Antimicrobial Activity of Protamine-Loaded Calcium Phosphates against Oral Bacteria
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of DCPA Powders
2.2. Adsorption of Protamine to DCPA Powders and Fabrication of Discs
2.3. Characterization of Protamine-Loaded DCPA
2.4. Microorganisms
2.5. Antibacterial Activity of Protamine-Loaded DCPA Discs against S. mutans
2.6. Biofilm Formation on the Protamine-Loaded DCPA Disc
2.7. Effect of pH against Antimicrobial Activity of Protamine
2.8. Statistical Analysis
3. Results
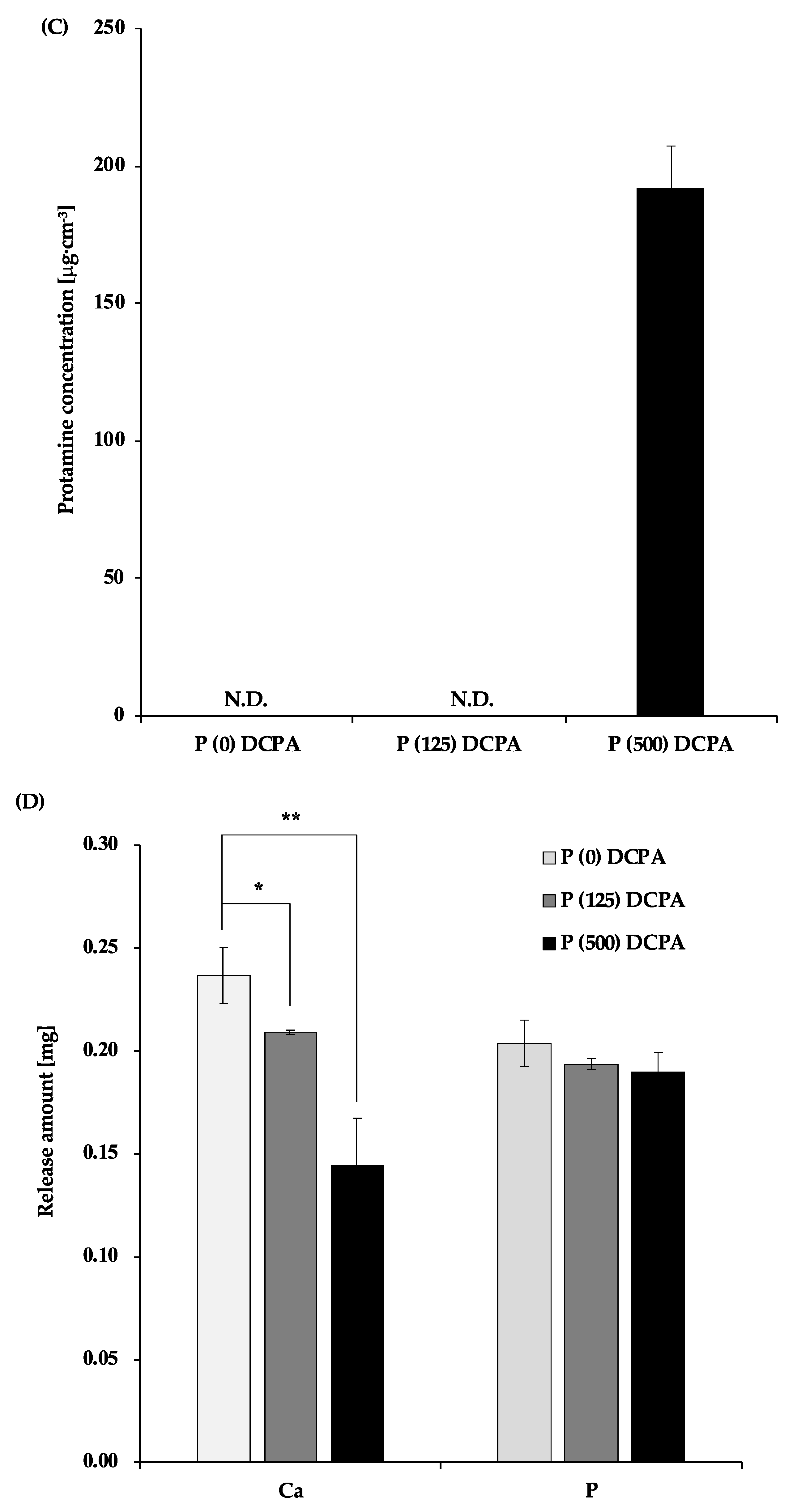
3.1. Characterization of Protamine-Loaded DCPA
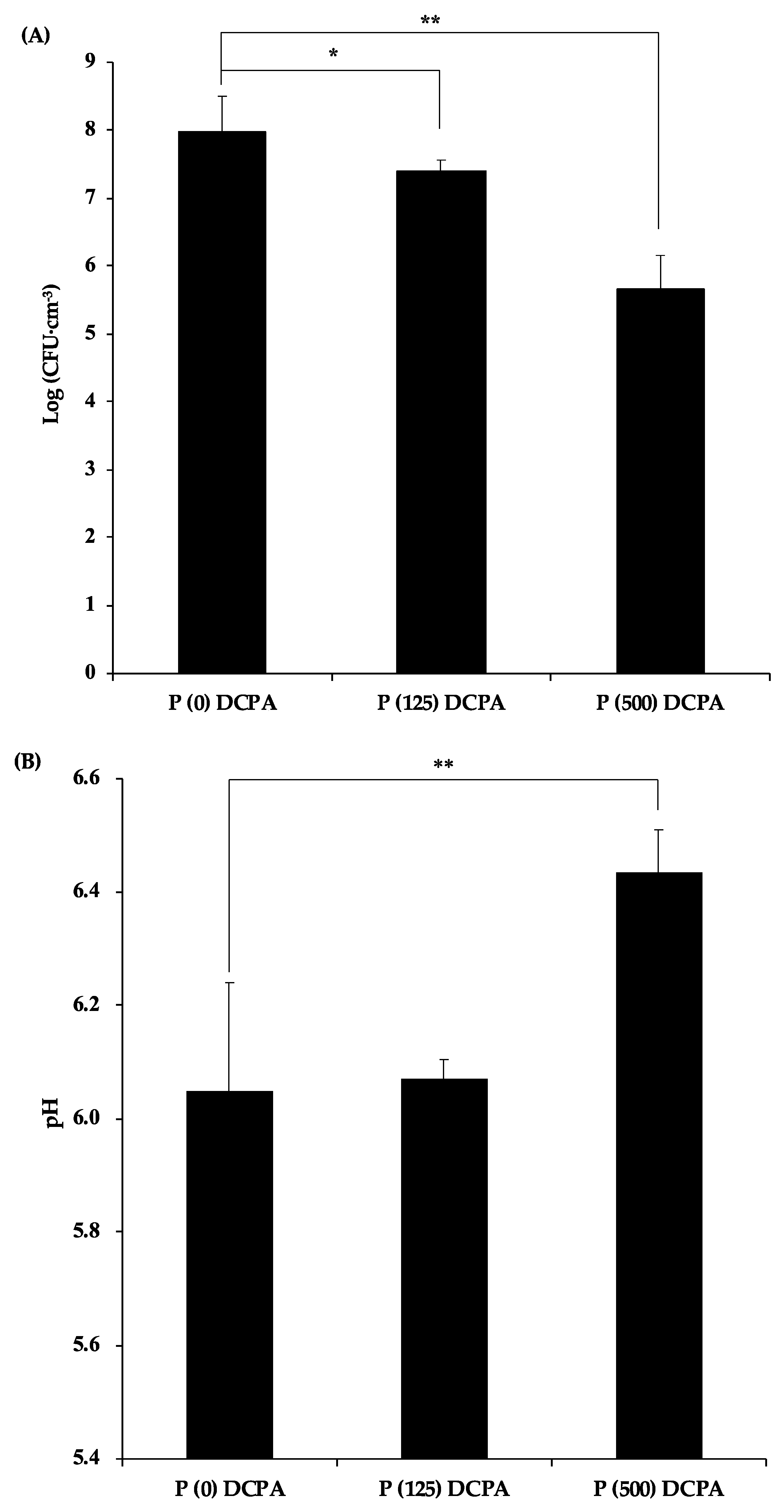
3.2. Antibacterial Activity of P-DCPA Discs against S. mutans
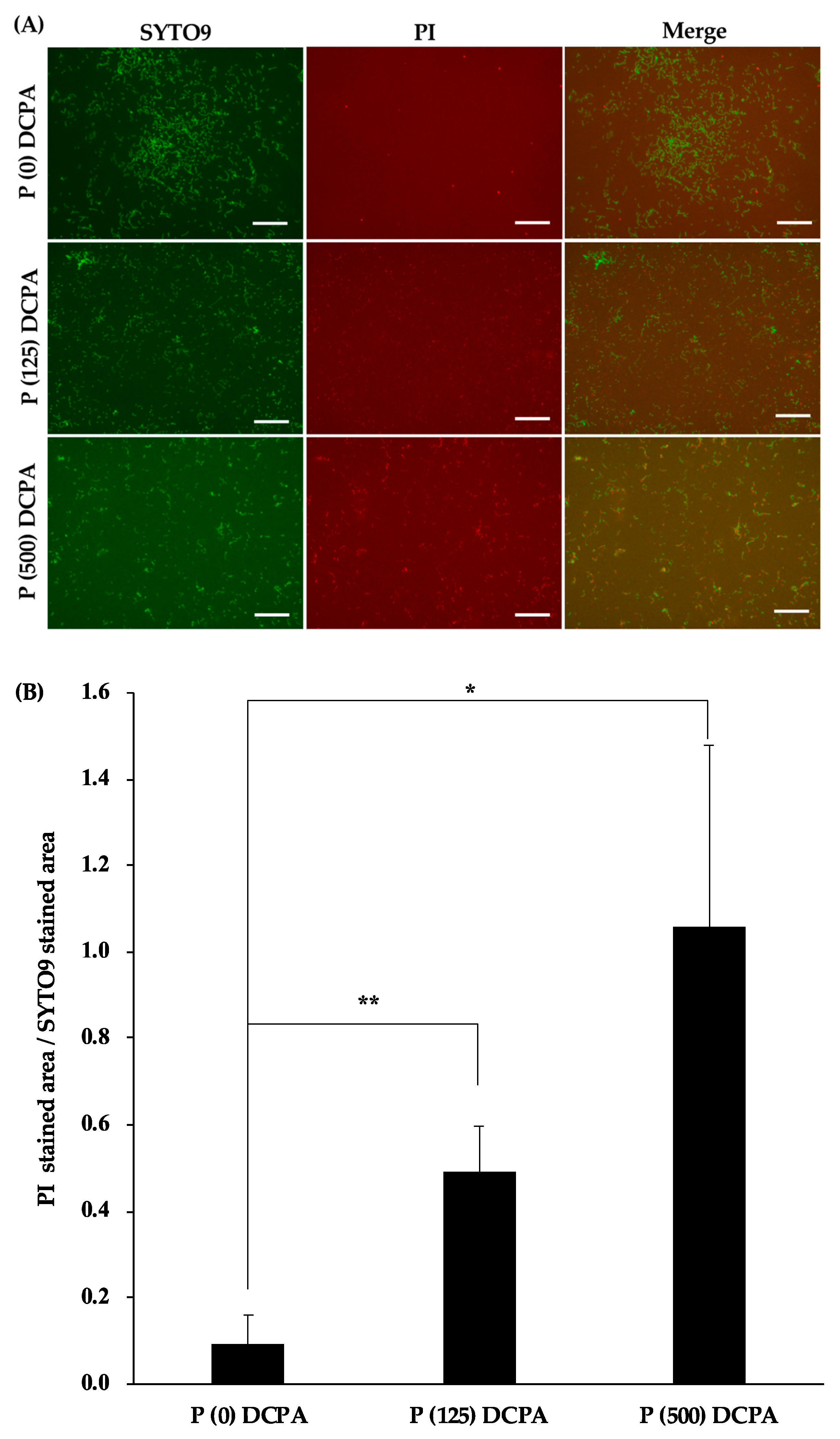
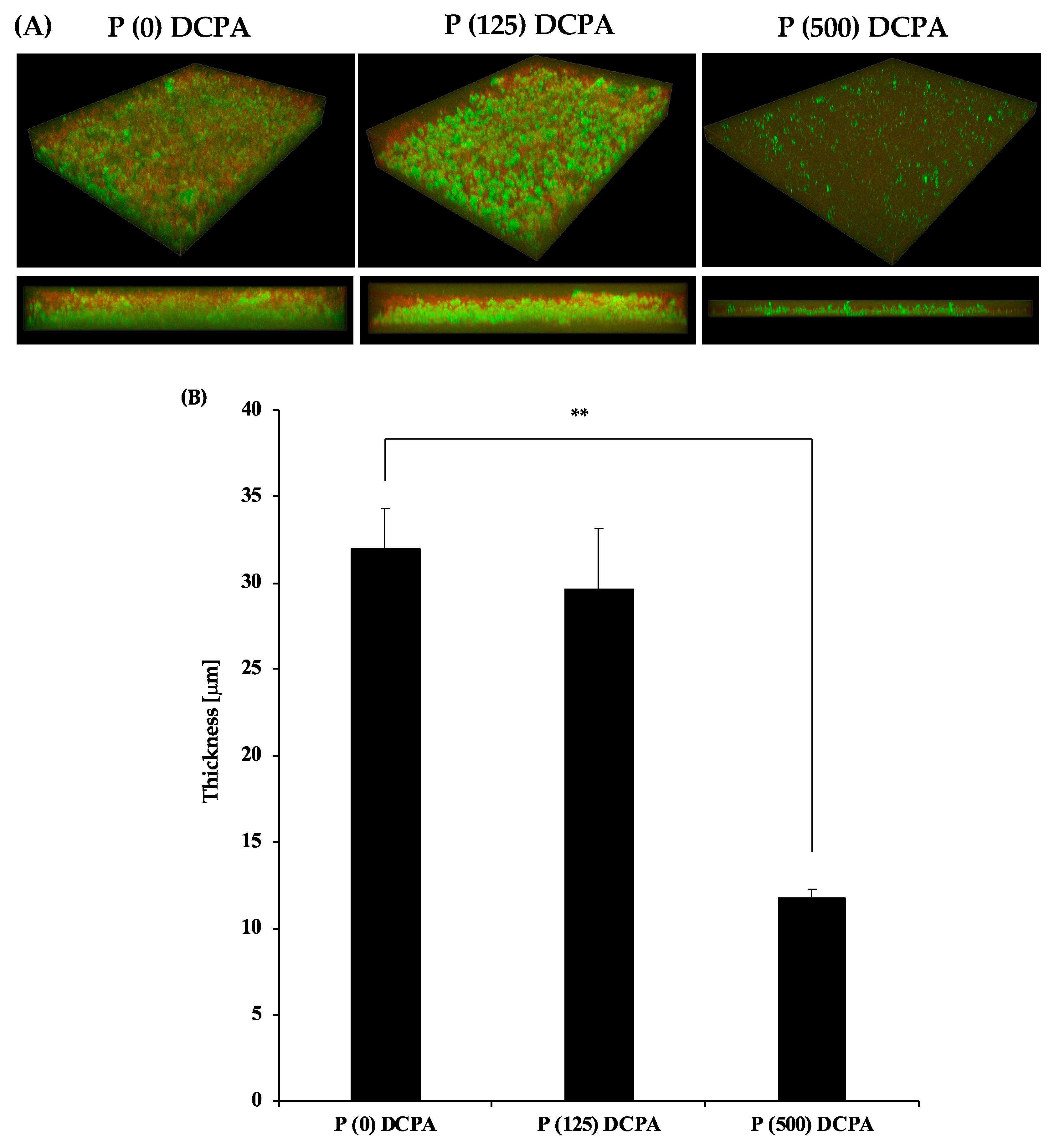
3.3. Biofilm Formation on the P-DCPA Discs
3.4. The Effect of pH against Antimicrobial Activity of Protamine
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kim, Y.-H.; Kim, S.M.; Lee, S.Y. Antimicrobial activity of protamine against oral microorganisms. Biocontrol Sci. 2015, 20, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Potter, R.; Truelstrup Hansen, L.; Gill, T.A. Inhibition of foodborne bacteria by native and modified protamine: Importance of electrostatic interactions. Int. J. Food Microbiol. 