A Planar Model of an Ankle Joint with Optimized Material Parameters and Hertzian Contact Pairs
Abstract
1. Introduction
1.1. Modeling the Ankle
1.2. Dimensionality of the Models
1.3. The Aim of the Study and Its Novel Aspects
2. Materials and Methods
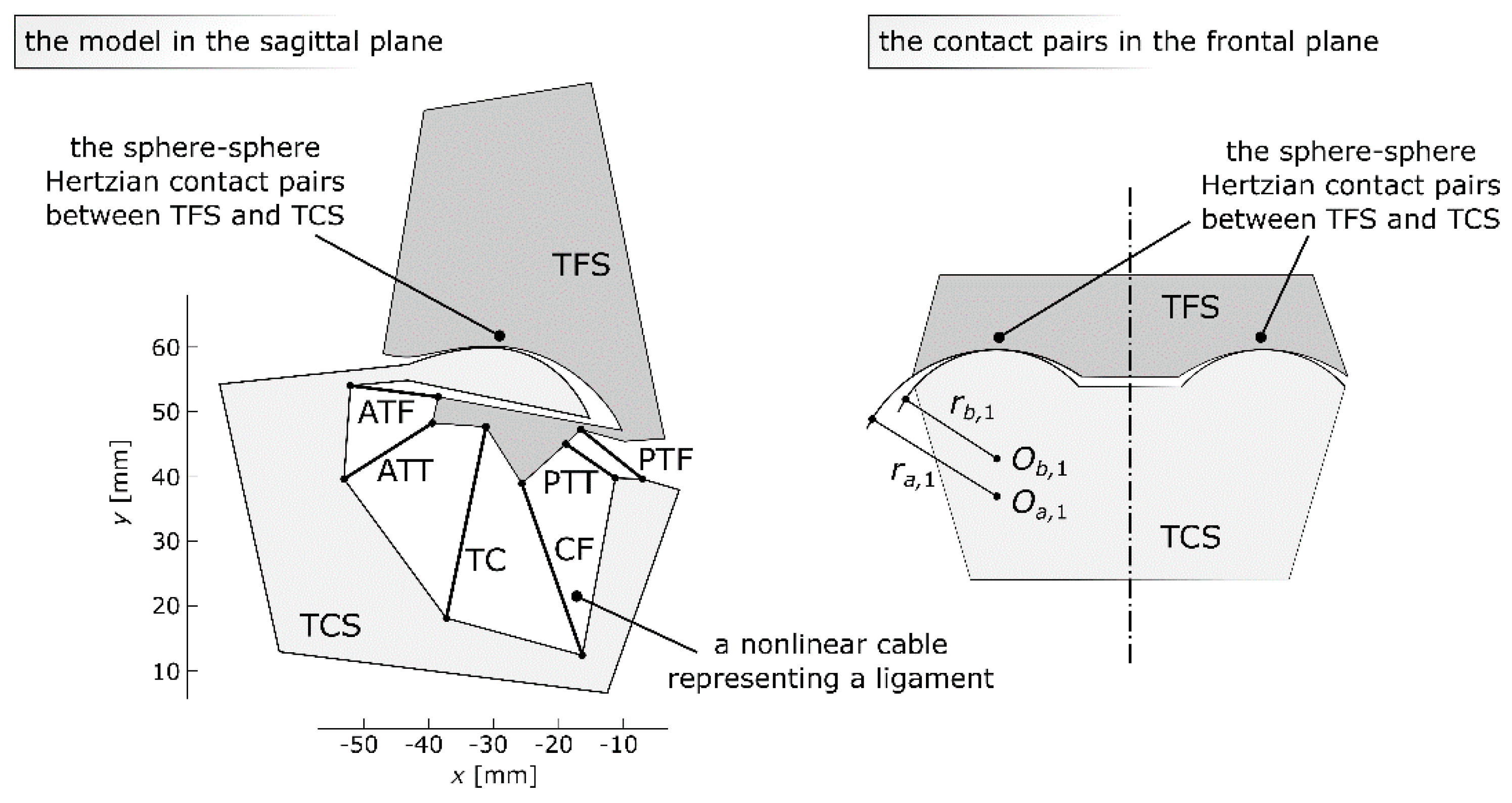
2.1. The Model
- Two rigid bodies: the basis - tibia-fibula segment (TFS), the moving body - talus-calcaneus segment (TCS);
- Six nonlinear planar cables, representing ATT, TC, PTT, ATF, CF, and PTF;
- Two symmetrical sphere–sphere Hertzian contact pairs representing the cartilage between the tibia and the talus;
- To specify the location of the TFS with regard to the TCS, three variables were used;
- One angular coordinate θ, used to compute the rotation matrix R from the TCS to TFS:
- Two linear coordinates, which formed the position vector between the frames p:
2.2. The Ligaments
2.3. Optimizing the Material Parameters of The Ligaments
2.4. The Cartilage
2.5. Solving Elastostatic Problems
3. Results
3.1. The Input Dataset
- Simulation #1: Mext = −0.20:0.20 Nm in 51 steps;
- Simulation #2: Mext = −5.00:5.00 Nm in 51 steps;
- Simulation #3: Mext = −5.00:5.00 Nm in 51 steps the geometry of PTT was modified to assess the sensitivity of the model.
