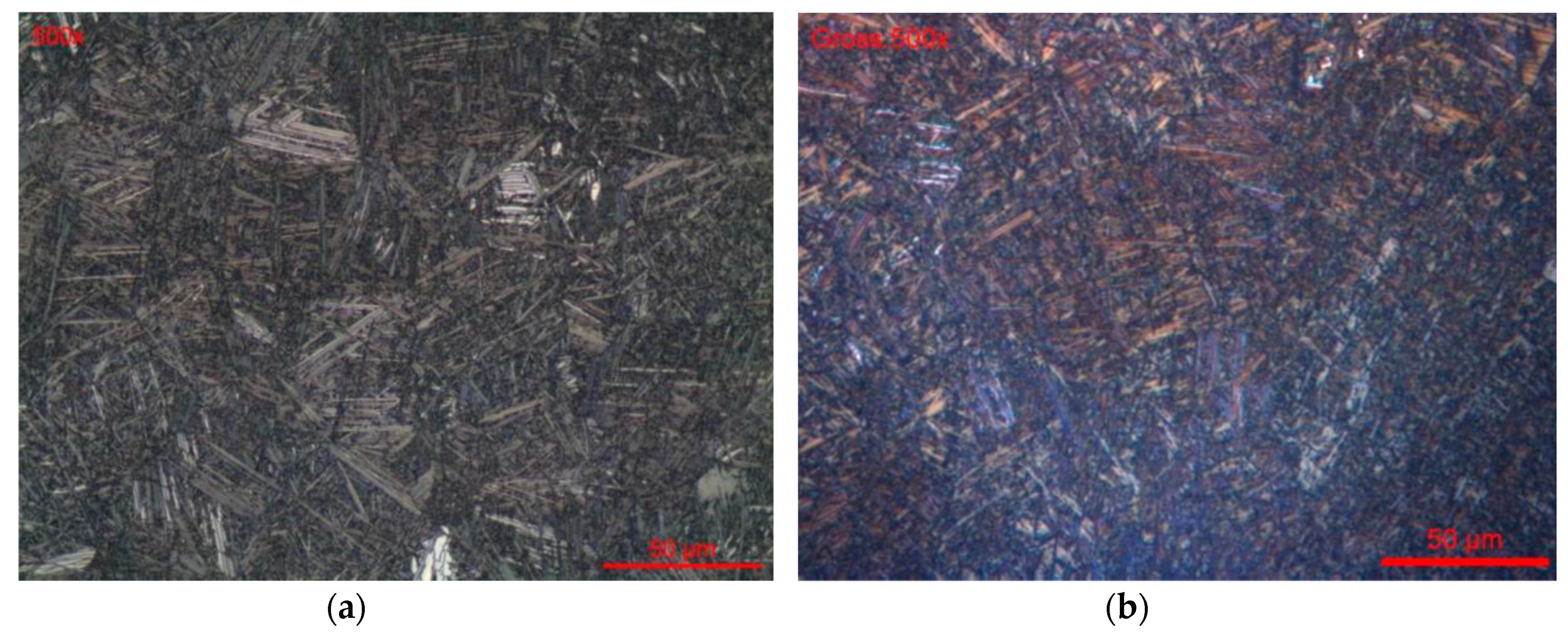
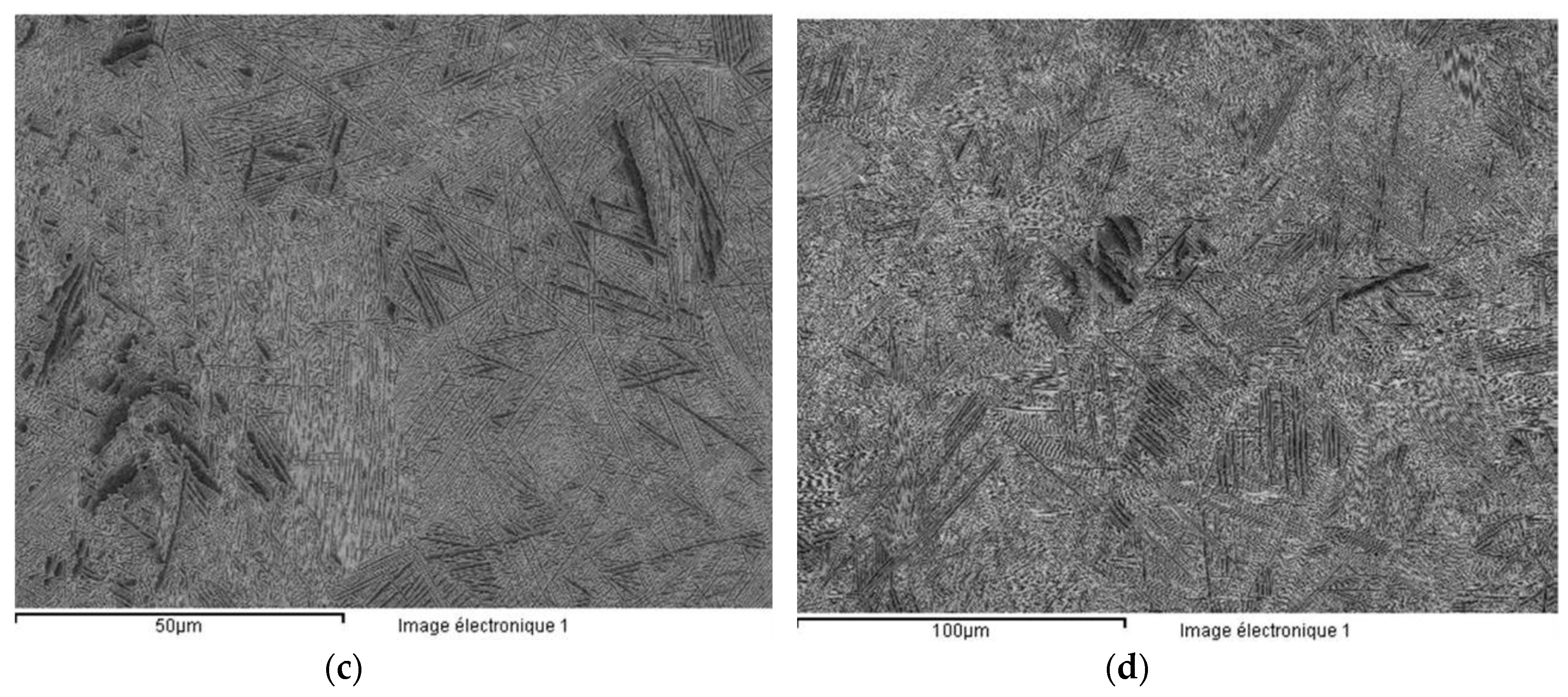
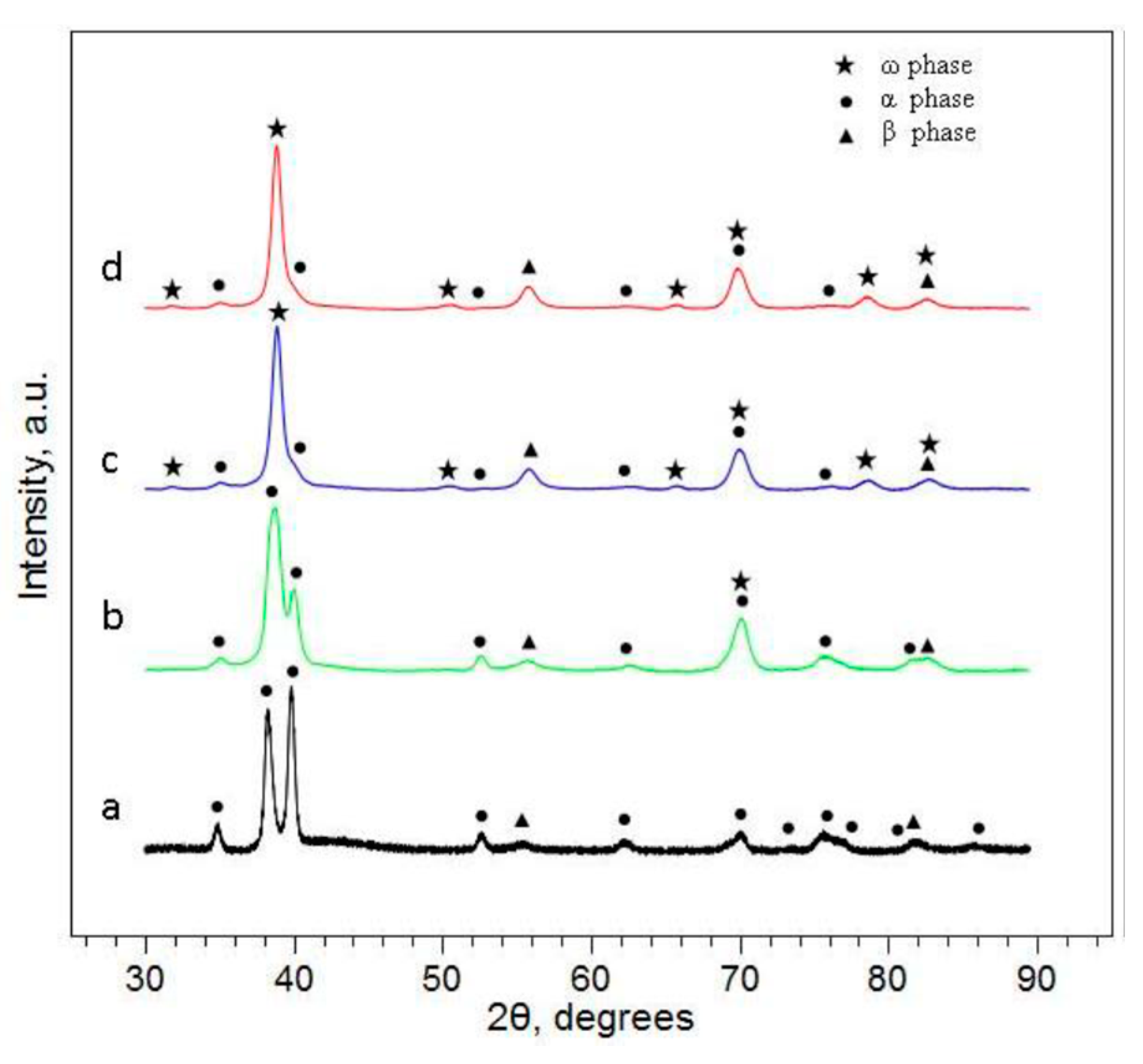
3.1. Metallographic Characterization of Ti-Based Alloys
3.2. The First Procedure—Corrosion Resistance Evaluation