2005, 103, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.; Karal, M.A.S.; Levadnyy, V.; Yamazaki, M. Mechanism of Initial Stage of Pore Formation Induced by Antimicrobial Peptide Magainin 2. Langmuir 2018, 34, 3349–3362. [Google Scholar] [CrossRef]
- Seo, M.D.; Won, H.S.; Kim, J.H.; Mishig-Ochir, T.; Lee, B.J. Antimicrobial peptides for therapeutic applications: A Review. Molecules 2012, 17, 12276–12286. [Google Scholar] [CrossRef] [PubMed]
- Thanatvarakorn, O.; Nakashima, S.; Sadr, A.; Prasansuttiporn, T.; Thitthaweerat, S.; Tagami, J. Effect of a calcium-phosphate based desensitizer on dentin surface characteristics. Dent. Mater. J. 2013, 32, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Pei, D.; Huang, C.; Liu, Y.; Du, X.; Sun, H. Effect of desensitising paste containing 8% arginine and calcium carbonate on biofilm formation of Streptococcus mutans in vitro. J. Dent. 2013, 41, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Shiau, H.J. Dentin Hypersensitivity. J. Evid. Based Dent. Pract. 2012, 12, 220–228. [Google Scholar] [CrossRef]
- Yu, J.; Yang, H.; Li, K.; Ren, H.; Lei, J.; Huang, C. Development of Epigallocatechin-3-gallate-Encapsulated Nanohydroxyapatite/Mesoporous silica for Therapeutic Management of Dentin Surface. ACS Appl. Mater. Interfaces 2017, 9, 25796–25807. [Google Scholar] [CrossRef]
- Matsumoto-Nakano, M. Role of Streptococcus mutans surface proteins for biofilm formation. Jpn. Dent. Sci Rev. 2018, 54, 22–29. [Google Scholar] [CrossRef]
- Hansen, L.T.; Gill, T.A. Solubility and antimicrobial efficacy of protamine on Listeria monocytogenes and Escherichia coli as influenced by pH. J. Appl. Microbiol. 2000, 88, 1049–1055. [Google Scholar] [CrossRef]
- Klein, M.I.; Duarte, S.; Xiao, J.; Mitra, S.; Foster, T.H.; Koo, H. Structural and molecular basis of the role of starch and sucrose in Streptococcus mutans biofilm development. Appl. Environ. Microbiol. 2009, 75, 837–841. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, E.C.; Wong, A. Effect of adsorbed protein on hydroxyapatite zeta potential and Streptococcus mutans adherence. Infect. Immun. 1983, 39, 1285–1290. [Google Scholar] [PubMed]
