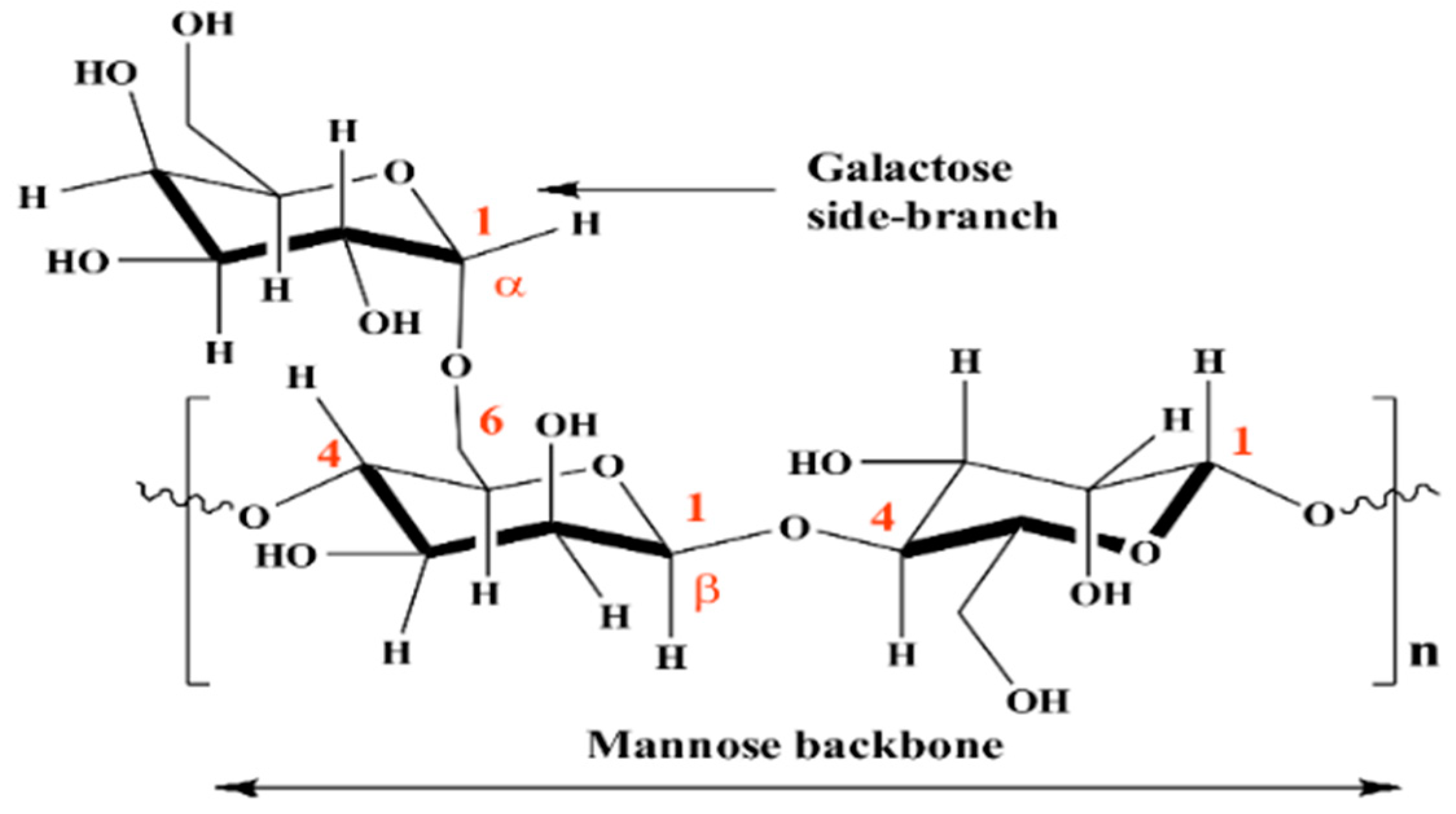
Guar Gum as an Eco-Friendly Corrosion Inhibitor for Pure Aluminium in 1-M HCl Solution
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Material and Solution
2.2. Gravimetric Measurement
2.3. Electrochemical Measurements
2.4. Surface Analysis
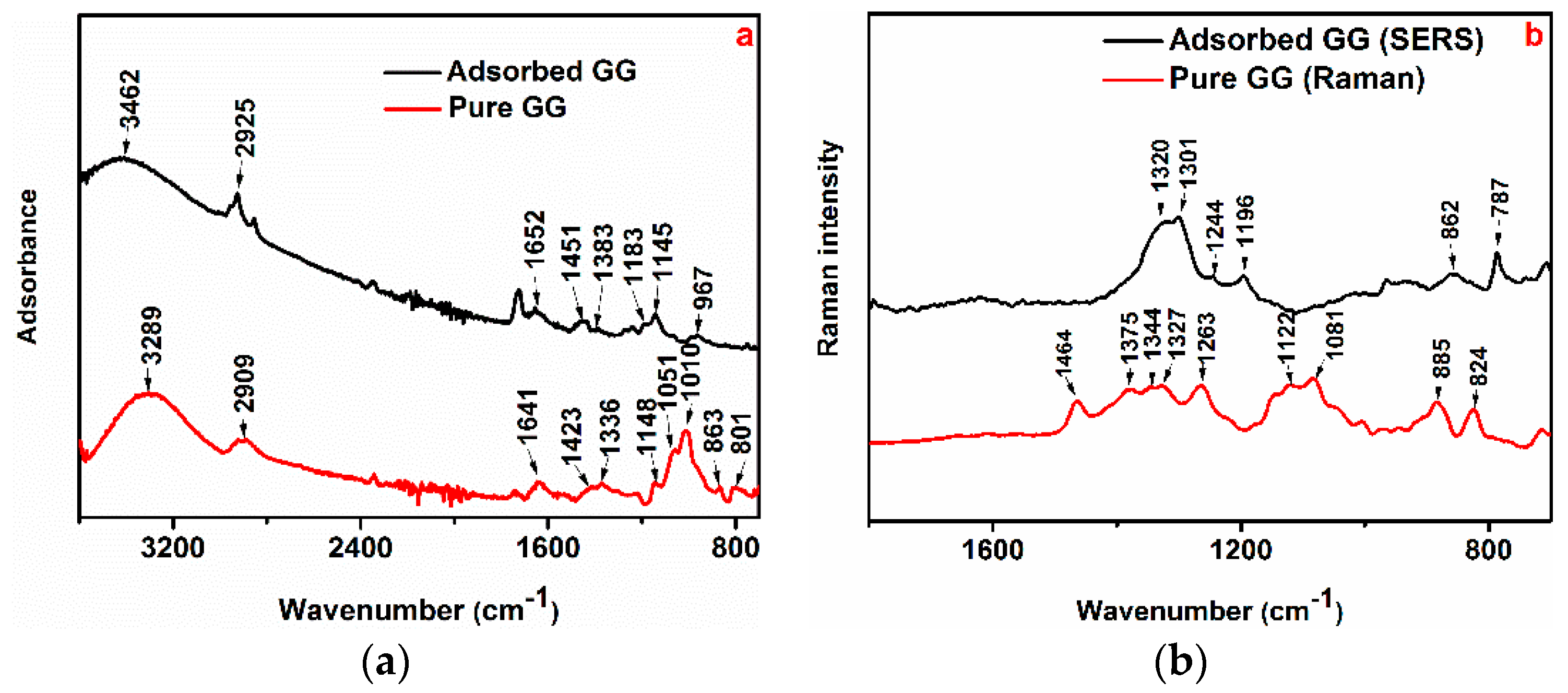
2.4.1. Fourier-Transform Infrared (FTIR) Spectroscopy
2.4.2. Raman and SERS Measurement
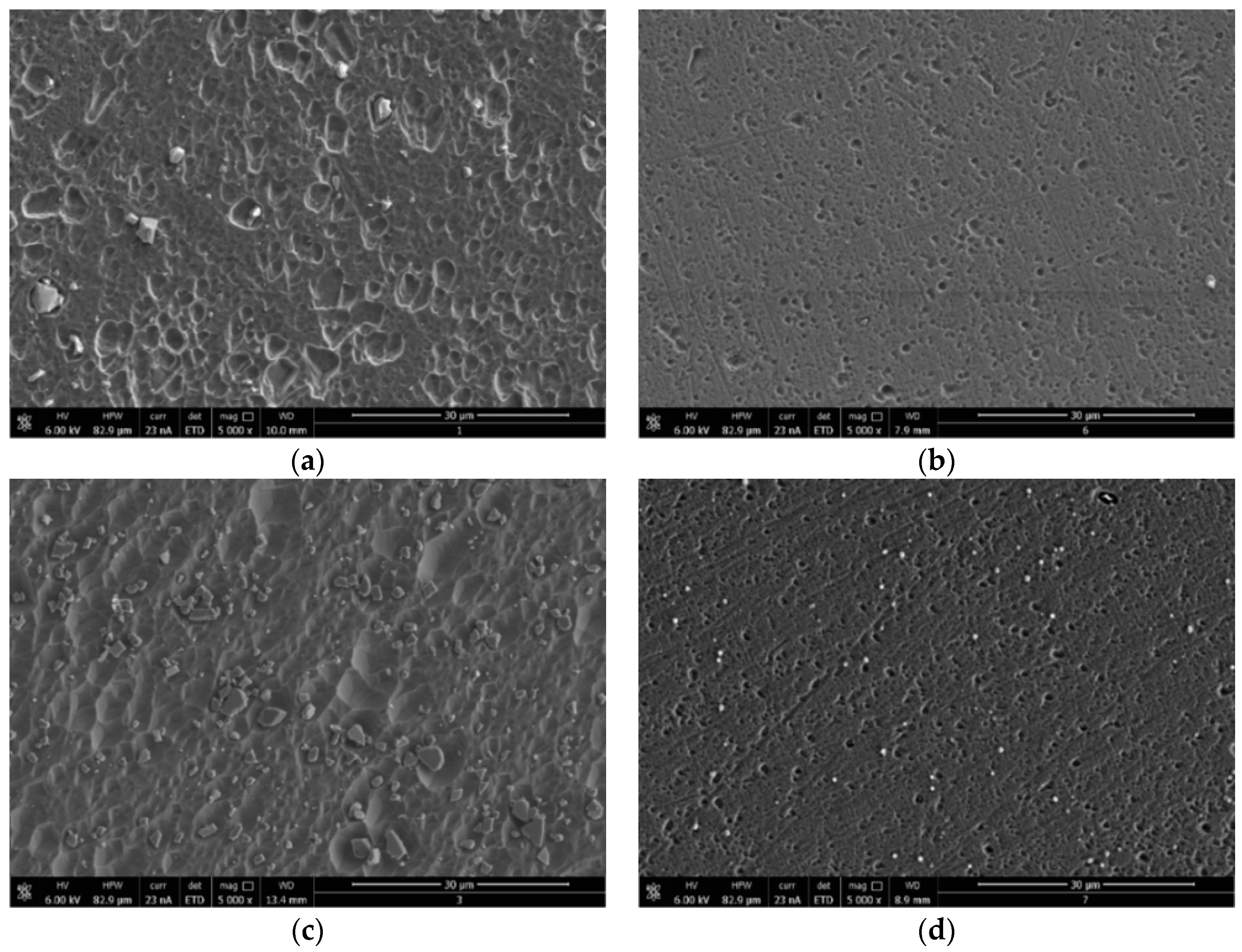
2.4.3. Scanning Electron Microscopy (SEM)
3. Results and Discussion