3.2.1. Evaluation of General Corrosion Behavior of Ti-Based Alloys
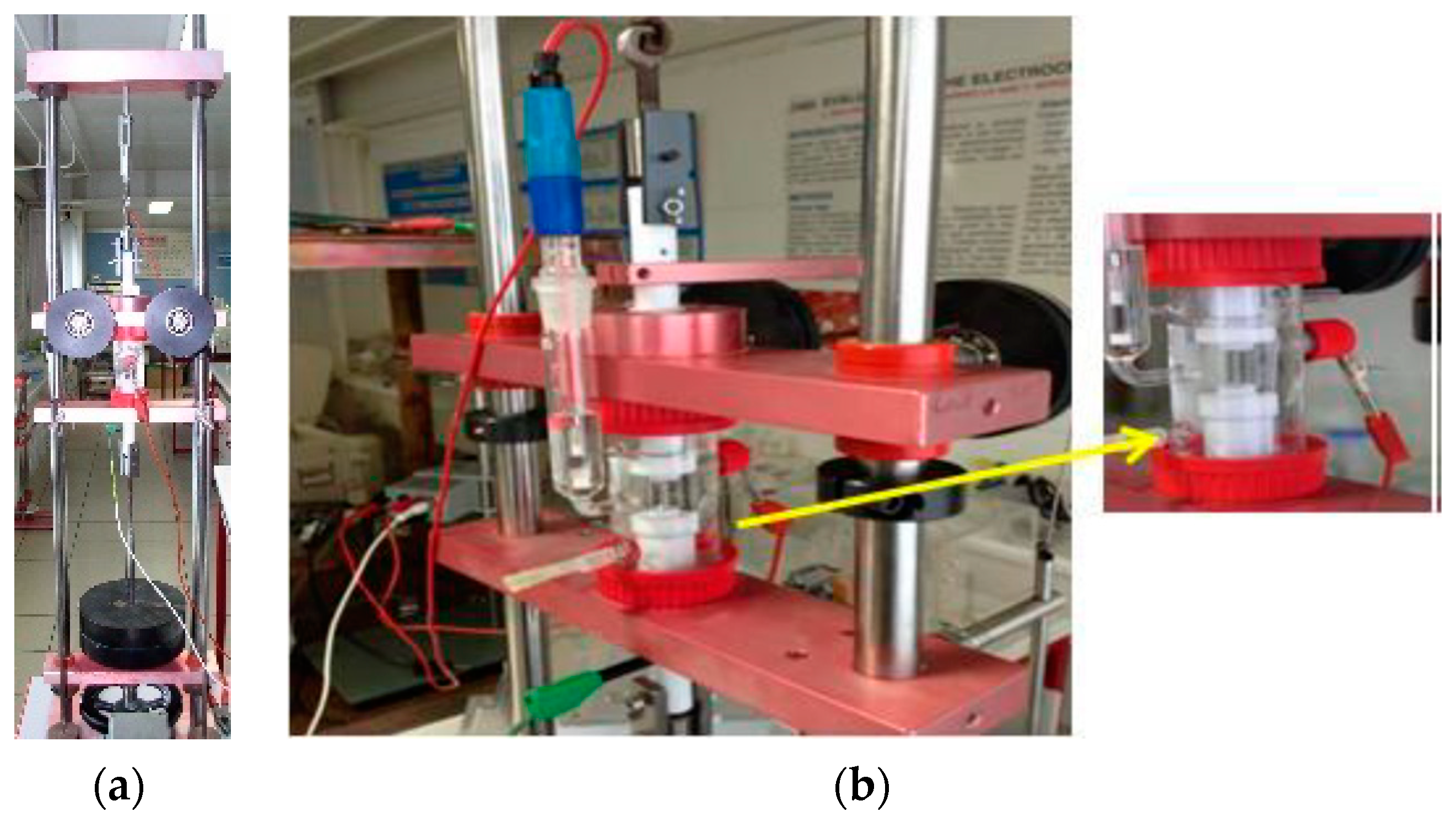
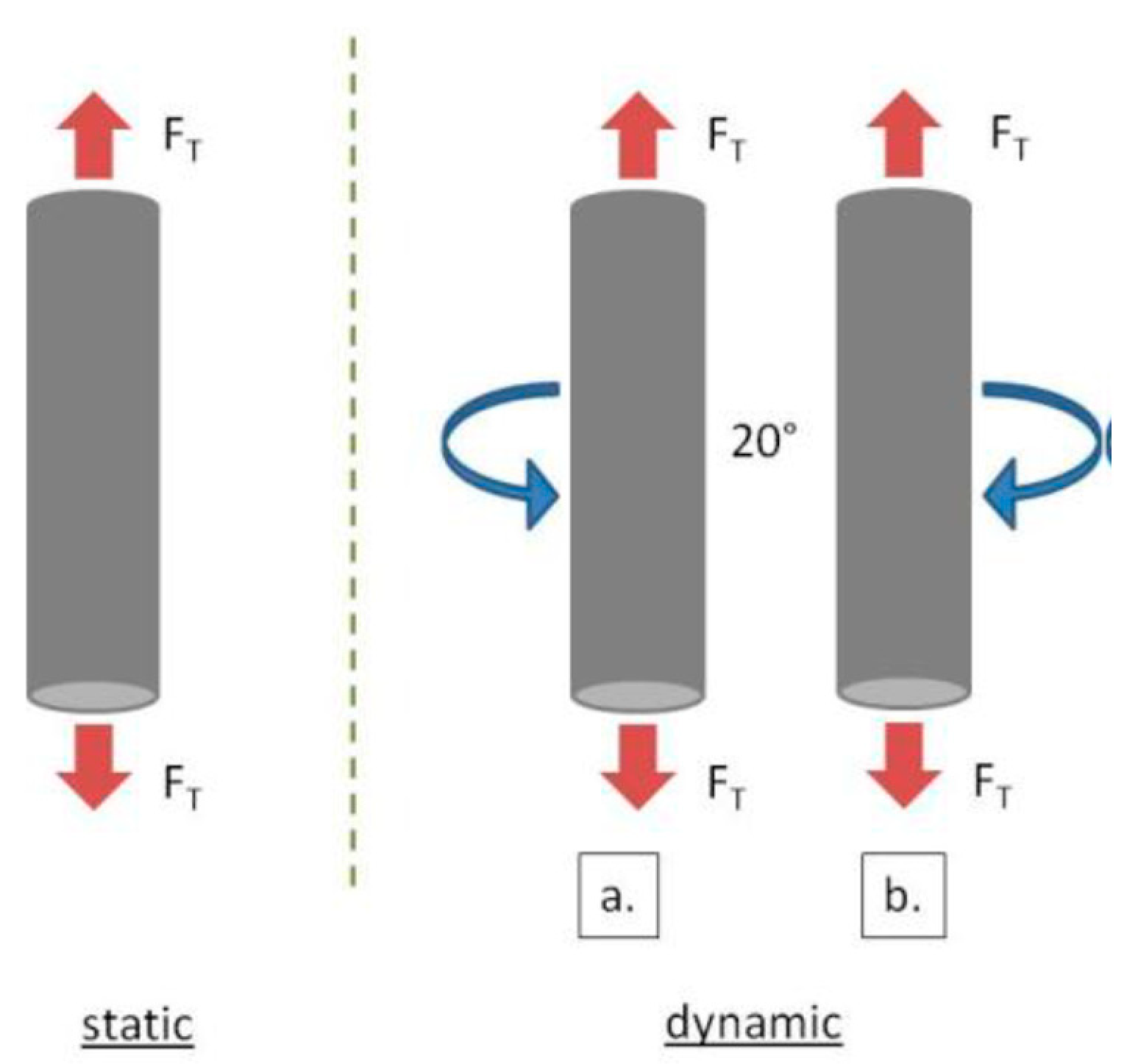
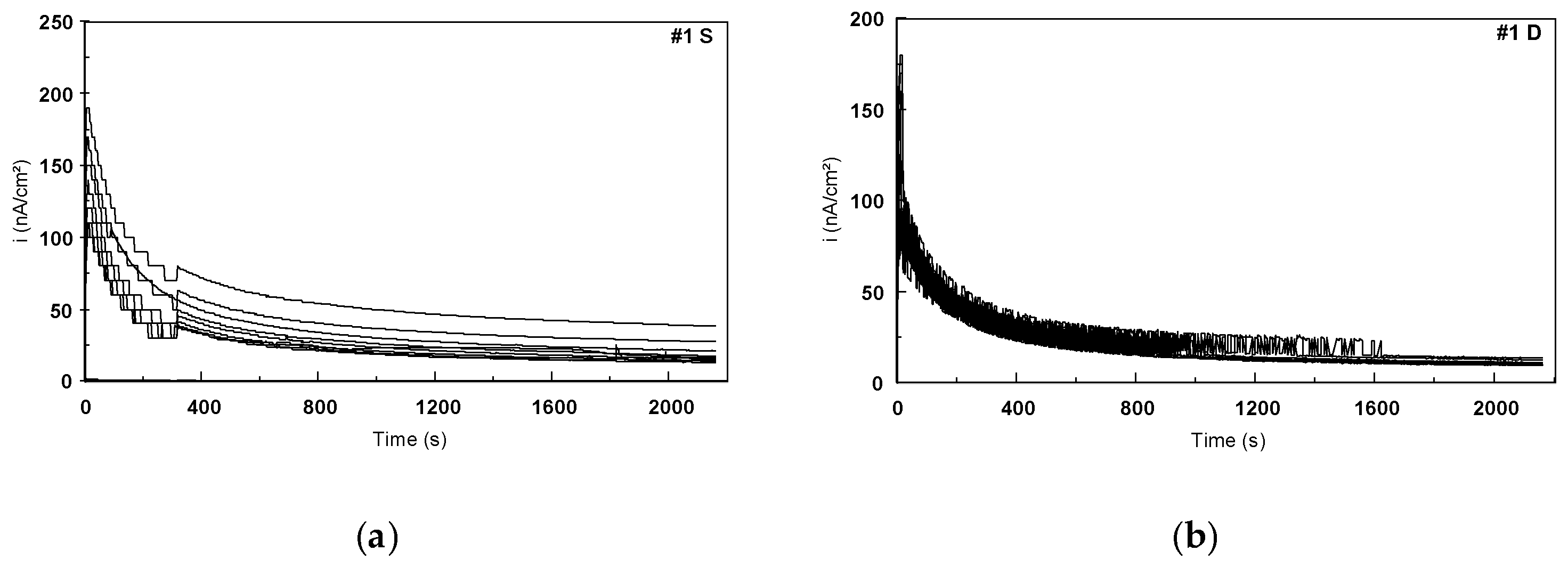
