Carbides and Nitrides of Zirconium and Hafnium
Abstract
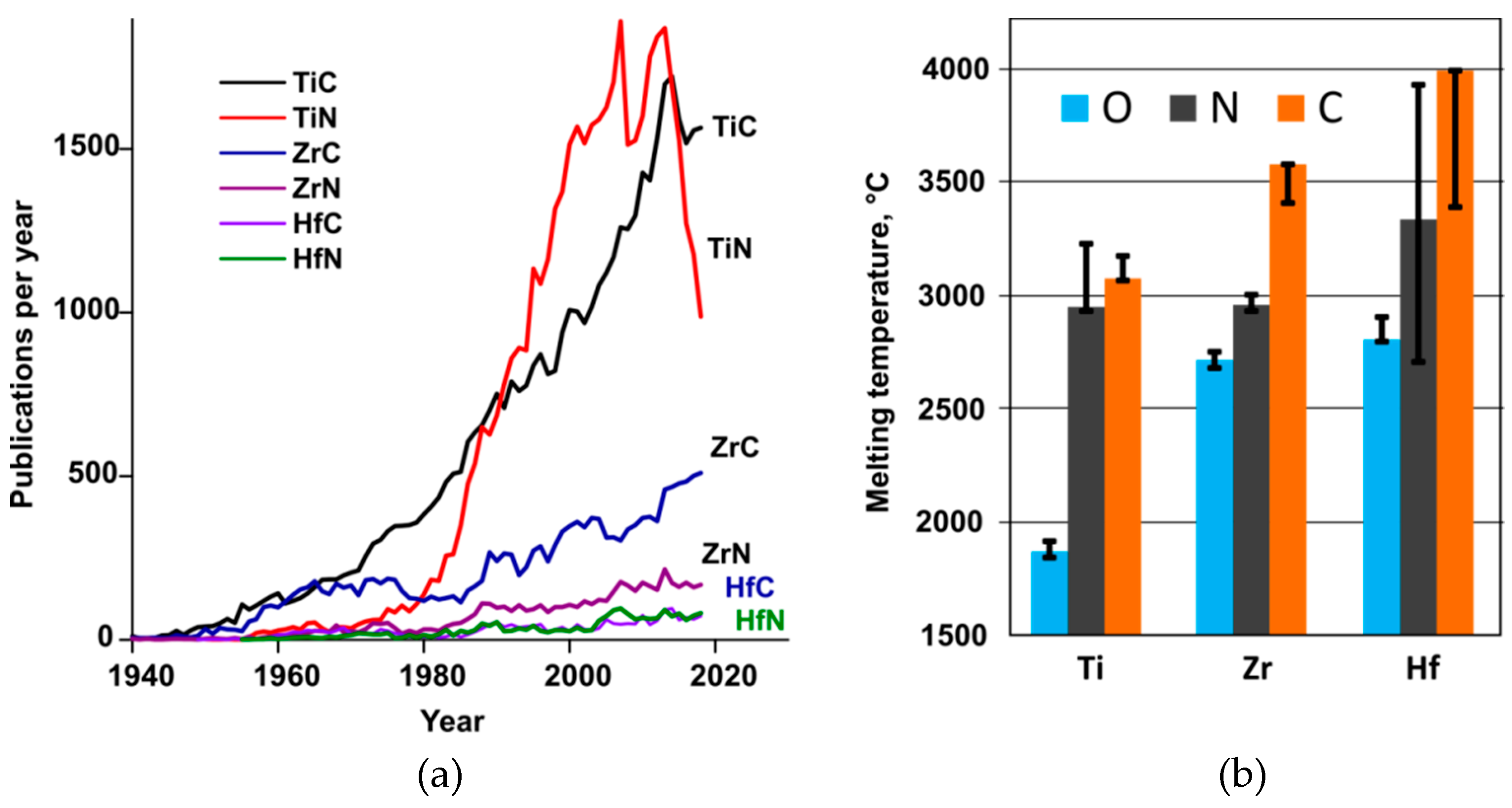
1. Introduction
2. Synthesis Methods
3. Phase Diagrams
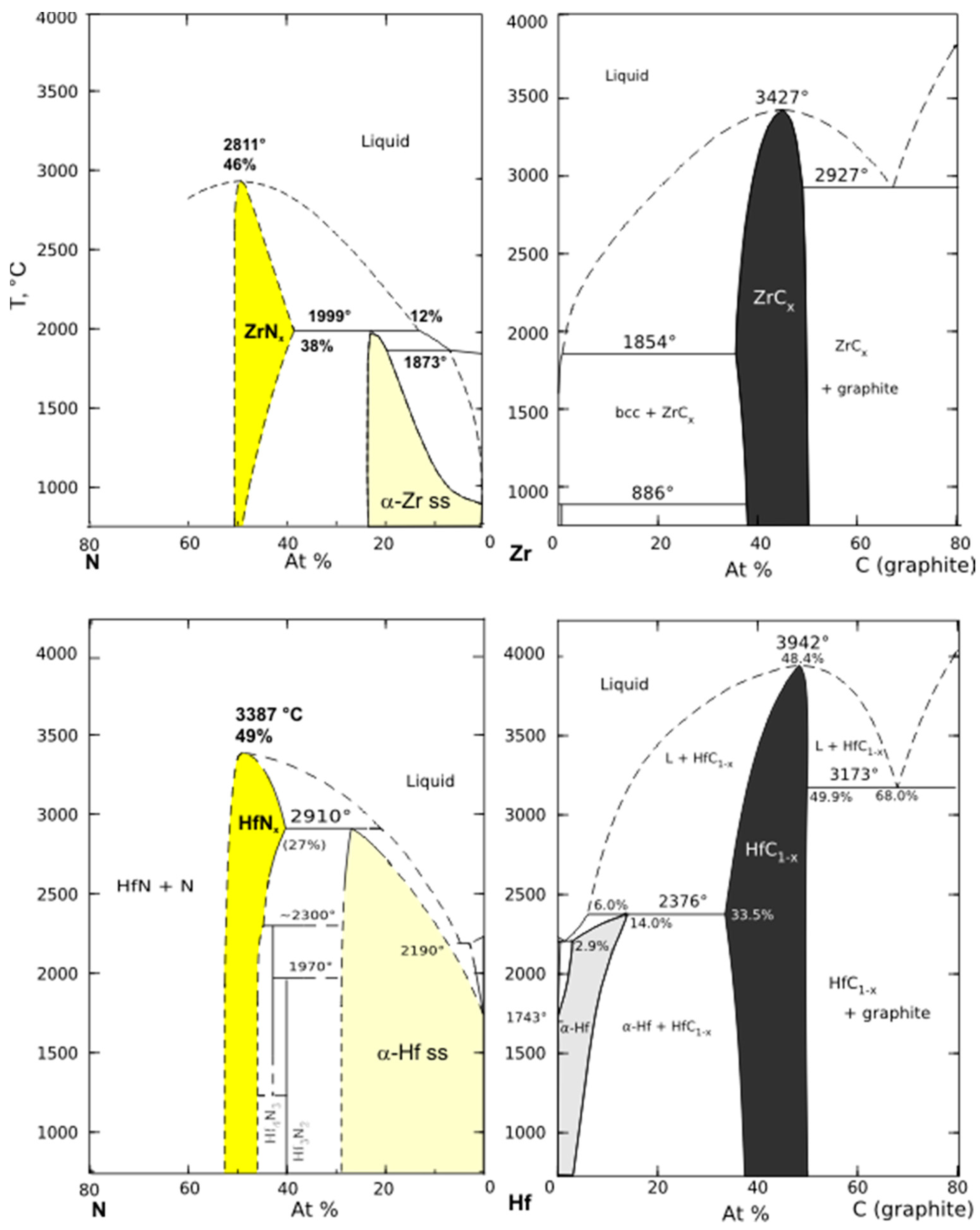
3.1. Zr-C, Hf-C, and Zr-Hf-C
Subcarbides
3.2. Zr-N and Hf-N
3.2.1. Subnitrides
3.2.2. Higher Nitrides
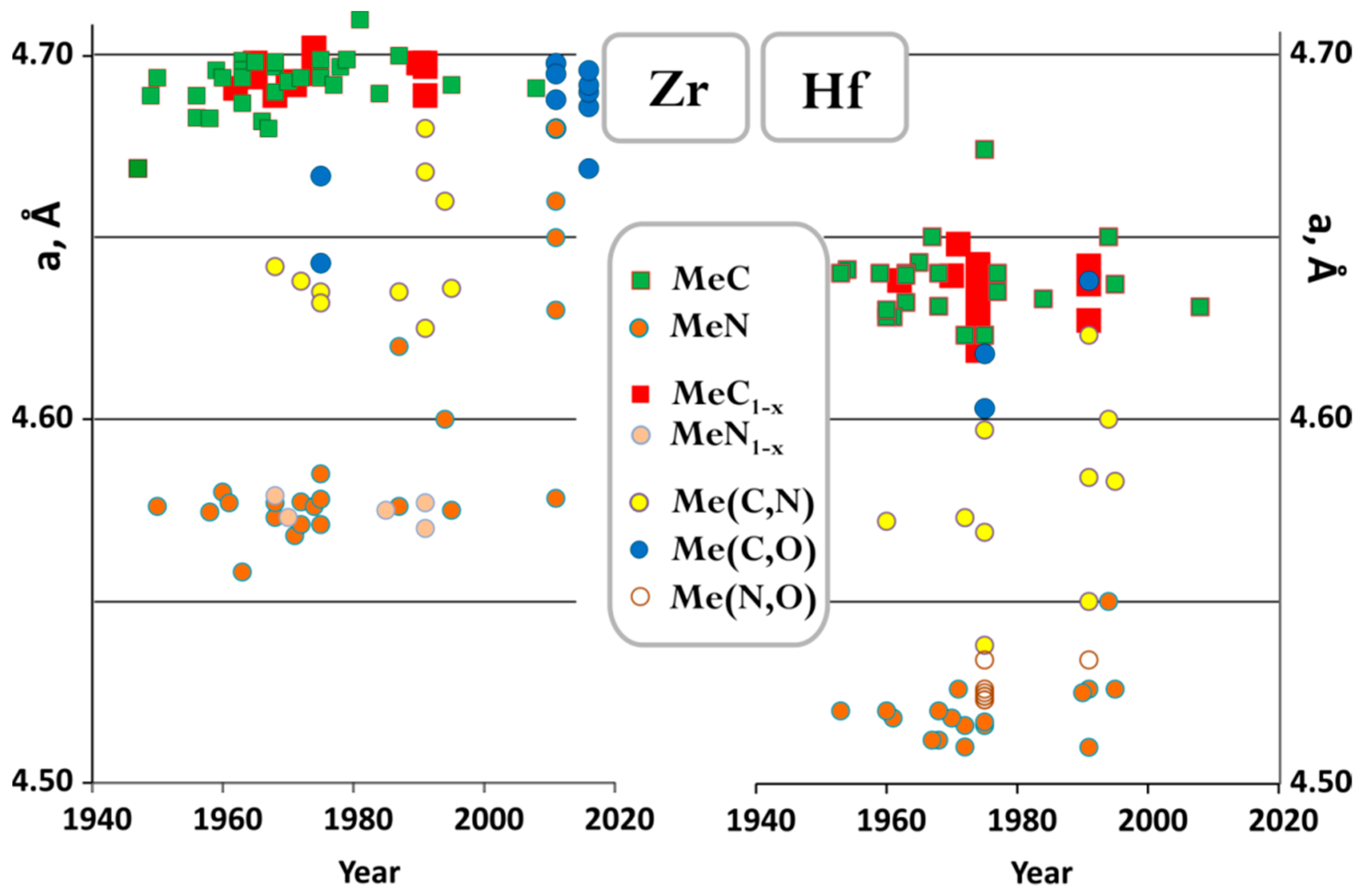
3.3. Zr Versus Hf in Binaries with Carbon and Nitrogen
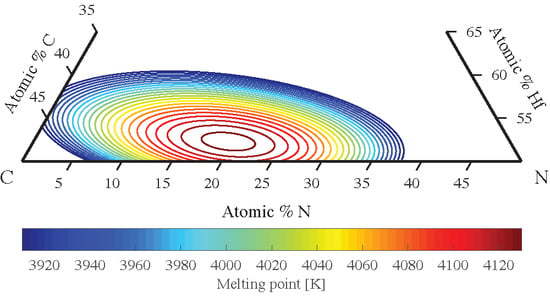
3.4. Zr-C-N, Hf-C-N, Zr-N-O, Hf-N-O Ternaries
4. Rocksalt (oxy)carbonitrides
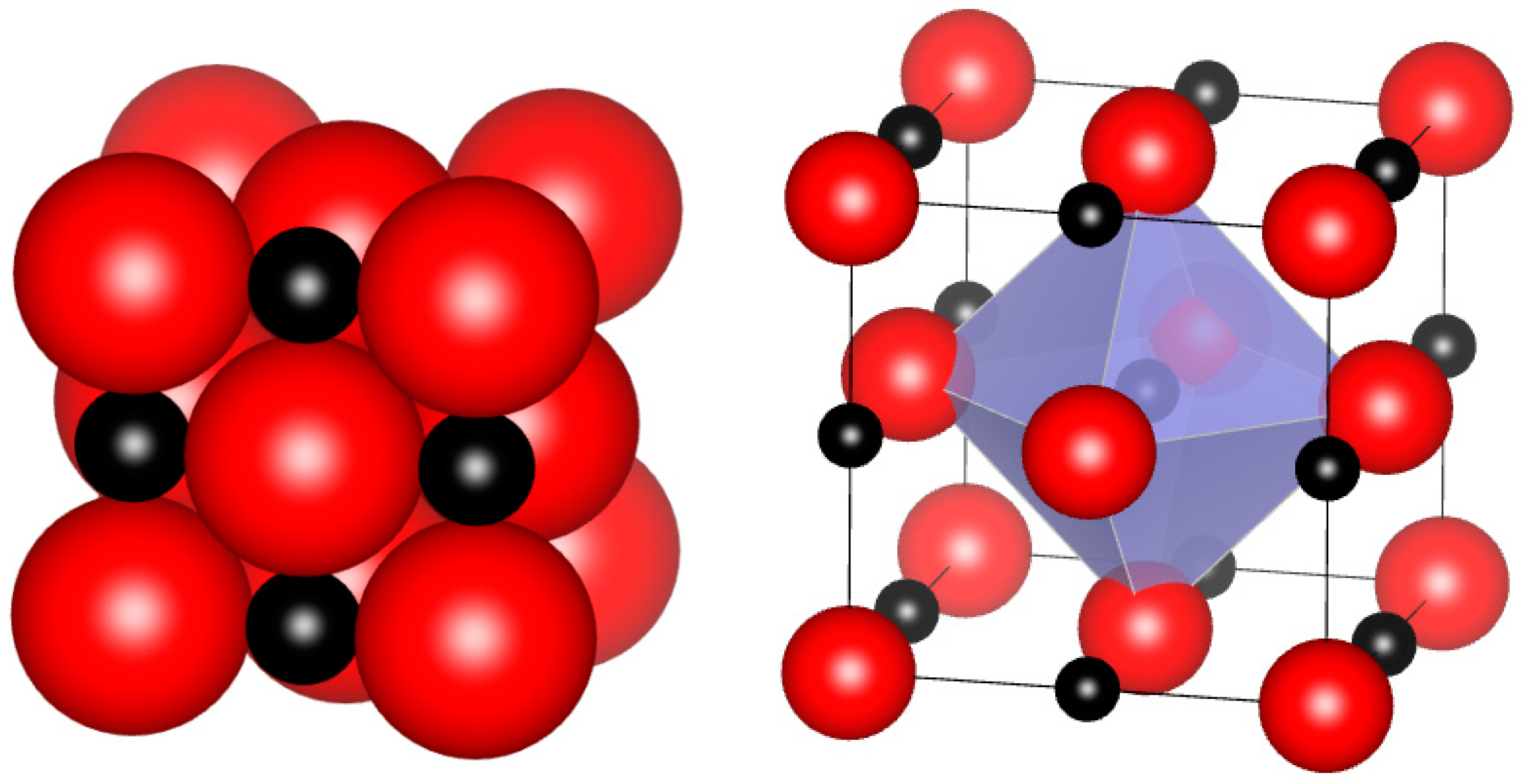
4.1. Structural Features
4.2. Stability Field of Rocksalt Carbonitrides in Hf-Zr-C-N-O System