3.1. Effect of Concentration and Temperature
3.1.1. Gravimetric Measurement
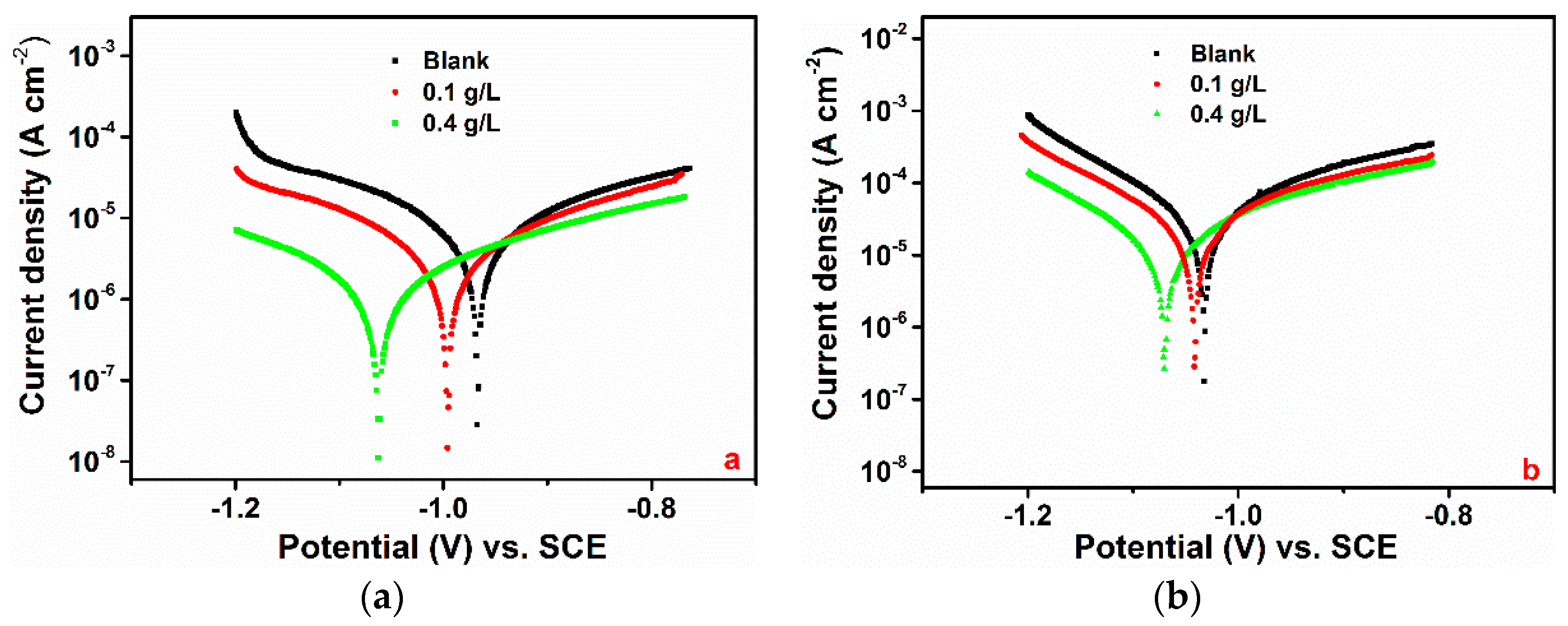
3.1.2. PDP Measurement
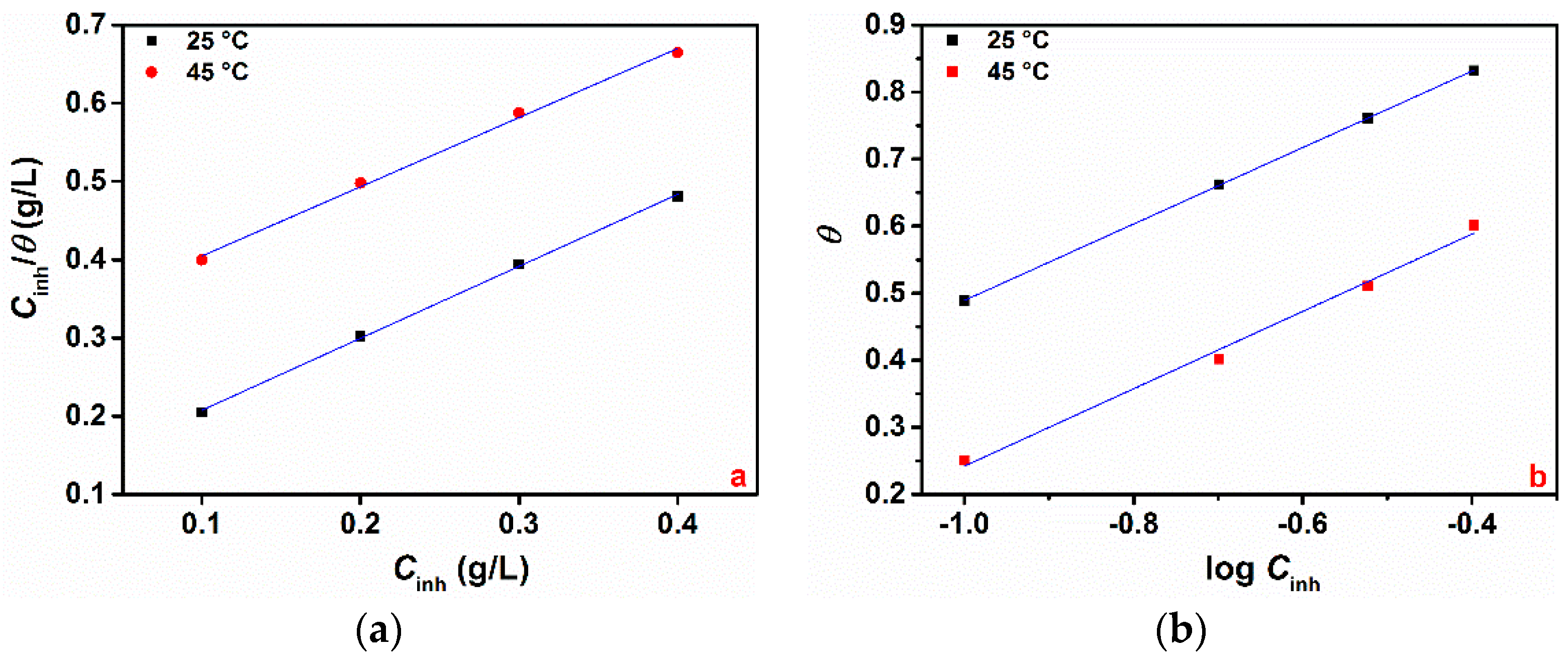
3.1.3. Adsorption Study and Standard Adsorption Free Energy
- (i)
- (ii)
- The value of ΔGads ranges between −22.48 and −21.32 kJ mol−1 for the Langmuir isotherm and between −27.73 and −26.92 kJ mol−1 for the Temkin isotherm. It should be noted that the adsorption is regarded as physisorption for values of ΔGads up to −20 kJ mol−1, whereas for values more negative than −40 kJ mol−1, the adsorption is said to be chemisorption [5,20]. This suggests that the adsorption of GG molecules on the metal surface occurs through a mixed-type adsorption process (i.e., physical and chemical adsorption). Other authors also reported this mixed interaction adsorption process of GG in acid media [14,16,17];
- (iii)
- The values of “a” determined from the Temkin isotherm are negative in all cases, indicating that repulsion forces exist between the adsorbed inhibitor molecules in the adsorption layer, also reported by Messali et al. [14].