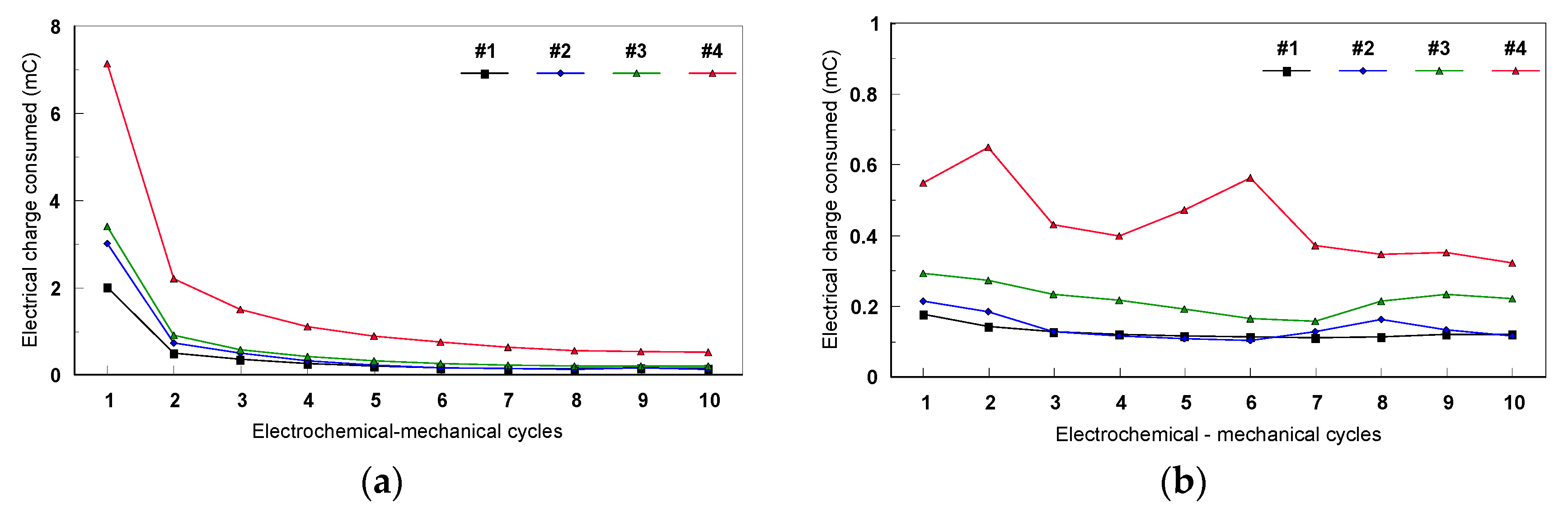
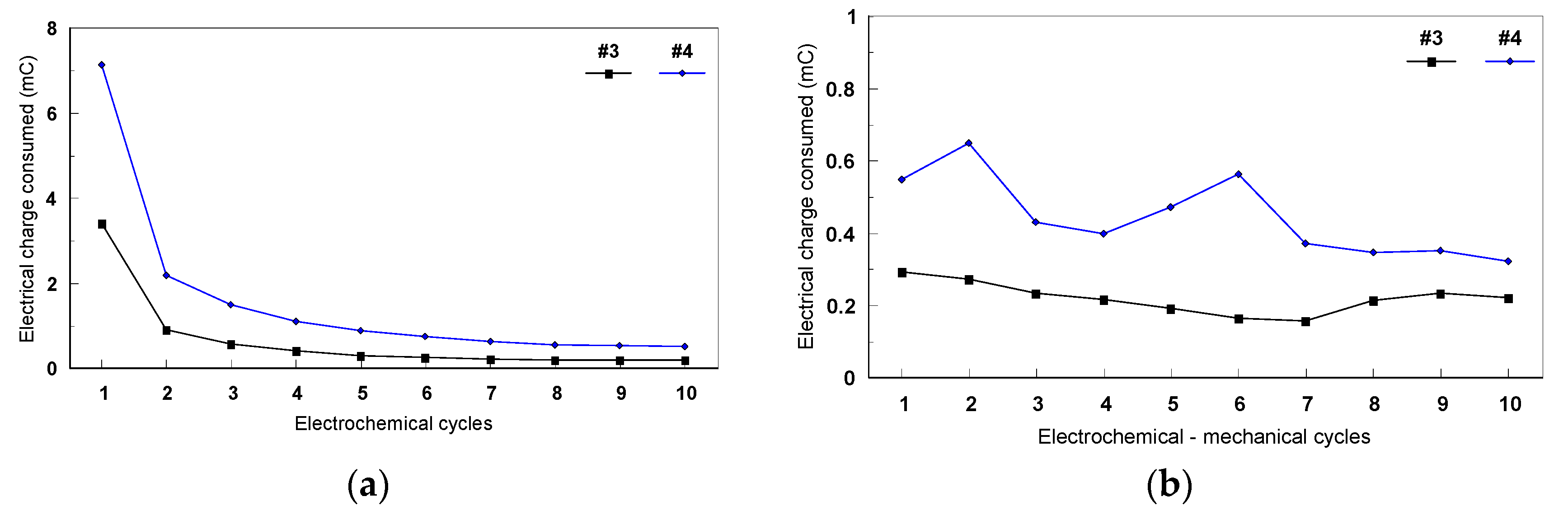
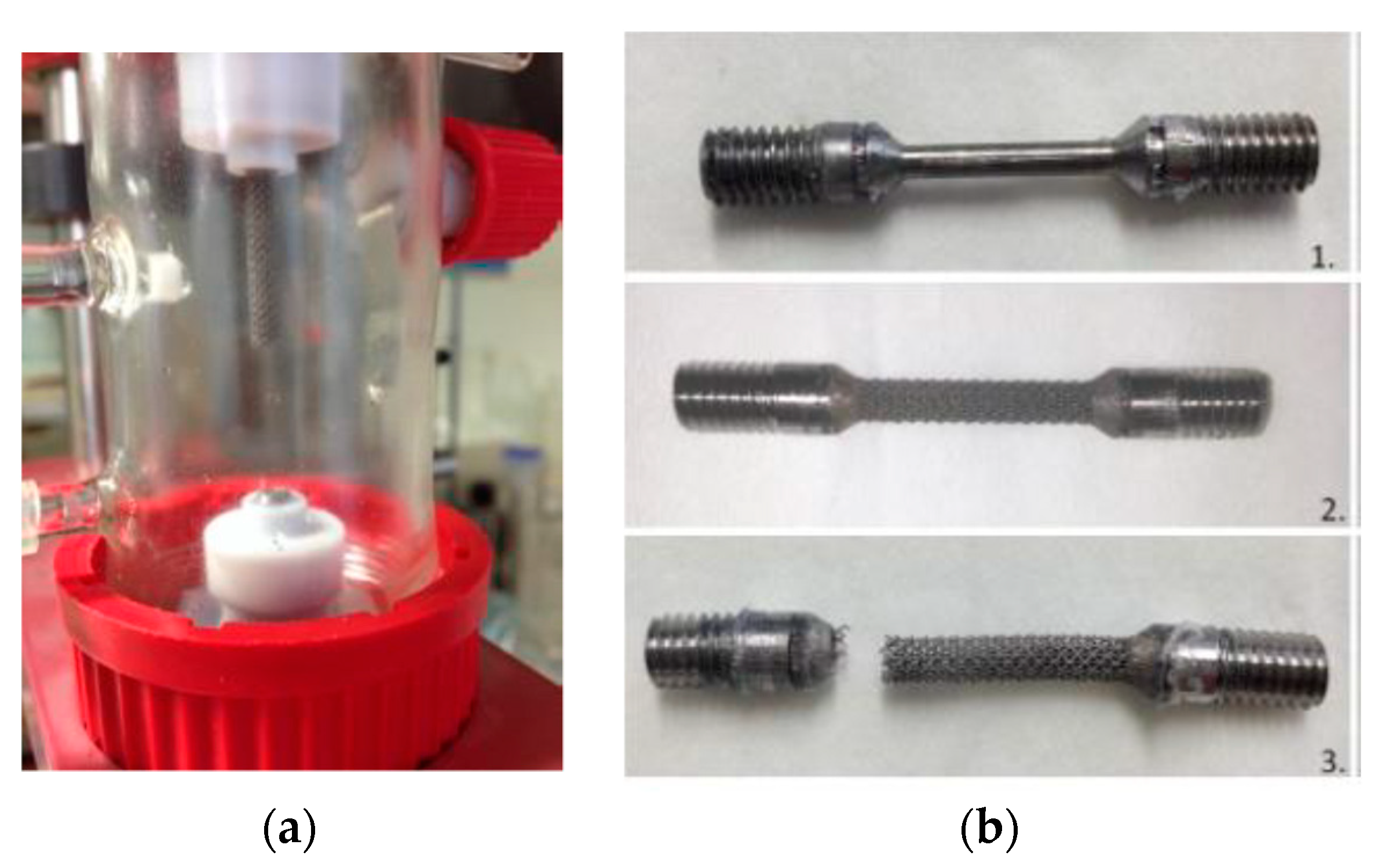
3.2.2. Evaluation of Fatigue Corrosion Behavior of Bulk Ti-Based Alloys
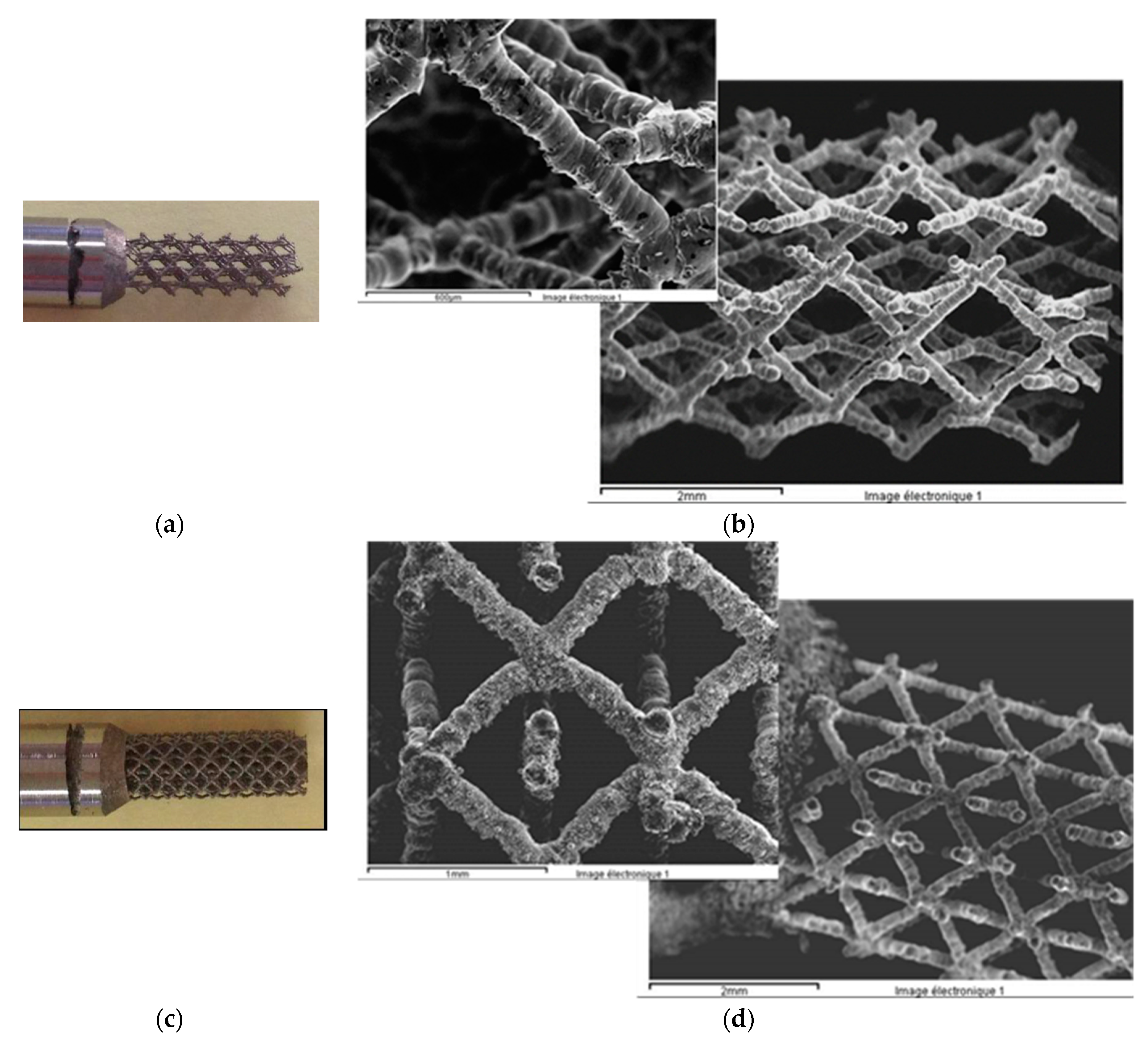