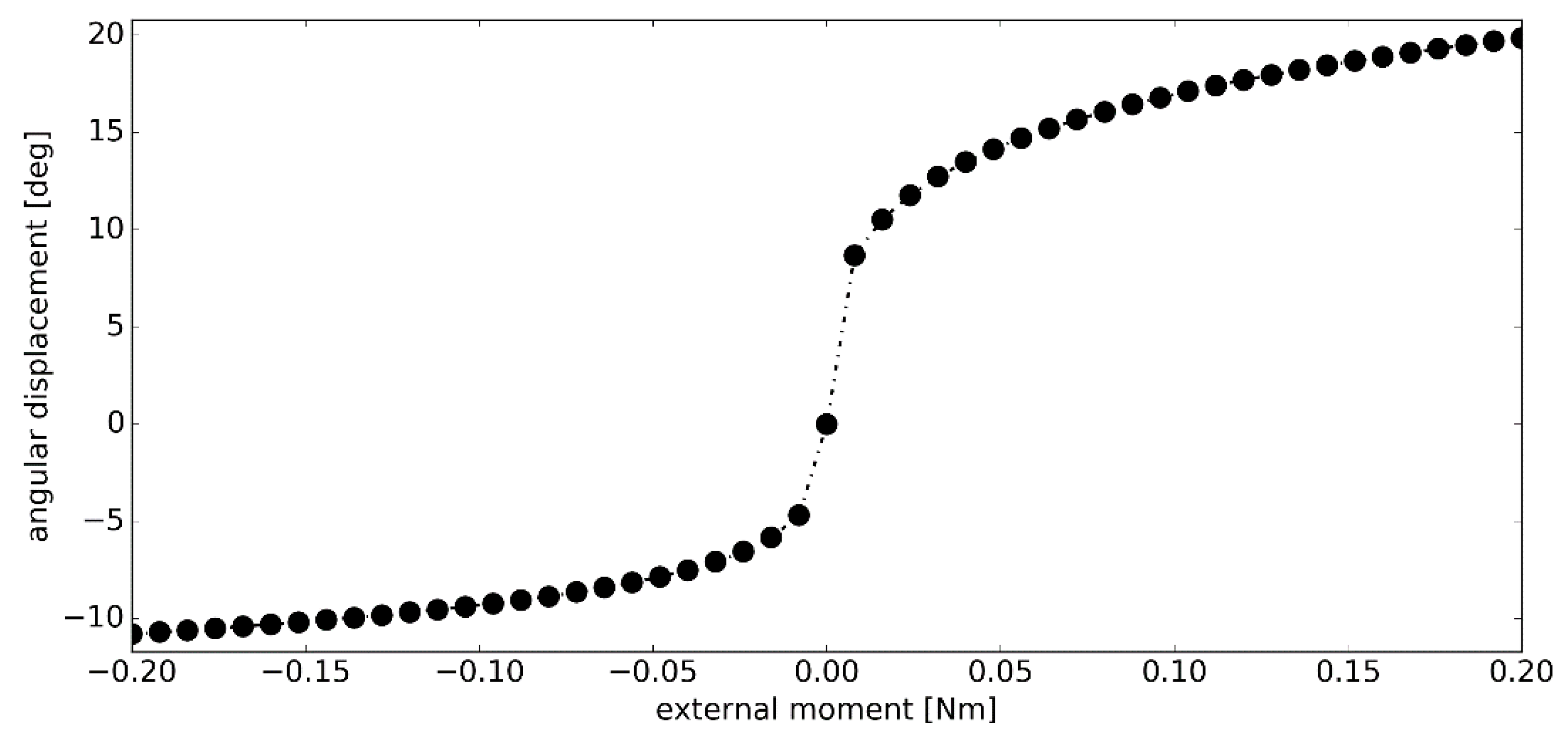
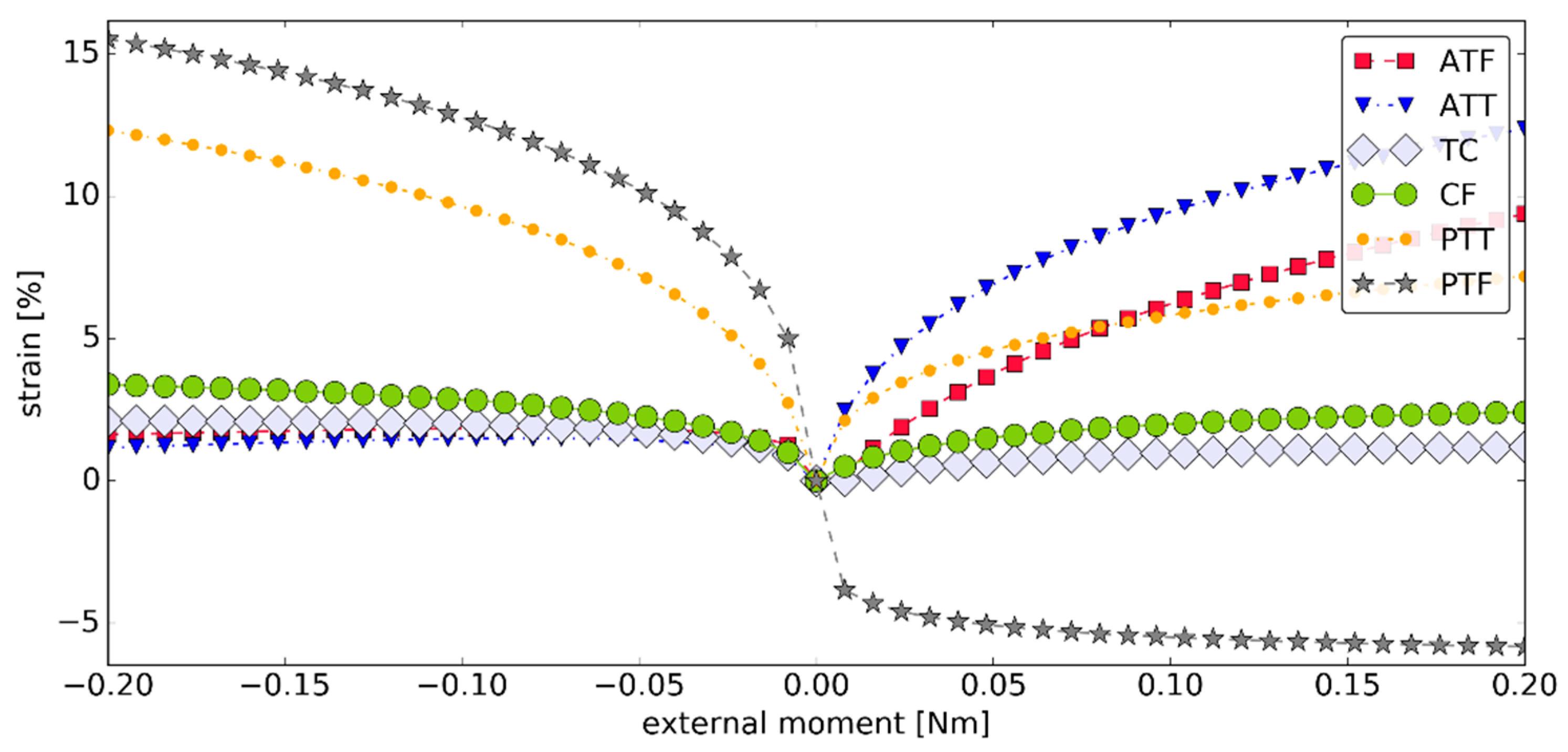
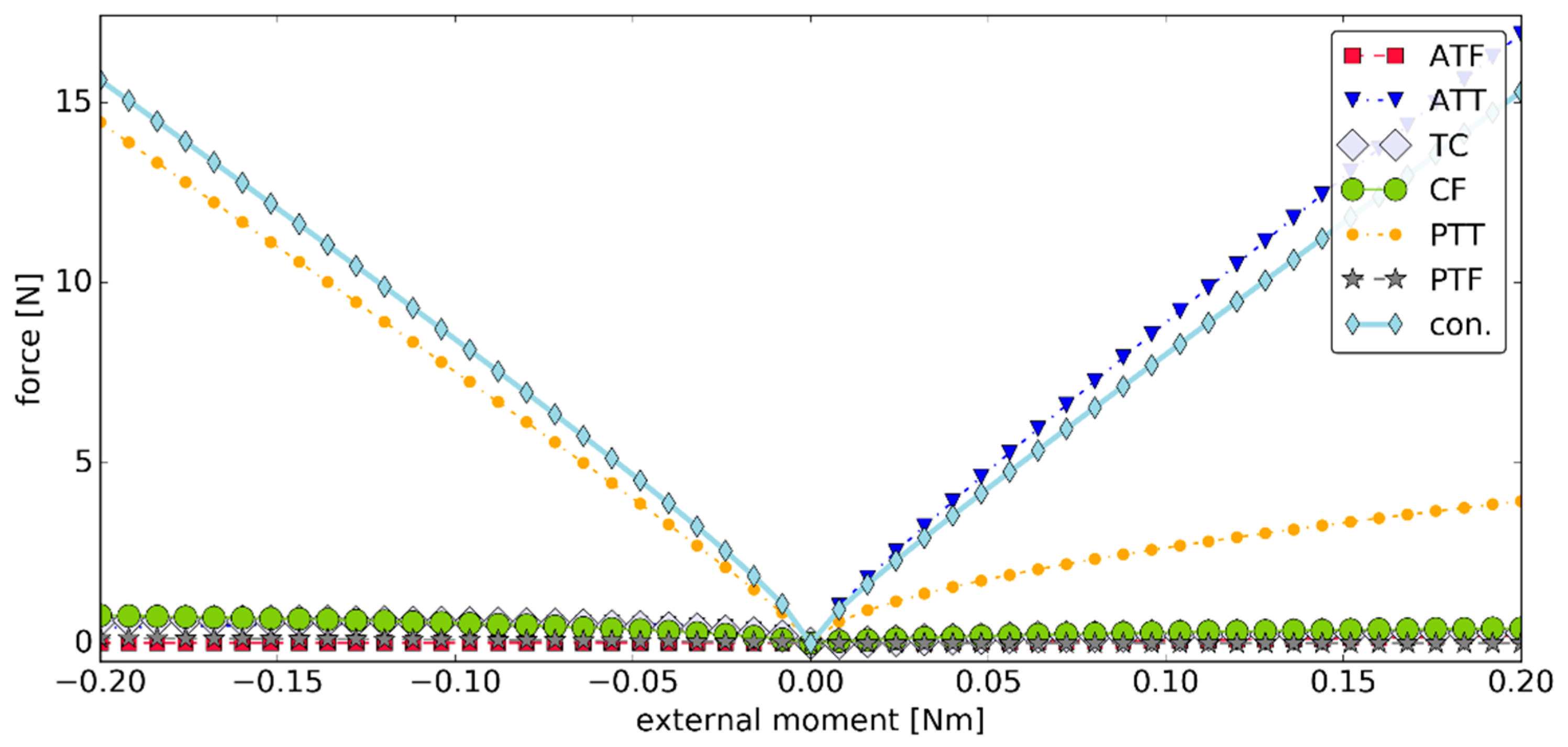
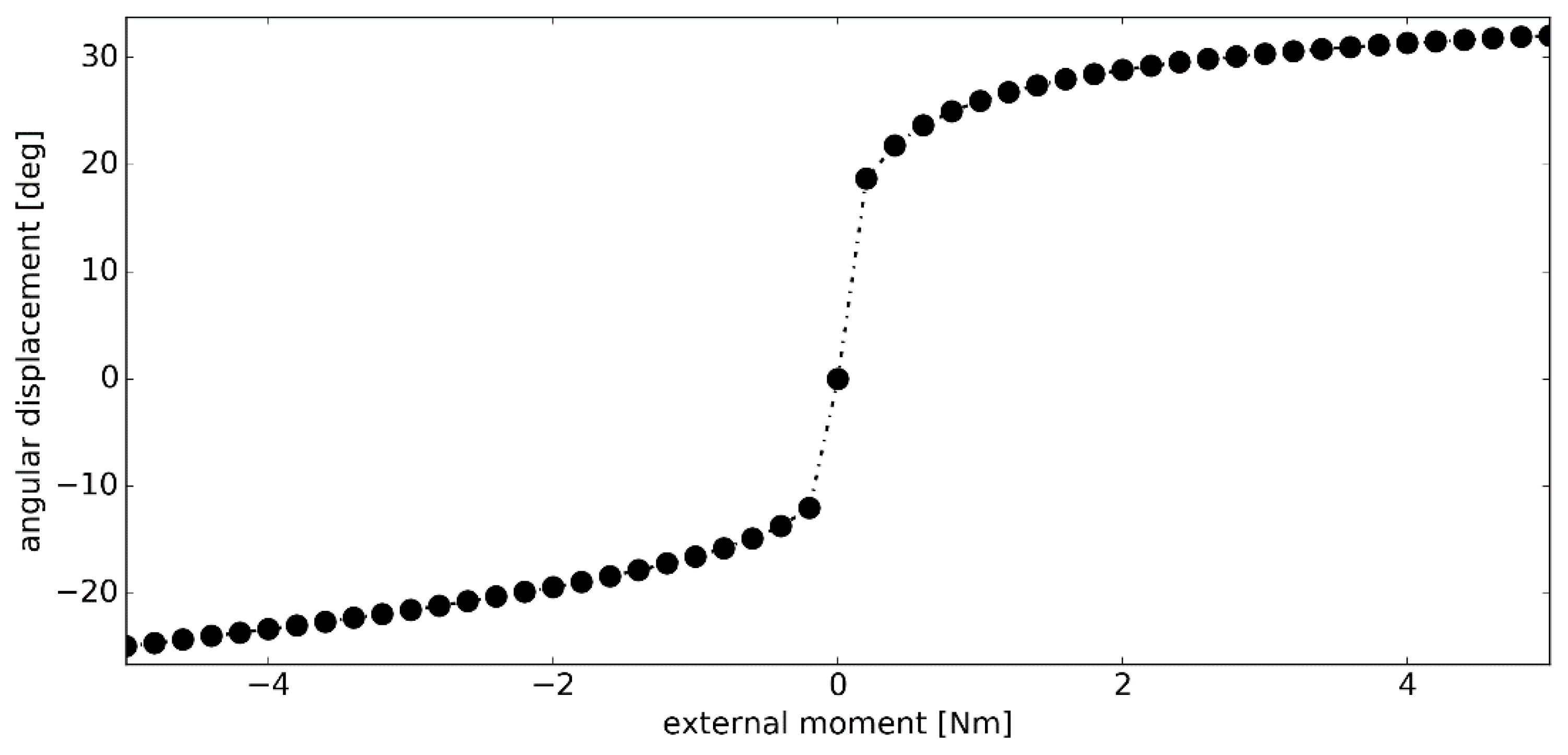
3.2. Simulation #1
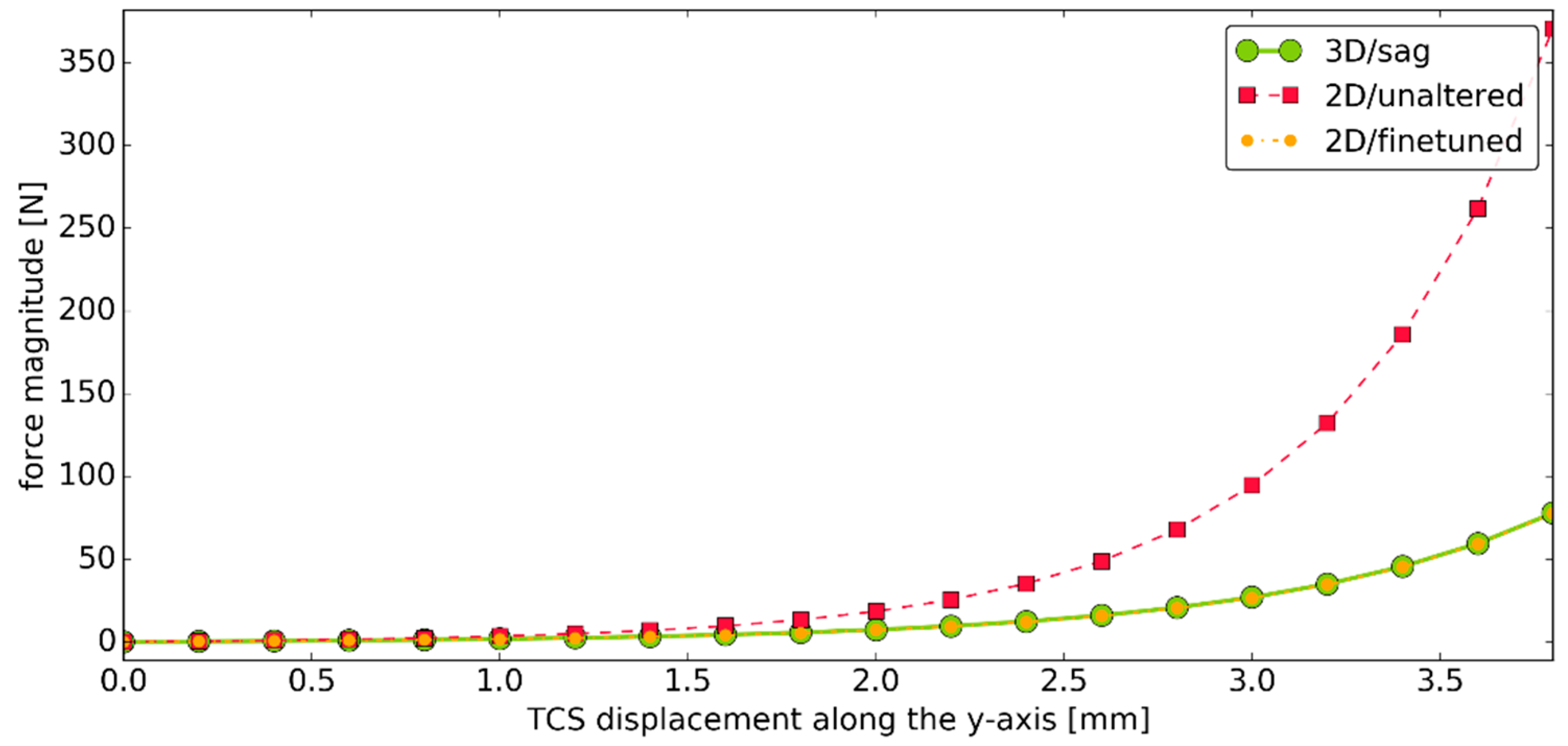
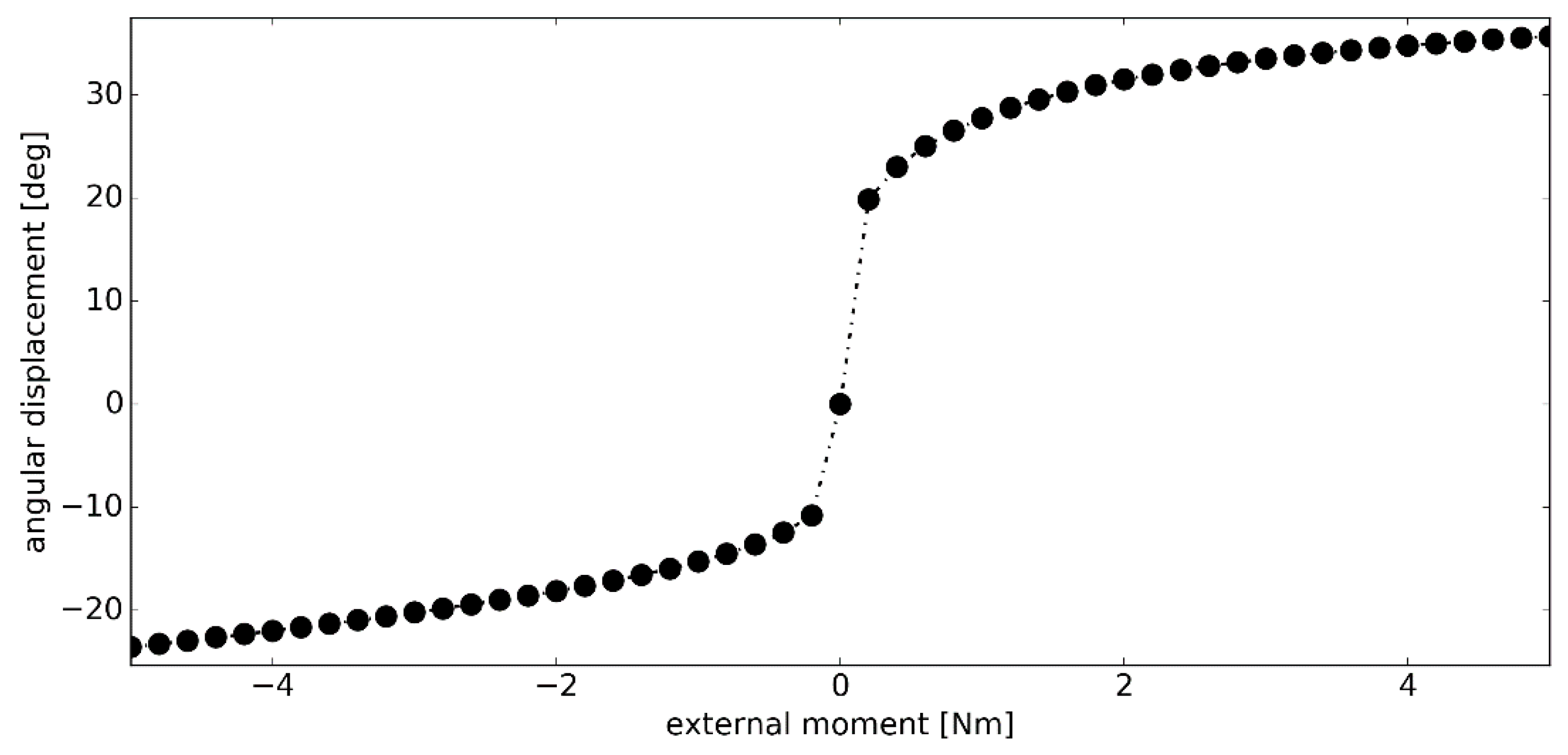
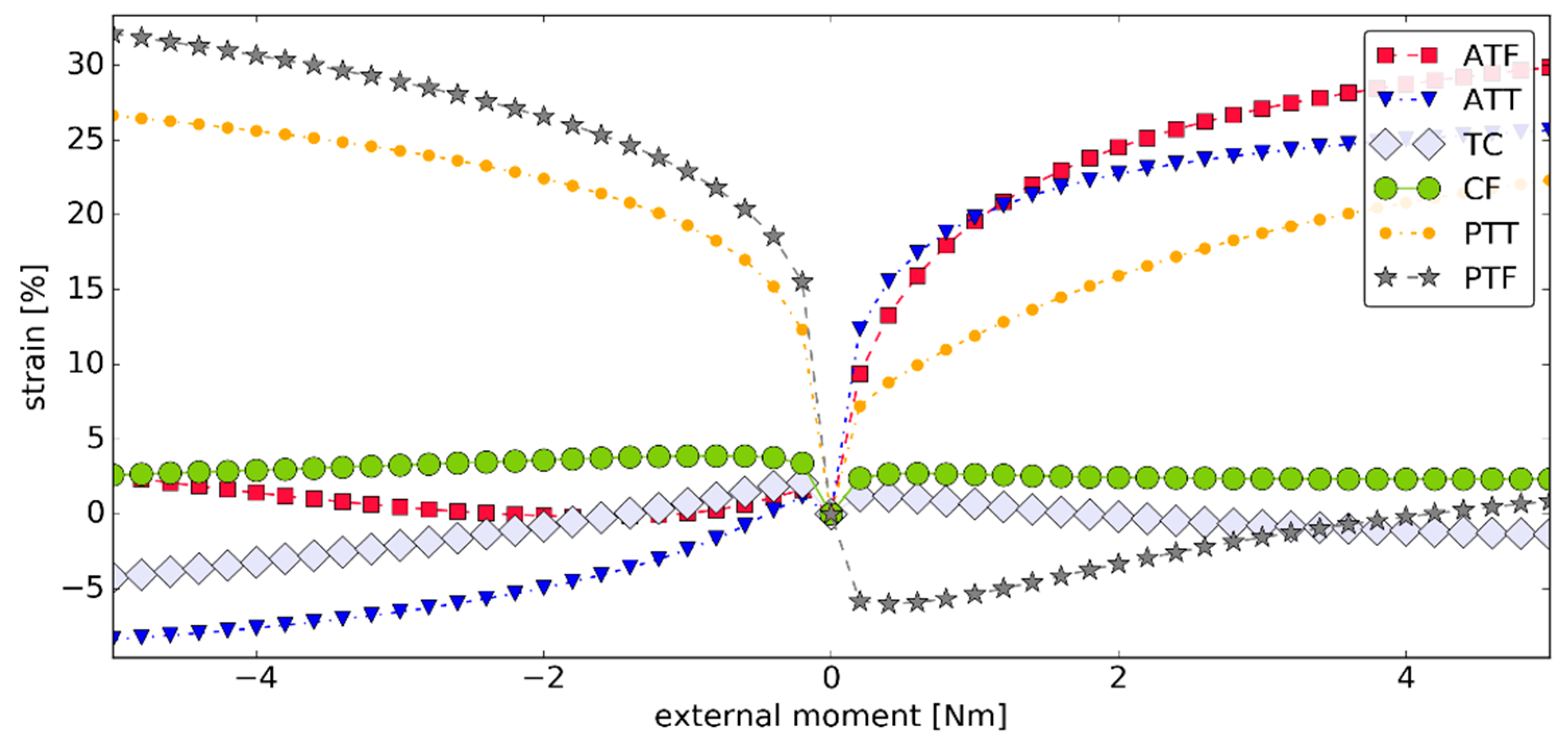
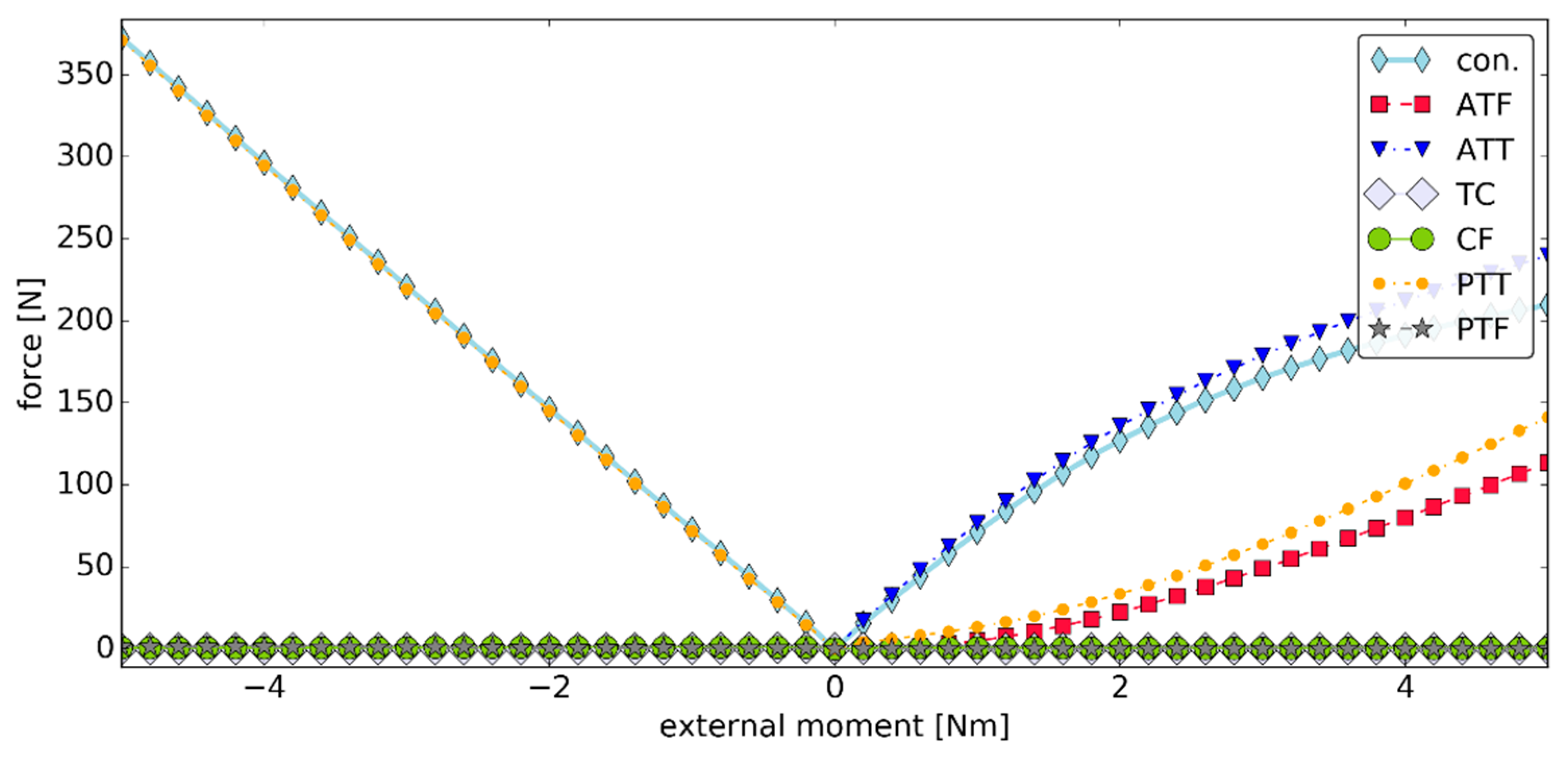
3.3. Simulation #2
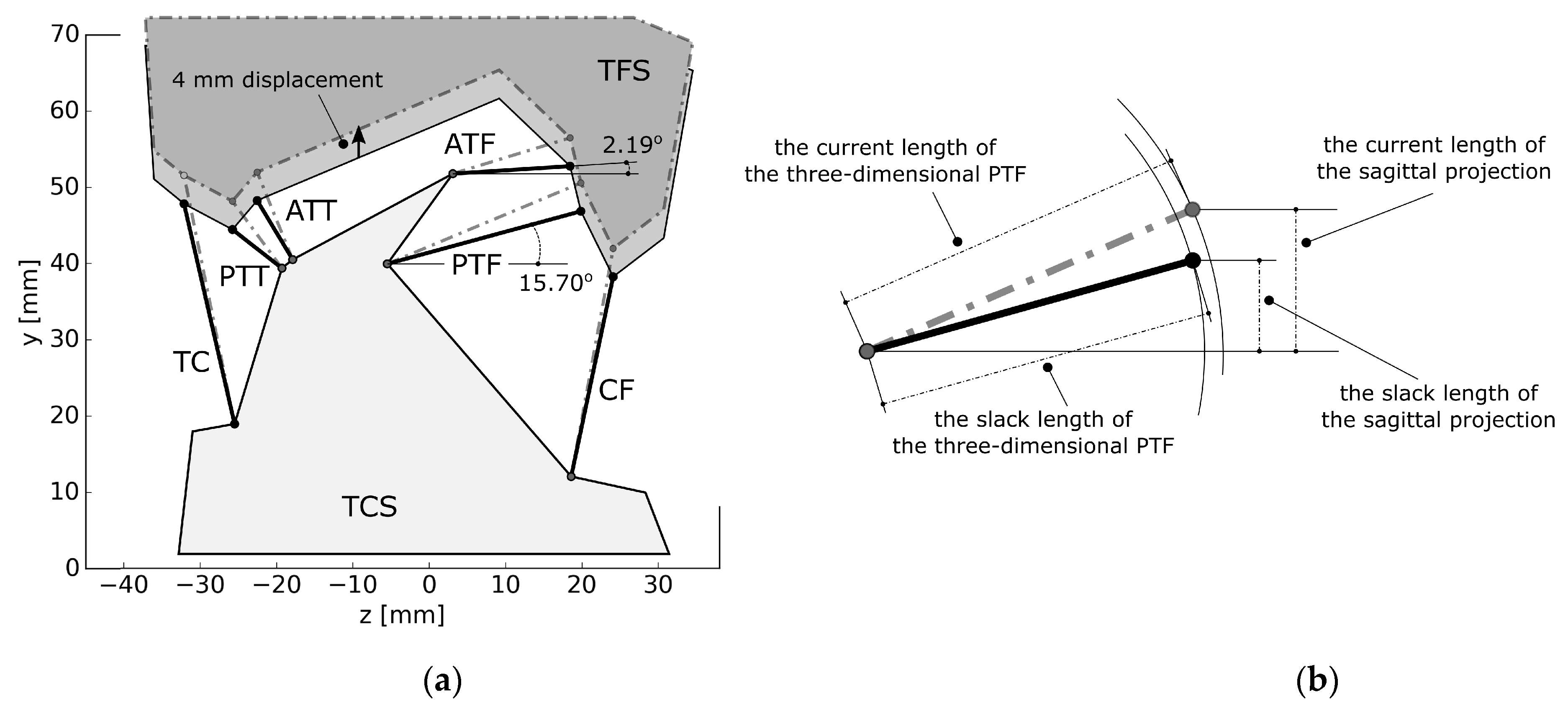
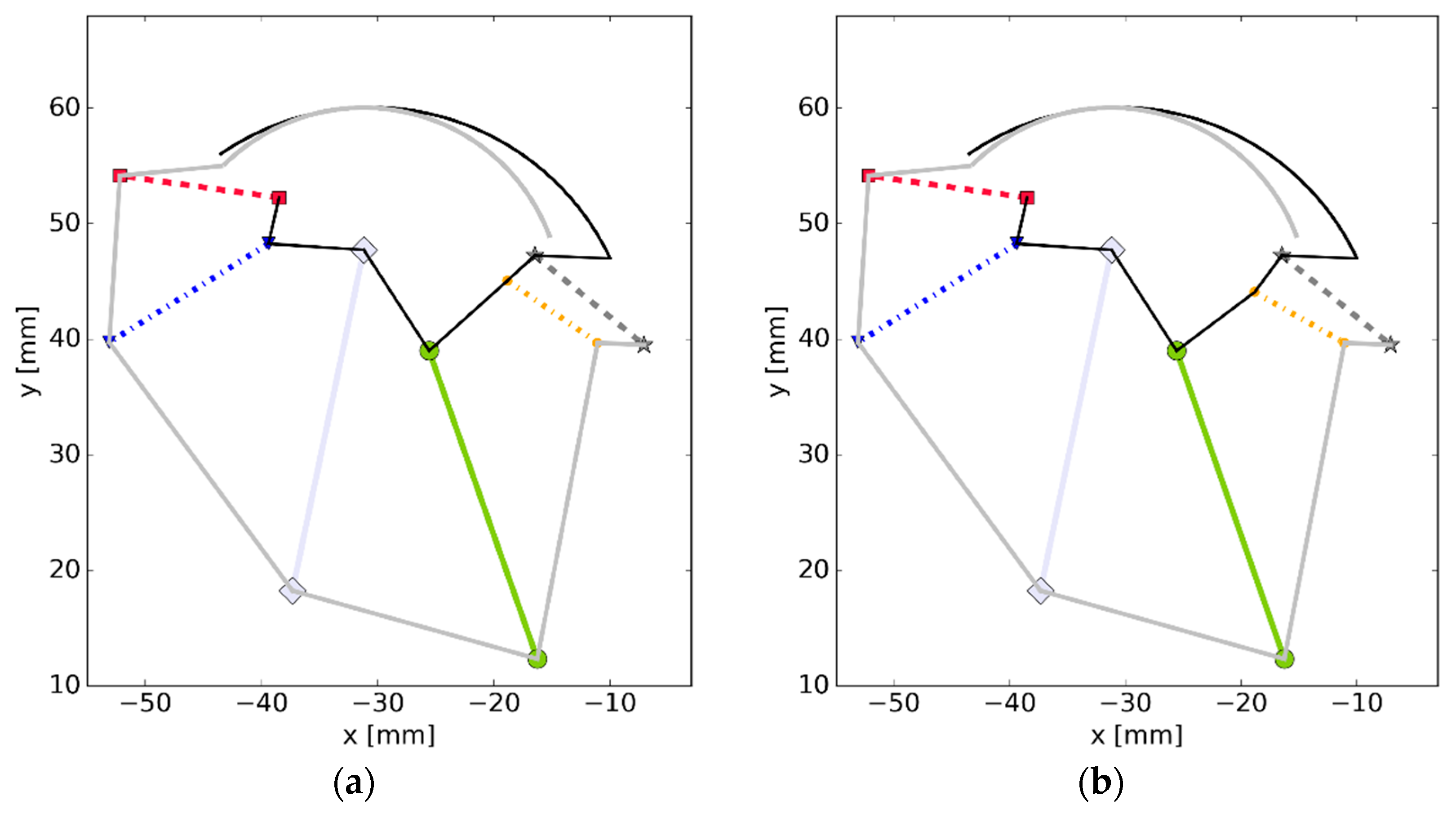
3.4. Simulation #3—Elements of a Sensitivity Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kudasik, T.; Libura, M.; Markowska, O.; Miechowicz, S. Methods for designing and fabrication large-size medical models for orthopaedics. Bull. Polish Acad. Sci. Tech. Sci. 2015, 63, 623–627. [Google Scholar] [CrossRef]
- Pietruski, P.; Majak, M.; Światek-Najwer, E.; Popek, M.; Jaworowski, J.; Zuk, M.; Nowakowski, F. Image-guided bone resection as a prospective alternative to cutting templates-A preliminary study. J. Craniomaxillofac. Surg. 2015, 43, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Ciszkiewicz, A.; Milewski, G. Path planning for minimally-invasive knee surgery using a hybrid optimization procedure. Comput. Methods Biomech. Biomed. Engin. 2018, 21, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Ciszkiewicz, A.; Lorkowski, J.; Milewski, G. A novel planning solution for semi-autonomous aspiration of Baker’s cysts. Int. J. Med. Robot. 2018, 14, e1882. [Google Scholar] [CrossRef] [PubMed]