3.1.4. Activation Parameters
- (i)
- The values of Ea are higher in the presence of GG than for the bank solution. It is said that higher values of Ea in the presence of the inhibitor compared to the free acid solution are an indication of the inhibitive action of the inhibitor as a result of the increase in energy barrier for the corrosion process [14,29];
- (ii)
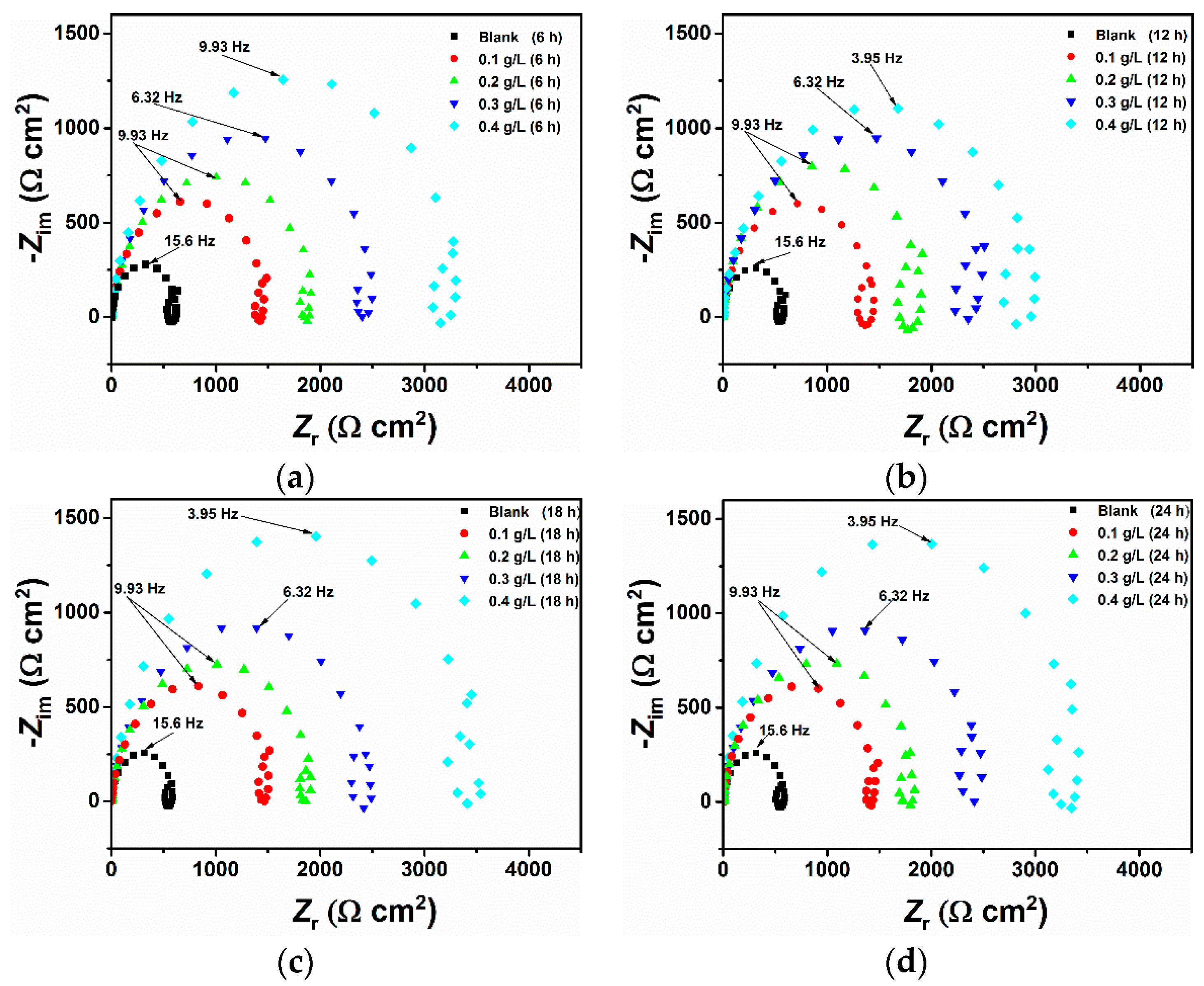
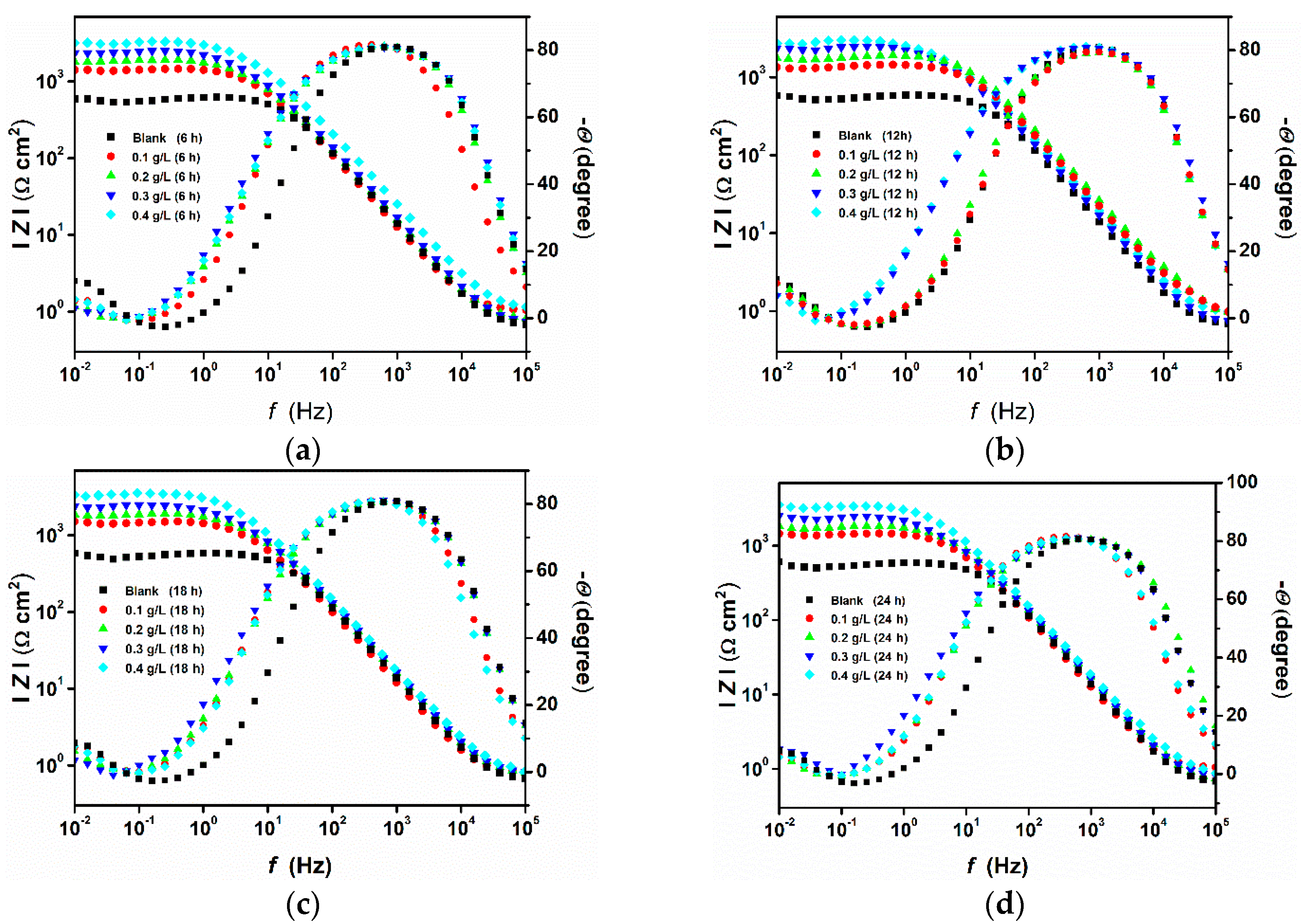
3.2. Effect of Time
3.3. Surface Analysis
3.3.1. FTIR and Raman/SERS Analysis
3.3.2. SEM Morphology
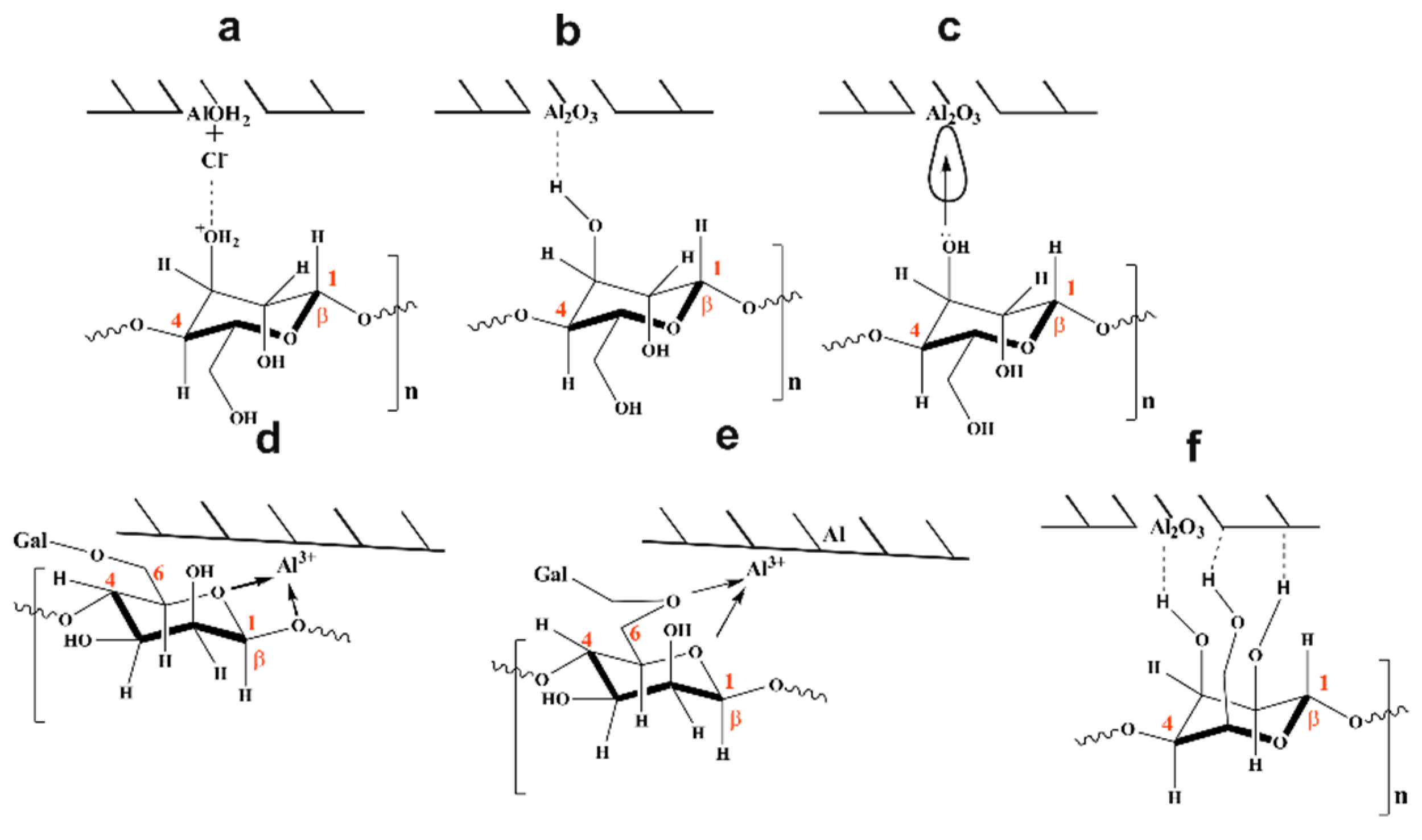
3.4. Mechanism of Inhibition
3.4.1. Electrostatic Interaction
3.4.2. Adsorption via H-Bond Formation
3.4.3. Chemical Adsorption
3.4.4. Chelate Formation
3.4.5. Mode of Adsorption (Horizontal Orientation)
4. Conclusions
- GG was found to be a good corrosion inhibitor for pure aluminium in the tested solution.
- The inhibition efficiency increases with an increase in inhibitor concentration but decreases as the temperature increases. The maximum inhibition efficiencies were 82.85 and 64.30 at 25 °C and 45 °C, respectively.
- Time has little effect on the corrosion inhibition efficiency of the tested inhibitor, suggesting that GG is able to protect the metal for long exposure periods.
- The inhibition action of Guar gum is attributed to its horizontal adsorption on the metallic surface.
- The adsorption of GG on the aluminium surface follows the Temkin adsorption isotherm model.
