Nanosilica Extracted from Hexafluorosilicic Acid of Waste Fertilizer as Reinforcement Material for Natural Rubber: Preparation and Mechanical Characteristics
Abstract
1. Introduction
2. Experimental Procedures
2.1. Materials
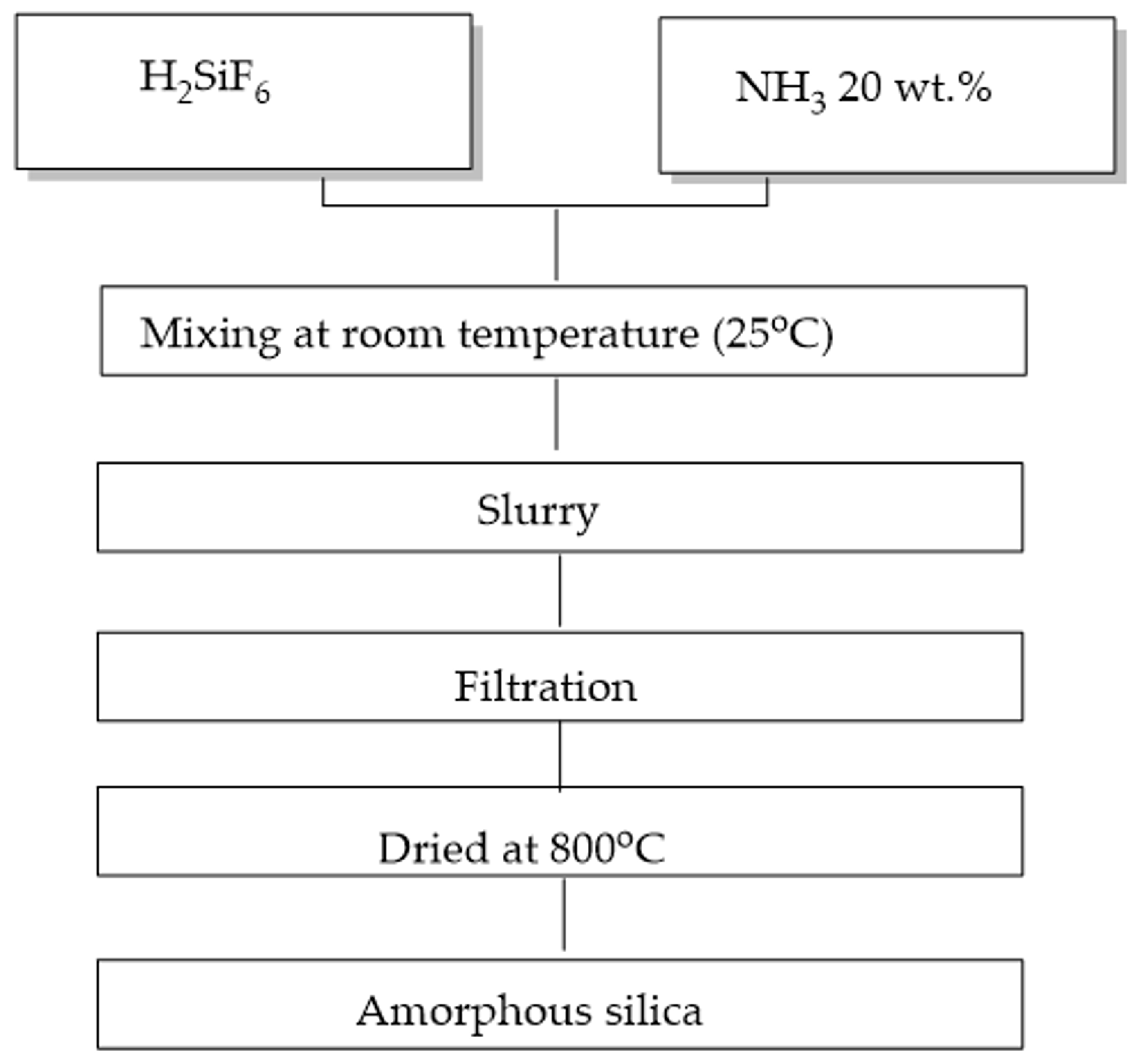
2.1.1. Recovery of Nanosilica
2.1.2. Rubber Compound Fabrication
2.2. Measurements
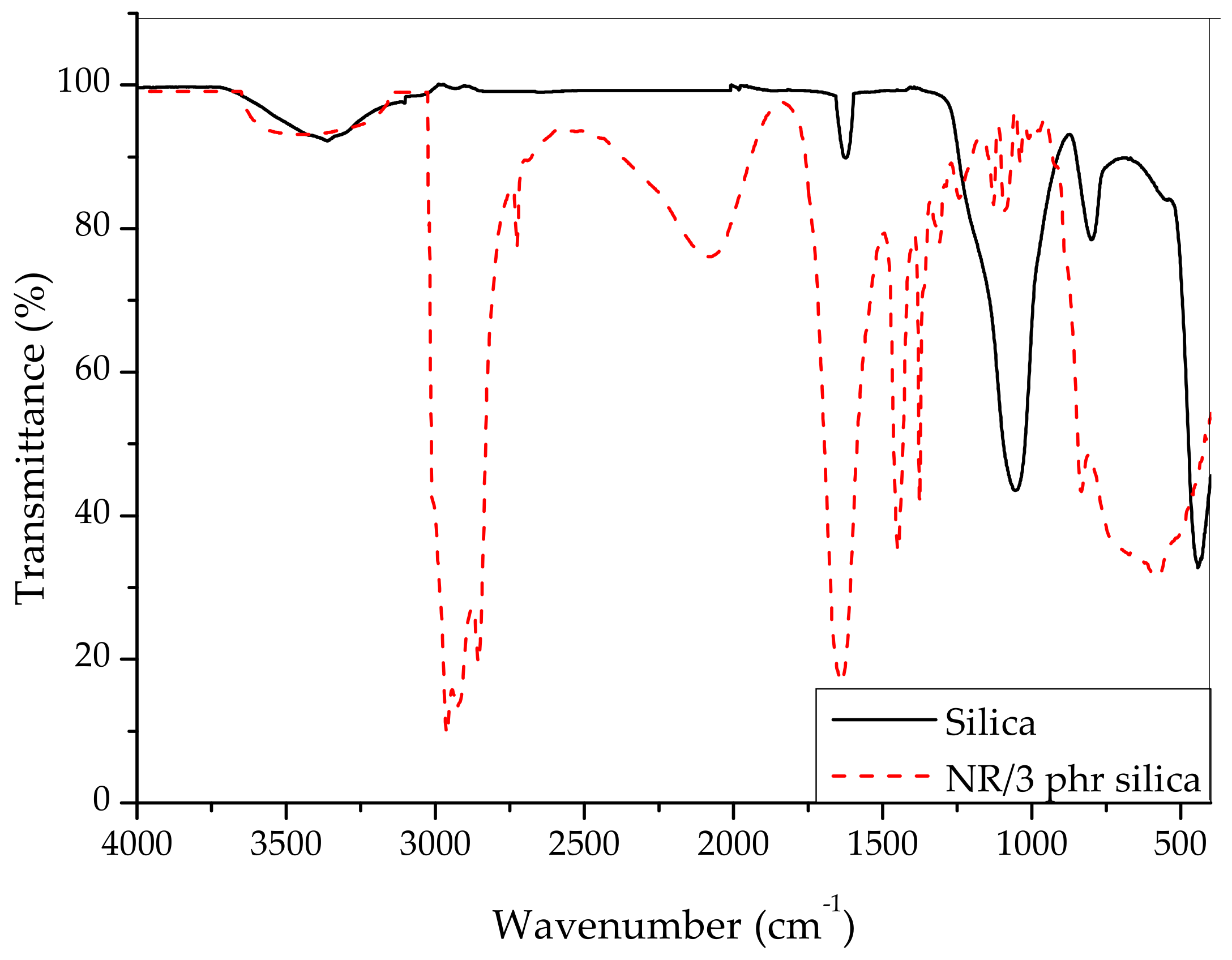
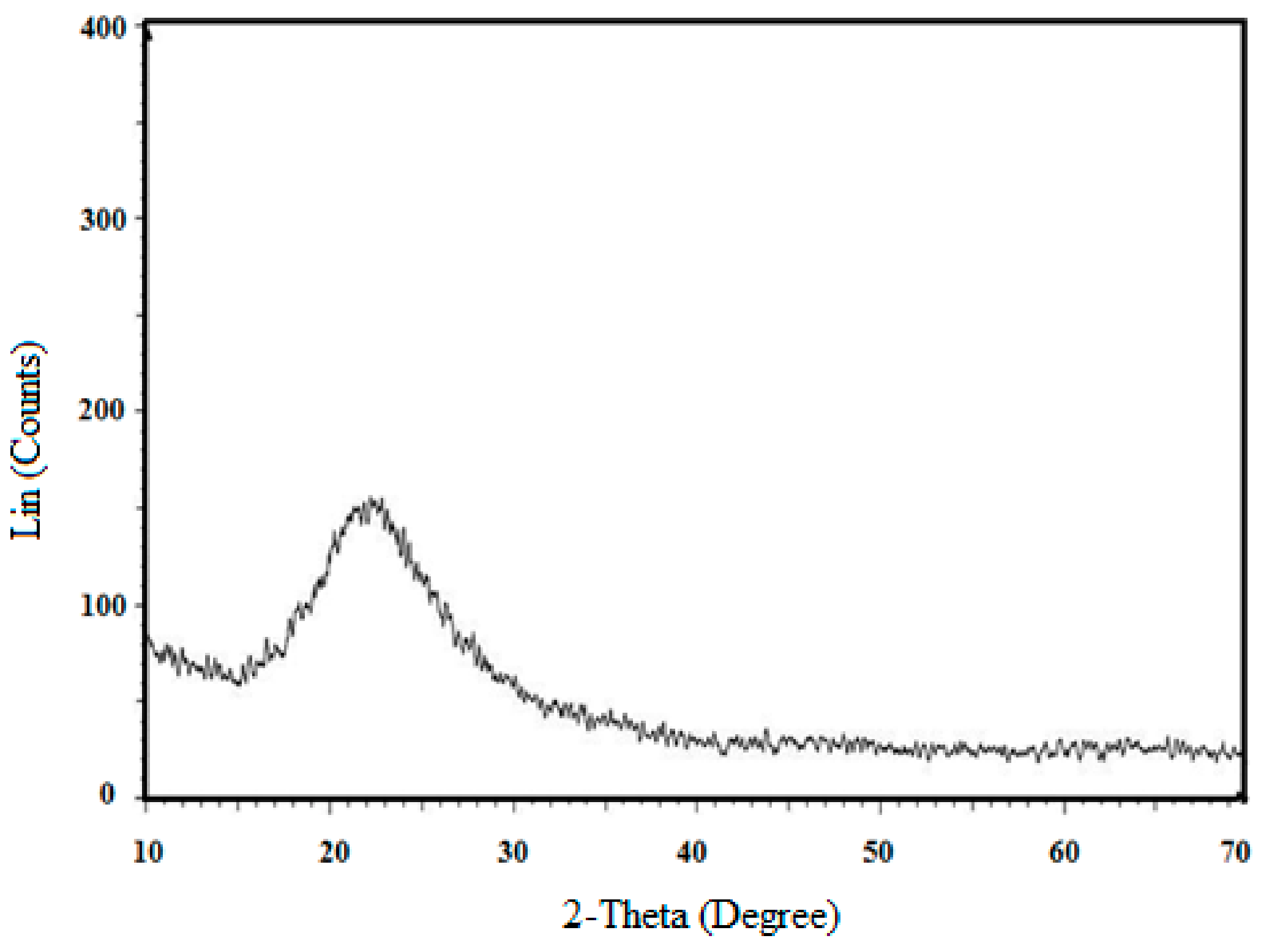
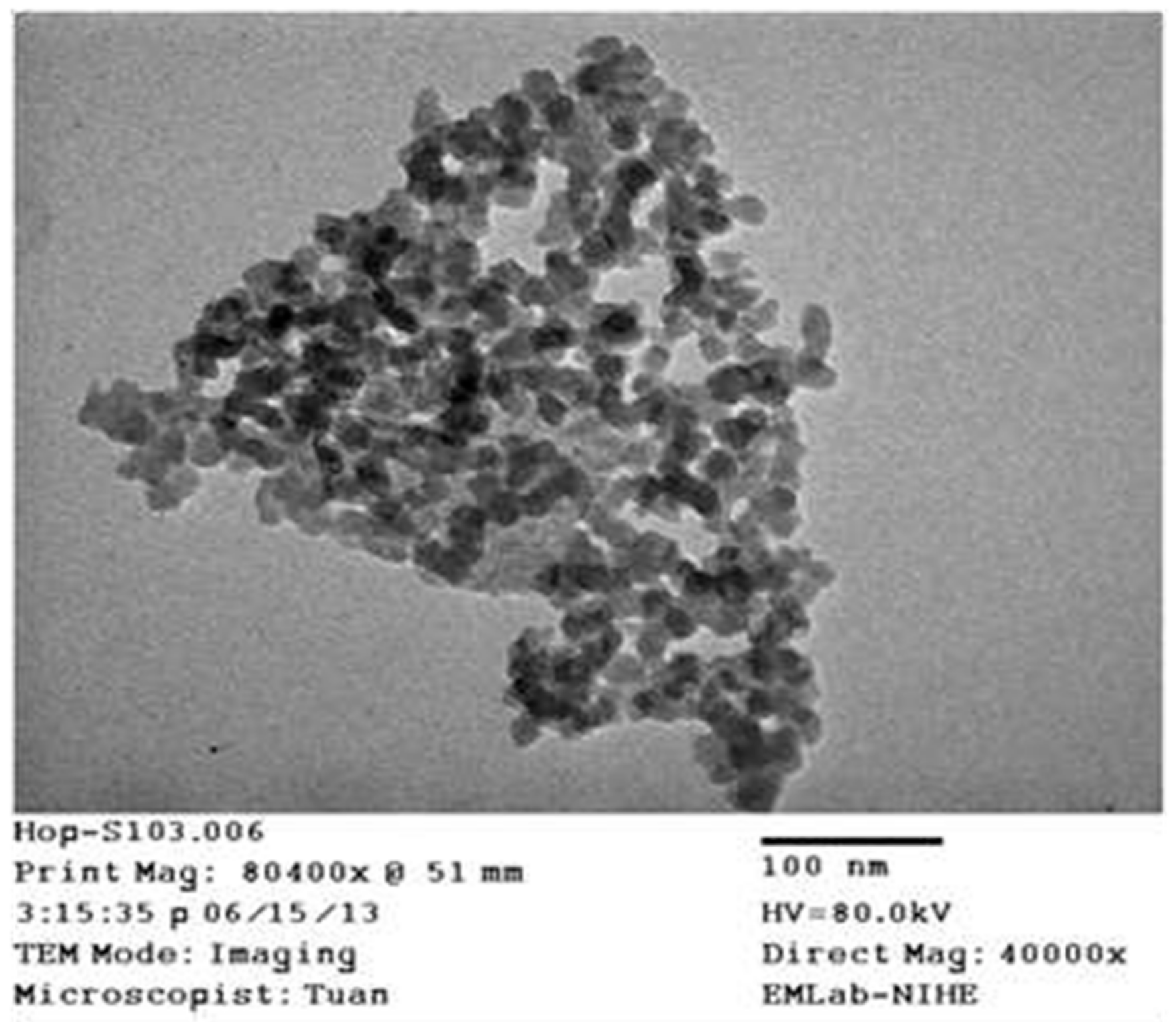
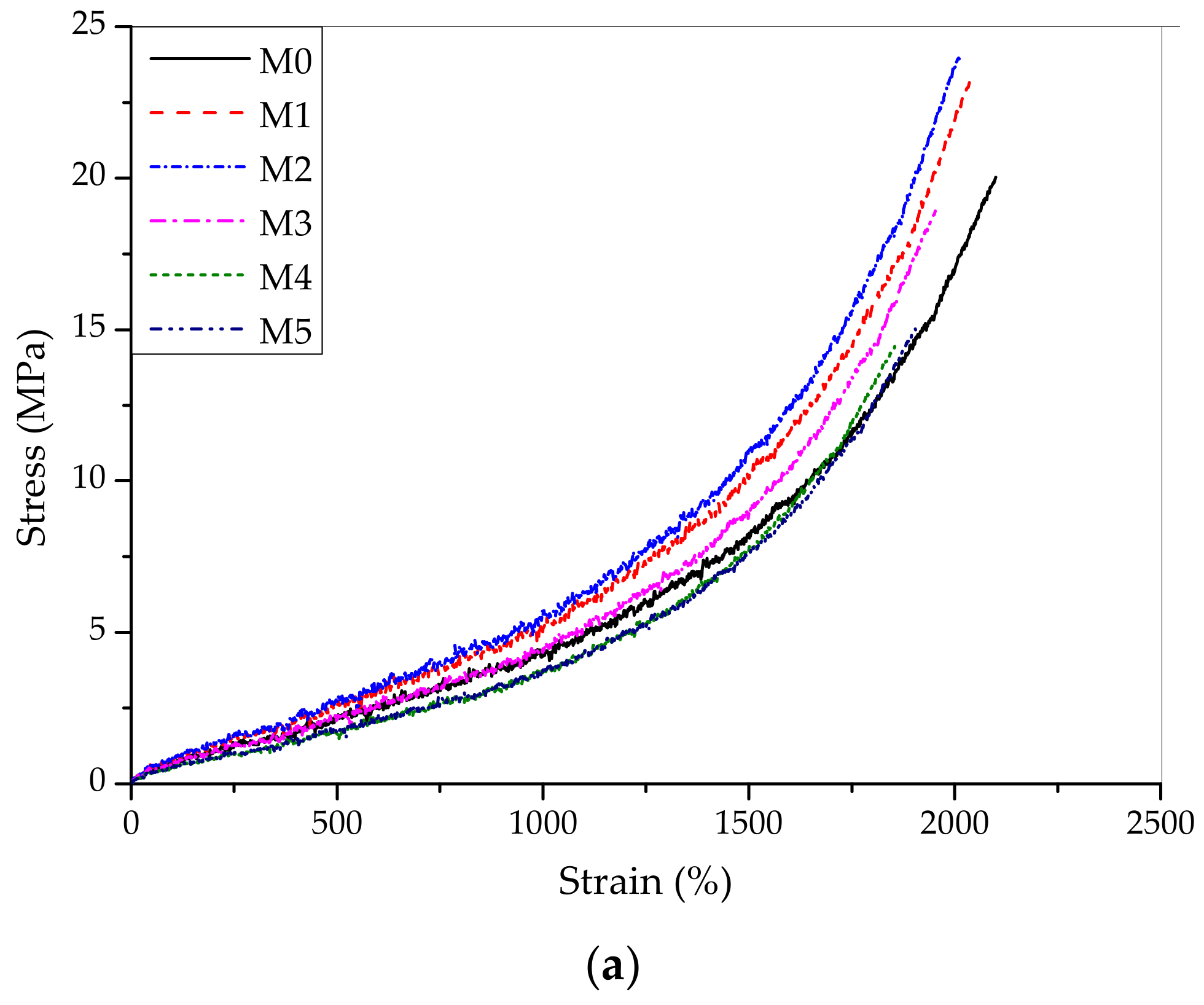
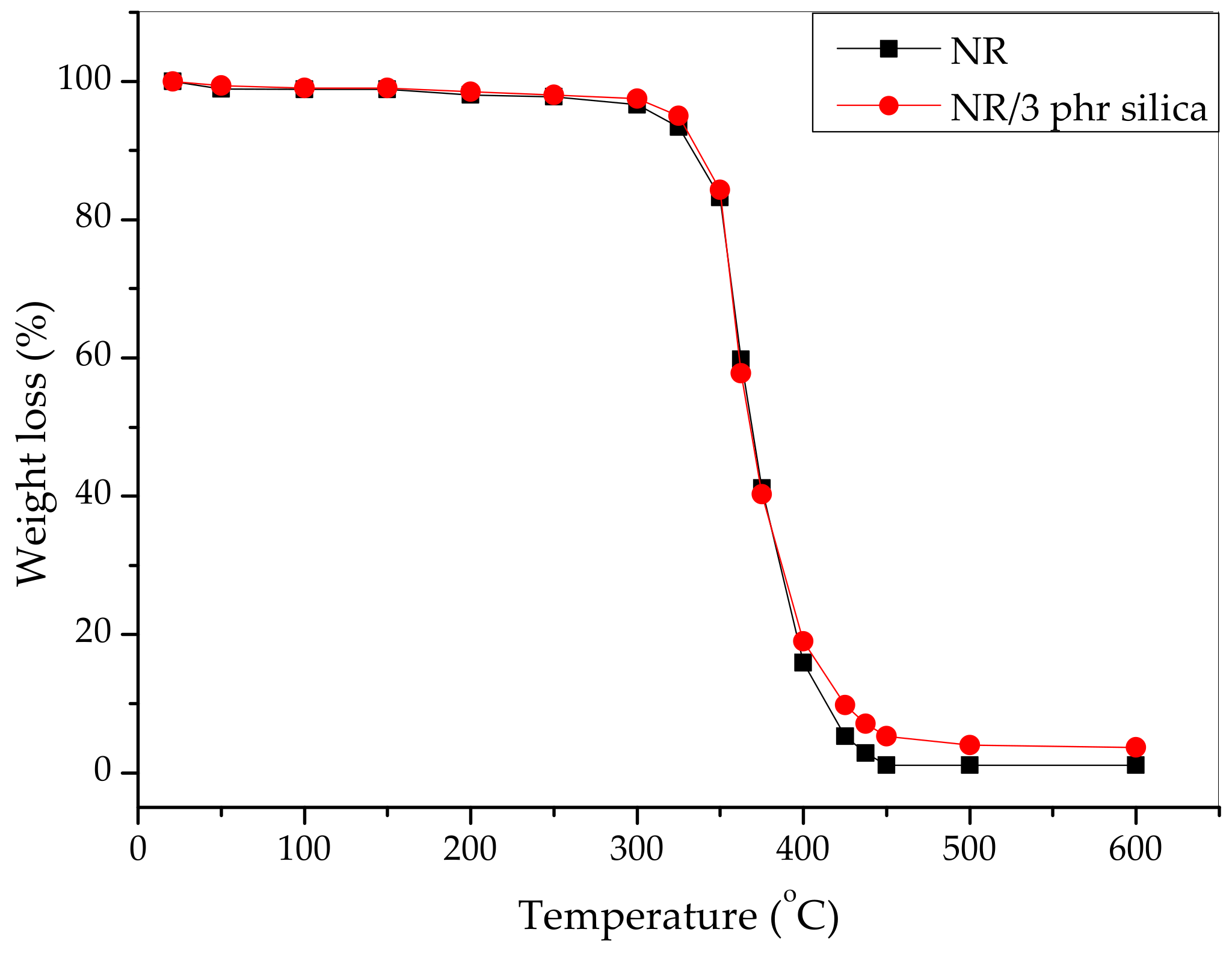
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chandra, C.S.J.; Bipinbal, P.K.; Sunil, K.N. Viscoelastic behaviour of silica filled natural rubber composites—Correlation of shear with elongational testing. Polym. Test. 2017, 60, 187–197. [Google Scholar] [CrossRef]
- Tchalla, S.T.; Gac, P.Y.L.; Maurin, R.; Creachcade, R. Polychloroprene behaviour in a marine environment: Role of silica fillers. Polym. Degrad. Stab. 2017, 139, 28–37. [Google Scholar] [CrossRef]
- Xu, T.; Jia, Z.; Wu, L.; Chen, Y.; Luo, Y.; Jia, D.; Peng, Z. Influence of acetone extract from natural rubber on the structure and interface interaction in NR/silica composites. Appl. Surf. Sci. 2017, 423, 43–52. [Google Scholar] [CrossRef]
- Zhang, C.; Tang, Z.; Guo, B.; Zhang, L. Significantly improved rubber-silica interface via subtly controlling surface chemistry of silica. Compos. Sci. Technol. 2018, 156, 70–77. [Google Scholar] [CrossRef]