- Baranowski, P.; Buka, J.; Damaziak, K.; Małachowski, J.; Mazurkiewicz, Ł.; Muszyński, A. Numerical analysis of child restraint system equipped with built-in belts pretensioner during frontal impact. In Proceedings of the Springer Proceedings in Mathematics & Statistics, Łódź, Poland, 7–10 December 2015; Springer: New York, NY, USA, 2016; pp. 27–38. [Google Scholar]
- Clin, J.; Aubin, C.É.; Parent, S.; Labelle, H. Biomechanical modeling of brace treatment of scoliosis: Effects of gravitational loads. Med. Biol. Eng. Comput. 2011, 49, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Szepietowska, K.; Magnain, B.; Lubowiecka, I.; Florentin, E. Sensitivity analysis based on non-intrusive regression-based polynomial chaos expansion for surgical mesh modelling. Struct. Multidiscip. Optim. 2018, 57, 1391–1409. [Google Scholar] [CrossRef]
- Szkoda-Poliszuk, K.; Żak, M.; Pezowicz, C. Finite element analysis of the influence of three-joint spinal complex on the change of the intervertebral disc bulge and height. Int. J. Numer. Method. Biomed. Eng. 2018, 34. [Google Scholar] [CrossRef]
- Sherman, M.A.; Seth, A.; Delp, S.L. Simbody: Multibody dynamics for biomedical research. Proc. IUTAM 2011, 2, 241–261. [Google Scholar] [CrossRef]
- Sensini, A.; Cristofolini, L. Biofabrication of Electrospun Scaffolds for the Regeneration of Tendons and Ligaments. Materials 2018, 11, 1963. [Google Scholar] [CrossRef]
- Beaugonin, M.; Haug, E.; Cesari, D. Improvement of numerical ankle/foot model: modeling of deformable bone. SAE Trans. 1997, 106, 3742–3754. [Google Scholar]
- Klekiel, T.; Będziński, R. Finite element analysis of large deformation of articular cartilage in upper ankle joint of occupant in military vehicles during explosion. Arch. Metall. Mater. 2015, 60, 2115–2121. [Google Scholar] [CrossRef][Green Version]
