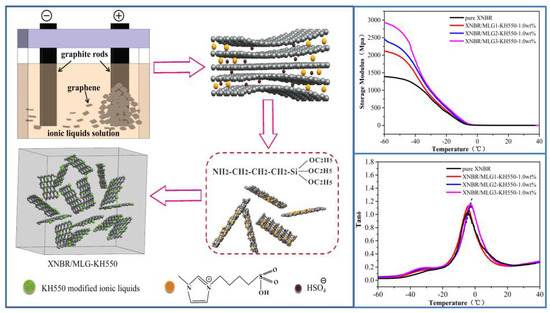
Improving the Dynamic Mechanical Properties of XNBR Using ILs/KH550-Functionalized Multilayer Graphene
Abstract
1. Introduction
2. Experimental
2.1. Materials
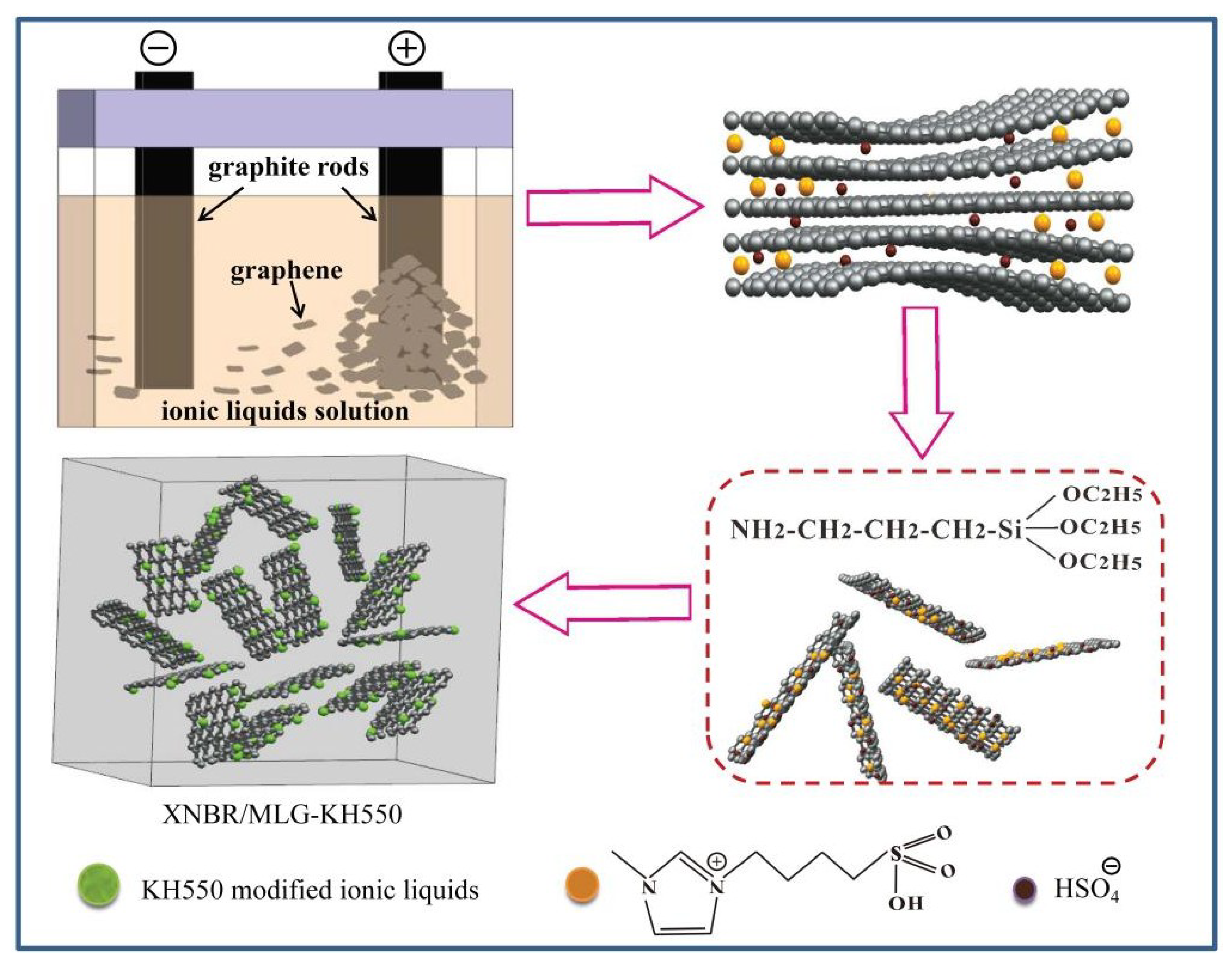
2.2. Surface Functionalization of Multilayer Graphene by (BSO3HMIm)(HSO4) and Silane Grafting
2.3. Characterization of Functionalized Multilayer Graphene
2.4. Preparation of Functionalized Multilayer Graphene Hybrid XNBR
3. Results and Discussion
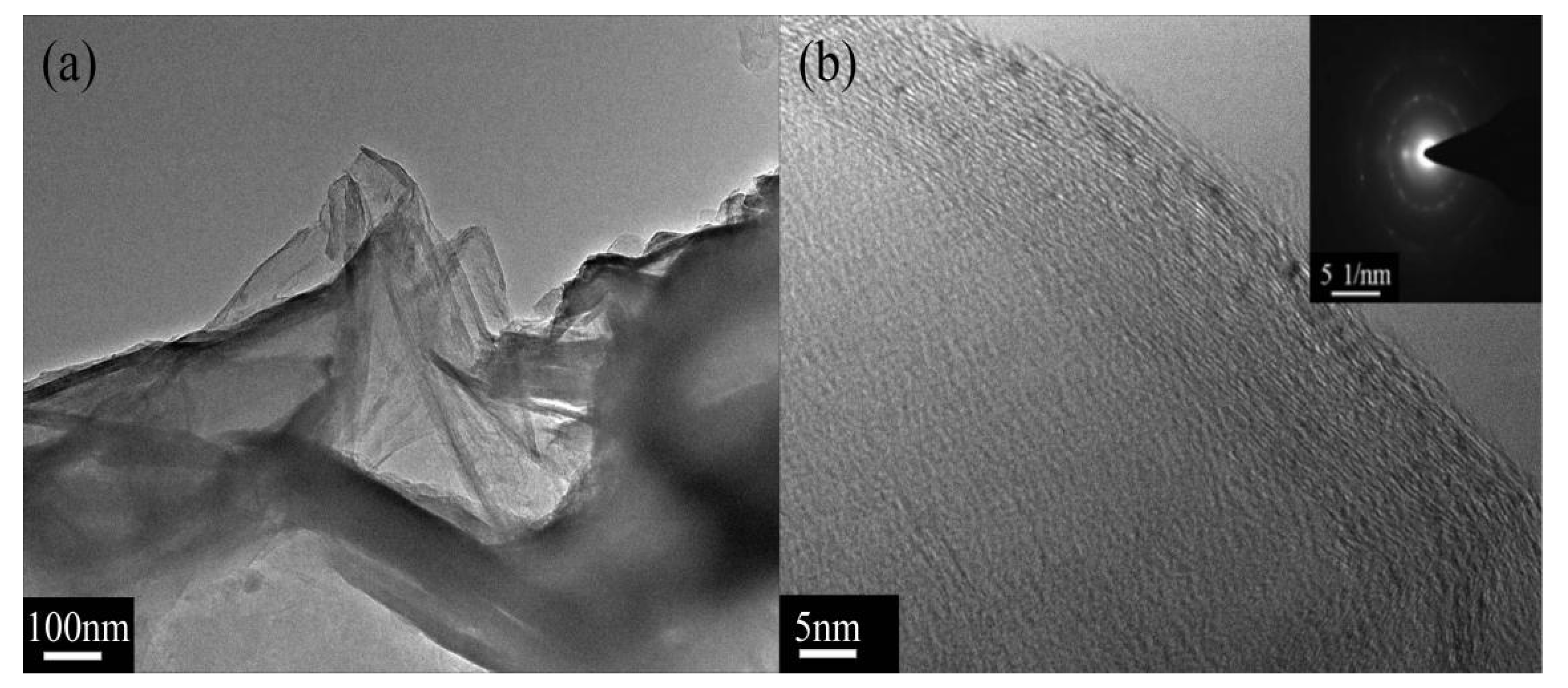
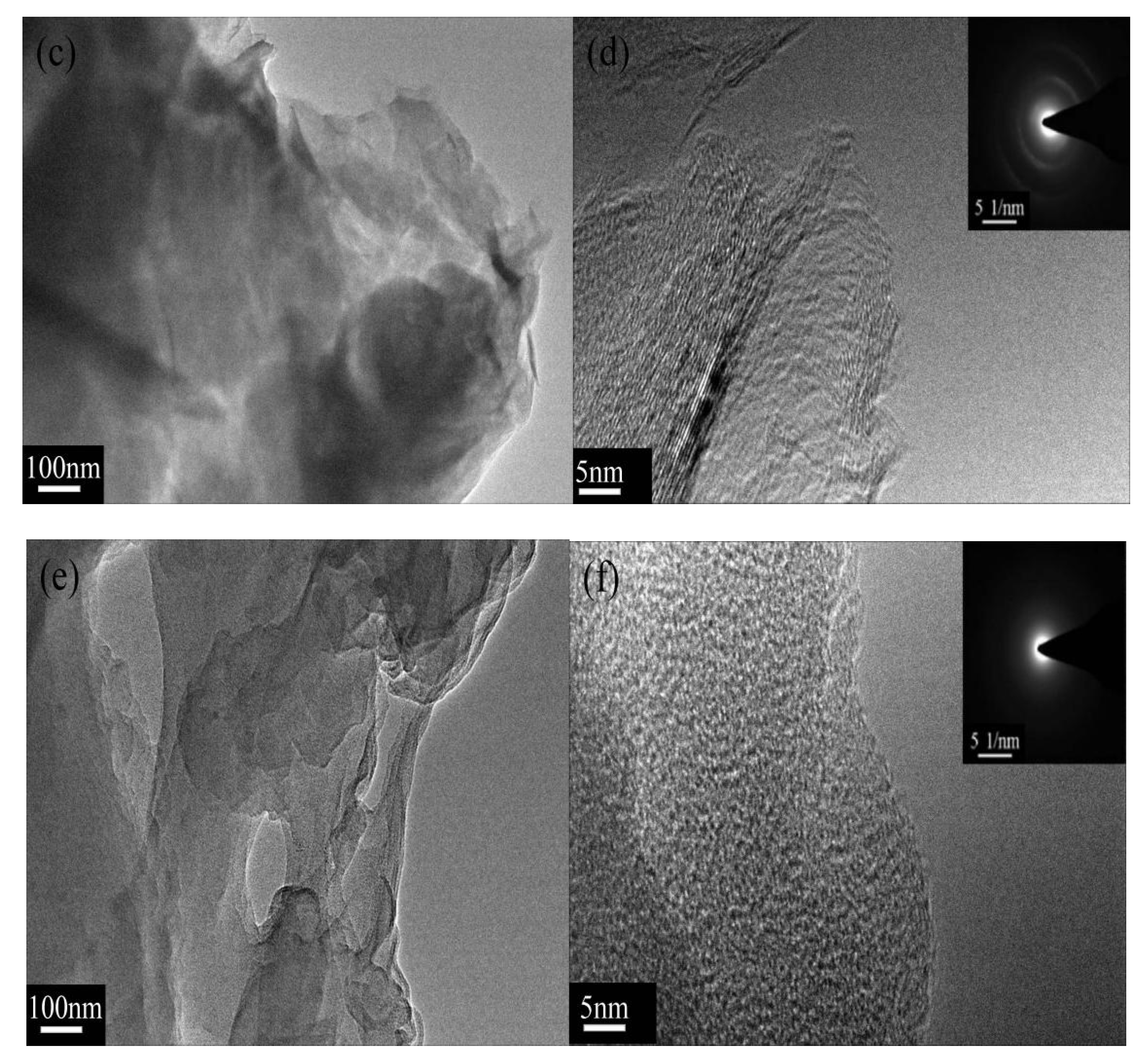
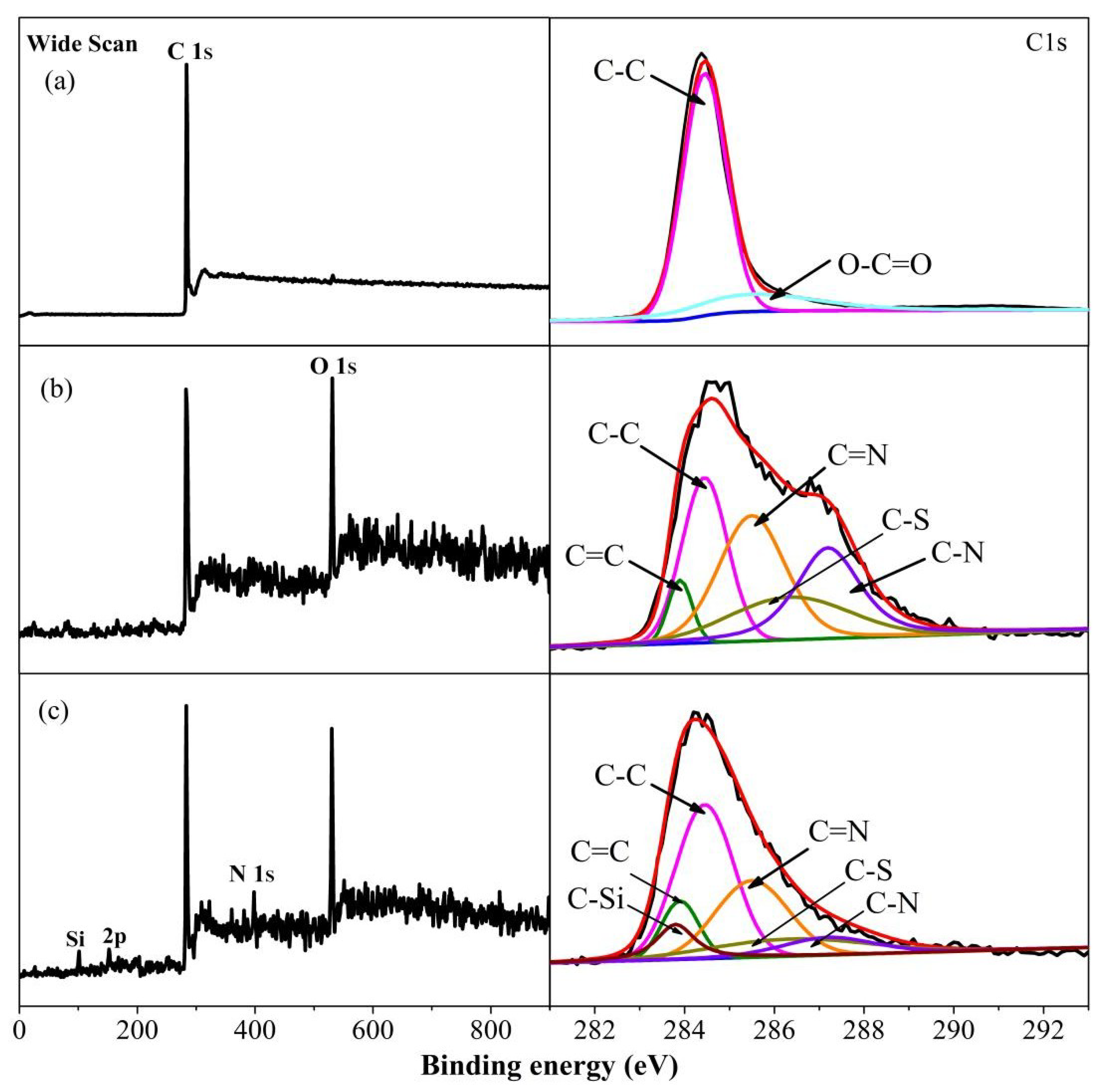
3.1. Surface Functionalization of Multilayer Graphene
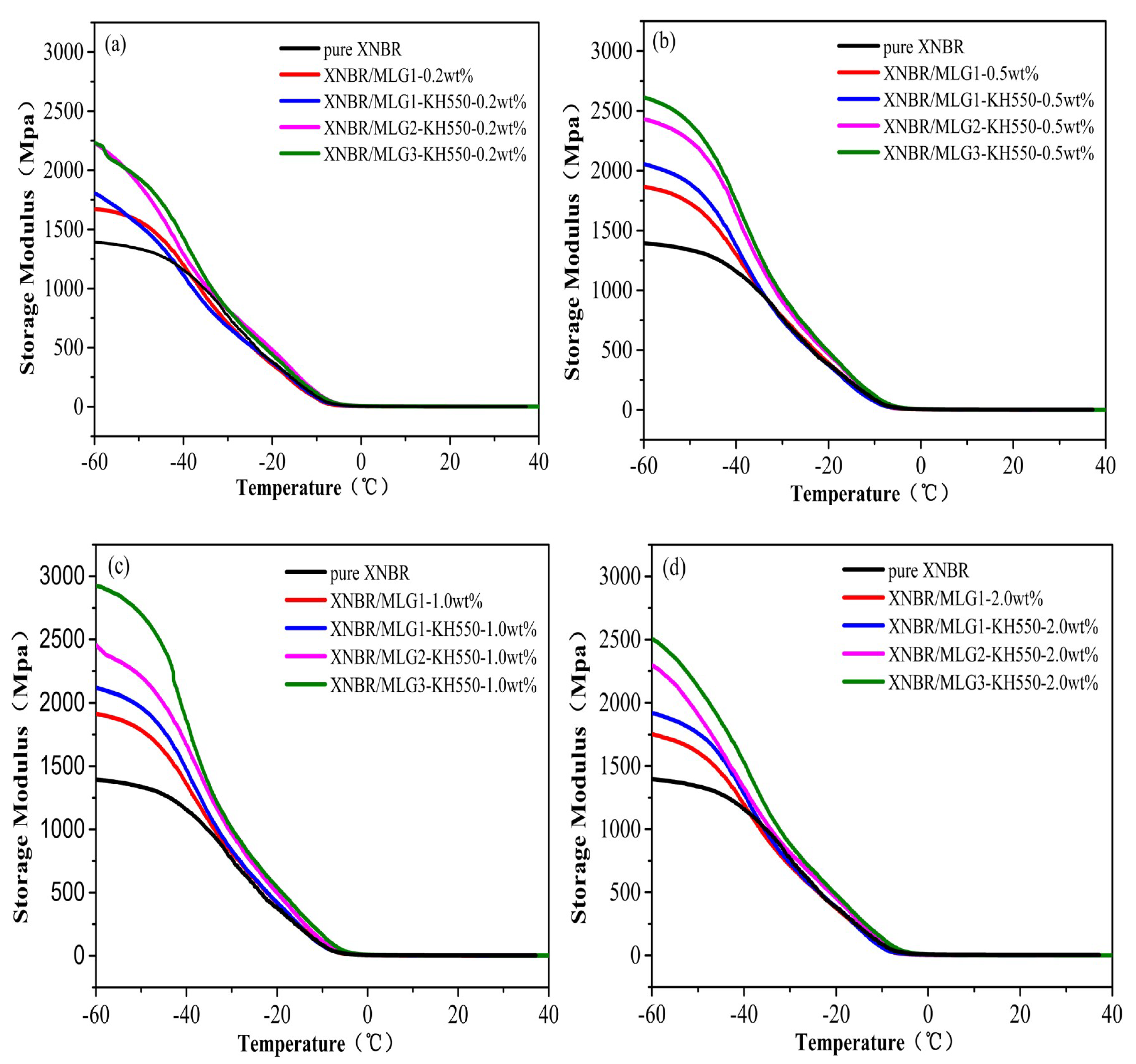
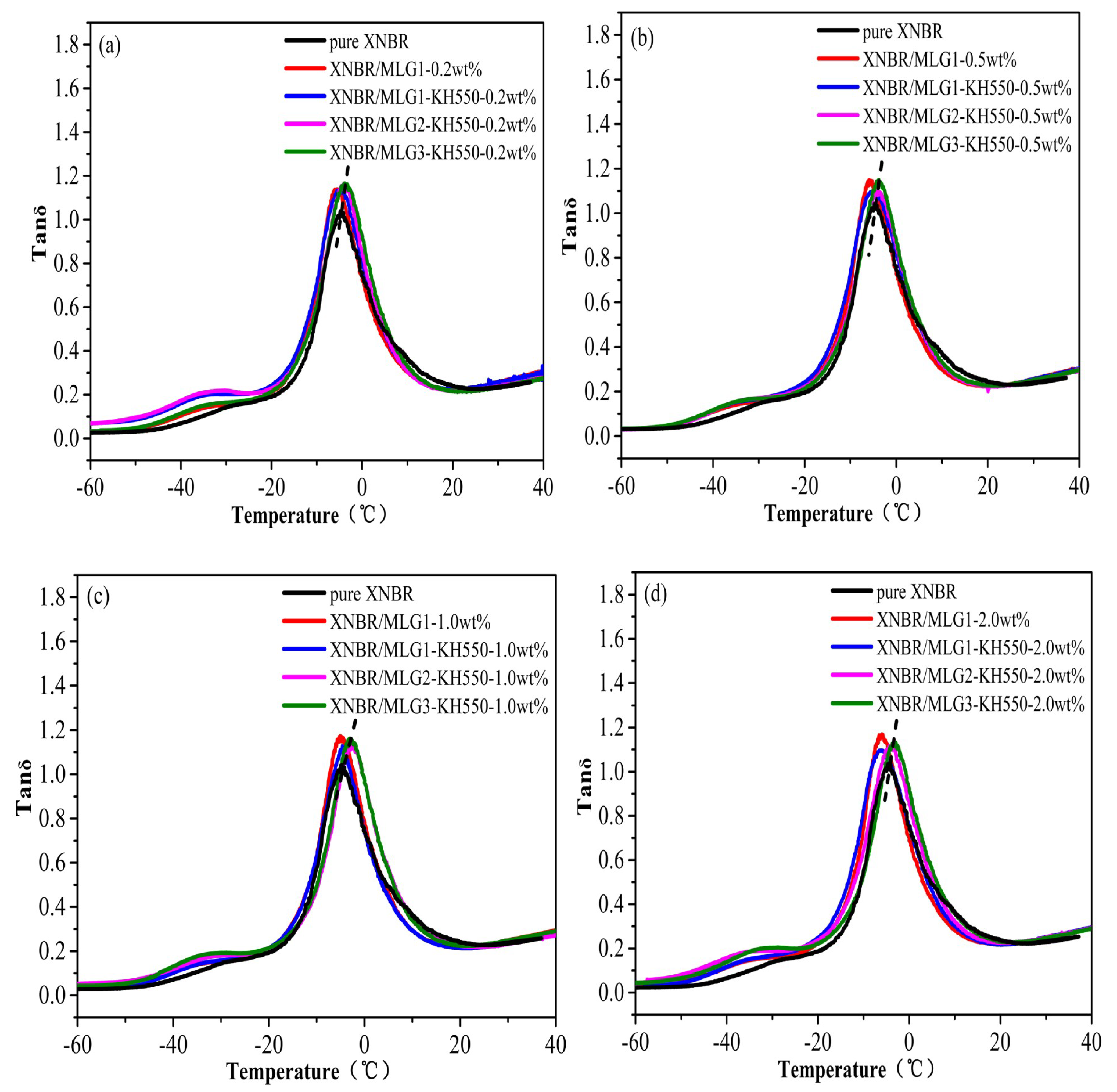
3.2. Dynamic Mechanical Properties
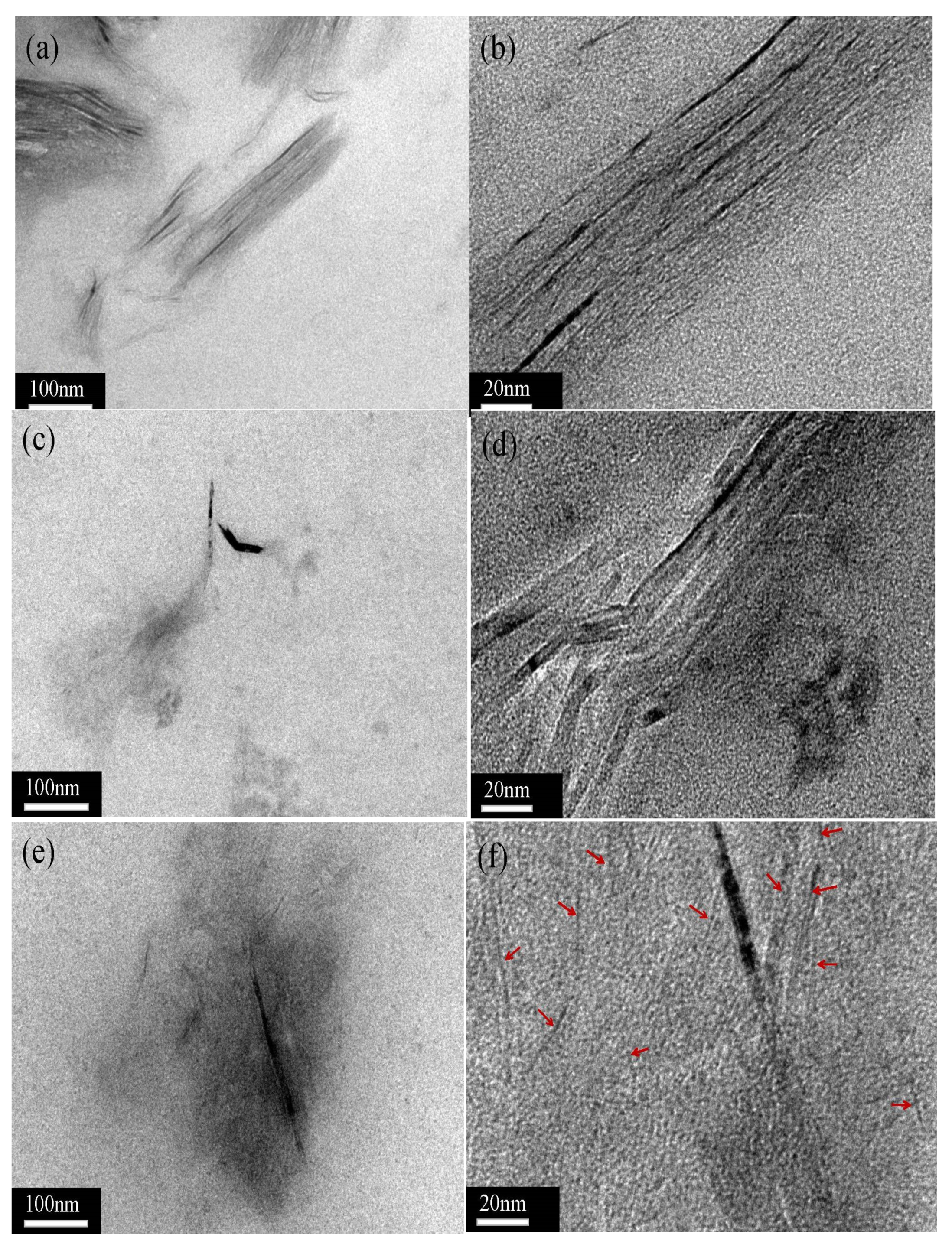
3.3. Morphology of XNBR Composites
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lv, X.S.; Huang, Z.X.; Huang, C.; Shi, M.X.; Gao, G.B.; Gao, Q.Q. Damping properties and the morphology analysis of the polyurethane/epoxy continuous gradient IPN materials. Compos. Part. B Eng. 2016, 88, 139–149. [Google Scholar] [CrossRef]
- Sasikumar, K.; Manoj, N.R.; Mukundan, T.; Khastgir, D. Hysteretic damping in XNBR−MWNT nanocomposites at low and high compressive strains. Part. B Eng. 2016, 92, 74–83. [Google Scholar] [CrossRef]
- Suhr, J.; Nikhil, K.; Pawel, K.; Pulickel, A. Viscoelasticity in carbon nanotube composites. Nat. Mater. 2005, 2, 134–137. [Google Scholar]
- Chen, B.Y.; Ma, N.; Xin, B.; Zhang, H.M.; Zhang, Y. Effects of graphene oxide on surface energy, mechanical, damping and thermal properties of ethylene-propylene-diene rubber/petroleum resin blends. RSC Adv. 2012, 2, 4683–4689. [Google Scholar] [CrossRef]