- The negative values of the free energy of adsorption indicate spontaneous adsorption of GG on the aluminium surface. Moreover, the values of ΔGads indicate that the adsorption process is a mixed-type interaction (i.e., physical and chemical adsorption). The values of Ea and Qads further support the mixed-type adsorption.
- The potentiostatic polarisation measurements indicate that GG acts as a cathodic inhibitor.
- FTIR and Raman spectroscopies suggested that the most probable inhibition action of GG is due to its adsorption of the metal surface via H-bond formation.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Singh, A.; Lin, Y.; Ebenso, E.E.; Liu, W.; Pan, J.; Huang, B. Gingko biloba fruit extract as an eco-Friendly corrosion inhibitor for J55 steel in CO2 saturated 3.5% NaCl solution. J. Ind. Eng. Chem. 2015, 24, 219–228. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, W.F.; Wang, H.L.; Hao, C. Adsorption and inhibitive properties of Apigenin derivatives as eco-Friendly corrosion inhibitors for brass in nitric acid solution. J. Adhes. Sci. Technol. 2019, 33, 1–25. [Google Scholar] [CrossRef]
- Ibrahim, T.H.; Gomes, E.E.; Obot, I.B.; Khamis, M.; Sabri, M.A. Mild steel green inhibition by Ficus carica leaves extract under practical field conditions. J. Adhes. Sci. Technol. 2017, 31, 2697–2718. [Google Scholar] [CrossRef]
- Amin, M.A.; Ei-Rehim, S.S.A.; El-Sherbini, E.E.F.; Hazzazi, O.A.; Abbas, M.N. Polyacrylic acid as a corrosion inhibitor for aluminium in weakly alkaline solutions. Part I: Weight loss, polarisation, impedance EFM and EDX studies. Corros. Sci. 2009, 51, 658–667. [Google Scholar] [CrossRef]
- Umoren, S.A. Inhibition of aluminium and mild steel corrosion in acidic medium using Gum Arabic. Cellulose 2008, 15, 751–761. [Google Scholar] [CrossRef]
- Abu-Dalo, M.A.; Othman, A.A.; Al-Rawashdeh, N.A.F. Exudate gum from acacia trees as green corrosion inhibitor for mild steel in acidic media. Int. J. Electrochem. Sci. 2012, 7, 9303–9324. [Google Scholar]
- Mobin, M.; Khan, M.A. Investigation on the adsorption and corrosion inhibition behavior of gum acacia and synergistic surfactants additives on mild steel in 0.1 M H2SO4. J. Dispers. Sci. Technol. 2013, 34, 1496–1506. [Google Scholar] [CrossRef]
- Umoren, S.A. Synergistic Influence of Gum Arabic and Iodide Ion on the Corrosion Inhibition of Aluminium in Alkaline Medium. Port. Electrochim. Acta 2009, 27, 565–577. [Google Scholar] [CrossRef]
- Umoren, S.A.; Obot, I.B.; Ebenso, E.E.; Okafor, P.C.; Ogbobe, O.; Oguzie, E.E. Gum arabic as a potential corrosion inhibitor for aluminium in alkaline medium and its adsorption characteristics. Anti Corros. Methods Mater. 2006, 53, 277–282. [Google Scholar] [CrossRef]
- Mobin, M.; Rizvi, M. Inhibitory effect of xanthan gum and synergistic surfactant additives for mild steel corrosion in 1 M HCl. Carbohydr. Polym. 2016, 136, 384–393. [Google Scholar] [CrossRef]
- Mobin, M.; Rizvi, M. Polysaccharide from Plantago as a green corrosion inhibitor for carbon steel in 1M HCl solution. Carbohydr. Polym. 2017, 160, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Mudgil, D.; Barak, S.; Khatkar, B.S. X-ray diffraction, IR spectroscopy and thermal characterization of partially hydrolyzed guar gum. Int. J. Biol. Macromol. 2012, 50, 1035–1039. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M. Guar gum as corrosion inhibitor for carbon steel in sulfuric acid solutions. Port. Electrochim. Acta 2004, 22, 161–175. [Google Scholar] [CrossRef]
- Messali, M.; Lgaz, H.; Dassanayake, R.; Salghi, R.; Jodeh, S.; Abidi, N.; Hamed, O. Guar gum as efficient non-Toxic inhibitor of carbon steel corrosion in phosphoric acid medium: Electrochemical, surface, DFT and MD simulations studies. J. Mol. Struct. 2017, 1145, 43–54. [Google Scholar] [CrossRef]
- Palumbo, G.; Banaś, J. Inhibition effect of guar gum on the corrosion behaviour of carbon steel (K-55) in fracturing fluid. Solid State Phenom. 2015, 227, 59–62. [Google Scholar] [CrossRef]
- Peter, A.; Sharma, S.K.; Obot, I.B. Anticorrosive efficacy and adsorptive study of guar gum with mild steel in acidic medium. J. Anal. Sci. Technol. 2016, 7, 26. [Google Scholar] [CrossRef]
- Roy, P.; Karfa, P.; Adhikari, U.; Sukul, D. Corrosion inhibition of mild steel in acidic medium by polyacrylamide grafted Guar gum with various grafting percentage: Effect of intramolecular synergism. Corros. Sci. 2014, 88, 246–253. [Google Scholar] [CrossRef]
- Biswas, A.; Pal, S.; Udayabhanu, G. Effect of chemical modification of a natural polysaccharide on its inhibitory action on mild steel in 15% HCl solution. J. Adhes. Sci. Technol. 2017, 31, 2468–2489. [Google Scholar] [CrossRef]