- Jing, Y.; Niu, H.; Li, Y. Improved ethylene-propylene rubber/silica interface via in-situ polymerization. Polymer 2019, 172, 117–125. [Google Scholar] [CrossRef]
- Jong, L. Improved mechanical properties of silica reinforced rubber with natural polymer. Polym. Test. 2019, 79, 106009. [Google Scholar] [CrossRef]
- Tian, Q.; Tang, Y.; Ding, T.; Li, X.; Zhang, Z. Effect of nanosilica surface-capped by bis[3-(triethoxysilyl)propyl] tetrasulfide on the mechanical properties of styrene-butadiene rubber/butadiene rubber nanocomposites. Compos. Commun. 2018, 10, 190–193. [Google Scholar] [CrossRef]
- Xu, H.; Xia, X.; Hussain, M.; Song, Y.; Zheng, Q. Linear and nonlinear rheological behaviors of silica filled nitrile butadiene rubber. Polymer 2018, 156, 222–227. [Google Scholar] [CrossRef]
- Liu, D.; Song, L.; Song, H.; Chen, J.; Tian, Q.; Chen, L.; Sun, L.; Lu, A.; Huang, C.; Sun, G. Correlation between mechanical properties and microscopic structures of an optimized silica fraction in silicone rubber. Compos. Sci. Technol. 2018, 165, 373–379. [Google Scholar] [CrossRef]
- Dong, B.; Liu, C.; Wu, Y.P. Fracture and fatigue of silica/carbon black/natural rubber composites. Polym. Test. 2014, 38, 40–45. [Google Scholar] [CrossRef]
- Spratte, T.; Plagge, J.; Wunde, M.; Klüppel, M. Investigation of strain-induced crystallization of carbon black and silica filled natural rubber composites based on mechanical and temperature measurements. Polymer 2017, 115, 12–20. [Google Scholar] [CrossRef]