- Liu, S.; Chen, F.; Zhang, Y.H.; Shen, Q.; Huang, Z.X.; Kemp, K.C.; Zhang, L.M. Interfacial bond dependence of damping properties of carbon nanotubes enhanced polymers. Polym. Compos. 2014, 35, 548–556. [Google Scholar] [CrossRef]
- Li, B.; Olson, E.; Perugini, A.; Zhong, W.H. Simultaneous enhancements in damping and static dissipation capability of polyetherimide composites with organosilane surface modified graphene nanoplatelets. Polymer 2011, 52, 5606–5614. [Google Scholar] [CrossRef]
- Zhang, C.M.; Chen, Y.J.; Li, H.; Liu, H.Z. Facile fabrication of polyurethane/ epoxy IPNs filled graphene aerogel with improved damping, thermal and mechanical properties. RSC Adv. 2018, 8, 27390–27399. [Google Scholar] [CrossRef]
- Jin, Z.; Lomeda, J.R.; Price, B.K.; Lu, W.; Zhu, Y.; Tour, J.M. Mechanically assisted exfoliation and functionalization of thermally converted graphene sheets. Chem. Mater. 2009, 21, 3045–3047. [Google Scholar] [CrossRef]
- Sharma, R.; Baik, J.H.; Perera, C.J.; Strano, M.S. Anomalously large reactivity of single graphene layers and edges toward electron transfer chemistries. Nano Lett. 2010, 10, 398–405. [Google Scholar] [CrossRef]
- Md, Z.H.; Walsh, M.A.; Hersam, M.C. Scanning tunneling microscopy, spectroscopy, and nanolithography of epitaxial graphene chemically modified with aryl moieties. J. Am. Chem. Soc. 2010, 132, 15399–15403. [Google Scholar]
- Strom, T.A.; Dillon, E.P.; Hamilton, C.E.; Barron, A.R. Nitrene addition to exfoliated graphene: A one-step route to highly functionalized graphene. Chem. Commun. 2010, 46, 4097–4099. [Google Scholar] [CrossRef] [PubMed]