- Cheung, T.M.J.; Zhang, M.; An, K.N. Effects of plantar fascia stiffness on the biomechanical responses of the ankle-foot complex. Clin. Biomech. 2004, 19, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Tannous, R.E.; Bandak, F.A.; Toridis, T.G.; Eppinger, R.H. A Three-Dimensional finite element model of the human ankle: Development and preliminary application to axial impulsive loading. In Proceedings of the 40th Stapp Car Crash Conference, Albuquerque, NM, USA, 1 November 1996; pp. 219–238. [Google Scholar]
- Delp, S.L.; Anderson, F.C.; Arnold, A.S.; Loan, P.; Habib, A.; John, C.T.; Guendelman, E.; Thelen, D.G. OpenSim: Open-source software to create and analyze dynamic simulations of movement. IEEE Trans. Biomed. Eng. 2007, 54, 1940–1950. [Google Scholar] [CrossRef] [PubMed]
- Dettwyler, M.; Stacoff, A.; Kramers-De Quervain, I.A.; Stüssi, E. Modelling of the ankle joint complex. Reflections with regards to ankle prostheses. Foot Ankle Surg. 2004, 10, 109–119. [Google Scholar] [CrossRef]
- Jamwal, P.K.; Hussain, S.; Tsoi, Y.H.; Ghayesh, M.H.; Xie, S.Q. Musculoskeletal modelling of human ankle complex: Estimation of ankle joint moments. Clin. Biomech. 2017, 44, 75–82. [Google Scholar] [CrossRef]
- Lewis, G.S.; Sommer, H.J.; Piazza, S.J. In Vitro assessment of a motion-based optimization method for locating the talocrural and subtalar joint axes. J. Biomech. Eng. 2006, 128, 596. [Google Scholar] [CrossRef]