- Cheng, Y.Z.; Zeng, X.; Cheng, D.B.; Xu, X.D.; Zhang, X.Z.; Zhuo, R.X.; He, F. Functional mesoporous silica nanoparticles (MSNs) for highly controllable drug release and synergistic therapy. Colloids Surf. B Biointerfaces 2016, 145, 217–225. [Google Scholar] [CrossRef]
- Geng, H.; Zhao, Y.; Liu, J.; Cui, Y.; Wang, Y.; Zhao, Q.; Wang, S. Hollow mesoporous silica as a high drug loading carrier for regulation insoluble drug release. Int. J. Pharm. 2016, 510, 184–194. [Google Scholar] [CrossRef]
- Jiao, J.; Li, X.; Zhang, S.; Liu, J.; Di, D.; Zhang, Y.; Zhao, Q.; Wang, S. Redox and pH dual-responsive PEG and chitosan-conjugated hollow mesoporous silica for controlled drug release. Mater. Sci. Eng. C 2016, 67, 26–33. [Google Scholar] [CrossRef]
- Anbarasu, G.; Malathy, M.; Karthikeyan, P.; Rajavel, R. Silica functionalized Cu(II) acetylacetonate Schiff base complex: An efficient catalyst for the oxidative condensation reaction of benzyl alcohol with amines. J. Solid State Chem. 2017, 253, 305–312. [Google Scholar] [CrossRef]
- Leckie, L.; Mapolie, S.F. Mesoporous silica as phase transfer agent in the biphasic oxidative cleavage of alkenes using triazole complexes of ruthenium as catalyst precursors. Appl. Catal. A Gen. 2018, 565, 76–86. [Google Scholar] [CrossRef]
- Shabbir, S.; Lee, S.; Lim, M.; Lee, H.; Ko, H.; Lee, Y.; Rhee, H. Pd nanoparticles on reverse phase silica gel as recyclable catalyst for Suzuki-Miyaura cross coupling reaction and hydrogenation in water. J. Organomet. Chem. 2017, 846, 296–304. [Google Scholar] [CrossRef]
- Zeng, K.; Huang, Z.; Yang, J.; Gu, Y. Silica-supported policresulen as a solid acid catalyst for organic reactions. Chin. J. Catal. 2015, 36, 1606–1613. [Google Scholar] [CrossRef]