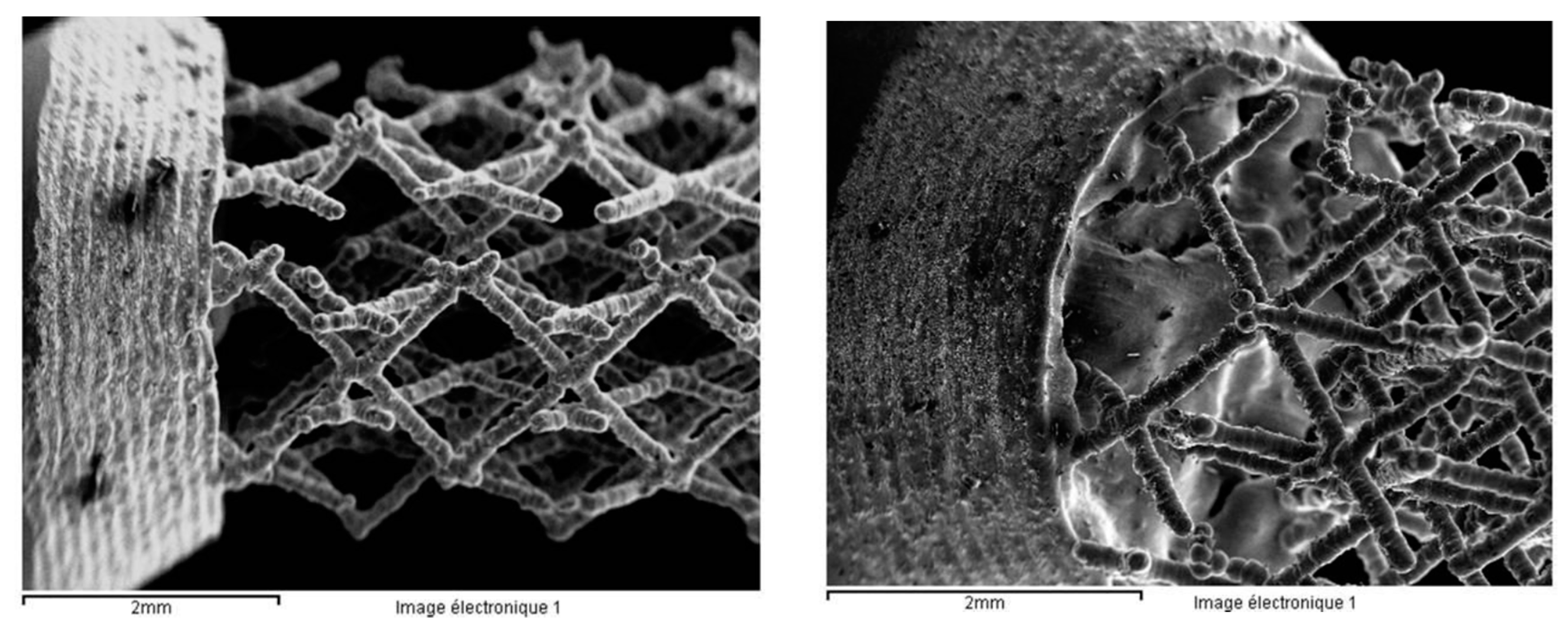
- The sample was not well melted on the bulk parts during the SLM process because of the specific design. Figure 26 presents the SEM images of the scaffold structure before testing.
