New Sulfur Organic Polymer-Concrete Composites Containing Waste Materials: Mechanical Characteristics and Resistance to Biocorrosion
Abstract
1. Introduction
2. Materials and Methods
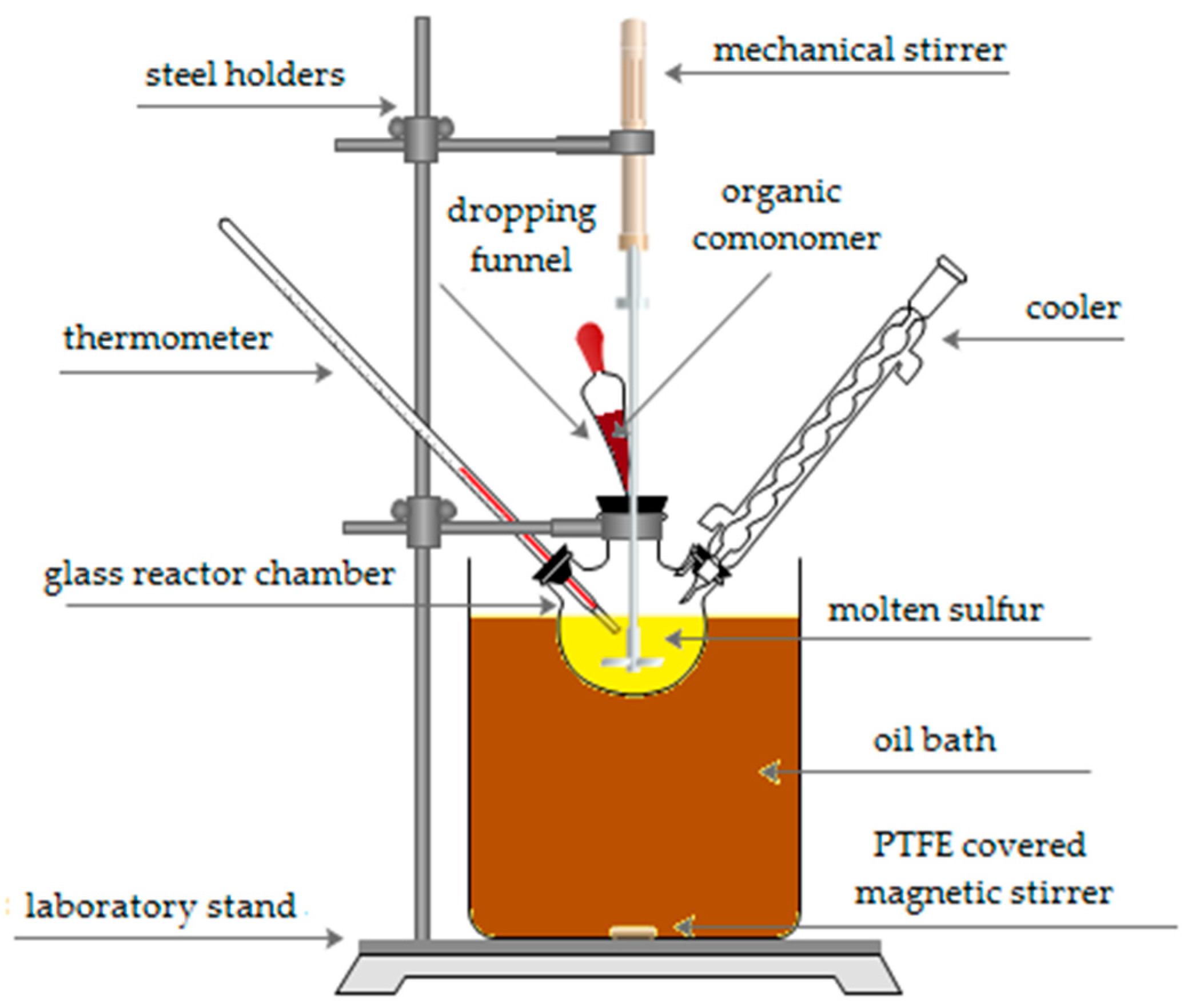
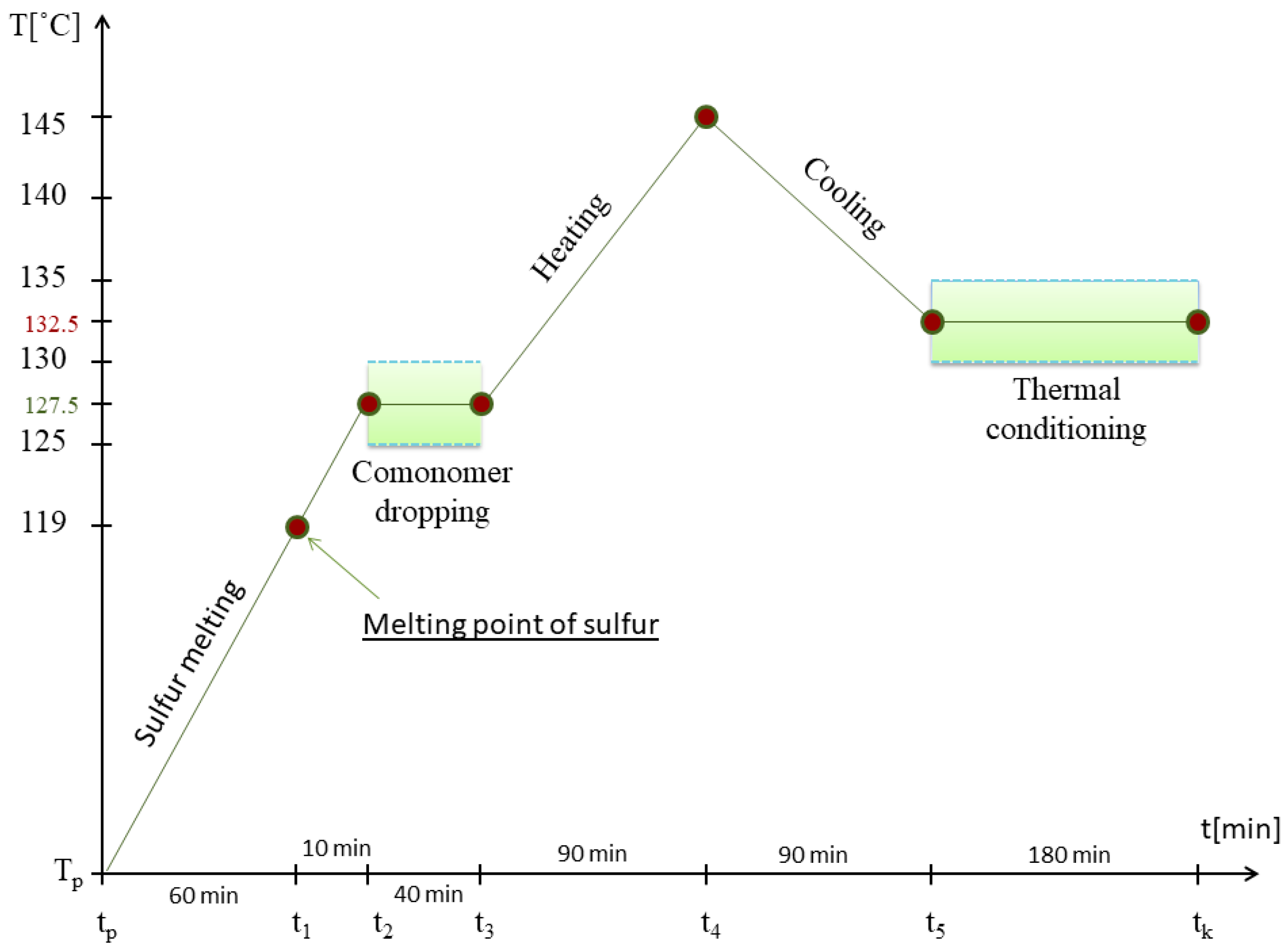
2.1. Synthesis of Sulfur–Organic Copolymers
2.2. Concrete Composites
2.3. Microorganisms
2.4. Testing the Sulfur–Organic Copolymers
2.4.1. The Softening Temperature
2.4.2. The Thermal Stability
2.4.3. The Melting Point of the Crystalline Phase
2.4.4. The Shore D Hardness
2.5. Testing of Concrete Composites
2.5.1. The Compressive Strength
2.5.2. Freeze–Thaw Resistance
2.5.3. Abrasion Resistance
2.6. Testing of Biocorrosion
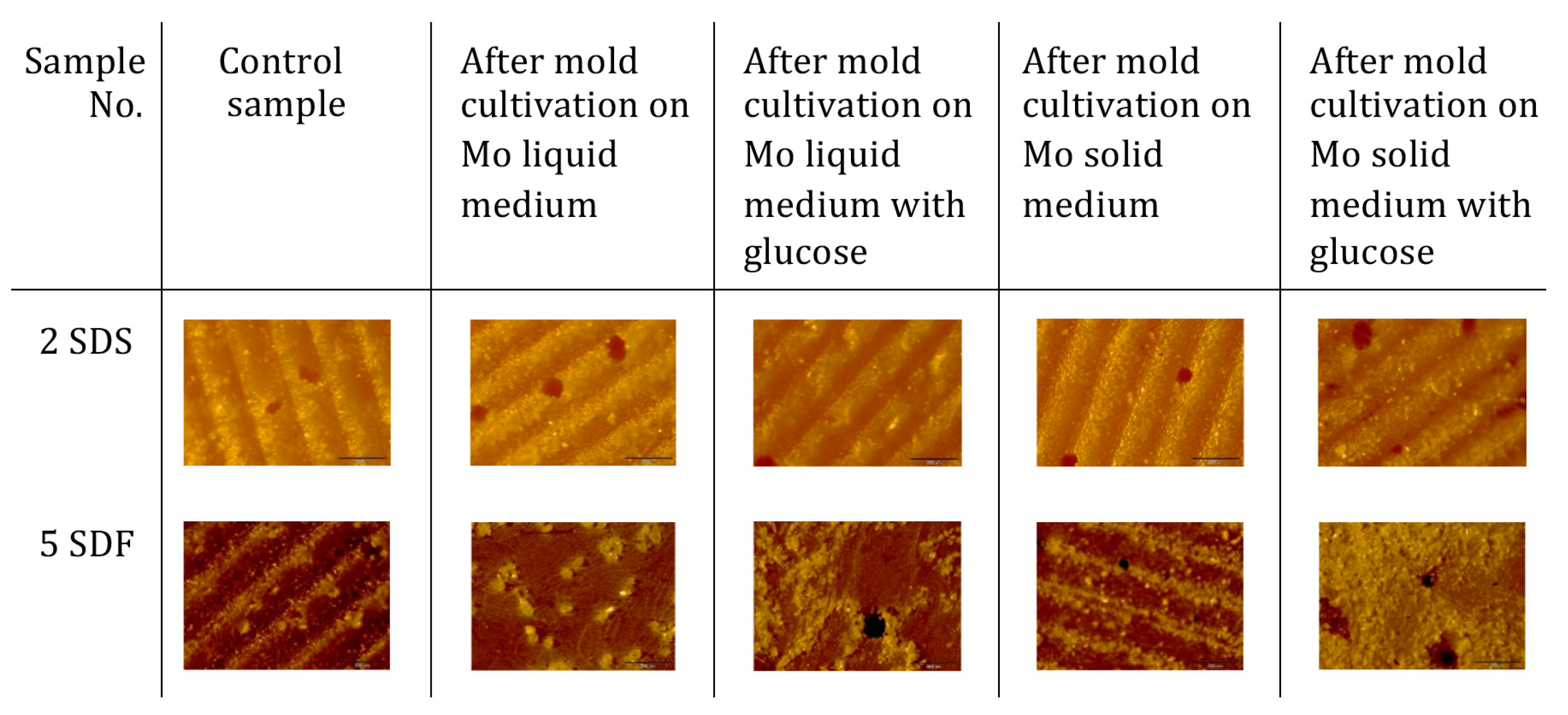
2.6.1. The Growth of Microorganisms on the Sulfur–Organic Copolymers
2.6.2. The Growth of Microorganisms on the Concrete Composites
2.6.3. The Acid Activity Measurement
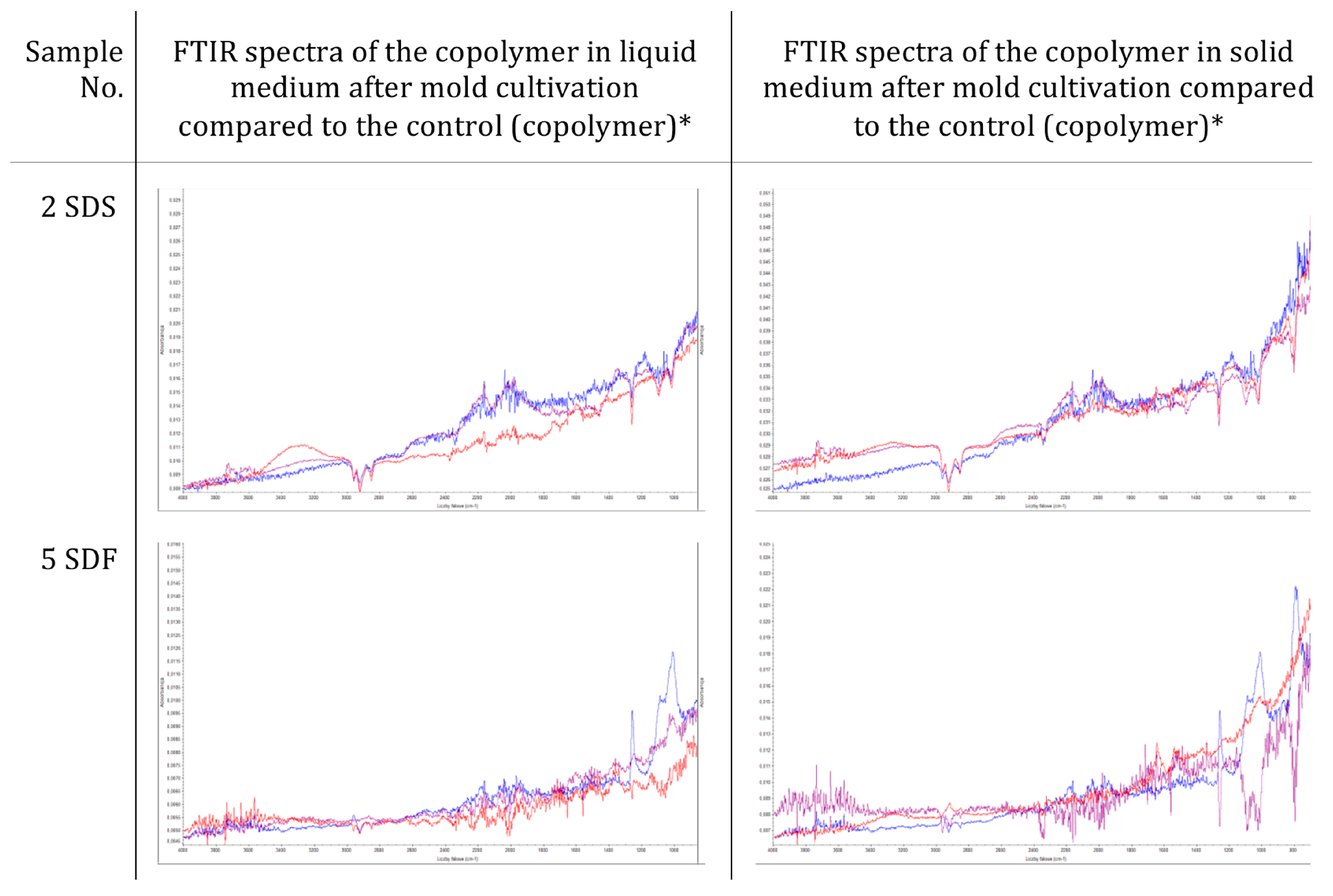
2.6.4. Morphological and Chemical Analysis of the Copolymers and Concrete Composites
3. Results and Discussion
3.1. Characteristics of the Sulfur–Organic Copolymers
3.2. Resistance of Sulfur–Organic Copolymers to Microbial Growth