- Abranches, J.; Zeng, L.; Kajfasz, J.K.; Palmer, S.R.; Chakraborty, B.; Wen, Z.T.; Richards, V.P.; Brady, L.J.; Lemos, J.A. Biology of Oral Streptococci. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Porto, I.C.C.M.; Andrade, A.K.M.; Montes, M.A.J.R. Diagnosis and treatment of dentinal hypersensitivity. J. Oral Sci. 2009, 51, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Guentsch, A.; Seidler, K.; Nietzsche, S.; Hefti, A.F.; Preshaw, P.M.; Watts, D.C.; Jandt, K.D.; Sigusch, B.W. Biomimetic mineralization: Long-term observations in patients with dentin sensitivity. Dent. Mater. 2012, 28, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Thanatvarakorn, O.; Nakashima, S.; Sadr, A.; Prasansuttiporn, T.; Ikeda, M.; Tagami, J. In vitro evaluation of dentinal hydraulic conductance and tubule sealing by a novel calcium-phosphate desensitizer. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, K.; Asaoka, K. Estimation of Ideal Mechanical Strength and Critical Porosity of Calcium Phosphate Cement. J. Biomed. Mater. Res. 1995, 29, 1537–1543. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.J.; Kim, Y.M.; Kwon, Y.H.; Kim, I.R.; Park, B.S.; Son, W.S.; Lee, S.M.; Kim, Y.I. Enamel Surface Remineralization Effect by Fluorinated Graphite and Bioactive Glass-Containing Orthodontic Bonding Resin. Materials 2019, 12, 1308. [Google Scholar] [CrossRef] [PubMed]
- Tamara, F.R.; Lin, C.; Mi, F.L.; Ho, Y.C. Antibacterial Effects of Chitosan/Cationic Peptide Nanoparticles. Nanomaterials 2018, 8, 88. [Google Scholar] [CrossRef] [PubMed]
- Kandori, K.; Oda, S.; Fukusumi, M.; Morisada, Y. Synthesis of positively charged calcium hydroxyapatite nano-crystals and their adsorption behavior of proteins. Coll. Surf. B Biointerfaces 2009, 73, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Tarafder, S.; Banerjee, S.; Bandyopadhyay, A.; Bose, S. Electrically polarized biphasic calcium phosphates: Adsorption and release of bovine serum albumin. Langmuir 2010, 26, 16625–16629. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.B.; Shi, X.; Mao, J.; Gong, S.Q. Design of a hydroxyapatite-binding antimicrobial peptide with improved retention and antibacterial efficacy for oral pathogen control. Sci. Rep. 2016, 6, 38410. [Google Scholar] [CrossRef] [PubMed]