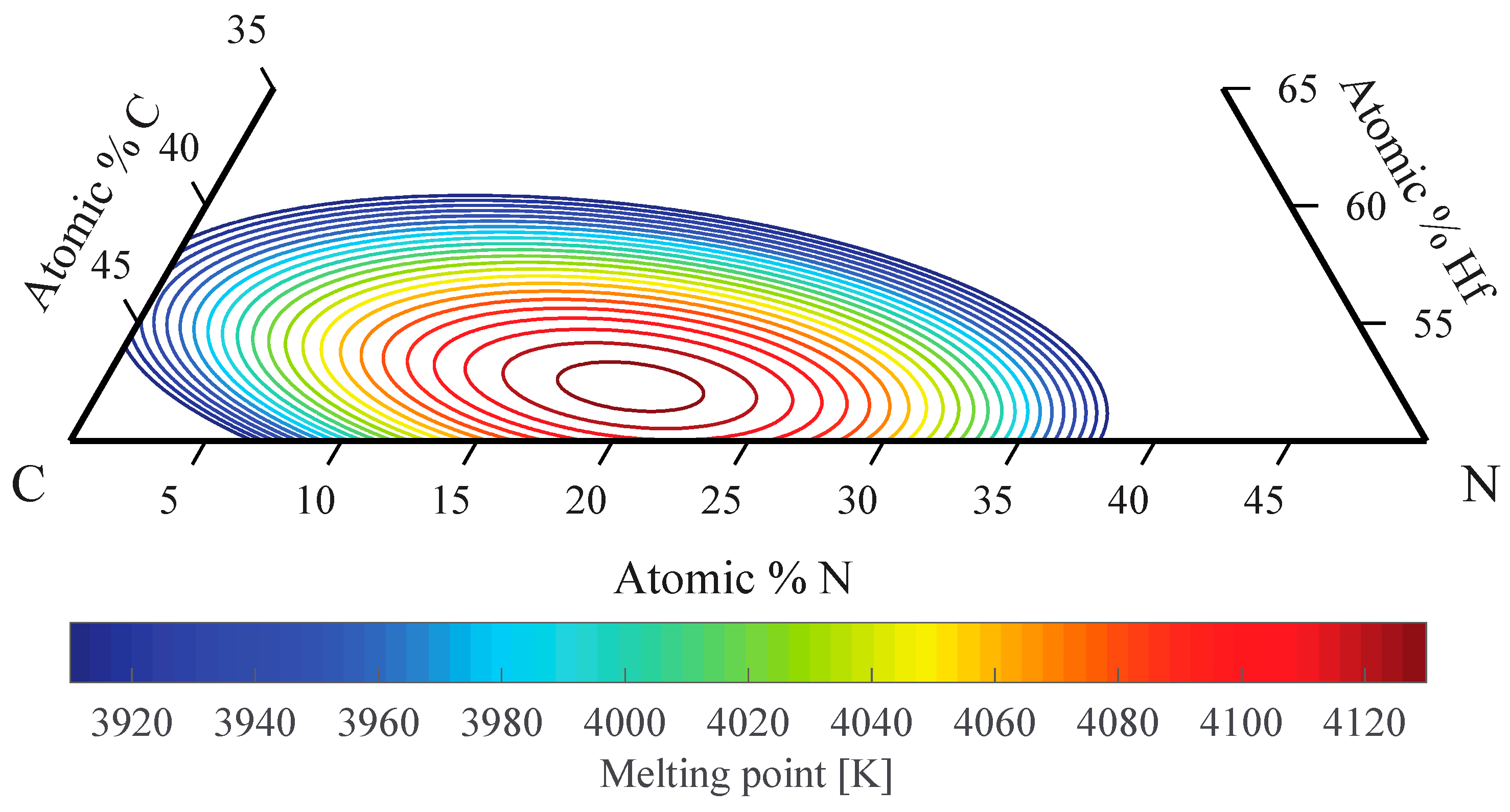
4.3. Melting Temperatures of Hafnium Carbides and Carbonitrides
4.4. Thermochemistry
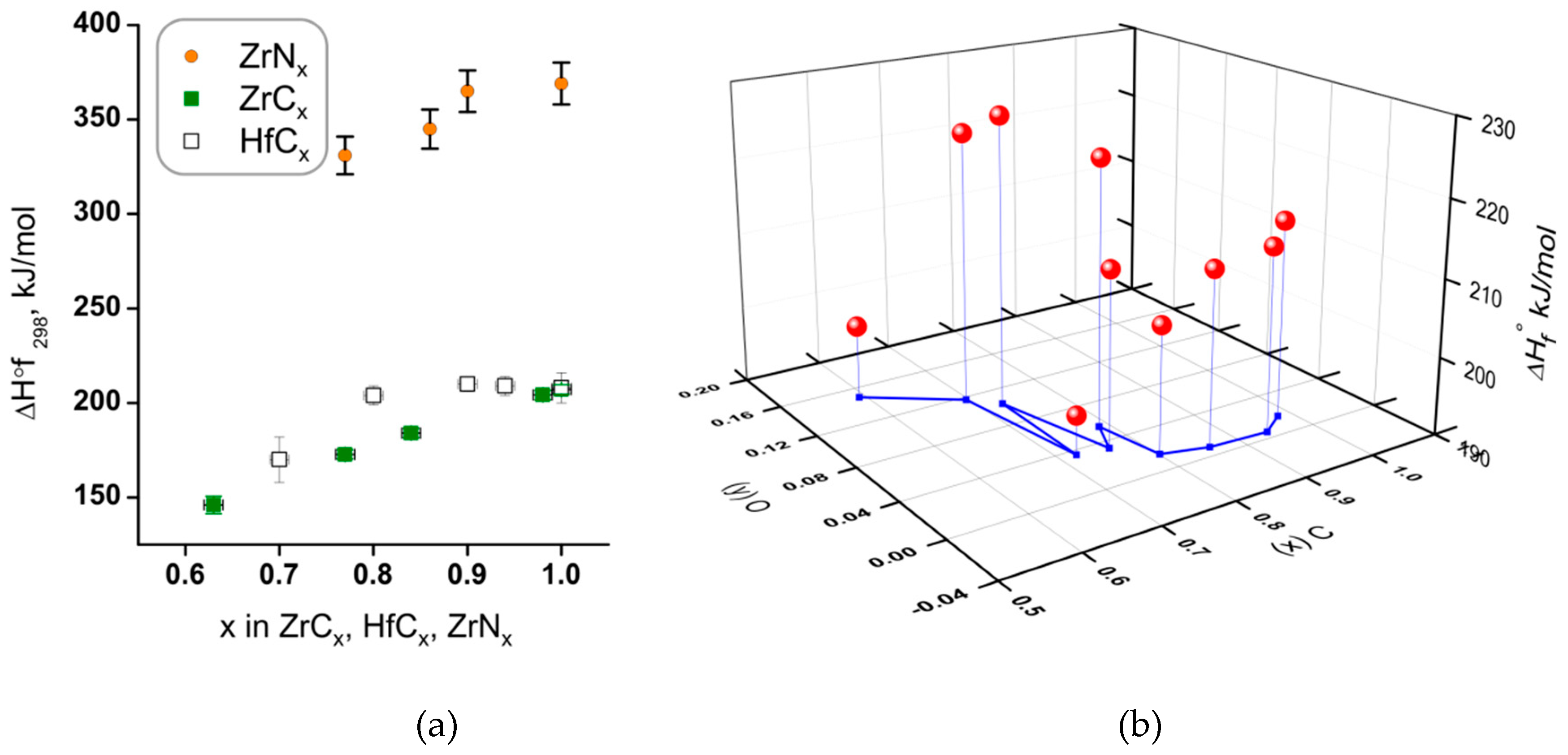
4.4.1. Enthalpies of Formation
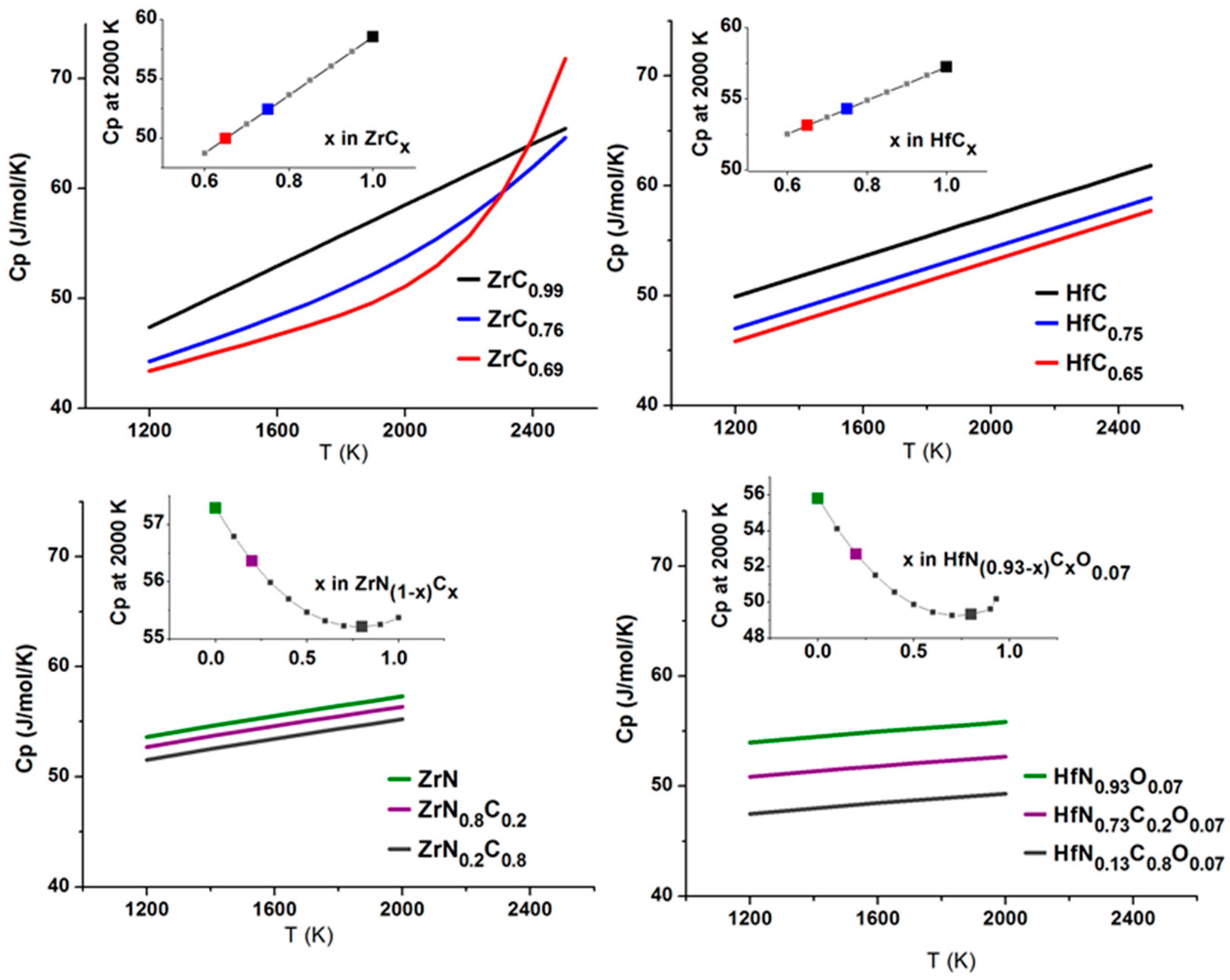
4.4.2. High Temperature Heat Capacities from Calorimetry
4.4.3. Fusion Enthalpies of ZrC and ZrN from Pulsed Heating
4.4.4. Fusion Enthalpies of HfC-HfN from ab Initio Computations
5. Summary and Future Directions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hong, Q.-J.; van de Walle, A. Prediction of the material with highest known melting point from ab initio molecular dynamics calculations. Phys. Rev. B 2015, 92, 020104. [Google Scholar] [CrossRef]
- Searcy, A.W.; Ragone, D.V.; Colombo, U.; Donegani, F. Chemical and Mechanical Behavior of Inorganic Materials; Wiley-Interscience: Hoboken, NJ, USA, 1970. [Google Scholar]
- Ettmayer, P.; Lengauer, W. Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Weinheim, Germany, 2012; Volume 24. [Google Scholar]
- Watson, C.W. Nuclear Rockets: High-Performance Propulsion for Mars; LA-12784-MS; Los Alamos National Laboratory: Los Alamos, NM, USA, 1994; INIS Volume 25, Available online: Inis.iaea.org/search/search.aspx?orig_q=RN:25070120 (accessed on 23 August 2019).