3.3. Characteristics of ConcreteCcomposites—Mechanical Properties and Durability
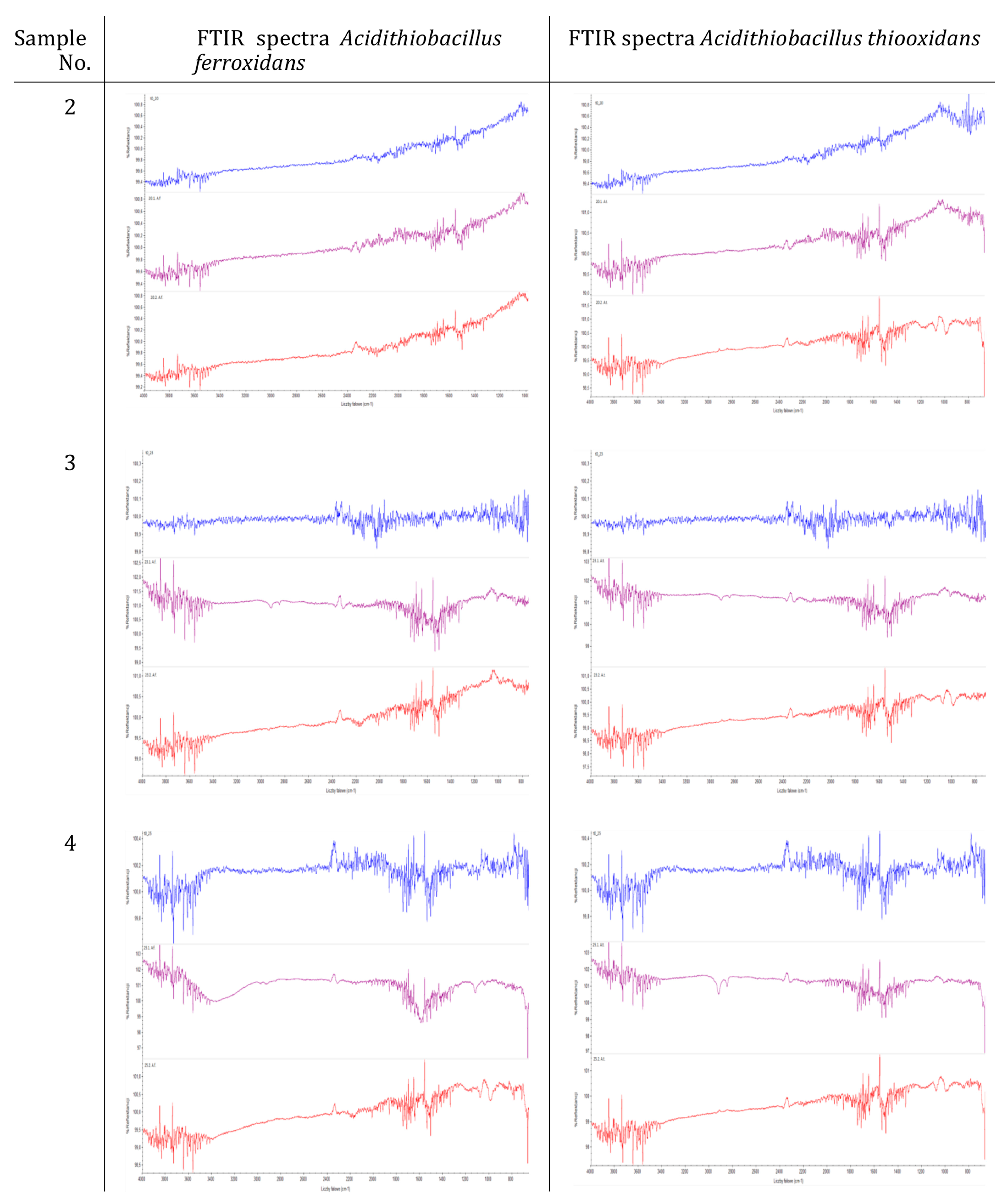
3.4. Resistance of Concrete Composites to Microbial Corrosion
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Datta, A.; Krishnan, V. Thermal behaviour of sulfur-selenium mixtures. J. Therm. Anal. 1979, 17, 31–38. [Google Scholar] [CrossRef]
- Sakaguchi, Y.; Tamura, K. Photo-induced structural changes in liquid sulfur. J. Non-Cryst. Solids. 1996, 205–207, 115–119. [Google Scholar] [CrossRef]
- Munejiri, S.; Shimojo, F.; Hoshino, K. Photo-induced polymerization in liquid sulfur studied by ab initio molecular-dynamics simulation. Comput. Phys. Commun. 2001, 142, 131–135. [Google Scholar] [CrossRef]
- Blight, L.B.; Currell, B.R.; Nash, B.J.; Scott, R.T.M.; Stillo, C. Chemistry of the modification of sulfur by the use of dicyclopentadiene and of styrene. Br. Polym. J. 2007, 12, 5–11. [Google Scholar] [CrossRef]
- Tsuda, T.; Takeda, A. Palladium-catalysed cycloaddition copolymerisation of diynes with elemental sulfur to poly(thiophene)s. Chem. Commun. 1996, 11, 1317–1318. [Google Scholar] [CrossRef]