- The structure was also weakened during etching, the machining process, and cleaning in the ultra sound bath.
3.3. The Second Procedure—Cations Extraction
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Graedel, T.E.; Harper, E.M.; Nassar, N.T.; Nuss, P.; Reck, B.K. Criticality of metals and metalloids. Proc. Natl. Acad. Sci. USA. 2015, 14, 4257–4262. [Google Scholar] [CrossRef] [PubMed]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Blessy, B.M.; Beeregowda, K.M. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 2, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Hamann, D.; Hamann, C.R.; Thyssen, J.P. The impact of common metal allergens in daily devices. Curr. Opin. Allergy Cl. 2013, 13, 525–530. [Google Scholar]
- Reclaru, L.; Ardelean, L.; Rusu, L.C. Toxic materials, allergens and mutagens and their impact on the dental field. Med. Evol. 2008, 14, 98–102. [Google Scholar]
- ECHA (European Chemical Agency). Available online: https://echa.europa.eu/home (accessed on 10 October 2018).
- Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), establishing a European Chemicals Agency, amending Directive 1999/45/EC and repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC. Available online: https://echa.europa.eu/regulations/reach/legislation (accessed on 10 October 2018).
- Substances of Very High Concern Identification. Available online: https://echa.europa.eu/substances-of-very-high-concern-identification (accessed on 10 October 2018).
- Diepgen, T.L.; Ofenloch, R.F.; Bruze, M.; Bertuccio, P.; Cazzaniga, S.; Coenraads, P.J.; Elsner, P.; Goncalo, M.; Svensson, A.; Naldi, L. Prevalence of contact allergy in the general population in different European regions. Br. J. Dermatol. 2016, 174, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Godrey, A.; Abdel-Moneim, A.; Sepúlveda, M.S. Acutemixturetoxicity of halogenated chemicals and their next generation counterparts on zebrafish embryos. Chemosphere 2017, 181, 710–712. [Google Scholar] [CrossRef]
- Meyer, O. Testing and assessment strategies, including alternative and new approaches. Toxicol. Lett. 2003, 140–141, 21–30. [Google Scholar]
- Commission Directive 2004/96/EC of 27 September 2004 amending Council Directive 76/769/EEC as regards Restrictions on The Marketing and use of Nickel for Piercing Post Assemblies. Available online: https://publications.europa.eu/en/publication-detail/-/publication/c64a48ae-c8db-48c8-9e88-0b8b94c87156/language-en (accessed on 17 June 2019).
- Commission Communication in the framework of the implementation of Regulation (EC) No 1907/2006 of the European Parliament and of the Council concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32016R2235 (accessed on 17 June 2019).
- EN 71-3:2013+A1 Safety of toys—Part 3: Migration of certain elements. Available online: https://law.resource.org/pub/eu/toys/en.71.3.2015.html (accessed on 17 June 2019).
- Annex XVII REACH, All The Restrictions adopted in The Framework of REACH And the Previous Legislation, Directive 76/769/EEC. Available online: https://echa.europa.eu/fr/substances-restricted-under-reach (accessed on 13 May 2019).
- ASTM Vol 13.012014, F136-13 Standard Specification for Wrought Titanium-6Aluminum-4Vanadium ELI (Extra Low Interstitial) Alloy for Surgical Implant Applications (UNS R56401). Available online: https://vpp-piercing.de/wp-content/uploads/ 2015/10/ASTMF13608.pdf (accessed on 17 June 2019).
- ASTM specification as Gr36, F-04.12.44, UNS R58450. Available online: http://www.hexonmetal.com/45nb-p-52.html (accessed on 13 May 2019).
- ASTM Vol 13.01 2014, F1713-08(2013) Standard Specification for Wrought Titanium-13Niobium-13Zirconium Alloy for Surgical Implant Applications (UNS R58130). Available online: https://www.astm.org/BOOKSTORE/BOS/TOCS_2018/13.01.html (accessed on 13 May 2019).
- ASTM Vol 13.01 2014, F1580-12 Standard Specification for Titanium and Titanium-6 Aluminum-4 Vanadium Alloy Powders for Coatings of Surgical Implants). Available online: https://www.astm.org/BOOKSTORE/BOS/TOCS_2018/13.01.html (accessed on 13 May 2019).
- ISO-10993, International Standard, Biological Evaluation of Medical Devices 15: Identification and Quantification of Degradation Products from Metals and Alloys. Available online: https://www.iso.org/standard/22694.html (accessed on 17 June 2019).
- Reclaru, L.; Zeigenhagen, R.; Eschler, P.Y.; Blatter, A.; Lüthy, H. Effects of crystallographic orientation of stainless steels on pitting and crevice corrosion and nickel release. In New Research on Biomaterials; Bloomington, D.R., Ed.; Nova Science Publishers: New York, NY, USA, 2007; pp. 237–281. [Google Scholar]
- Reclaru, L.; Lüthy, H.; Ziegenhagen, R.; Eschler, P.Y.; Blatter, A. Anisotropy of nickel release and corrosion in austenitic stainless steels. Acta Biomater. 2008, 4, 680–685. [Google Scholar]
- ASTM G3-84 standard practice for conventions applicable to electrochemical measurements in corrosion testing. In Annual Book of ASTM Standards, Section 3, Metals Tests Methods and Analytical Procedures, volume 03.02 Corrosion of Metals; Wear and Erosion; ASTM International: West Conshohocken, PA, USA, 2010.
- ASTM G5-87 Standard Reference Test Method for Making Potentiostatic and Potentiodynamic Anodic Polarization Measurements. In Annual Book of ASTM standards, Section 3, Metals Tests Methods and Analytical Procedures, volume 03.02 Corrosion of Metals; Wear and Erosion; ASTM International: West Conshohocken, PA, USA, 2010.
- ASTM G59-97 Standard Test Method for Conducting Potentiodynamic Polarization Resistance Measurements. In Annual Book of ASTM Standards, Section 3, Metals Tests Methods and Analytical Procedures, volume 03.02 Corrosion of Metals; Wear and Erosion; ASTM International: West Conshohocken, PA, USA, 2010.
- ASTM F746-87 Standard Test Method for Pitting or Crevice Corrosion of Metallic Surgical Implant Materials. In Annual Book of ASTM Standards, Section 13, Medical Devices and Services, volume 13.01 Medical Devices, Emergency Medical Services; ASTM International: West Conshohocken, PA, USA, 2010.
- Corrosion of Titanium Alloys in Physiological Solutions. Available online: https://cdn.ymaws.com/titanium.org/resource/resmgr/ZZ-WCTP1973-VOL4/1973_Vol.4-3-Corrosion_of_Ti.pdf (accessed on 13 May 2019).
- Solar, R. Corrosion resistance of titanium surgical implant alloys: A review. In Corrosion and Degradation of Implant Materials; Syrett, B., Acharya, A., Eds.; ASTM International: West Conshohocken, PA, USA, 1979; pp. 259–273. [Google Scholar]
- Koike, M.; Fujii, H. The corrosion resistance of pure titanium in organic acids. Biomaterials 2001, 22, 2931–2936. [Google Scholar] [CrossRef]
- Reclaru, L.; Brooks, R.A.; Zuberbühler, M.; Eschler, P.Y.; Constantin, F.; Tomoaia, G. Evaluation of taper joints with combined fatigue and crevice corrosion testing: Comparison to human explanted modular prostheses. Mater. Sci. Eng. C 2014, 3, 69–77. [Google Scholar]
- EN1811-2011, Reference Test Method for Release of Nickel from All Post Assemblies which are inserted into Pierced Parts of The Human Body and Articles intended to come into direct and prolonged contact with The Skin. Available online: https://www.teg.co.uk/nickel/en1811.php (accessed on 13 May 2019).