- Abou, R.M.; Faouzi, H. Synthesis and charaterization of amorphous silica nanoparticles from aqueous silicates using cationic Surfactants. J. Met. Mater. Miner. 2014, 24, 37–42. [Google Scholar]
- Elineema, G.; Kim, J.K.; Hilonga, A.; Shao, G.N.; Kim, Y.N.; Quang, D.V.; Sarawade, P.B.; Kim, H.T. Quantitative recovery of high purity nanoporous silica from waste products of the phosphate fertilizer industry. J. Ind. Eng. Chem. 2013, 19, 63–67. [Google Scholar] [CrossRef]
- Gustafsson, H.; Holmberg, K. Emulsion-based synthesis of porous silica. Adv. Colloid Interface Sci. 2017, 247, 426–434. [Google Scholar] [CrossRef]
- Kang, K.K.; Oh, H.S.; Kim, D.Y.; Shim, G.; Lee, C.S. Synthesis of Silica Nanoparticles Using Biomimetic Mineralization with Polyallylamine Hydrochloride. J. Colloid Interface Sci. 2017, 507, 145–153. [Google Scholar] [CrossRef]
- Kerdlap, W.; Thongpitak, C.; Keawmaungkom, S.; Warakulwit, C.; Klangprapan, S.; Choowongkomon, K.; Chisti, Y.; Hansupalak, N. Natural rubber as a template for making hollow silica spheres and their use as antibacterial agents. Micropor. Mesopor. Mater. 2019, 273, 10–18. [Google Scholar] [CrossRef]
- Zulfiqar, U.; Subhani, T.; Husain, S.W. Synthesis and characterization of silica nanoparticles from clay. J. Asian Ceramic Soc. 2016, 4, 91–96. [Google Scholar] [CrossRef]
- Sarawade, P.B.; Kim, J.K.; Hilonga, A.; Kim, H.T. Recovery of high surface area mesoporous silica from waste hexafluorosilicic acid (H2SiF6) of fertilizer industry. J. Hazard. Mater. 2010, 173, 576–580. [Google Scholar] [CrossRef]