- Ding, Y.; Hay, A.S. Copolymerization of elemental sulfur with cyclic (arylene disulfide) oligomers. J Polym. Sci. A. 1997, 35, 2961–2968. [Google Scholar] [CrossRef]
- Chung, W.I.; Griebel, J.J.; Kim, E.T.; Yoon, H.; Simmonds, A.G.; Ji, H.J.; Dirlam, P.T.; Glass, R.S.; Wie, J.J.; Nguyen, N.A.; et al. The use of elemental sulfur as an alternative feedstock for polymeric materials. Nat. Chem. 2013, 5, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, A.G.; Griebel, J.J.; Park, J.; Kim, K.R.; Chung, W.J.; Oleshko, V.P.; Kim, J.; Kim, E.T.; Glass, R.S.; Soles, C.L.; et al. Inverse vulcanization of elemental sulfur to prepare polymeric electrode materials for Li-S batteries. ACS Macro Lett. 2014, 3, 229–232. [Google Scholar] [CrossRef]
- Griebel, J.J.; Namnabat, S.; Kim, E.T.; Himmelhuber, R.; Moronta, D.H.; Chung, W.J.; Simmonds, A.G.; Kim, K.J.; van der Laan, J.; Nguyen, N.A.; et al. New infrared transmitting material via inverse vulcanization of elemental sulfur to prepare high refractive index polymers. Adv. Mater. 2014, 26, 3014–3018. [Google Scholar] [CrossRef]