- Kugler, R.; Bouloussa, O.; Rondelez, F. Evidence of a charge-density threshold for optimum efficiency of biocidal cationic surfaces. Microbiology 2005, 151, 1341–1348. [Google Scholar] [CrossRef] [PubMed]
- Islam, N.M.D.; Oda, H.; Motohiro, T. Changes in the Cell Morphology and the Release of Soluble Constituents from the Washed Cells of Bacillus subtilis by the Action of Protamine. Nippon Suisan. Gakkaishi 1987, 53, 297–303. [Google Scholar] [CrossRef][Green Version]
- Johansen, C.; Verheul, A.; Gram, L.; Gill, T.; Abee, T. Protamine-induced permeabilization of cell envelopes of gram-positive and gram-negative bacteria. Appl. Environ. Microbiol. 1997, 63, 1155–1159. [Google Scholar]
- Pink, D.A.; Hasan, F.M.; Quinn, B.E.; Winterhalter, M.; Mohan, M.; Gill, T.A. Interaction of protamine with gram-negative bacteria membranes: Possible alternative mechanisms of internalization in Escherichia coli, Salmonella typhimurium and Pseudomonas aeruginosa. J. Pept. Sci. 2014, 20, 240–250. [Google Scholar] [CrossRef]
- Pink, D.A.; Truelstrup Hansen, L.; Gill, T.A.; Quinn, B.E.; Jericho, M.H.; Beveridge, T.J. Divalent Calcium Ions Inhibit the Penetration of Protamine through the Polysaccharide Brush of the Outer Membrane of Gram-Negative Bacteria. Langmuir 2003, 19, 8852–8858. [Google Scholar] [CrossRef]
- Rodrigues, M.C.; Natale, L.C.; Arana-Chaves, V.E.; Braga, R.R. Calcium and phosphate release from resin-based materials containing different calcium orthophosphate nanoparticles. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 103, 1670–1678. [Google Scholar] [CrossRef]
- Xu, H.; Weir, M.; Sun, L. Nanocomposites with Ca and PO4 release: Effects of reinforcement, dicalcium phosphate particle size and silanization. Dent. Mater. 2007, 23, 1482–1491. [Google Scholar] [CrossRef]

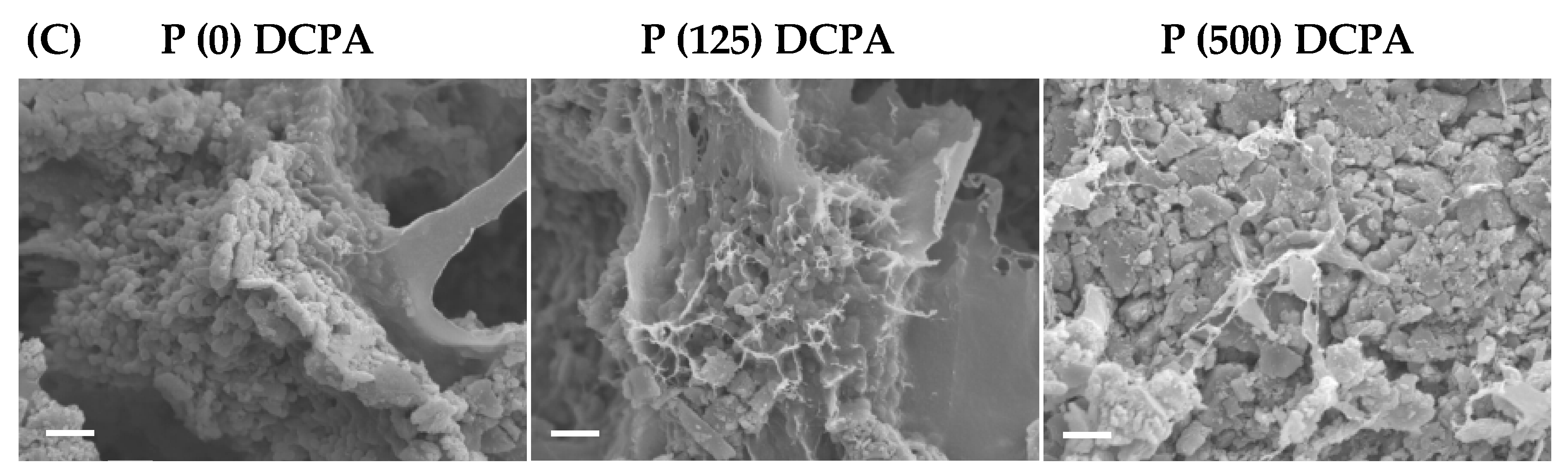
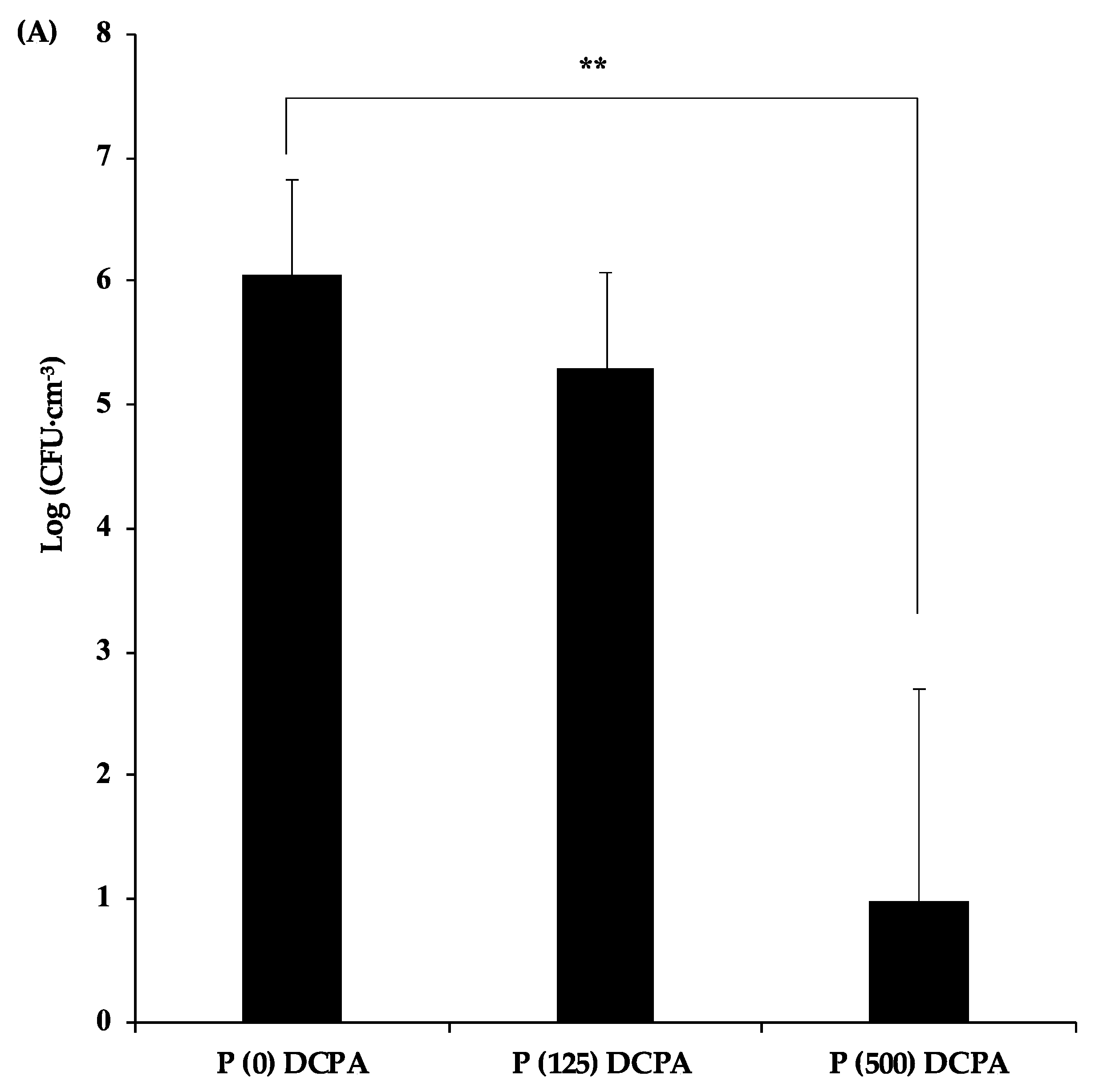
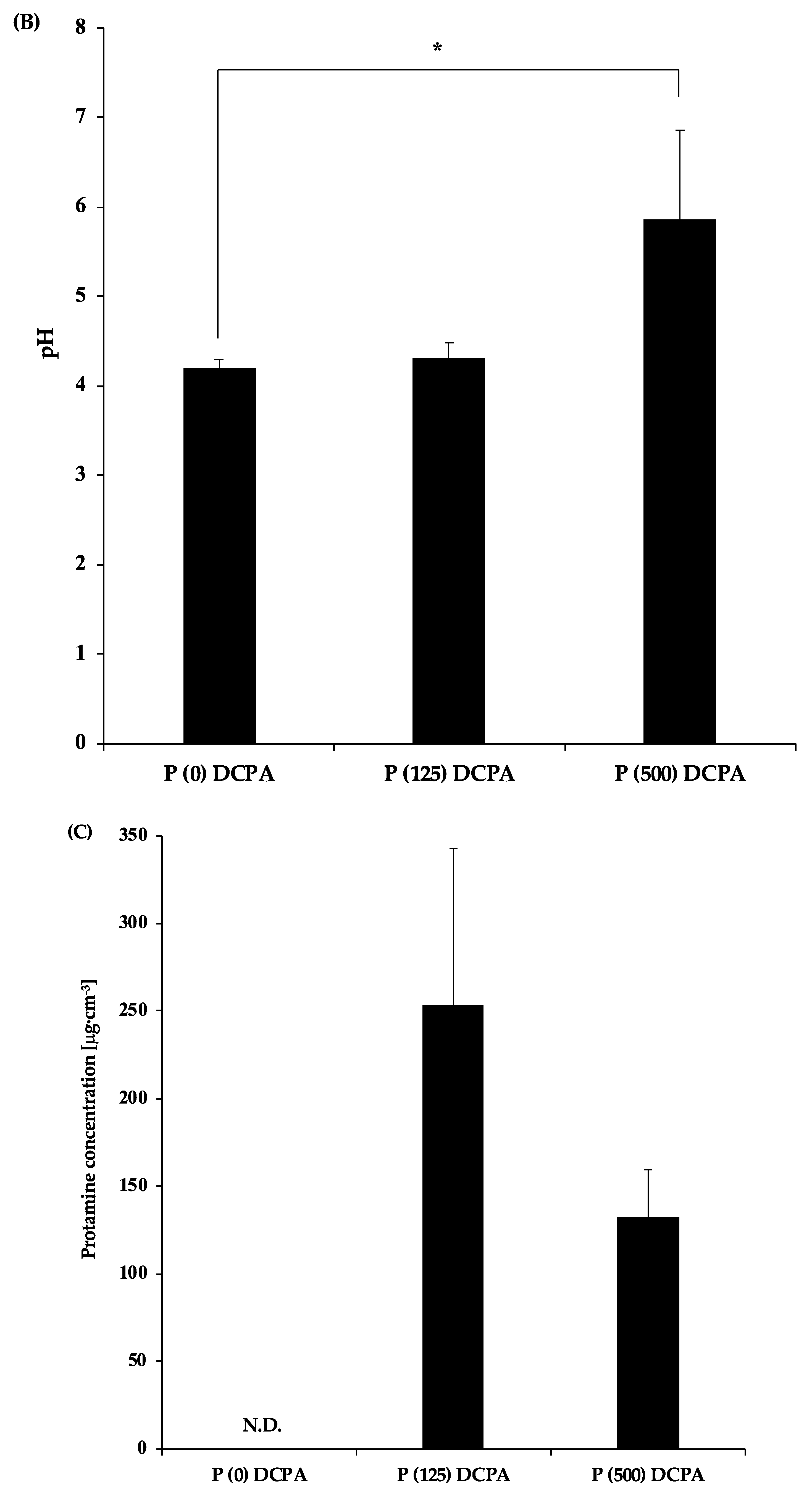


| Sample | Charged-Protamine | Loaded-Protamine | Zeta Potential | Median Size |
|---|---|---|---|---|
| μg·cm−3 | mg·m−2 | mV | μm | |
| P (0) DCPA | 0 | 0 | −22.34 ± 2.36 | 1.596 ± 0.166 |
| P (125) DCPA | 125 | 0.289 ± 0.022 | 2.95 ± 2.11 | 1.792 ± 0.157 |