- Satisk, K.W. Amorphous Precipitated Siliceous Pigment for Cosmetic or Dentifrice Use and Method for Their Production. USA Patent 3,928,541, 23 December 1975. [Google Scholar]
- Ui, S.W.; Lim, S.J.; Sang, H.L.; Choi, S.C. Control of size and morphology of nanosize silica particles using a sodium silicate solution. J. Ceram. Process. Res. 2009, 10, 553–558. [Google Scholar]
- Patel, B.H.; Patel, P.N. Synthesis and charaterization of silica nano particles by acid leaching technique. Res. J. Chem. Sci. 2014, 4, 52–55. [Google Scholar]
- Jin, F.; Wang, X.; Liu, T.; Wu, Y.; Xiao, L.; Yuan, M.; Fan, Y. Synthesis of ZSM-5 with the Silica Source from Industrial Hexafluorosilicic Acid as Transalkylation Catalyst. Chin. J. Chem. Eng. 2016, 25, 1303–1313. [Google Scholar] [CrossRef]
- Krysztafkiewicz, A.; Rager, B.; Maik, M. Silica recovery from waste obtained in hydrofluoric acid and aluminum fluoride production from fluosilicic acid. J. Hazard. Mater. 1996, 48, 31–49. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Suh, J.K.; Lee, J.M.; Kwon, O.Y. Preparation of silica-based mesoporous materials from fluorosilicon compounds: Gelation of H2SiF6 in ammonia surfactant solution. J. Colloid Interface Sci. 1997, 192, 156–161. [Google Scholar] [CrossRef]
- Panasyuk, G.P.; Azarova, L.A.; Privalov, V.L.; Belan, V.N.; Voroshilov, I.G.; Shpigun, L.K. Preparation of Silicon Dioxide with a Fixed Content of Fluorine from Fluorosilicic Acid. Theor. Found. Chem. Eng. 2018, 52, 607–611. [Google Scholar] [CrossRef]