- Katoh, Y.; Vasudevamurthy, G.; Nozawa, T.; Snead, L.L. Properties of zirconium carbide for nuclear fuel applications. J. Nucl. Mater. 2013, 441, 718–742. [Google Scholar] [CrossRef]
- Ciriello, A.; Rondinella, V.V.; Staicu, D.; Somers, J.; Benes, O.; Jardin, R.; Bouexiere, D.; Wastin, F.; Colineau, E. Thermophysical characterization of ZrN and (Zr, Pu)N. J. Alloys Compd. 2009, 473, 265–271. [Google Scholar] [CrossRef]
- Berg, G.; Friedrich, C.; Broszeit, E.; Berger, C. Data Collection of Properties of Hard Material. In Handbook of Ceramic Hard Materials; Riedel, R., Ed.; Wiley: Darmstadt, Germany, 2000. [Google Scholar] [CrossRef]
- Hans, K.; Latha, S.; Bera, P.; Barshilia, H.C. Hafnium carbide based solar absorber coatings with high spectral selectivity. Sol. Energy Mater. Sol. Cells 2018, 185, 1–7. [Google Scholar] [CrossRef]
- Chung, S.; Shrestha, S.; Wen, X.; Feng, Y.; Gupta, N.; Xia, H.; Yu, P.; Tang, J.; Conibeer, G. Hafnium nitride for hot carrier solar cells. Sol. Energy Mater. Sol. Cells 2016, 144, 781–786. [Google Scholar] [CrossRef]
- Xu, J.; Xu, S.; Munroe, P.; Xie, Z.-H. A ZrN nanocrystalline coating for polymer electrolyte membrane fuel cell metallic bipolar plates prepared by reactive sputter deposition. RSC Adv. 2015, 5, 67348–67356. [Google Scholar] [CrossRef]
- Randhawa, H. Hard coatings for decorative applications. Surf. Coat. Technol. 1988, 36, 829–836. [Google Scholar] [CrossRef]
- Klumdoung, P.; Buranawong, A.; Chaiyakun, S.; Limsuwan, P. Variation of color in zirconium nitride thin films prepared at high Ar flow rates with reactive dc magnetron sputtering. Procedia Eng. 2012, 32, 916–921. [Google Scholar] [CrossRef]
- Zheng, C.Y.; He, G.; Chen, X.F.; Liu, M.; Lv, J.G.; Gao, J.; Zhang, J.W.; Xiao, D.Q.; Jin, P.; Jiang, S.S.; et al. Modification of band alignments and optimization of electrical properties of in GaZnO MOS capacitors with high-k HfOxNy gate dielectrics. J. Alloys Compd. 2016, 679, 115–121. [Google Scholar] [CrossRef]
- Wallace, R.M.; Stoltz, R.A.; Wilk, G.D. Fabricating a Field-Effect Device Having a Zirconium and/or Hafnium Oxynitride Gate Dielectric and Integrated Circuit Using the Device. US Patent US6013553A, 11 January 2000. [Google Scholar]
- Tsai, L.-S.; Wang, C.-H.; Chen, W.-Y.; Wang, W.-C.; Hwang, J.-C. Low-voltage organic thin-film transistors with hydrophobic hafnium oxynitride film as gate insulator. Org. Electron. 2010, 11, 123–126. [Google Scholar] [CrossRef]
- Thangadurai, P.; Mikhelashvili, V.; Eisenstein, G.; Kaplan, W.D. Microstructure and chemical analysis of Hf-based high-k dielectric layers in metal-insulator-metal capacitors. Thin Solid Film. 2010, 518, 4467–4472. [Google Scholar] [CrossRef]
- Skaja, K.; Schönbohm, F.; Weier, D.; Lühr, T.; Keutner, C.; Berges, U.; Westphal, C. Thermal stability of an ultrathin hafnium oxide film on plasma nitrided Si(100). Surf. Sci. 2013, 616, 104–109. [Google Scholar] [CrossRef]
- Fahrenholtz, W.G.; Hilmas, G.E. Ultra-high temperature ceramics: Materials for extreme environments. Scr. Mater. 2017, 129, 94–99. [Google Scholar] [CrossRef]
- Fahrenholtz, W.G.; Wuchina, E.J.; Lee, W.E.; Zhou, Y. Ultra-High Temperature Ceramics: Materials for Extreme Environment Applications; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar]
- Goldschmidt, H.J. Interstitial Alloys; Butterworth Co.: London, UK, 1967. [Google Scholar]
- Storms, E. The Refractory Carbides; Academic Press: New York, NY, USA, 1967; p. 144. [Google Scholar]
- Toth, L. Transition Metal Carbides and Nitrides; Academic Press: New York, NY, USA; London, UK, 1971. [Google Scholar]
- Pierson, H.O. Handbook of Refractory Carbides and Nitrides: Properties, Characteristics, Processing, and Applications; William Andrew: Norwich, NY, USA, 1996. [Google Scholar]
- Freer, R. The Physics and Chemistry of Carbides, Nitrides and Borides; Springer: Dordrecht, The Netherlands, 1990; Volume 185. [Google Scholar]
- Samsonov, G.V. Refractory Carbides; Springer: Boston, MA, USA, 1995. [Google Scholar]
- Oyama, S.T. The Chemistry of Transition Metal Carbides and Nitrides; Springer: Dordrecht, The Netherlands, 1996. [Google Scholar]
- Gogotsi, Y.G.; Andrievski, R.A. Materials Science of Carbides, Nitrides and Borides; Springer: Dordrecht, The Netherlands, 1999; Volume 68. [Google Scholar]
- Levy, R.B. Properties of Carbides, Nitrides and Borides: Implications for Catalysis. In Advanced Materials in Catalysis; Burton, J.J., Garten, R.L., Eds.; Academic Press: New York, NY, USA, 1977; pp. 101–127. [Google Scholar]
- Ham, D.; Lee, J. Transition Metal Carbides and Nitrides as Electrode Materials for Low Temperature Fuel Cells. Energies 2009, 2, 873. [Google Scholar] [CrossRef]
- Turchanin, A.G.; Turchanin, M.A. Thermodynamics of Refractory Carbides and Carbonitrides; Metallurgiya: Moscow, Russia, 1991; p. 352. (In Russian) [Google Scholar]
- Turchanin, A.G.; Babenko, S.A. Thermodynamic properties of zirconium carbonitride (ZrCxN1−x) at 298–1500 K. Izv. Akad. Nauk SSSR Neorg. Mater. 1985, 21, 1325–1328. [Google Scholar]
- Turchanin, A.G.; Babenko, S.A.; Mitrofanov, B.V.; Ivenko, N.V. Thermodynamic properties of hafnium oxycarbonitride at 298–1500 K. Zh. Fiz. Khim. 1985, 59, 1847–1849. [Google Scholar]
- Turchanin, A.G.; Fesenko, V.V. Enthalpy and heat capacity of zirconium carbide in the homogeneity region over the temperature range 1300–2500 K. Poroshk. Metall. 1968, 8, 88–90. [Google Scholar]
- Turchanin, A.G.; Polyakov, A.E. Thermodynamic properties of hafnium carbide in the 0–3000 K range. Izv. Akad. Nauk SSSR Neorg. Mater. 1982, 18, 404–406. [Google Scholar]
- Samsonov, G.V.; Upadhyaya, G.S.; Neshpor, V.S. Physical Materials Science of Carbides; Naukova Dumka: Kiev, Ukraine, 1974. (In Russian) [Google Scholar]
- Upadhyaya, G.S. Nature and Properties of Refractory Carbides; Nova Science Publishers: Commack, NY, USA, 1996; p. 545. [Google Scholar]
- Lengauer, W. Multiphase reaction diffusion in transition metal-carbon and transition metal-nitrogen systems. J. Alloys Compd. 1995, 229, 80–92. [Google Scholar] [CrossRef]
- Lengauer, W. Transition Metal Carbides, Nitrides, and Carbonitrides. In Handbook of Ceramic Hard Materials; Riedel, R., Ed.; Wiley-VCH: Weinheim, Germany, 2000; pp. 202–252. [Google Scholar]
- Lengauer, W. Carbides: Transition Metal Solid-State Chemistry. In Encyclopedia of Inorganic and Bioinorganic Chemistry; John Wiley Sons Ltd.: Hoboken, NJ, USA, 2012. [Google Scholar] [CrossRef]
- Lengauer, W.; Binder, S.; Aigner, K.; Ettmayer, P.; Guillou, A.; Debuigne, J.; Groboth, G. Solid state properties of group IVb carbonitrides. J. Alloys Compd. 1995, 217, 137–147. [Google Scholar] [CrossRef]
- Lengauer, W.; Bohn, M. Thermochemical Basis of the Preparation of Well-Defined Transition Metal Carbide, Nitride and Carbonitride Reference Materials for Electron-Probe Microanalysis (EPMA). Solid State Prop. Nitrides Carbonitrides Carbonitride-Based Cermets 2018, 274, 20. [Google Scholar] [CrossRef]
- Shabalin, I.L. Ultra-High Temperature Materials II: Refractory Carbides I (Ta, Hf, Nb and Zr Carbides); Springer: Dordrecht, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Juza, R.; Rabenau, A.; Nitschke, I. Über ein braunes Zirkonnitrid Zr3N4. Z. Anorg. Allg. Chem. 1964, 332, 1–4. [Google Scholar] [CrossRef]