- Montefiori, E.; Modenese, L.; Di Marco, R.; Magni-Manzoni, S.; Malattia, C.; Petrarca, M.; Ronchetti, A.; de Horatio, L.T.; van Dijkhuizen, P.; Wang, A.; et al. An image-based kinematic model of the tibiotalar and subtalar joints and its application to gait analysis in children with Juvenile Idiopathic Arthritis. J. Biomech. 2019, 85, 27–36. [Google Scholar] [CrossRef]
- Vandenbogert, A.; Smith, G. In vivo determination of anatomical axes of ankle joint complex. J. Biomech. 1994, 27, 1477–1488. [Google Scholar] [CrossRef]
- Wright, I.C.; Bogert, A.J. Van Den The influence of foot position on ankle sprain. J. Biomech. 2000, 33, 513. [Google Scholar] [CrossRef]
- Leardini, A.; O’Connor, J.J.; Catani, F.; Giannini, S. A geometric model of the human ankle joint. J. Biomech. 1999, 32, 585–591. [Google Scholar] [CrossRef]
- Baldisserri, B. New mechanisms for modelling the motion of the human ankle complex. Ph.D. Thesis, University of Bologna, Bologna Italy, 2012. [Google Scholar]
- Franci, R.; Parenti-Castelli, V. A 5-5 one-degree-of-freedom fully parallel mechanism for the modeling of passive motion at the human ankle joint. In Proceedings of the ASME 2007 International Design Engineering Technical Conferences & Computers and Information in Engineering Conference IDETC/CIE 2007, Las Vegas, NV, USA, 4–7 September 2007; pp. 637–644. [Google Scholar]
- Gregorio, R.; Parenti-Castelli, V.; O’Connor, J.J.; Leardini, A. Mathematical models of passive motion at the human ankle joint by equivalent spatial parallel mechanisms. Med. Biol. Eng. Comput. 2007, 45, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Ciszkiewicz, A.; Milewski, G. A novel kinematic model for a functional spinal unit and a lumbar spine. Acta Bioeng. Biomech. 2016, 18, 87–95. [Google Scholar] [PubMed]