- Sikdar, S.K.; Moore, J.H. Recovery of Hydrofluoric Acid from Fluosilicic Acid with High pH Hydrolysis. USA Patent 4,213,951, 22 July 1980. [Google Scholar]
- Sikdar, S.K.; Moore, J.H. Process for Producing Fluorine Compounds and Amorphous Silica. USA Patent 4,308,244, 29 December 1981. [Google Scholar]
- Spijker, R. Process for the Preparation of Pure Silicon Dioxide. Eur. Patent 1983, 94, 139. [Google Scholar]
- Toki, S.; Hsiao, B.S. Nature of Strain-Induced Structures in Natural and Synthetic Rubbers under Stretching. Macromolecules 2003, 36, 5915–5917. [Google Scholar] [CrossRef]
- Zorya, L.; Krot, V. Method of high-purity silica production from hexafluorosilicic acid. React. Kinet. Catal. Lett. 1993, 50, 349–354. [Google Scholar] [CrossRef]
- Dragicevic, T.; Hraste, M. Surface area of silica produced by neutralization of fluosilicic acid. Chem. Biochem. Eng. 1994, 8, 141–143. [Google Scholar]
- Cicala, G.; Bruno, G.; Capezzuto, P. Plasma deposition of amorphous silicon alloys from fluorinated gases. Pure Appl. Chem. 1996, 5, 1143–1149. [Google Scholar] [CrossRef]
- Guzeev, V.V.; D’yachenko, A.N.; Grishkov, V.N. Integrated utilization of silicon tetrafluoride and zirconium dioxide. Russ. J. Appl. Chem. 2003, 76, 1952–1955. [Google Scholar] [CrossRef]
- Liu, T.; Jin, F.; Wang, X.; Fan, Y.; Yuan, M. Synthesis of titanium containing MCM-41 from industrial hexafluorosilicic acid as epoxidation catalyst. Catal. Today 2017, 297, 316–323. [Google Scholar] [CrossRef]