- Namnabat, S.; Gabriel, J.J.; Pyun, J.; Norwood, R.A. Optical properties of sulfur copolymers for infrared applications. Proc. SPIE 2014, 8983. [Google Scholar] [CrossRef]
- Trofimov, B.; Parshina, L.; Gusarova, N.; Ivanova, N.; Myachina, G.; Kovalev, I.; Skotheim, T. Sulfur-rich copolymers of sulfur with 5-vinylobicyclo[2.2.1]hept-2-ene and tricyclo[5.2.1.0.2.6]deca-3,8-diene as prospective cathode materials for lithium cells. Sulfur. Lett. 2002, 25, 219–227. [Google Scholar] [CrossRef]
- Rucinski, J.; Slusarski, L.; Przybyl, W. The way for preparation of copolymers of sulfur and styrene (in Polish). Poland. Patent PL 81369, 16 October 1975. [Google Scholar]
- Bobrowska-Krajewska, K.; Dojka, M.; Dufaj-Zemla, H.; Matyka, J.; Slezak, M. The way of synthesis of stable polymeric sulfur for production of sulfur-concretes and waste protection (in Polish). Poland Patent PL 190343, 30 November 2005. [Google Scholar]
- Blasik, I.; Luszczyk, L.; Zwiruk, S.; Dojka, M. Application of modified sulfur for production of sulfur-concretes (in Polish). Chemik. 1988, 41, 243–245. [Google Scholar]
- Ciak, N.; Harasymiuk, J. Sulfur concrete’s technology and its application to the building industry. Tech. Sci. 2013, 16, 323–331. [Google Scholar]