- Ottoboni, A.; Parenti-Castelli, V.; Sancisi, N.; Belvedere, C.; Leardini, A. Articular surface approximation in equivalent spatial parallel mechanism models of the human knee joint: An experiment-based assessment. Proc. Inst. Mech. Eng. H. 2010, 224, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Sancisi, N.; Parenti-Castelli, V. A novel 3D parallel mechanism for the passive motion simulation of the patella-femur-tibia complex. Meccanica 2011, 46, 207–220. [Google Scholar] [CrossRef]
- Sancisi, N.; Parenti-Castelli, V. A 1-Dof parallel spherical wrist for the modelling of the knee passive motion. Mech. Mach. Theory 2010, 45, 658–665. [Google Scholar] [CrossRef]
- Ciszkiewicz, A.; Knapczyk, J. Load analysis of a patellofemoral joint by a quadriceps muscle. Acta Bioeng. Biomech. 2016, 18, 111–119. [Google Scholar] [PubMed]
- Liacouras, P.C.; Wayne, J.S. Computational modeling to predict mechanical function of joints: application to the lower leg with simulation of two cadaver studies. J. Biomech. Eng. 2007, 129, 811. [Google Scholar] [CrossRef] [PubMed]
- Forlani, M.; Sancisi, N.; Parenti-Castelli, V. A Three-Dimensional Ankle Kinetostatic Model to Simulate Loaded and Unloaded Joint Motion. J. Biomech. Eng. 2015, 137. [Google Scholar] [CrossRef]
- Machado, M.; Moreira, P.; Flores, P.; Lankarani, H.M. Compliant contact force models in multibody dynamics: Evolution of the Hertz contact theory. Mech. Mach. Theory 2012, 53, 99–121. [Google Scholar] [CrossRef]
- Machado, M.; Flores, P.; Claro, J.C.P.; Ambrósio, J.; Silva, M.; Completo, A.; Lankarani, H.M. Development of a planar multibody model of the human knee joint. Nonlinear Dyn. 2009, 60, 459–478. [Google Scholar] [CrossRef]