- Lerch, M.; Füglein, E.; Wrba, J. Synthesis, Crystal Structure, and High Temperature Behavior of Zr3N4. Z. Anorg. Allg. Chem. 1996, 622, 367–372. [Google Scholar] [CrossRef]
- Zerr, A.; Miehe, G.; Riedel, R. Synthesis of cubic zirconium and hafnium nitride having Th3P4 structure. Nat. Mater. 2003, 2, 185–189. [Google Scholar] [CrossRef]
- Schwarz, K.; Williams, A.R.; Cuomo, J.J.; Harper, J.H.E.; Hentzell, H.T.G. Zirconium nitride—A new material for Josephson junctions. Phys. Rev. B Condens. Matter 1985, 32, 8312–8316. [Google Scholar] [CrossRef]
- Johansson, B.O.; Hentzell, H.T.G.; Harper, J.M.E.; Cuomo, J.J. Higher nitrides of hafnium, zirconium, and titanium synthesized by dual ion beam deposition. J. Mater. Res. 1986, 1, 442–451. [Google Scholar] [CrossRef]
- Tsuchida, T.; Kawaguchi, M.; Kodaira, K. Synthesis of ZrC and ZrN in air from mechanically activated Zr-C powder mixtures. Solid State Ion. 1997, 101–103, 149–154. [Google Scholar] [CrossRef]
- Lerch, M. Nitridation of zirconia. J. Am. Ceram. Soc. 1996, 79, 2641–2644. [Google Scholar] [CrossRef]
- Yamamura, H.; Emoto, S.; Mori, T. Factors affecting the formation rate of ZrN by the carbothermal nitridation method. J. Ceram. Soc. Jpn. 1998, 106, 650–653. [Google Scholar] [CrossRef][Green Version]
- David, J.; Trolliard, G.; Volkringer, C.; Loiseau, T.; Masson, O.; Maitre, A. Study of the reaction mechanisms involved in the formation of zirconium oxycarbide from Metal-Organic Frameworks (MOFs) precursors. J. Alloys Compd. 2016, 680, 571–585. [Google Scholar] [CrossRef]
- Yin, L.; Jones, M.I. Synthesis of ZrN powders by aluminum-reduction nitridation of ZrO2 powders with CaCO3 additive. Ceram. Int. 2017, 43, 3183–3189. [Google Scholar] [CrossRef]
- Li, Y.; Li, N.; Ruan, G.; Li, J.; Li, X. Effects of technical factors on MgAl2O4-TiN composites produced by aluminothermic reduction and nitridation. Mater. Des. 2006, 28, 969–972. [Google Scholar] [CrossRef]
- Perry, A.J.; Schlapbach, L.; Sproul, W.D. Non-stoichiometry effects in the XPS spectra of HfN films. Solid State Commun. 1987, 62, 23–26. [Google Scholar] [CrossRef]
- Réjasse, F.; Rapaud, O.; Trolliard, G.; Masson, O.; Maître, A. Experimental investigation and thermodynamic evaluation of the C-O-Zr ternary system. RSC Adv. 2016, 6, 100122–100135. [Google Scholar] [CrossRef]
- Samsonow, G.W.; Morosow, W.W. Carbohydride der Übergangsmetalle (ICSD 77008). Mon. Chem./Chem. Mon. 1971, 102, 1667–1678. [Google Scholar] [CrossRef]
- Ma, X.; Li, C.; Bai, K.; Wu, P.; Zhang, W. Thermodynamic assessment of the Zr–N system. J. Alloys Compd. 2004, 373, 194–201. [Google Scholar] [CrossRef]
- Fernández Guillermet, A. Analysis of thermochemical properties and phase stability in the zirconium-carbon system. J. Alloys Compd. 1995, 217, 69–89. [Google Scholar] [CrossRef]
- Okamoto, H. The Hf-N (Hafnium-Nitrogen) system. Bull. Alloy Phase Diagr. 1990, 11, 146–149. [Google Scholar] [CrossRef]
- Bittermann, H.; Rogl, P. Critical assessment and thermodynamic calculation of the binary system hafnium-carbon (Hf-C). J. Phase Equilibria 1997, 18, 344–356. [Google Scholar] [CrossRef]
- Savvatimskiy, A.I.; Onufriev, S.V.; Muboyadzhyan, S.A. Thermophysical properties of the most refractory carbide Ta0.8Hf0.2C under high temperatures (2000–5000 K). J. Eur. Ceram. Soc. 2019, 39, 907–914. [Google Scholar] [CrossRef]
- Sheindlin, M.; Falyakhov, T.; Petukhov, S.; Valyano, G.; Vasin, A. Recent advances in the study of high-temperature behaviour of non-stoichiometric TaCx, HfCx and ZrCx carbides in the domain of their congruent melting point. Adv. Appl. Ceram. 2018, 117 (Suppl. 1), s48–s55. [Google Scholar] [CrossRef]
- Savvatimskiy, A.I.; Onufriev, S.V.; Muboyajan, S.A.; Tsygankov, P.A. Pulsed Heating of Carbides. Bull. Russ. Acad. Sci. Phys. 2018, 82, 363–368. [Google Scholar] [CrossRef]
- Savvatimskiy, A.I.; Onufriev, S.V.; Muboyadzhyan, S.A. Measurement of ZrC properties up to 5000 K by fast electrical pulse heating method. J. Mater. Res. 2017, 32, 1287–1294. [Google Scholar] [CrossRef]
- Onufriev, S.V.; Kondratiev, A.M.; Savvatimskiy, A.I.; Val’yano, G.E.; Muboyajan, S.A. Investigation of the high-temperature properties of zirconium nitride by impulse current heating method. High Temp. 2015, 53, 455–457. [Google Scholar] [CrossRef]
- Kondratyev, A.; Muboyajan, S.; Onufriev, S.; Savvatimskiy, A. The application of the fast pulse heating method for investigation of carbon-rich side of Zr–C phase diagram under high temperatures. J. Alloys Compd. 2015, 631, 52–59. [Google Scholar] [CrossRef]
- Lv, Z.; Hu, H.; Wu, C.; Cui, S.; Zhang, G.; Feng, W. First-principles study of structural stability, electronic and elastic properties of ZrC compounds. Phys. B Condens. Matter 2011, 406, 2750–2754. [Google Scholar] [CrossRef]
- Weinberger, C.R.; Thompson, G.B. Review of phase stability in the group IVB and VB transition-metal carbides. J. Am. Ceram. Soc. 2018, 101, 4401–4424. [Google Scholar] [CrossRef]
- Weinberger, C.R.; Yu, H.; Wang, B.; Thompson, G.B. A computational investigation into the microstructures and stability of the zeta phase in transition metal carbides and nitrides. Adv. Appl. Ceram. 2018, 117 (Suppl. 1), s26–s33. [Google Scholar] [CrossRef]
- Van de Walle, A.; Sun, R.; Hong, Q.-J.; Kadkhodaei, S. Software tools for high-throughput CALPHAD from first-principles data. Calphad 2017, 58, 70–81. [Google Scholar] [CrossRef]
- Sundman, B.; Kattner, U.R.; Palumbo, M.; Fries, S.G. OpenCalphad—A free thermodynamic software. Integr. Mater. Manuf. Innov. 2015, 4. [Google Scholar] [CrossRef]
- Liu, Z.-K. First-Principles Calculations and CALPHAD Modeling of Thermodynamics. J. Phase Equilibria Diffus. 2009, 30, 517. [Google Scholar] [CrossRef]
- Kaufman, L.; Ågren, J. CALPHAD, first and second generation—Birth of the materials genome. Scr. Mater. 2014, 70, 3–6. [Google Scholar] [CrossRef]
- Gallob, R.; Jaeger, H.; Pottlacher, G. A submicrosecond pulse heating system for the investigation of thermophysical properties of metals at high temperatures. Int. J. Thermophys. 1986, 7, 139–147. [Google Scholar] [CrossRef]
- Frohberg, M.G. Thirty years of levitation melting calorimetry—A balance. Thermochim. Acta 1999, 337, 7–17. [Google Scholar] [CrossRef]
- Dinsdale, A.T. SGTE data for pure elements. Comput. Coupling Phase Diagr. Thermochem. 1991, 15, 317–425. [Google Scholar] [CrossRef]
- Luo, A.A. Material design and development: From classical thermodynamics to CALPHAD and ICME approaches. Calphad 2015, 50, 6–22. [Google Scholar] [CrossRef]
- Olson, G.B.; Kuehmann, C.J. Materials genomics: From CALPHAD to flight. Scr. Mater. 2014, 70, 25–30. [Google Scholar] [CrossRef]
- Sara, R.V. The system zirconium-carbon. J. Am. Ceram. Soc. 1965, 48, 243–247. [Google Scholar] [CrossRef]
- Rudy, E. Ternary Phase Equilibria in Transition Metal-Boron-Carbon-Silicon Systems. V. Compendium of Phase Diagram Data; Aerojet-General Corp.: Sacramento, CA, USA, 1969; p. 731. Available online: www.osti.gov/biblio/4754828 (accessed on 23 August 2019).