- Al-Ansary, M. Innovative solutions for sulfur in Quatar. In Proceedings of the Sulfur World Symposium, Doha, Quatar, 12–15 April 2010. [Google Scholar]
- Strickland, D.; Chughtai, M.J.; May, R.W. Bitominous composition. Shell Oil Company. US Patent WO 2012/138860 A1, 11 October 2012. [Google Scholar]
- Hanumanthgari, R.; D’Melo, D.J.; Fitts, G.; Soubigou, C. Process for the manufacture of an asphalt composition. Shell Technol. Centre Bangalore. India Patent WO 2014/2016986 A1, 31 December 2014. [Google Scholar]
- Verbist, G.L.M.M.; Hamelink, C.P.; Dixon, E.R.; Hommes, A.E. Sulfur cement products. Shell Oil Company. US Patent WO 2015/014953 A1, 5 February 2015. [Google Scholar]
- Mohamed, A.-M.; El-Gamal, M. Sulfur Concrete for the Construction Industry A Sustainable Development Approach; Mohamed, A.-M.O., El-Gamal, M., Eds.; Ross Publishing Inc.: Plantation, FL, USA, 2010; pp. 109–220. ISBN 978-1-60427-005-1. [Google Scholar]
- Vlahovic, M.M.; Martinovic, S.P.; Boljanac, T.D.; Jovanic, P.B.; Volkov-Husovic, T.D. Durability of sulfur concrete in various aggressive environments. Constr. Build. Mater. 2011, 25, 3926–3934. [Google Scholar] [CrossRef]