- Umoren, S.A.; Ogbobe, O.; Igwe, I.O.; Ebenso, E.E. Inhibition of mild steel corrosion in acidic medium using synthetic and naturally occurring polymers and synergistic halide additives. Corros. Sci. 2008, 50, 1998–2006. [Google Scholar] [CrossRef]
- Bentrah, H.; Rahali, Y.; Chala, A. Gum Arabic as an eco-Friendly inhibitor for API 5L X42 pipeline steel in HCl medium. Corros. Sci. 2014, 82, 426–431. [Google Scholar] [CrossRef]
- Li, X.; Deng, S.; Fu, H.; Li, T. Adsorption and inhibition effect of 6-Benzylaminopurine on cold rolled steel in 1.0 M HCl. Electrochim. Acta 2009, 54, 4089–4098. [Google Scholar] [CrossRef]
- Umoren, S.A.; Obot, I.B.; Obi-Egbedi, N.O. Raphia hookeri gum as a potential eco-Friendly inhibitor for mild steel in sulfuric acid. J. Mater. Sci. 2009, 44, 274–279. [Google Scholar] [CrossRef]
- Tiwari, A.; Terada, D.; Kobayash, H. Polyvinyl Modified Guar-gum Bioplastics for Packaging Applications. In Handbook of Bioplastics and Biocomposites Engineering Applications; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Fares, M.M.; Maayta, A.K.; Al-Qudah, M.M. Pectin as promising green corrosion inhibitor of aluminum in hydrochloric acid solution. Corros. Sci. 2012, 60, 112–117. [Google Scholar] [CrossRef]
- Dračínský, M. Chapter One-The Chemical Bond: The Perspective of NMR Spectroscopy. In Annual Reports on NMR Spectroscopy; Webb, G.A., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 90, pp. 1–40. [Google Scholar]
- Liu, Y. Is the Free Energy Change of Adsorption Correctly Calculated? J. Chem. Eng. Data 2009, 54, 1981–1985. [Google Scholar] [CrossRef]
- ASTM. G102-89 Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements; ASTM: West Conshohocken, PA, USA, 2015. [Google Scholar]
- Nnanna, L.A.; Owate, I.O.; Nwadiuko, O.C.; Nneka, D.E.; Oji, W.J. Adsorption and Corrosion Inhibtion of Gnetum Africana Leaves Extract on Carbon Steel. Int. J. Mater. Chem. 2013, 3, 10–16. [Google Scholar]
- Noor, E.A.; Al-Moubaraki, A.H. Thermodynamic study of metal corrosion and inhibitor adsorption processes in mild steel/1-Methyl-4[4 × (-X)-Styryl pyridinium iodides/hydrochloric acid systems. Mater. Chem. Phys. 2008, 110, 145–154. [Google Scholar] [CrossRef]
- Brett, C.M.A. The application of electrochemical impedance techniques to aluminium corrosion in acidic chloride solution. J. Appl. Electrochem. 1990, 20, 1000–1003. [Google Scholar] [CrossRef]
- Frers, S.E.; Stefenel, M.M.; Mayer, C.; Chierchie, T. AC-Impedance measurements on aluminium in chloride containing solutions and below the pitting potential. J. Appl. Electrochem. 1990, 20, 996–999. [Google Scholar] [CrossRef]
- Brett, C.M.A. On the electrochemical behaviour of aluminium in acidic chloride solution. Corros. Sci. 1992, 33, 203–210. [Google Scholar] [CrossRef]
- Umoren, S.A.; Li, Y.; Wang, F.H. Effect of polyacrylic acid on the corrosion behaviour of aluminium in sulphuric acid solution. J. Solid State Electrochem. 2010, 14, 2293–2305. [Google Scholar] [CrossRef]
- Khaled, K.F.; Amin, M.A. Electrochemical and molecular dynamics simulation studies on the corrosion inhibition of aluminum in molar hydrochloric acid using some imidazole derivatives. J. Appl. Electrochem. 2009, 39, 2553–2568. [Google Scholar] [CrossRef]
- Mansfeld, F.; Lin, S.; Kim, K.; Shih, H. Pitting and surface modification of SIC/Al. Corros. Sci. 1987, 27, 997–1000. [Google Scholar] [CrossRef]
- Mansfeld, F.; Lin, S.; Kim, S.; Shih, H. Electrochemical impedance spectroscopy as a monitoring tool for passivation and localized Corrosion of aluminum alloys. Mater. Corros. Werkst. Korros. 1988, 39, 487. [Google Scholar] [CrossRef]
- Li, X.; Deng, S.; Fu, H. Sodium molybdate as a corrosion inhibitor for aluminium in H3PO4 solution. Corros. Sci. 2011, 53, 2748–2753. [Google Scholar] [CrossRef]
- Lorenz, W.J.; Mansfeld, F. Determination of corrosion rates by electrochemical DC and AC methods. Corros. Sci. 1981, 21, 647–672. [Google Scholar] [CrossRef]
- Şafak, S.; Duran, B.; Yurt, A.; Türkoğlu, G. Schiff bases as corrosion inhibitor for aluminium in HCl solution. Corros. Sci. 2012, 54, 251–259. [Google Scholar] [CrossRef]
- McCafferty, E.; Hackerman, N. Double layer capacitance of iron and corrosion inhibition with polymethylene diamines. Electrochem. Soc. 1972, 119, 146–154. [Google Scholar] [CrossRef]
- Bataillon, C.; Brunet, S. Electrochemical impedance spectroscopy on oxide films formed on zircaloy 4 in high temperature water. Electrochim. Acta 1994, 39, 455–465. [Google Scholar] [CrossRef]
- Yuen, S.N.; Choi, S.M.; Phillips, D.L.; Ma, C.Y. Raman and FTIR spectroscopic study of carboxymethylated non-Starch polysaccharides. Food Chem. 2009, 114, 1091–1098. [Google Scholar] [CrossRef]
- Hohl, H.; Stumm, W. Interaction of Pb2+ with hydrous γ-Al2O3. J. Colloid Interface Sci. 1976, 55, 281–288. [Google Scholar] [CrossRef]