- Jackson, H.F.; Lee, W.E. Properties and characteristics of ZrC. In Material Properties. Oxide Fuels for Light Water Reactors and Fast Neutron Reactors; Konings, R.J.M., Ed.; Elsevier B.V.: Amsterdam, The Netherlands; Waltham, MA, USA, 2012; Volume 2, pp. 339–372. [Google Scholar] [CrossRef]
- Zhou, P.; Peng, Y.; Du, Y.; Wang, S.; Wen, G. Thermodynamic modeling of the C-W-Zr system. Int. J. Refract. Met. Hard Mater. 2015, 50, 274–281. [Google Scholar] [CrossRef]
- Pirani, M.; Alterthum, H. Method for the determination of the melting point of metals which fuse at high temperatures. Z. Elektrochem. Angew. Phys. Chem. 1923, 29, 5–8. [Google Scholar]
- Okamoto, H. C-Hf (carbon-hafnium). J. Phase Equilibria 2001, 22, 510. [Google Scholar] [CrossRef]
- Bittermann, H.; Rogl, P. Critical assessment and thermodynamic calculation of the ternary system C-Hf-Zr (carbon-zirconium-hafnium). J. Phase Equilibria 2002, 23, 218–235. [Google Scholar] [CrossRef]
- Brukl, C.E.; Harmon, D.P. Ternary Phase Equilibriums in Transition Metal-Boron-Carbon-Silicon Systems. II. Ternary Systems. 4. Titanium-Zirconium-Carbon, Titanium-Hafnium-Carbon, and Zirconium-Hafnium-Carbon Systems; CAN70:81391; Materials Research Laboratory, Aerojet-General Corp.: Sacramento, CA, USA, 1966; p. 92. [Google Scholar]
- Adjaoud, O.; Steinle-Neumann, G.; Burton, B.P.; van de Walle, A. First-principles phase diagram calculations for the HfC-TiC, ZrC-TiC, and HfC-ZrC solid solutions. Phys. Rev. B 2009, 80, 134112. [Google Scholar] [CrossRef]
- Obata, N.; Nakazawa, N. Superlattice formation in zirconium-carbon system. J. Nucl. Mater. 1976, 60, 39–42. [Google Scholar]
- Hu, W.; Xiang, J.; Zhang, Y.; Liu, S.; Chen, C.; Wang, P.; Wang, H.; Wen, F.; Xu, B.; He, J.; et al. Superstructural nanodomains of ordered carbon vacancies in nonstoichiometric ZrC0.61. J. Mater. Res. 2012, 27, 1230–1236. [Google Scholar] [CrossRef]
- Domagala, R.F.; McPherson, D.J.; Hansen, M. System Zirconium-Nitrogen. JOM 1956, 8, 98–105. [Google Scholar] [CrossRef]
- Sridar, S.; Kumar, R.; Hari Kumar, K.C. Thermodynamic modelling of Ti-Zr-N system. Comput. Coupling Phase Diagr. Thermochem. 2017, 56, 102–107. [Google Scholar] [CrossRef]
- Gal’braikh, É.I.; Kulik, O.P.; Kuznetsov, A.A.; Lyutaya, M.D.; Morozova, M.P. Enthalpy of formation of the nitrogen solid solution in α-zirconium and of zirconium nitride in their homogeneity regions. Sov. Powder Metall. Met. Ceram. 1970, 9, 748–751. [Google Scholar] [CrossRef]
- Humphrey, G.L. Heats of formation of hafnium oxide and hafnium nitride. J. Am. Chem. Soc. 1953, 75, 2806–2807. [Google Scholar] [CrossRef]
- Rudy, E. Crystal structures of Hf3N2 and Hf4N3. Met. Trans. 1970, 1, 1249–1252. [Google Scholar] [CrossRef]
- Lengauer, W.; Rafaja, D.; Täubler, R.; Kral, C.; Ettmayer, P. Preparation of binary single-phase line compounds via diffusion couples: The subnitride phases η-Hf3N2−x and ζ-Hf4N3−x. Acta Metall. Mater. 1993, 41, 3505–3514. [Google Scholar] [CrossRef]
- Xu, M.; Wang, S.; Yin, G.; Li, J.; Zheng, Y.; Chen, L.; Jia, Y. Optical properties of cubic Ti3N4, Zr3N4, and Hf3N4. Appl. Phys. Lett. 2006, 89. [Google Scholar] [CrossRef]
- Yablonskikh, M.; Dzivenko, D.; Bourguille, J.; Riedel, R.; Magnano, E.; Parmigiani, F.; Zerr, A. Electronic structure and band gap of oxygen bearing c-Zr3N4 and of c-Hf3N4 by soft X-ray spectroscopy. Phys. Status Solidi A 2014, 211, 835–842. [Google Scholar] [CrossRef]
- Yu, S.; Zeng, Q.; Oganov, A.R.; Frapper, G.; Huang, B.; Niu, H.; Zhang, L. First-principles study of Zr-N crystalline phases: Phase stability, electronic and mechanical properties. RSC Adv. 2017, 7, 4697–4703. [Google Scholar] [CrossRef]
- Zhang, J.-D.; Yang, K. Theoretical study of the thermodynamic properties of cubic Zr3N4 and Hf3N4 under high pressures. J. Alloys Compd. 2014, 608, 90–94. [Google Scholar] [CrossRef]
- Zhang, J.-P.; Zhu, X.-L.; Zhang, Y.-Y.; Gao, J.-X. First principles study on structural and electronic properties and defect formation enthalpies of cubic Hf3N4 under high pressure. Can. J. Phys. 2014, 92, 1581–1586. [Google Scholar] [CrossRef]
- Zheng, F.; Fang, Y.; Yu, S.; Wu, S.; Zhu, Z.-Z. Exploration of crystal structures and phase transitions in Hf3N4. CrystEngComm 2017, 19, 2608–2613. [Google Scholar] [CrossRef]
- Oganov, A.R.; Pickard, C.J.; Zhu, Q.; Needs, R.J. Structure prediction drives materials discovery. Nat. Rev. Mater. 2019, 4, 331–348. [Google Scholar] [CrossRef]
- Zhang, J.; Oganov, A.R.; Li, X.; Niu, H. Pressure-stabilized hafnium nitrides and their properties. Phys. Rev. B 2017, 95, 020103. [Google Scholar] [CrossRef]
- Gu, Z.; Hu, C.; Huang, H.; Zhang, S.; Fan, X.; Wang, X.; Zheng, W. Identification and thermodynamic mechanism of the phase transition in hafnium nitride films. Acta Mater. 2015, 90, 59–68. [Google Scholar] [CrossRef]
- Sui, Y.R.; Xu, Y.; Yao, B.; Xiao, L.; Liu, B. Preparation, characterization and properties of N-rich Zr-N thin film with Th3P4 structure. Appl. Surf. Sci. 2009, 255, 6355–6358. [Google Scholar] [CrossRef]
- Tham, A.T.; Roedel, C.; Lerch, M.; Wang, D.; Su, D.S.; Klein-Hoffmann, A.; Schloegl, R. A TEM study on ZrO2-rich phases in the quasibinary system ZrO2-Zr3N4: Comparison between fast and slowly cooled samples. Cryst. Res. Technol. 2005, 40, 193–198. [Google Scholar] [CrossRef][Green Version]
- Walter, D.; Lerch, M.; Laqua, W. Thermal stability of the β” phase in the ZrO2-Zr3N4 system. J. Therm. Anal. 1997, 48, 709–716. [Google Scholar] [CrossRef]
- Orlovskii, V.P.; Rudenko, N.V.; Ivanov-Emin, B.N. Thermal stability of zirconium tetrachloride ammines. Zh. Neorg. Khim. 1967, 12, 2305–2308. [Google Scholar]
- Chhowalla, M.; Unalan, H.E. Thin films of hard cubic Zr3N4 stabilized by stress. Nat. Mater. 2005, 4, 317–322. [Google Scholar] [CrossRef]
- Kroll, P. Assessment of the Hf-N, Zr-N and Ti-N phase diagrams at high pressures and temperatures: Balancing between MN and M3N4 (M = Hf, Zr, Ti). J. Phys. Condens. Matter 2004, 16, S1235–S1244. [Google Scholar] [CrossRef]