- El-Gamal, M.; El-Dieb, A.; El-Sawy, K.M.E.; Mohamed, A.M. Durability of modified sulfur concrete in sewerage environment. Environ. Geotech. 2015, 2, 95–103. [Google Scholar]
- Fazli, A.; Fathi, S.; Fazli, M. Collation the durability of modified and non-modified sulfur concrete in erosive environments. In Proceedings of the CSCE, Roma, Italy, 7–8 June 2014. [Google Scholar]
- Mohamed, A.M.; El-Gamal, M.E. Hydro-mechanical behavior of a newly developed sulfur polymer concrete. Cem. Concr. Compos. 2009, 31, 186–194. [Google Scholar] [CrossRef]
- Ochrona Środowiska 2015. pp. 40–42; 328–350, (In Polish). Available online: http://stat.gov.pl/download/gfx/portalinformacyjny/pl/defaultaktualnosci/5484/1/16/1/ochrona_srodowiska_2015.pdf (accessed on 15 July 2019).
- Maeda, T.; Negishi, A.; Komoto, H.; Oshima, Y.; Kamimura, K.; Sugio, T. Isolation of iron-oxidazing bacteria from corroded concretes of sewage treatment plants. J. Biosci. Bioenginineering. 1999, 88, 300–305. [Google Scholar] [CrossRef]