- Reclaru, L.; Unger, R.E.; Kirkpatrik, C.J.; Susz, C.; Eschler, P.Y.; Zuercher, M.H.; Antoniac, I.V.; Lüthy, H. Ni-Cr based dental alloys; Ni release, corrosion and biological evaluation. Mater. Sci. Eng. C 2012, 32, 1452–1460. [Google Scholar] [CrossRef] [PubMed]
- Donachie, M.J. Titanium: A Technical Guide, 2nd ed.; ASM International: Materials Park, OH, USA, 2010; p. 469. [Google Scholar]
- Gammon, M.; Briggs, R.D.; Packard, J.M.; Batson, K.W.; Boyer, R.; Domby, C.W. Metallographgy and microstructure of titanium and its alloys. In ASM Handbook 9: Metallography and Microstructures; Voort, G.F.V., Ed.; ASM International: Materials Park, OH, USA, 2004; pp. 899–917. [Google Scholar]
- Massalski, T.B.; Murray, J.L.; Bennett, L.H.; Baker, H. Binary Alloy Phase Diagrams, vol. 2: Fe-Ru to Zn-Zr; American Society for Metals: Metal Park, OH, USA, 1986; pp. 1699–1703. [Google Scholar]
- Kruth, J.P.; Vandenbroucke, B.; Van Vaerenbergh, J.; Mercelis, P. Benchmarking of different SLS/SLM processes as rapid manufacturing techniques. In Proceedings of the International Conference Polymers & Moulds Innovations PMI 2005, Gent, Belgium, 20–23 April 2005. [Google Scholar]
- Wilkes, J.; Hagedor, Y.C.; Ocylok, S.; Meiners, W.; Wissenbach, K. Rapid manufacturing of ceramic parts by selective laser melting. In Advanced Processing and Manufacturing Technologies for Structural and Multifunctional Materials IV: Ceramic Engineering and Science Proceedings; Ohji, T., Singh, M., Mathur, S., Eds.; Wiley: Hoboken, NJ, USA, 2010; pp. 137–148. [Google Scholar]
- Pyka, G.; Burakowski, A.; Kerckhofs, G.; Moesen, M.; Van Bael, S.; Schrooten, J.; Wevers, M. Surface modification of Ti6Al4V open porous structures produced by additive manufacturing. Adv. Eng. Mater. 2012, 14, 363–370. [Google Scholar] [CrossRef]
- Huang, Y.; Kawasaki, M.; Langdon, T.G. Influence of anvil alignment on shearing patterns in High-Pressure Torsion. Adv. Eng. Mater. 2013, 15, 747–755. [Google Scholar] [CrossRef]
- Hoar, T.P.; Mears, D.C.; Ewans, U.R. Corrosion—resistant alloys in chloride solutions materials for surgical implants. P. Roy. Soc. A-Math. Phys. 1966, 294, 486–510. [Google Scholar]
- Fraker, A.C.; Ruff, A.W. Studies of oxide film formation on titanium alloys in saline water. Corros. Sci. 1971, 11, 763–765. [Google Scholar] [CrossRef]
- Okazaki, Y.; Gotoh, E. Comparison of metal release from various metallic biomaterials in vitro. Biomaterials 2005, 26, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Stern, M.; Geary, A.L. Electrochemical polarization I: A theoretical analysis of the slope of polarization curves. J. Electrochem. Soc. 1957, 104, 559–563. [Google Scholar] [CrossRef]
- Reclaru, L.; Zeigenhagen, R.; Eschler, P.Y.; Blatter, A.; Lemaître, J. Comparative corrosion study of “Ni-free‘‘ austenitic stainless steels in view of medical applications. Acta Biomater. 2006, 2, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Reclaru, L.; Meyer, J.M. Effects of fluorides on titanium and other dental alloys in dentistry. Biomaterials 1998, 19, 85–92. [Google Scholar] [CrossRef]