- Kroll, P. Hafnium Nitride with Thorium Phosphide Structure: Physical Properties and an Assessment of the Hf-N, Zr-N, and Ti-N Phase Diagrams at High Pressures and Temperatures. Phys. Rev. Lett. 2003, 90. [Google Scholar] [CrossRef]
- Binder, S.; Lengauer, W.; Ettmayer, P.; Bauer, J.; Debuigne, J.; Bohn, M. Phase equilibria in the systems Ti-C-N, Zr-C-N and Hf-C-N. J. Alloys Compd. 1995, 217, 128–136. [Google Scholar] [CrossRef]
- Réjasse, F.; Rapaud, O.; Trolliard, G.; Masson, O.; Maître, A. Experimental investigation and thermodynamic evaluation of the C–Hf–O ternary system. J. Am. Ceram. Soc. 2017, 100, 3757–3770. [Google Scholar] [CrossRef]
- Constant, K.; Kieffer, R.; Ettmayer, P. Pseudoternary hafnium oxide-hafnium mononitride-hafnium monocarbide system. Monatsh. Chem. 1975, 106, 973–981. [Google Scholar] [CrossRef]
- Salamat, A.; Hector, A.L.; Kroll, P.; McMillan, P.F. Nitrogen-rich transition metal nitrides. Coord. Chem. Rev. 2013, 257, 2063–2072. [Google Scholar] [CrossRef]
- Bredow, T.; Lerch, M. On the Anion Distribution in Zr7O8N4. Z. Anorg. Allg. Chem. 2007, 633, 2598–2602. [Google Scholar] [CrossRef]
- Locherer, T.; Dubrovinsky, L.; Fuess, H. Isothermal compression of nitrogen doped zirconia/zirconium oxonitride Zr7O11N2 and equation of states. Solid State Commun. 2007, 143, 408–411. [Google Scholar] [CrossRef]
- Lerch, M. Phase relationships in the ZrO2-Zr3N4 system. J. Mater. Sci. Lett. 1998, 17, 441–443. [Google Scholar] [CrossRef]
- Clarke, S.J.; Michie, C.W.; Rosseinsky, M.J. Structure of Zr2ON2 by Neutron Powder Diffraction: The Absence of Nitride–Oxide Ordering. J. Solid State Chem. 1999, 146, 399–405. [Google Scholar] [CrossRef]
- Michie, C.W.; Claridge, J.B.; Clarke, S.J.; Rosseinsky, M.J. Zr4O5N2−Intergrowth of Fluorite and Bixbyite Anion Layers Formed by Coupled Site-Selective Anion and Vacancy Ordering. Chem. Mater. 2003, 15, 1547–1553. [Google Scholar] [CrossRef]
- Razumovskiy, V.I.; Ghosh, G. A first-principles study of cementite (Fe3C) and its alloyed counterparts: Structural properties, stability, and electronic structure. Comput. Mater. Sci. 2015, 110, 169–181. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, B.; Wang, J.; Wang, J. Theoretical investigations of the effects of ordered carbon vacancies in ZrC1−x on phase stability and thermo-mechanical properties. Acta Mater. 2016, 111, 232–241. [Google Scholar] [CrossRef]
- Kim, C.; Pilania, G.; Ramprasad, R. Machine Learning Assisted Predictions of Intrinsic Dielectric Breakdown Strength of ABX3 Perovskites. J. Phys. Chem. C 2016, 120, 14575–14580. [Google Scholar] [CrossRef]
- Aigner, K.; Lengauer, W.; Rafaja, D.; Ettmayer, P. Lattice parameters and thermal expansion of Ti(CxN1−x), Zr(CxN1−x), Hf(CxN1−x) and TiN1−x from 298 to 1473 K as investigated by high-temperature X-ray diffraction. J. Alloys Compd. 1994, 215, 121–126. [Google Scholar] [CrossRef]
- Kornilov, A.N.; Chelovskaya, N.V.; Zhelankin, V.I.; Shveikin, G.P. Enthalpies of formation of hafnium carbides. J. Chem. Thermodyn. 1977, 9, 629–642. [Google Scholar] [CrossRef]
- Brundiers, G.D. Herstellung, Aufbau und Eigenschaften von Hafniumverbindungen im System Hf-C-N-O. KFK-2161. Thesis/Dissertation, Karlsruhe Univ., Karlsruhe, Germany, 1975; p. 278. Available online: http://inis.iaea.org/search/search.aspx?orig_q=RN:07230513 (accessed on 23 August 2019).
- Banus, M.D.; Reed, T.B.; Strauss, A.J. Electrical and Magnetic Properties of TiO and VO. Phys. Rev. B 1972, 5, 2775–2784. [Google Scholar] [CrossRef]
- Adachi, G.-Y.; Imanaka, N.; Zhang, F. Rare earth carbides. In Handbook on the Physics and Chemistry of Rare Earths; Gschneidner, K.A., Jr., Eyring, L., Eds.; Elsevier: Amsterdam, The Netherlands, 1991; Volume 15, pp. 161–189. [Google Scholar] [CrossRef]
- Butherus, A.D.; Eick, H.A. Preparation and some properties of the lanthanide oxide carbides, Ln4O3C. J. Am. Chem. Soc. 1968, 90, 1715–1718. [Google Scholar] [CrossRef]
- Losego, M.D.; Mita, S.; Collazo, R.; Sitar, Z.; Maria, J.-P. Epitaxial growth of the metastable phase ytterbium monoxide on gallium nitride surfaces. J. Cryst. Growth 2008, 310, 51–56. [Google Scholar] [CrossRef]
- Losego, M.D.; Maria, J.-P. Synthesis of polycrystalline ytterbium monoxide thin films by molecular beam deposition. J. Vac. Sci. Technol. B 2006, 24, 2111–2114. [Google Scholar] [CrossRef]
- Leger, J.M.; Yacoubi, N.; Loriers, J. Synthesis of rare earth monoxides. J. Solid State Chem. 1981, 36, 261–270. [Google Scholar] [CrossRef]
- Katz, J.J.; Morss, L.R.; Edelstein, N.M.; Fuger, J. The Chemistry of the Actinide and Transactinide Elements; Springer: Dordrecht, The Netherlands, 2007. [Google Scholar] [CrossRef]
- Mazzoni, A.D.; Conconi, M.S. Study of carbonitriding reactions of zirconia. Synthesis of Zr(C,N,O) phases and β-type zirconium oxynitrides. Ceram. Int. 2004, 30, 23–29. [Google Scholar] [CrossRef]
- Constant, K.; Kieffer, R.; Ettmayer, P. Pseudoternary zirconium monoxide-zirconium nitride-zirconium carbide system. Monatsh. Chem. 1975, 106, 823–832. [Google Scholar] [CrossRef]
- Kornilov, A.N.; Chelovskaya, N.V.; Zhelankin, V.I. Heat of formation of zirconium carbides. Zh. Fiz. Khim. 1975, 49, 1341. [Google Scholar]
- Pipon, Y.; Toulhoat, N.; Moncoffre, N.; Gutierrez, G.; Maitre, A.; Gendre, M. Influence of the Oxygen content on the thermal migration of Xenon in ZrCxO1−x. J. Nucl. Mater. 2013, 440, 546–552. [Google Scholar] [CrossRef]
- Agte, C.; Alterthum, H. Systems of high-melting carbides; contributions to the problem of carbon fusion. Z. Tech. Phys. 1930, 11, 182–191. [Google Scholar]
- Kato, H.; Copeland, M.I. Report of Investigations; U.S. Bureau of Mines: Washington, DC, USA, 1962.
- Adams, R.P.; Beall, R.A. Preparation and Evaluation of Fused Hafnium Carbide. 1066–5552; U.S. Department of the Interior; Bureau of Mines, 1963; p. 17. Available online: https://catalog.hathitrust.org/Record/005981077 (accessed on 23 August 2019).