- Vadukumpully, S.J.; Paul, J.; Manta, N.; Valiyaveettil, S. Flexible conductive graphene/poly(vinyl chloride) composite thin films with high mechanical strength and thermal stability. Carbon 2011, 49, 198–205. [Google Scholar] [CrossRef]
- He, H.K.; Gao, C. General approach to individually dispersed, highly soluble, and conductive graphene nanosheets functionalized by nitrene chemistry. Chem. Mater. 2010, 22, 5054–5064. [Google Scholar] [CrossRef]
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.T.; Holland, B.; Byrne, M.; Gun’Ko, Y.K.; et al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol. 2008, 3, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.P.; Deepa, M.; Joshi, A.G.; Bhandari, S.; Srivastava, A.K. Poly(3,4-ethylenedioxythiophene)-ionic liquid functionalized graphene/reduced graphene oxide nanostructures: Improved conduction and electrochromism. ACS Appl. Mater. Interfaces 2011, 3, 1115–1126. [Google Scholar] [CrossRef] [PubMed]
- An, X.H.; Butler, T.W.; Washington, M.; Nayak, S.K.; Kar, S. Optical and sensing properties of 1-pyrenecarboxylic acid-functionalized graphene films laminated on polydimethylsiloxane membranes. ACS Nano 2011, 5, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.X.; Bai, H.; Lu, G.W.; Li, C.; Shi, G.Q. Flexible graphene films via the filtration of water-soluble noncovalent functionalized graphene sheets. J. Am. Chem. Soc. 2008, 130, 5856–5857. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.C.; Shiue, R.J.; Tsai, C.C.; Wang, W.H.; Chen, Y.T. High-quality graphene p-n junctions via resist-free fabrication and solution-based noncovalent functionalization. ACS Nano. 2011, 5, 2051–2059. [Google Scholar] [CrossRef]
- Lu, J.; Yang, J.X.; Wang, J.Z.; Lim, A.; Wang, S.; Loh, K.P. One-pot synthesis of fluorescent carbon nanoribbons, nanoparticles, and graphene by the exfoliation of graphite in ionic liquids. ACS Nano. 2009, 3, 2367–2375. [Google Scholar] [CrossRef]