- Solar, R.J. In vitro corrosion testing of titanium surgical implants alloys: An approach to understanding titanium release from implants. J. Biomed. Mater. Res. 1979, 13, 217–250. [Google Scholar] [CrossRef]
- Sourdot, A. La Corrosion du Titane en Milieu Buccal. Ph.D. Thesis, Université Henri Poincare-Nancy, Nancy, France, 2 March 2007. [Google Scholar]
- Takemoto, S.; Hattori, M.; Yoshinari, M.; Kawada, E.; Oda, Y. Tarnish and surface characterization of titanium alloys immersed in an alkaline and peroxide containing denture cleanser. J. Jap. Soc. Dent. Mat. Dev. 2013, 32, 213–219. [Google Scholar]
- Song, Y.; Xu, J.; Ma, X. The study of tarnish induced by Actinobacillusactinomycetemcomitans serotype b (Aay4) on titanium and Ti-75 alloys. Zhonghua Kou Qiang YiXue ZaZhi 2000, 35, 256–258. [Google Scholar]







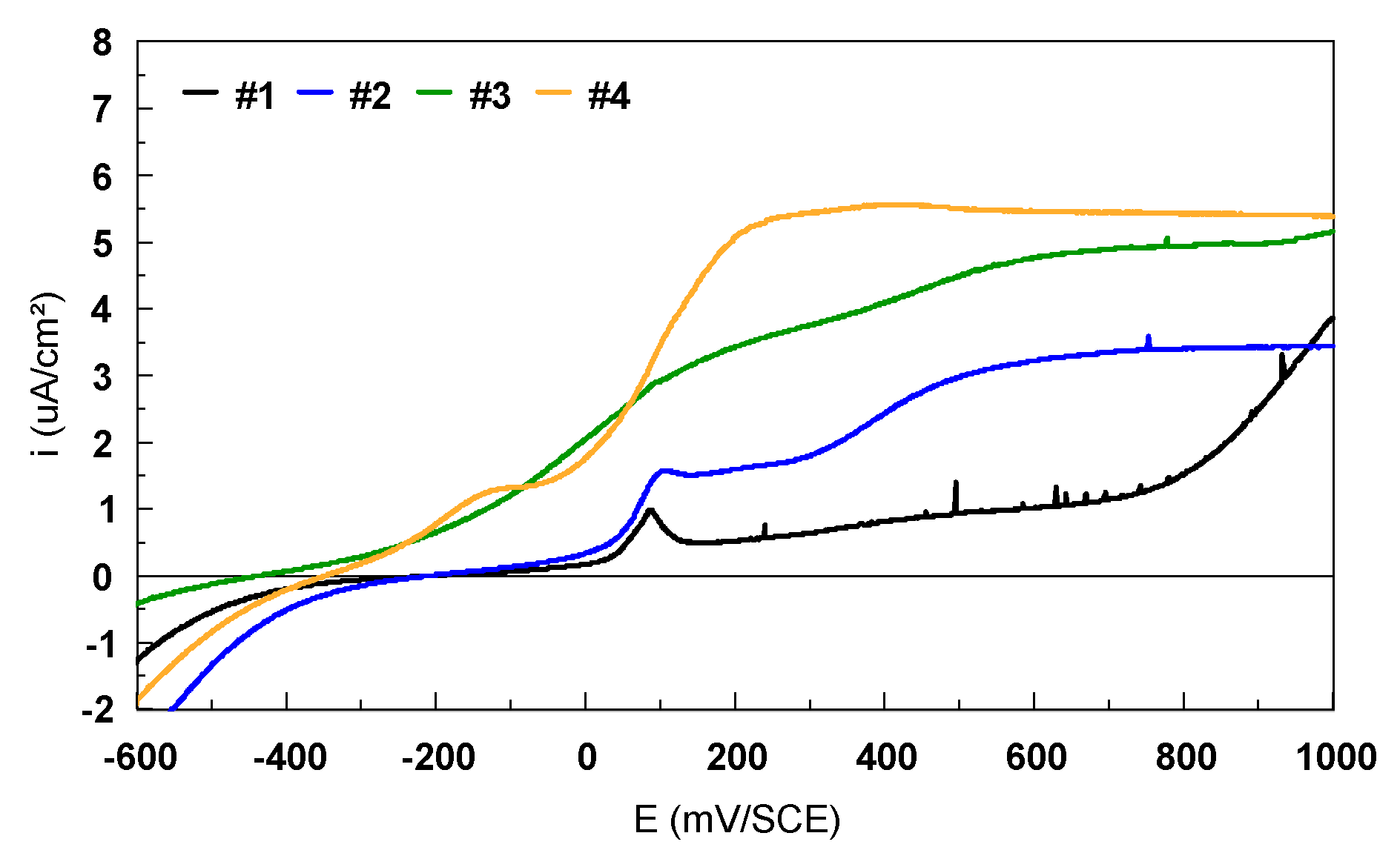
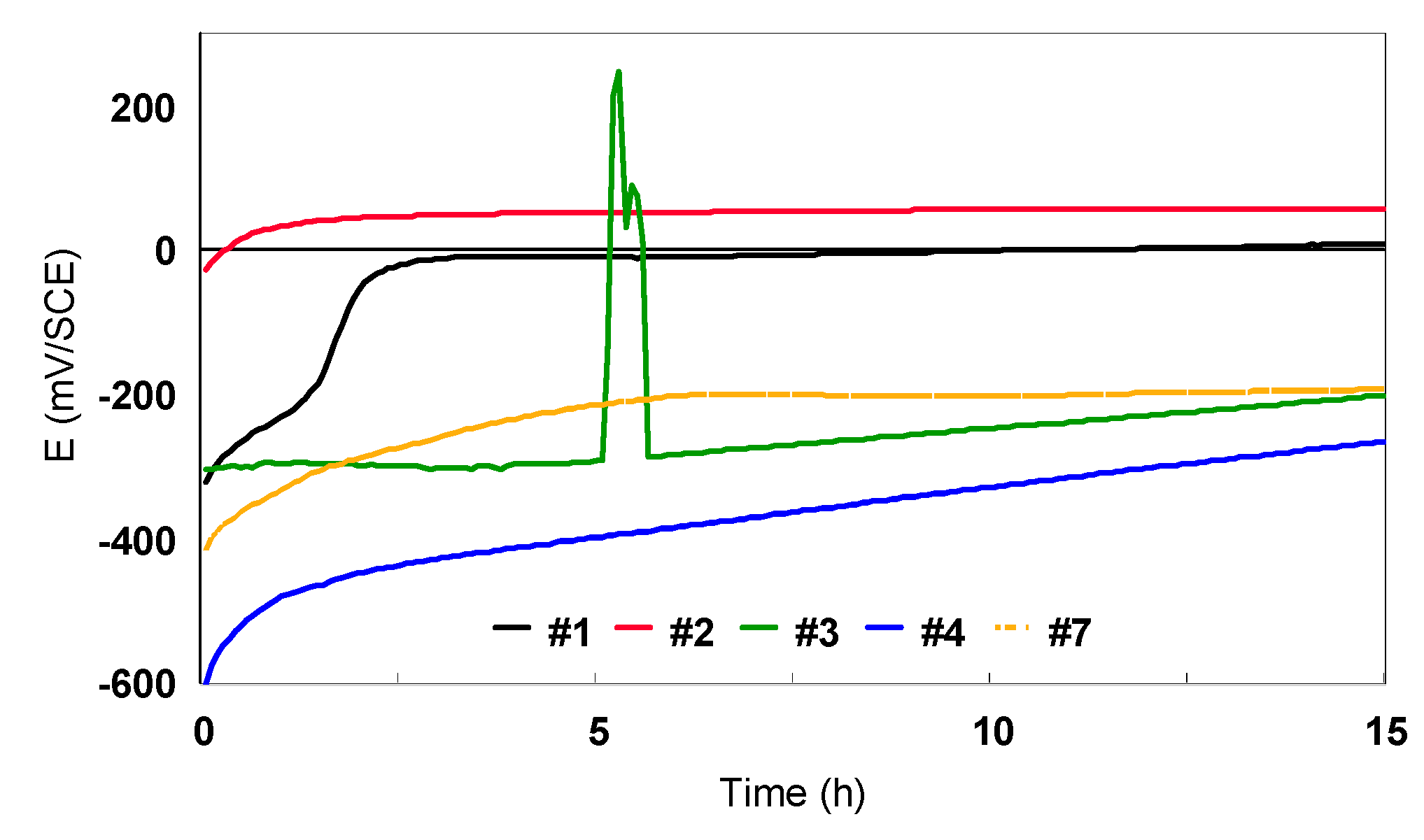
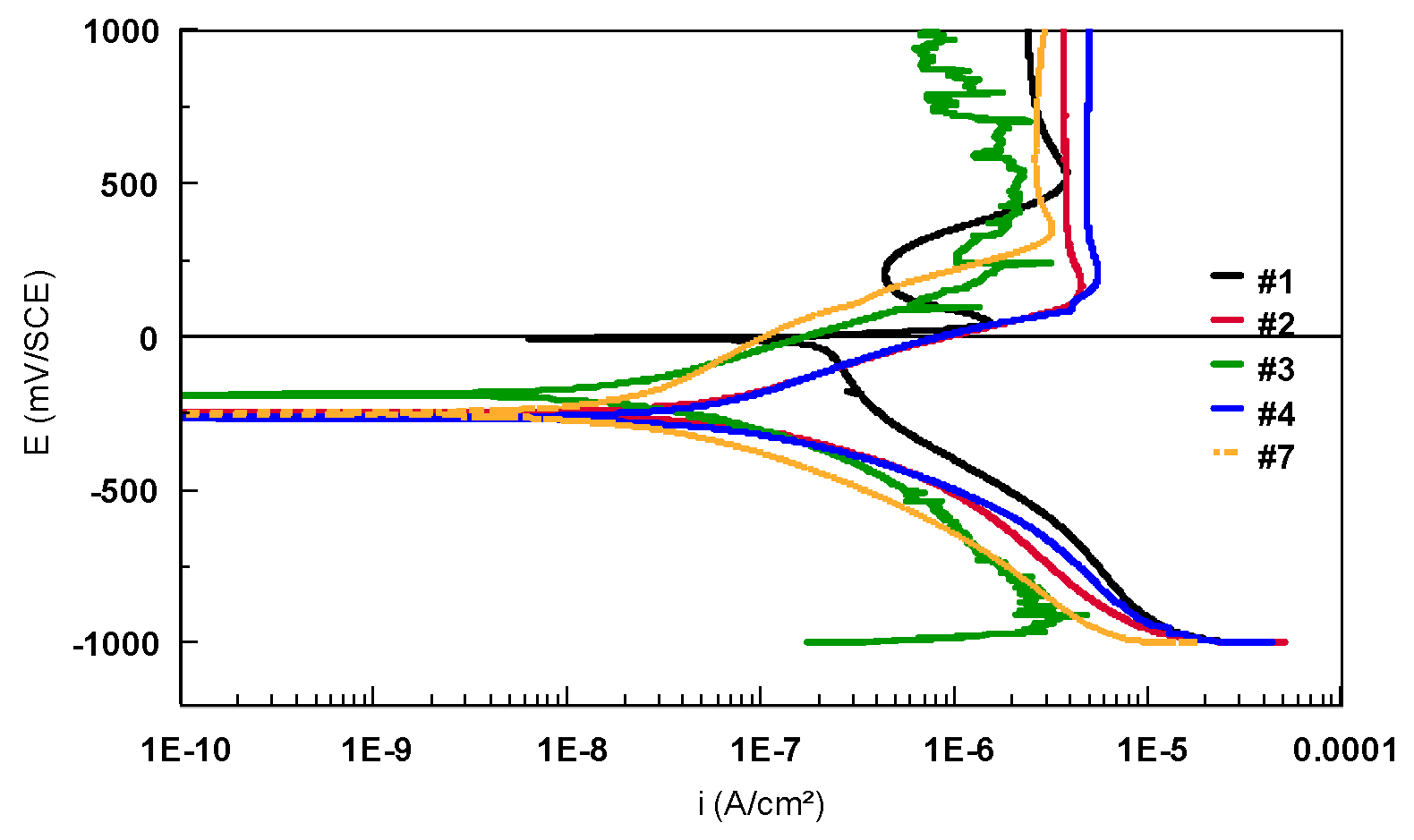
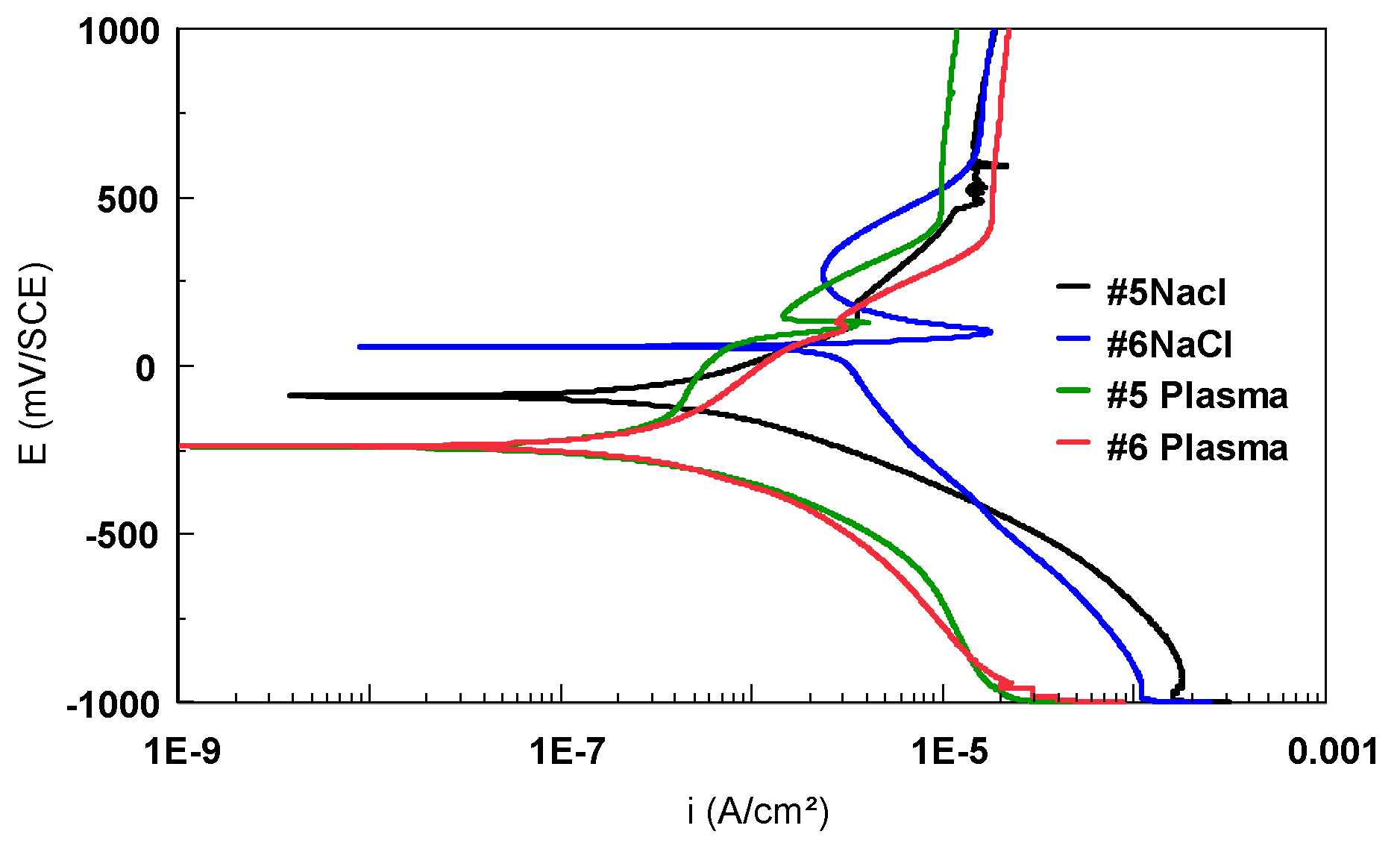





| Alloy | C | N | O | H | Fe | Al | V | Nb | Zr | Ti |
|---|---|---|---|---|---|---|---|---|---|---|
| Ti6Al4V | 0.0110 | 0.0060 | 0.1100 | 0.0034 | 0.1200 | 6.0800 | 3.9300 | - | - | rest |
| Ti45Nb | 0.0200 | 0.0200 | 0.1600 | 0.0050 | 0.2500 | - | - | 45.5200 | - | rest |
| Ti13Nb13Zr | 0.0500 | 0.0150 | 0.1100 | 0.0010 | 0.0550 | - | - | 13.7300 | 13.7300 | rest |
| Code | Alloy | Sample Characteristics |
|---|---|---|
| #1 | Ti6Al4V | bulk, longitudinal section (L) |
| #2 | Ti13Nb13Zr | bulk, longitudinal section (L) |
| #3 | Ti45Nb | bulk, longitudinal section (L) |
| #4 | Ti45Nb | bulk, transversal section (T) |
| #5 | Ti6Al4V | scaffold structure with 1000 μm pores size |
| #6 | Ti6Al4V | scaffold structure with750 μm pores size |
| #7 | Ti13Nb13Zr | nanostructured by HPT process |
| No. | Step | Potential (mV) | Time (min) |
|---|---|---|---|
| 1. | Excitation | 800 | 1 |
| 2. | Potentiostatic level | 600 | 36 |
| 3. | Excitation | 800 | 1 |
| 4. | Potentiostatic level | 650 | 36 |
| 5. | Excitation | 800 | 1 |
| 6. | Potentiostatic level | 700 | 36 |
| 7. | Excitation | 800 | 1 |
| 8. | Potentiostatic level | 750 | 36 |
| 9. | Potentiostatic level | −700 | 20 |
| Alloy | Hardness (HV) |
|---|---|
| #1 | 337 ± 4 |
| #2 | 258 ± 8 |
| #3 | 187 ± 4 |
| #4 | 189 ± 3 |
| #7 | 362 ± 11 |
| Sample | Eoc mV | Ecorr mV | icorr nA/cm2 | ba mV/dec | bc mV/dec | Rp kΩ.cm2 | Coulometric Analysis | |
|---|---|---|---|---|---|---|---|---|
| Zone I mC/cm2 | Zone II mC/cm2 | |||||||
| #1 | –24 | –10.75 | 2.83 | 49.34 | 150.50 | 1105.7 | 0.354 | 0.518 |
| #2 | –44 | –17.60 | 13.22 | 77.59 | 134.10 | 359.8 | 0.801 | 1.585 |
| #3 | –290 | –305.5 | 26.62 | 153.80 | 196.40 | 247.8 | 1.854 | 2.568 |
| #4 | –240 | –232.2 | 29.66 | 146.50 | 171.50 | 254.6 | 2.416 | 3.308 |
| #7 | –259 | –124.3 | 14.02 | 134.30 | 151.80 | 8431.5 | 0.728 | 1.477 |
| Sample | Eoc mV | Ecorr mV | icorr nA/cm2 | ba mV/dec | bc mV/dec | Rp kΩcm2 | Coulometric Analysis | |
|---|---|---|---|---|---|---|---|---|
| Zone I mC/cm2 | Zone II mC/cm2 | |||||||
| #1 | 10 | 8.9 | 218.2 | 54.53 | 303.80 | 100.3 | 0.418 | 1.542 |
| #2 | 58 | −235.9 | 34.14 | 163.20 | 166.30 | 271.5 | 2.189 | 2.320 |
| #3 | −200 | −145.6 | 12.61 | 113.70 | 190.90 | 652.3 | 546.8 | 1.165 |
| #4 | −266 | −205.5 | 19.35 | 1.970 | 212.80 | 332.5 | 2.554 | 3.005 |