- Sara, R.V. The hafnium-carbon system. Trans. Am. Inst. Min. Metall. Pet. Eng. 1965, 233, 1683–1691. [Google Scholar]
- Rudy, E.; Windisch, S. Ternary Phase Equilibriums in Transition Metal-Boron-Carbon-Silicon Systems. Part 2 Vol. I.; AFML-TR-65-2; Aerojet-Gen. Corp.: Sacramento, CA, USA, 1965; p. 53. [Google Scholar]
- Rudy, E.; Progulski, J. Pirani furnace for the precision determination of the melting temperatures of refractory metallic substances. Planseeber. Pulvermetall. 1967, 15, 13–45. [Google Scholar]
- Deardorff, D.K.; Copeland, M.I.; Adams, R.P. Hafnium-carbon phase diagram. US Bur. Mines Rep. Invest. 1967, 6983, 16. [Google Scholar]
- Cedillos-Barraza, O.; Manara, D.; Boboridis, K.; Watkins, T.; Grasso, S.; Jayaseelan, D.D.; Konings, R.J.M.; Reece, M.J.; Lee, W.E. Investigating the highest melting temperature materials: A laser melting study of the TaC-HfC system. Sci. Rep. 2016, 6, 37962. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Heyd, J.; Scuseria, G.E.; Ernzerhof, M. Hybrid functionals based on a screened Coulomb potential. J. Chem. Phys. 2003, 118, 8207. [Google Scholar] [CrossRef]
- Baker, F.B.; Storms, E.K.; Holley, C.E., Jr. Enthalpy of formation of zirconium carbide. J. Chem. Eng. Data 1969, 14, 244–246. [Google Scholar] [CrossRef]
- Yungman, V.S.; Glushko, V.P.; Medvedev, V.A.; Gurvich, L.V. Thermal Constants of Substances; Wiley: Hoboken, NJ, USA, 1974; p. 343. [Google Scholar]
- Ettmayer, P.; Kieffer, R.; Hattinger, F. Determination of the melting point of metal nitrides under nitrogen pressure. Metall 1974, 28, 1151–1156. [Google Scholar]
- Chase, M.W.J. NIST-JANAF Thermochemical Tables; American Inst. of Physics: University Park, MD, USA, 1998. Available online: https://janaf.nist.gov/ (accessed on 23 August 2019). [CrossRef]
- Meschel, S.V.; Kleppa, O.J. Thermochemistry of alloys of transition metals and lanthanide metals with some IIIA and IVA elements in the periodic table. J. Alloys Compd. 2001, 321, 183–200. [Google Scholar] [CrossRef]
- Kornilov, A.N.; Ushakova, I.M.; Huber, E.J., Jr.; Holley, C.E., Jr. Enthalpy of formation of hafnium dioxide. J. Chem. Thermodyn. 1975, 7, 21–26. [Google Scholar] [CrossRef]
- Kim, S.; Szlufarska, I.; Morgan, D. Ab initio study of point defect structures and energetics in ZrC. J. Appl. Phys. 2010, 107, 053521. [Google Scholar] [CrossRef]
- Ushakov, S.V.; Navrotsky, A. Experimental approaches to the thermodynamics of ceramics above 1500 °C. J. Am. Ceram. Soc. 2012, 95, 1463–1482. [Google Scholar] [CrossRef]
- Dworkin, A.S.; Bredig, M.A. Diffuse transition and melting in fluorite and antifluorite type of compounds. Heat content of potassium sulfide from 298 to 1260 K. J. Phys. Chem. 1968, 72, 1277–1281. [Google Scholar] [CrossRef]
- Hong, Q.-J.; Ushakov, S.V.; Kapush, D.; Benmore, C.J.; Weber, R.J.K.; van de Walle, A.; Navrotsky, A. Combined computational and experimental investigation of high temperature thermodynamics and structure of cubic ZrO2 and HfO2. Sci. Rep. 2018, 8, 14962. [Google Scholar] [CrossRef]
- Schroeer, W.; Pottlacher, G. Estimation of critical data and phase diagrams of pure molten metals. High Temp. High Press. 2014, 43, 201–215. [Google Scholar]
- McGrath, J.R. Exploding Wire Research 1774-1963. NRL Memorandum Report 1698; AD0633623. US Naval Research Laboratory: Washington, DC, USA, 1966. Available online: http://www.dtic.mil/docs/citations/AD0633623 (accessed on 23 August 2019).
- Onufriev, S.V.; Savvatimskiy, A.I. Measuring the Specific Heat of Conducting Substances in Conditions of Microsecond Heating with a Current Pulse. High Temp. 2018, 56, 678–684. [Google Scholar] [CrossRef]
- Knyazkov, A.M.; Kurbakov, S.D.; Savvatimskiy, A.I.; Sheindlin, M.A.; Yanchuk, V.I. Melting of carbides by electrical pulse heating. High Temp. High Press. 2011, 40, 349–358. [Google Scholar]
- Hong, Q.-J.; van de Walle, A. Solid-liquid coexistence in small systems: A statistical method to calculate melting temperatures. J. Chem. Phys. 2013, 139, 094114. [Google Scholar] [CrossRef]
- Van de Walle, A.; Ceder, G. The effect of lattice vibrations on substitutional alloy thermodynamics. Rev. Mod. Phys. 2002, 74, 11–45. [Google Scholar] [CrossRef]
| Phases | SG | Str. Type | Comments, [Refs] |
|---|---|---|---|
| Reported from the experiments at 1 Atm | |||
| δ-Hf,Zr(C,N,O) | Fm-3m | NaCl (B1) | [38,55,112,113,114] |
| η-Hf3N2 | R-3m | Ta2VC2 | [94,95] |
| ζ-Hf4N3 | R-3m | Hf4N3 | [94,95] |
| Zr3N4 | Pnma | [115] | |
| Zr3N4 | I-43d | Th3P4 | [109] |
| β-Zr7O8N4 | R-3H | Pr7O12 | [116] |
| β’-Zr7O11N2 | R-3H | Zr5Sc2O13 | [117] |
| β”-Zr7O9.5N3 | [118] | ||
| γ-Zr2ON2, Hf2ON2 | Ia-3 | Mn2O3 | [119] |
| Zr4O5N2 | I4cm | Flr-deriv | [120] |
| Predicted from computations to be stable at 1 atm | |||
| Zr3C, Hf3C, | Pnma | In3Ir | [121] |
| Zr3C2 Zr8C7 | [122] | ||
| (Zr,Hf)2ON2 | C1m1 | Pv-deriv | [123] |
| Hf6N | P-31c | [103] | |
| Hf2N | Pnnm | [103] | |
| HfN (ZrN) | P63/mmc | TiAs | [103] |
| Lattice parameter a, Å [35] | ΔH°f 298 kJ/mol [150] | D0 kJ/mol [150] | S298 J/mol/K [150] | Cp298 J/mol/K [150] | Cp2000 J/mol/K [30] | Tm, °C | α 10−6/K [23] | |
|---|---|---|---|---|---|---|---|---|
| TiC | 4.33 | −209 ± 21 § | 1388 ± 20 | 24.7 ± 0.2 | 34.3 ± 0.3 | 60.5 | 3067 ± 25 [143] | 7.4 |
| TiN | 4.24 | −338 ± 4 | 1261 | 30.3 ± 0.2 | 37.1 ± 0.1 | 61.2 | 2945 ± 30 [151] | 9.3 |
| ZrC | 4.70 | −207 ± 3 | 1508 ± 7 | 33.3 ± 0.1 | 37.9 ± 0.8 | 55.4 ǁ | 3572 ± 30 [62] | 6.7 |
| ZrN | 4.57 | −372 ± 2 † | 1438 ± 6 | 38.9 ± 0.2 | 40.4 ± 0.1 | 57.3 | 2955 ± 30 [151] | 7.2 |
| HfC | 4.64 | −208 ± 8 | 1537 ± 9 | 40.1 ± 0.2 | 38.1 ± 0.2 | 50.2§ | 3982 ± 30 [62] | 6.6 |
| HfN | 4.52 | −374 ± 2 ‡ | 1461 ± 5 | 45 ± 1 | 41 ± 2 | 55.8 * | 3330 ± 50 [151] § | 6.9 |
| HfCx | Fusion Enthalpy | HfCxNy | Fusion enthalpy | ||
|---|---|---|---|---|---|
| (eV/atom) | (kJ/mol) | (eV/atom) | (kJ/mol) | ||
| HfC | 0.67 | 130 | HfC0.75N0.22 | 0.79 | 150 |
| HfC0.97 | 0.68 | 130 | HfC0.62N0.19 | 0.71 | 124 |
| HfC0.94 | 0.76 | 141 | HfC0.56N0.25 | 0.73 | 127 |
| HfC0.91 | 0.72 | 133 | HfC0.56N0.38 | 0.75 | 141 |
| HfC0.88 | 0.73 | 131 | HfC0.44N0.5 | 0.74 | 139 |
| HfC0.84 | 0.73 | 130 | HfC0.31N0.62 | 0.69 | 130 |
| HfC0.81 | 0.72 | 126 | |||
| HfC0.78 | 0.69 | 118 | |||
| HfC0.75 | 0.69 | 117 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ushakov, S.V.; Navrotsky, A.; Hong, Q.-J.; van de Walle, A. Carbides and Nitrides of Zirconium and Hafnium. Materials 2019, 12, 2728. https://doi.org/10.3390/ma12172728
Ushakov SV, Navrotsky A, Hong Q-J, van de Walle A. Carbides and Nitrides of Zirconium and Hafnium. Materials. 2019; 12(17):2728. https://doi.org/10.3390/ma12172728
Chicago/Turabian StyleUshakov, Sergey V., Alexandra Navrotsky, Qi-Jun Hong, and Axel van de Walle. 2019. "Carbides and Nitrides of Zirconium and Hafnium" Materials 12, no. 17: 2728. https://doi.org/10.3390/ma12172728
APA StyleUshakov, S. V., Navrotsky, A., Hong, Q.-J., & van de Walle, A. (2019). Carbides and Nitrides of Zirconium and Hafnium. Materials, 12(17), 2728. https://doi.org/10.3390/ma12172728