- Liu, N.; Luo, F.; Wu, H.X.; Liu, Y.H.; Zhang, C.; Chen, J. One-step ionic-liquid-assisted electrochemical synthesis of ionic-liquid-functionalized graphene sheets directly from graphite. Adv. Funct. Mater. 2008, 18, 1518–1525. [Google Scholar] [CrossRef]
- Hana, P.; Zhang, H.M.; Qiu, X.P.; Ji, X.L.; Gao, L.X. Palladium within ionic liquid functionalized mesoporous silica SBA-15 and its catalytic application in room-temperature Suzuki coupling reaction. J. Mol. Catal. A-Chem. 2008, 295, 57–67. [Google Scholar] [CrossRef]
- Shim, H.L.; Udayakumar, S.; Yu, J.I.; Kim, I.; Park, D.W. Synthesis of cyclic carbonate from allyl glycidyl ether and carbon dioxide using ionic liquid-functionalized amorphous silica. Catal. Today 2009, 148, 350–354. [Google Scholar] [CrossRef]
- Li, X.Y.; Liu, Y.X.; Zheng, L.C.; Dong, M.J.; Xue, Z.H.; Lu, X.Q.; Liu, X.H. A novel nonenzymatic hydrogen peroxide sensor based on silver nanoparticles and ionic liquid functionalized multiwalled carbon nanotube composite modified electrode. Electrochim. Acta. 2013, 113, 170–175. [Google Scholar] [CrossRef]
- Wang, Z.J.; Zhang, Q.X.; Kuehner, D.; Xu, X.Y.; Ivask, A.; Niu, L. The synthesis of ionic-liquid-functionalized multiwalled carbon nanotubes decorated with highly dispersed Au nanoparticles and their use in oxygen reduction by electrocatalysis. Carbon 2008, 46, 1687–1692. [Google Scholar] [CrossRef]
- Fan, X.Q.; Wang, L.P. Ionic liquids gels with in situ modified multiwall carbon nanotubes towards high-performance lubricants. Tribol. Int. 2015, 88, 179–188. [Google Scholar] [CrossRef]