| #7 | −193 | −180.0 | 12.29 | 337.40 | 180.70 | 1563 | 0.458 | 1.741 |
| Sample | Eoc mV | Ecorr mV | icorr nA/cm2 | ba mV/dec | bc mV/dec | Rp kΩcm2 | Coulometric Analysis | |
|---|---|---|---|---|---|---|---|---|
| Zone I mC/cm2 | Zone II mC/cm2 | |||||||
| in 9 g/L NaCl | ||||||||
| #5 | −86 | −96.53 | 338.9 | 314.10 | 163.50 | 125.0 | 2.027 | 7.108 |
| #6 | 38 | 3.258 | 21.93 | 154.20 | 109.40 | 670.1 | 2.360 | 4.039 |
| in artificial plasma bone | ||||||||
| #5 | −120 | −138.7 | 49.61 | 265.60 | 113.90 | 442.2 | 1.154 | 5.204 |
| #6 | −136 | −117.2 | 106.4 | 174.50 | 117.50 | 187.9 | 2.364 | 10.24 |
| Cation | Blanc | #1 | #2 | #3 | #4 | #5 | #6 | #7 |
|---|---|---|---|---|---|---|---|---|
| As | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 |
| Ba | 2.4 | 2.2 | 2.0 | 27.2 | 2.1 | 1.66 | 2.0 | 1.63 |
| Be | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| Cd | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | < 0.2 | <0.2 | <0.2 |
| Co | 0.3 | <0.2 | <0.2 | 0.85 | <0.2 | <0.2 | <0.2 | <0.2 |
| Hg | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 |
| Li | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 |
| Mo | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 |
| Nb | <0.02 | <0.02 | <0.02 | <0.02 | <0.02 | <0.02 | <0.02 | <0.02 |
| Pb | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 |
| Sb | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 |
| Sn | <0.2 | < 0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 |
| Sr | 12.0 | 21.6 | 18.6 | 16.6 | 17.3 | 15.0 | 20.30 | 16.0 |
| Zn | 11.0 | 116.60 | 120.0 | 510.0 | 120.0 | 110.0 | 116.60 | 120.0 |
| Al | 8.20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 |
| Cr | 0.9 | <2 | <2 | <2 | <2 | <2 | <2 | <2 |
| Cu | <4 | <8 | <8 | <8 | <8 | <8 | <8 | <8 |
| Fe | <4 | <8 | <8 | <8 | <8 | <8 | <8 | <8 |
| Ni | <4 | <8 | <8 | <8 | <8 | <8 | <8 | <8 |
| Ti | <2 | <2 | <2 | <2 | <2 | <2 | <2 | <2 |
| V | <0.8 | <0.8 | <0.8 | <0.8 | <0.8 | <0.8 | <0.8 | <0.8 |
| Zr | <4 | <8 | <8 | <8 | <8 | <8 | <8 | <8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ionescu, F.; Reclaru, L.; Ardelean, L.C.; Blatter, A. Comparative Analysis of the Corrosion Resistance of Titanium Alloys Intended to Come into Direct or Prolonged Contact with Live Tissues. Materials 2019, 12, 2841. https://doi.org/10.3390/ma12172841
Ionescu F, Reclaru L, Ardelean LC, Blatter A. Comparative Analysis of the Corrosion Resistance of Titanium Alloys Intended to Come into Direct or Prolonged Contact with Live Tissues. Materials. 2019; 12(17):2841. https://doi.org/10.3390/ma12172841
Chicago/Turabian StyleIonescu, Florina, Lucien Reclaru, Lavinia Cosmina Ardelean, and Andreas Blatter. 2019. "Comparative Analysis of the Corrosion Resistance of Titanium Alloys Intended to Come into Direct or Prolonged Contact with Live Tissues" Materials 12, no. 17: 2841. https://doi.org/10.3390/ma12172841
APA StyleIonescu, F., Reclaru, L., Ardelean, L. C., & Blatter, A. (2019). Comparative Analysis of the Corrosion Resistance of Titanium Alloys Intended to Come into Direct or Prolonged Contact with Live Tissues. Materials, 12(17), 2841. https://doi.org/10.3390/ma12172841