- Vu, C.M.; Vu, H.T.; Choi, H.J. Fabrication of Natural Rubber/Epoxidized Natural Rubber/Nanosilica Nanocomposites and Their Physical Characteristics. Macromol. Res. 2015, 23, 284–290. [Google Scholar] [CrossRef]
- Chen, L.; Guo, X.; Luo, Y.; Jia, Z.; Bai, J.; Chen, Y.; Jia, D. Effect of novel supported vulcanizing agent on the interfacial interaction and strain-induced crystallization properties of natural rubber nanocomposites. Polymer 2018, 148, 390–399. [Google Scholar] [CrossRef]
- Chenal, J.M.; Chazeau, L.; Guy, L.; Bomal, Y.; Gauthier, C. Molecular weight between physical entanglements in natural rubber: A critical parameter during strain-induced crystallization. Polymer 2007, 48, 1042–1046. [Google Scholar] [CrossRef]





| Ingredients (phr) | M0 | M1 | M2 | M3 | M4 | M5 |
|---|---|---|---|---|---|---|
| Natural Rubber Zinc Oxide Stearic Acid Parafin RD CBS DM Sulfur Silica | 100.0 5.0 3.0 1.0 2.5 1.5 0.5 2.0 0.0 | 100.0 5.0 3.0 1.0 2.5 1.5 0.5 2.0 1.0 | 100.0 5.0 3.0 1.0 2.5 1.5 0.5 2.0 3.0 | 100.0 5.0 3.0 1.0 2.5 1.5 0.5 2.0 5.0 | 100.0 5.0 3.0 1.0 2.5 1.5 0.5 2.0 7.0 | 100.0 5.0 3.0 1.0 2.5 1.5 0.5 2.0 10.0 |
| Samples | Curing Properties | ||||
|---|---|---|---|---|---|
| Minimum Torque, ML (dN·m) | Maximum Torque, MH (dN·m) | △M = MH − ML (dN·m) | Scorch Time, ts2 (min:s) | Cure Time, t90 (min:s) | |
| M0 | 0.135 | 6.000 | 5.865 | 4:11 | 15:89 |
| M1 | 0.144 | 6.400 | 6.256 | 4:25 | 16:15 |
| M2 | 0.148 | 6.600 | 6.452 | 4:39 | 16:41 |
| M3 | 0.154 | 6.850 | 6.696 | 4:41 | 16:83 |
| M4 | 0.169 | 7.535 | 7.366 | 4:54 | 16:92 |