- Liao, H.G.; Wu, H.; Wang, J.; Liu, J.; Jiang, Y.X.; Sun, S.G.; Lin, Y.H. Direct electrochemistry and electrocatalysis of myoglobin immobilized on graphene-ctab-ionic liquid nanocomposite film. Electroanal 2010, 22, 2297–2302. [Google Scholar] [CrossRef]
- Qiu, Y.Y.; Qu, X.J.; Dong, J.; Ai, S.Y.; Han, R.X. Electrochemical detection of DNA damage induced by acrylamide and its metabolite at the graphene-ionic liquid-Nafion modified pyrolytic graphite electrode. J. Hazard. Mater. 2011, 190, 480–485. [Google Scholar] [CrossRef]
- Chai, J.; Li, F.H.; Hu, Y.W.; Zhang, Q.X.; Hana, D.X.; Niu, L. Hollow flower-like AuPd alloy nanoparticles: one step synthesis, self-assembly on ionic liquid-functionalized graphene, and electrooxidation of formic acid. J. Mater. Chem. 2011, 21, 17922. [Google Scholar] [CrossRef]
- Gu, L.L.; Li, T.; Xu, Y.J.; Sun, C.H.; Yang, Z.Y.; Zhu, D.L.; Chen, D.L. Effects of the particle size of BaTiO3 fillers on fabrication and dielectric properties of BaTiO3/Polymer/Al films for capacitor energy-storage application. Materials 2019, 12, 439. [Google Scholar] [CrossRef]
- Ge, M.L.; Wang, X.B.; Du, M.Y.; Liang, G.D.; Hu, G.Q.; Jahangir Alam, S.M. Adsorption analyses of phenol from aqueous solutions using magadiite modified with organo-functional groups: Kinetic and equilibrium studies. Materials 2019, 12, 96. [Google Scholar] [CrossRef]
- Liu, X.X.; Chen, X.F.; Ren, J.L.; Zhang, C.H. TiO2-KH550 nanoparticle- reinforced PVA/xylan composite films with multifunctional properties. Materials 2018, 11, 1589. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.H.; Yang, Q.S.; Qi, X.D.; Xu, Y.Y.; Zhang, D.S.; Wang, Y.J.; Zhao, X.Q. A novel hydroxylamine ionic liquid salt resulting from the stabilization of NH2OH by a SO3H-functionalized ionic liquid. Chem. Commun. 2012, 51, 1930–1932. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.G.; Chang, Q.; Bao, C.X.; Dai, L.; Zhang, G.Z.; Wu, F.P. Surface modification of filter medium particles with silane coupling agent KH550. Colloid. Surf. A 2013, 434, 276–280. [Google Scholar] [CrossRef]
- Fan, X.Q.; Wang, L.P. High-performance lubricant additives based on modified graphene oxide by ionic liquids. Colloid. Interface Sci. 2015, 452, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.Q.; Wang, L.P.; Li, W. In situ fabrication of low-friction sandwich sheets through functionalized graphene crosslinked by Ionic Liquids. Tribol. Lett. 2015, 58, 1–12. [Google Scholar] [CrossRef]
- Sa, R.; Yan, Y.; Wei, Z.H.; Zhang, L.Q.; Wang, W.C.; Tian, M. Surface modification of aramid fibers by bio-Inspired poly(dopamine) and epoxy functionalized silane grafting. ACS Appl. Mater. Interfaces 2014, 6, 21730–21738. [Google Scholar] [CrossRef] [PubMed]
- Zangmeister, C.D.; Ma, X.F.; Zachariah, M.R. Restructuring of graphene oxide sheets into monodisperse nanospheres. Chem. Mater. 2012, 24, 2554–2557. [Google Scholar] [CrossRef]
- Wu, J.J.; Wu, C.T.; Liao, Y.C.; Lu, T.R.; Chen, L.C.; Chen, K.H.; Hwa, L.G.; Kuo, C.T.; Ling, K.J. Deposition of silicon carbon nitride films by ion beam sputtering. Thin Solid Films. 1999, 355, 417–422. [Google Scholar] [CrossRef]
- Eustatiu, I.G.; Tyliszczak, T.; Cooper, G.; Hitchcock, A.P.; Turci, C.C.; Rocha, A.B.; Barbatti, M.; Bielschowsky, C.E. Experimental and theoretical study of S 2p and C 1s generalized oscillator strengths in CS2. J Electron. Spectrosc. 2007, 156, 158–163. [Google Scholar] [CrossRef]
- Ni, Z.H.; Wang, Y.Y.; Yu, T.; Shen, Z.X. Raman spectroscopy and imaging of graphene. Nano Res. 2008, 1, 273–291. [Google Scholar] [CrossRef]