- Hertz, H. On the contact of solids—on the contact of rigid elastic solids and on hardness. In Miscellaneous Papers; Macmillan: London, UK, 1896; pp. 146–183. [Google Scholar]
- Moeinzadeh, M.H.; Engin, A.E.; Akkas, N. Two-dimensional dynamic modelling of human knee joint. J. Biomech. 1983, 16, 253–264. [Google Scholar] [CrossRef]
- Funk, J.R.; Hall, G.W.; Crandall, J.R.; Pilkey, W.D. Linear and Quasi-Linear Viscoelastic Characterization of Ankle Ligaments. J. Biomech. Eng. 2002, 122, 15. [Google Scholar] [CrossRef]
- Ciszkiewicz, A.; Milewski, G. Ligament-based spine-segment mechanisms. Bull. Polish Acad. Sci. Tech. Sci. 2018, 66, 705–712. [Google Scholar]
- Ciszkiewicz, A.; Milewski, G. Structural and material optimization for automatic synthesis of spine-segment mechanisms for humanoid robots with custom sti ff ness profiles. Materials 2019, 12, 1982. [Google Scholar] [CrossRef]
- Van der Walt, S.; Colbert, S.C.; Varoquaux, G. The numpy array: A structure for efficient numerical computation. Comput. Sci. Eng. 2011, 13, 22–30. [Google Scholar] [CrossRef]
- Standring, S. Gray’s Anatomy: The Anatomical Basis of Medicine and Surgery, 39th ed.; Elsevier Churchill Livingstone: London, UK, 2005; pp. 1430–1445. [Google Scholar]
- Chen, J.; Siegler, S.; Schneck, C.D. The Three-dimensional kinematics and flexibility characteristics of the human ankle and subtalar joint—Part II: Flexibility characteristics. J. Biomech. Eng. 1988, 110, 374–385. [Google Scholar] [CrossRef]