| M5 | 0.185 | 8.220 | 8.035 | 4:60 | 17:01 |
| Samples | Mechanical Properties | ||
|---|---|---|---|
| Tensile Strength (MPa) | Elongation at Break (%) | Hardness (Shore A) | |
| M0 | 20.02 | 2100.12 | 38.51 |
| M1 | 23.18 | 2036.85 | 39.72 |
| M2 | 24.15 | 2014.52 | 41.24 |
| M3 | 18.93 | 1953.77 | 42.51 |
| M4 | 15.13 | 1909.49 | 43.22 |
| M5 | 14.45 | 1855.26 | 45.06 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, V.-H.; Vu, C.M.; Choi, H.J.; Kien, B.X. Nanosilica Extracted from Hexafluorosilicic Acid of Waste Fertilizer as Reinforcement Material for Natural Rubber: Preparation and Mechanical Characteristics. Materials 2019, 12, 2707. https://doi.org/10.3390/ma12172707
Nguyen V-H, Vu CM, Choi HJ, Kien BX. Nanosilica Extracted from Hexafluorosilicic Acid of Waste Fertilizer as Reinforcement Material for Natural Rubber: Preparation and Mechanical Characteristics. Materials. 2019; 12(17):2707. https://doi.org/10.3390/ma12172707
Chicago/Turabian StyleNguyen, Van-Huy, Cuong Manh Vu, Hyoung Jin Choi, and Bui Xuan Kien. 2019. "Nanosilica Extracted from Hexafluorosilicic Acid of Waste Fertilizer as Reinforcement Material for Natural Rubber: Preparation and Mechanical Characteristics" Materials 12, no. 17: 2707. https://doi.org/10.3390/ma12172707
APA StyleNguyen, V.-H., Vu, C. M., Choi, H. J., & Kien, B. X. (2019). Nanosilica Extracted from Hexafluorosilicic Acid of Waste Fertilizer as Reinforcement Material for Natural Rubber: Preparation and Mechanical Characteristics. Materials, 12(17), 2707. https://doi.org/10.3390/ma12172707