- Cancado, L.G.; Reina, A.; Kong, J.; Dresselhaus, M.S. Geometrical approach for the study of G’ band in the Raman spectrum of monolayer graphene, bilayer graphene, and bulk graphite. Phys. Rev. B 2008, 77, 245408. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Ni, Z.H.; Yu, T.; Wang, H.M.; Wu, Y.H.; Chen, W.; Wee, A.T.S.; Shen, Z.X. Raman studies of monolayer graphene: the substrate effect. J. Phys. Chem. C 2008, 112, 10637–10640. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef]
- Fu, H.M.; Fan, X.Q.; Li, W.; Zhu, M.H.; Peng, J.F.; Li, H. In situ modified multilayer graphene toward high-performance lubricating additive. RSC Adv. 2017, 7, 24399. [Google Scholar] [CrossRef]
- Xiong, Y.; Chen, G.S.; Guo, S.Y. The preparation of core-shell CaCO3 particles and its effect on mechanical property of PVC composites. J. Appl. Polym. Sci. 2006, 102, 1084–1091. [Google Scholar] [CrossRef]
- Luo, Y.; Zhao, Y.; Cai, J.; Duan, Y.; Du, S. Effect of amino-functionalization on the interfacial adhesion of multi-walled carbon nanotubes/epoxy nanocomposites. Mater. Des. 2012, 33, 405–412. [Google Scholar] [CrossRef]
- Chen, S.S.; Cao, Y.W.; Feng, J.C. Polydopamine as an efficient and robust platform to functionalize carbon fiber for high-performance polymer composites. ACS Appl. Mater. Interfaces 2014, 6, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Brocks, T.; Cioffi, M.O.H.; Voorwald, H.J.C. Effect of fiber surface on flexural strength in carbon fabric reinforced epoxy composites. Appl. Surf. Sci. 2013, 274, 210–216. [Google Scholar] [CrossRef]