- ISO 4649: 2017—Rubber, Vulcanized or Thermoplastic—Determination of Abrasion Resistance Using a Rotating Cylindrical Drum Device; International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 57:1975/AMD 1:1996—Life Cycle; International Organization for Standardization: Geneva, Switzerland, 1996.
- ISO 87: 1959—Simple Blend Testing of Steel Sheet and Strip Not Less Than 3 mm Thick; International Organization for Standardization: Geneva, Switzerland, 1959.
- ISO 179-1:2010—Plastics—Determination of Charpy Impact Properties—Part 1: Non-Instrumented Impact Test; International Organization for Standardization: Geneva, Switzerland, 2010.
- PN-EN 12390-3:2011—Testing Hardened Concrete—Part 3: Compressive Strength of Test Specimens; The Polish Committee for Standardization: Warszawa, Poland, 2011.
- PN-EN 196-1:2016-07—Methods of Testing Cement. Part 1: Determination of Strength; The Polish Committee for Standardization: Warszawa, Poland, 2016.
- PN-B-06250:1988—Ordinary Concrete (in Polish); The Polish Committee for Standardization: Warszawa, Poland, 1988.
- PN-EN 1338:2004—Concrete Paving Blocks. Requirements and Test Methods; The Polish Committee for Standardization: Warszawa, Poland, 2004.