- De Asla, R.J.; Kozánek, M.; Wan, L.; Rubash, H.E.; Li, G. Function of anterior talofibular and calcaneofibular ligaments during in-vivo motion of the ankle joint complex. J. Orthop. Surg. Res. 2009, 6, 1–6. [Google Scholar]
- Muc, A.; Gurba, W. Genetic algorithms and finite element analysis in optimization of composite structures. Compos. Struct. 2001, 54, 275–281. [Google Scholar] [CrossRef]
- Muc, A.; Muc-Wierzgoń, M. An evolution strategy in structural optimization problems for plates and shells. Compos. Struct. 2012, 94, 1461–1470. [Google Scholar] [CrossRef]
- Bukala, J.; Malachowski, J.; Szafranski, T. Numerical optimization and design study of small wind turbine mast structure. In Proceedings of the IECON 2016-42nd Annual Conference of the IEEE Industrial Electronics Society, Piscataway, NJ, USA, 23–26 October 2016; 2016; pp. 5562–5567. [Google Scholar]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borucka, A.; Ciszkiewicz, A. A Planar Model of an Ankle Joint with Optimized Material Parameters and Hertzian Contact Pairs. Materials 2019, 12, 2621. https://doi.org/10.3390/ma12162621
Borucka A, Ciszkiewicz A. A Planar Model of an Ankle Joint with Optimized Material Parameters and Hertzian Contact Pairs. Materials. 2019; 12(16):2621. https://doi.org/10.3390/ma12162621
Chicago/Turabian StyleBorucka, Aleksandra, and Adam Ciszkiewicz. 2019. "A Planar Model of an Ankle Joint with Optimized Material Parameters and Hertzian Contact Pairs" Materials 12, no. 16: 2621. https://doi.org/10.3390/ma12162621
APA StyleBorucka, A., & Ciszkiewicz, A. (2019). A Planar Model of an Ankle Joint with Optimized Material Parameters and Hertzian Contact Pairs. Materials, 12(16), 2621. https://doi.org/10.3390/ma12162621