- Wood, R.; Fornasiero, D.; Ralston, J. Electrochemistry of the boehmite—Water interface. Colloids Surf. 1990, 51, 389–403. [Google Scholar] [CrossRef]
- Oguzie, E.E.; Li, Y.; Wang, F.H. Effect of 2-Amino-3-Mercaptopropanoic acid (cysteine) on the corrosion behaviour of low carbon steel in sulphuric acid. Electrochim. Acta 2007, 53, 909–914. [Google Scholar] [CrossRef]
- El-Haddad, M.N.; Fouda, A.S. Inhibition effect and adsorption behavior of new azodye derivatives on corrosion of carbon steel in acid medium. J. Dispers. Sci. Technol. 2013, 34, 1471–1480. [Google Scholar] [CrossRef]
- Abbasov, V.M.; Abd El-Lateef, H.M.; Aliyeva, L.I.; Qasimov, E.E.; Ismayilov, I.T.; Khalaf, M.M. A study of the corrosion inhibition of mild steel C1018 in CO2-Saturated brine using some novel surfactants based on corn oil. Egypt. J. Pet. 2013, 22, 451–470. [Google Scholar] [CrossRef]
- Jiang, X.; Zheng, Y.G.; Ke, W. Effect of flow velocity and entrained sand on inhibition performances of two inhibitors for CO2 corrosion of N80 steel in 3% NaCl solution. Corros. Sci. 2005, 47, 2636–2658. [Google Scholar] [CrossRef]
| Cinh (g L−1) | Corrosion Rate (mg cm−2 d−1) | IE (%) | ||
|---|---|---|---|---|
| - | 25 °C | 45 °C | 25 °C | 45 °C |
| Blank | 5.65 | 18.50 | - | - |
| 0.1 | 2.89 | 13.87 | 48.85 | 25.03 |
| 0.2 | 1.91 | 11.07 | 66.19 | 40.16 |
| 0.3 | 1.35 | 9.05 | 76.11 | 51.08 |
| 0.4 | 0.95 | 7.37 | 83.19 | 60.16 |
| Cinh (g L−1) | βa (V dec−1) | βc (V dec−1) | Icorr (µA cm−2) | Ecorr (V/SCE) | IE (%) |
|---|---|---|---|---|---|
| 25 °C | |||||
| Blank | 0.294 | 0.280 | 8.98 | −0.967 | - |
| 0.1 | 0.251 | 0.212 | 3.96 | −0.996 | 55.90 |
| 0.4 | 0.215 | 0.220 | 1.42 | −1.049 | 84.19 |
| 45 °C | |||||
| Blank | 0.293 | 0.185 | 29.9 | −1.03 | - |
| 0.1 | 0.161 | 0.137 | 22.4 | −1.04 | 25.08 |
| 0.4 | 0.164 | 0.124 | 14.4 | −1.07 | 51.84 |
| Temperature (°C) | R2 | Intercept | Slope | a | K | ΔGads (kJ mol−1) |
|---|---|---|---|---|---|---|
| Langmuir | ||||||
| 25 | 0.999 | 0.115 | 0.920 | - | 8.70 | −22.48 |
| 45 | 0.996 | 0.316 | 0.885 | - | 3.16 | −21.32 |
| Temkin | ||||||
| 25 | 0.999 | 1.059 | 0.570 | −2.02 | 72.09 | −27.73 |
| 45 | 0.989 | 0.819 | 0.577 | −2.00 | 26.27 | −26.92 |
| Cinh (g L−1) | Ea (kJ mol−1) | Qads (kJ mol−1) |
|---|---|---|
| Blank | 46.78 | - |
| 0.1 | 61.86 | −41.45 |
| 0.2 | 69.30 | −42.22 |
| 0.3 | 75.04 | −43.99 |
| 0.4 | 80.80 | −46.81 |
| Time (h) | Rs (Ω cm2) | Q (μΩ−1 sn cm−2) | n | R (Ω cm2) | Cdl (μF cm−2) | IE (%) |
|---|---|---|---|---|---|---|
| Blank | ||||||
| 6 | 0.63 | 19.8 | 0.94 | 624 | 16.15 | - |
| 12 | 0.63 | 20.2 | 0.94 | 589 | 17.11 | - |
| 18 | 0.63 | 20.7 | 0.93 | 585 | 17.23 | - |
| 24 | 0.64 | 20.6 | 0.93 | 589 | 17.11 | - |
| 0.1 g L−1 | ||||||
| 6 | 0.63 | 23.5 | 0.93 | 1442 | 11.85 | 56.73 |
| 12 | 0.63 | 19.4 | 0.92 | 1429 | 11.96 | 58.78 |
| 18 | 0.76 | 23.8 | 0.93 | 1481 | 11.54 | 60.50 |
| 24 | 0.76 | 23.5 | 0.93 | 1455 | 11.74 | 59.52 |
| 0.2 g L−1 | ||||||
| 6 | 0.76 | 18.8 | 0.93 | 1836 | 13.72 | 66.01 |
| 12 | 0.70 | 16.9 | 0.92 | 1893 | 13.31 | 68.89 |
| 18 | 0.78 | 17.9 | 0.93 | 1910 | 13.19 | 69.37 |
| 24 | 0.66 | 20.5 | 0.92 | 1799 | 14.01 | 67.26 |
| 0.3 g L−1 | ||||||
| 6 | 0.65 | 22.0 | 0.91 | 2498 | 10.09 | 75.02 |
| 12 | 0.66 | 28.2 | 0.90 | 2582 | 9.76 | 77.19 |
| 18 | 0.77 | 23.6 | 0.91 | 2480 | 10.16 | 76.41 |
| 24 | 0.77 | 17.5 | 0.93 | 2490 | 10.12 | 76.35 |
| 0.4 g L−1 | ||||||
| 6 | 0.65 | 14.2 | 0.91 | 3166 | 12.73 | 80.29 |
| 12 | 0.76 | 19.5 | 0.92 | 3116 | 12.94 | 81.10 |
| 18 | 1.05 | 22.5 | 0.92 | 3338 | 12.08 | 82.47 |
| 24 | 1.03 | 23.3 | 0.92 | 3431 | 11.75 | 82.83 |
| Element | Weight % | |||
|---|---|---|---|---|
| - | 25 °C | 45 °C | ||
| - | Blank | GG | Blank | GG |
| C | - | 5.7 | - | 4.9 |
| O | 1.6 | 2.9 | 1.2 | 2.6 |
| Si | 1.1 | 1.3 | 0.9 | 1.1 |
| Cl | 0.4 | 0.2 | 0.5 | 0.2 |
| Al | 96.9 | 89.9 | 97.4 | 91.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palumbo, G.; Berent, K.; Proniewicz, E.; Banaś, J. Guar Gum as an Eco-Friendly Corrosion Inhibitor for Pure Aluminium in 1-M HCl Solution. Materials 2019, 12, 2620. https://doi.org/10.3390/ma12162620
Palumbo G, Berent K, Proniewicz E, Banaś J. Guar Gum as an Eco-Friendly Corrosion Inhibitor for Pure Aluminium in 1-M HCl Solution. Materials. 2019; 12(16):2620. https://doi.org/10.3390/ma12162620
Chicago/Turabian StylePalumbo, Gaetano, Katarzyna Berent, Edyta Proniewicz, and Jacek Banaś. 2019. "Guar Gum as an Eco-Friendly Corrosion Inhibitor for Pure Aluminium in 1-M HCl Solution" Materials 12, no. 16: 2620. https://doi.org/10.3390/ma12162620
APA StylePalumbo, G., Berent, K., Proniewicz, E., & Banaś, J. (2019). Guar Gum as an Eco-Friendly Corrosion Inhibitor for Pure Aluminium in 1-M HCl Solution. Materials, 12(16), 2620. https://doi.org/10.3390/ma12162620