| P (500) DCPA | 500 | 0.632 ± 0.003 | 19.02 ± 3.23 | 1.888 ± 0.271 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujiki, M.; Abe, K.; Hayakawa, T.; Yamamoto, T.; Torii, M.; Iohara, K.; Koizumi, D.; Togawa, R.; Aizawa, M.; Honda, M. Antimicrobial Activity of Protamine-Loaded Calcium Phosphates against Oral Bacteria. Materials 2019, 12, 2816. https://doi.org/10.3390/ma12172816
Fujiki M, Abe K, Hayakawa T, Yamamoto T, Torii M, Iohara K, Koizumi D, Togawa R, Aizawa M, Honda M. Antimicrobial Activity of Protamine-Loaded Calcium Phosphates against Oral Bacteria. Materials. 2019; 12(17):2816. https://doi.org/10.3390/ma12172816
Chicago/Turabian StyleFujiki, Masashi, Kodai Abe, Tohru Hayakawa, Takatsugu Yamamoto, Mana Torii, Keishi Iohara, Daisuke Koizumi, Rie Togawa, Mamoru Aizawa, and Michiyo Honda. 2019. "Antimicrobial Activity of Protamine-Loaded Calcium Phosphates against Oral Bacteria" Materials 12, no. 17: 2816. https://doi.org/10.3390/ma12172816
APA StyleFujiki, M., Abe, K., Hayakawa, T., Yamamoto, T., Torii, M., Iohara, K., Koizumi, D., Togawa, R., Aizawa, M., & Honda, M. (2019). Antimicrobial Activity of Protamine-Loaded Calcium Phosphates against Oral Bacteria. Materials, 12(17), 2816. https://doi.org/10.3390/ma12172816