- Ning, N.Y.; Ma, Q.; Liu, S.; Tian, M.; Zhang, L.Q.; Nishi, T. Tailoring dielectric and actuated properties of elastomer composites by bioinspired poly(dopamine) encapsulated graphene oxide. ACS Appl. Mater. Interfaces 2015, 7, 10755–10762. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, Y.Z.; Zeng, Z.K.; Zhu, J.R.; Wei, Y.; Li, F.C.; Liu, L. Effect of ZnO nanoparticles doped graphene on static and dynamic mechanical properties of natural rubber composites. Compos. Part A appl. Sci. Manuf. 2015, 70, 35–44. [Google Scholar] [CrossRef]




| Samples | Element Content (at %) | Ratio of Elements | ||||
|---|---|---|---|---|---|---|
| C | O | N | Si | N/O | Si/C | |
| MLG1-KH550 | 78.54 | 12.89 | 3.45 | 5.12 | 0.267 | 0.065 |
| MLG2-KH550 | 69.83 | 17.16 | 6.12 | 6.89 | 0.357 | 0.099 |
| MLG3-KH550 | 68.26 | 17.83 | 6.65 | 7.26 | 0.373 | 0.106 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, D.; Gan, C.; Fan, X.; Zhang, L.; Li, W.; Zhu, M.; Quan, X. Improving the Dynamic Mechanical Properties of XNBR Using ILs/KH550-Functionalized Multilayer Graphene. Materials 2019, 12, 2800. https://doi.org/10.3390/ma12172800
Chen D, Gan C, Fan X, Zhang L, Li W, Zhu M, Quan X. Improving the Dynamic Mechanical Properties of XNBR Using ILs/KH550-Functionalized Multilayer Graphene. Materials. 2019; 12(17):2800. https://doi.org/10.3390/ma12172800
Chicago/Turabian StyleChen, Duoli, Chaoliang Gan, Xiaoqiang Fan, Lin Zhang, Wen Li, Minhao Zhu, and Xin Quan. 2019. "Improving the Dynamic Mechanical Properties of XNBR Using ILs/KH550-Functionalized Multilayer Graphene" Materials 12, no. 17: 2800. https://doi.org/10.3390/ma12172800
APA StyleChen, D., Gan, C., Fan, X., Zhang, L., Li, W., Zhu, M., & Quan, X. (2019). Improving the Dynamic Mechanical Properties of XNBR Using ILs/KH550-Functionalized Multilayer Graphene. Materials, 12(17), 2800. https://doi.org/10.3390/ma12172800