- PN-EN 1339:2004—Concrete Paving Flags. Requirements and Test Methods; The Polish Committee for Standardization: Warszawa, Poland, 2004.
- PN-EN 1340:2004—Concrete Kerb Units. Requirements and Test Methods; The Polish Committee for Standardization: Warszawa, Poland, 2004.
- PN-EN ISO 846:2002—Plastics—Evaluation of the Action of Microorganisms; The Polish Committee for Standardization: Warszawa, Poland, 2004.
- Gutarowska, B.; Żakowska, Z. Elaboration and application of mathematical model for estimation of mould contamination of some building materials based on ergosterol content determination. Int. Biodeterior. Biodegrad. 2002, 49, 299–305. [Google Scholar] [CrossRef]
- Gutarowska, B.; Żakowska, Z. Mathematical models of mycelium growth and ergosterol synthesis in stationary mould culture. Lett. Appli. Microbiol. 2009, 48, 605–610. [Google Scholar] [CrossRef]
- Roberts, D.J.; Nica, D.; Zuo, G.; Davis, J.L. Quantifying microbially induced deterioration of concrete:initial studies. Int. Biodeterior. Biodegrad. 2002, 49, 227–234. [Google Scholar] [CrossRef]
- Yamanaka, T.; Aso, I.; Togashi, S.; Tanigawa, M.; Shoji, K.; Watanabe, T.; Watanabe, N.; Maki, K.; Suzuki, H. Corrosion by bacteria of concreto in sewerage systems and inhibitory effects of formates on their growth. Water Res. 2002, 36, 2636–2642. [Google Scholar] [CrossRef]
- Gutarowska, B.; Czyżowska, A. The ability of filamentous fungi to produce acids on indoor building materials. Annals of Microbiol. 2009, 59, 807–813. [Google Scholar] [CrossRef]
- Grengg, C.; Mittermayr, F.; Baldermann, A.; Böttcher, M.E.; Leis, A.; Koraimann, G.; Grunert, P.; Dietzel, M. Microbiologically induced concrete corrosion: A case study from a combined sewer network. Cem. Concr. Res. 2015, 77, 16–25. [Google Scholar] [CrossRef]
- Cwalina, B. Biodeterioration of concrete. Archit. Civ. Eng. Environ. 2008, 4, 133–140. [Google Scholar]
| Sample Number | Content (%) | |||
|---|---|---|---|---|
| DCPD | Sulfur | Organic Additives | ||
| 1 control | - | 100 | - | - |
| 2 SDS | 5 | 90 | styrene | 5 |
| 3 SDT | 5 | 90 | turpentine | 5 |
| 4 SDD | 5 | 90 | 1-decene | 5 |
| 5 SDF | 5 | 90 | furfural | 5 |
| Sample Number | Content (%) | |||
|---|---|---|---|---|
| Sulfur–Organic Copolymers | Aggregate Type | Additives Type | ||
| 1 control | Sulfur | 30.0 | Sand 60.0 | Fly ash 10.0 |
| 2 | Sulfur DCPD SDS | 21.1 0.55 0.55 | Sand 15.6 Gravel 54.4 | Fly ash 7.8 |
| 3 | Sulfur DCPD SDS | 24.5 0.65 0.65 | Sand 6.4 Gravel 58.7 | Phosphogypsum 9.1 |
| 4 | Sulfur DCPD SDF | 24.5 0.65 0.65 | Sand 13.1 Gravel 52.0 | Phosphogypsum 9.1 |
| Sample Number | Parameter | ||||
|---|---|---|---|---|---|
| Softening Point (°C) | Melting Temp. Range (°C) | Melting Enthalpy (J/g) | Thermal Stability T5 (°C) | Thermal Stability T50 (°C) | |
| 2 SDS | 63.0 ± 2.0 | 80–115 | 37.79 | 238 | 295 |
| 3 SDT | 105.0 ± 1.0 | 85–120 | 33.77 | 226 | 285 |
| 4 SDD | 75.0 ± 2.0 | 95–120 | 41.27 | 221 | 272 |
| 5 SDF | 90.0 ± 1.0 | 95–125 | 47.31 | 220 | 288 |
| Sample Number | Compression Strength (N) | Flexural Strength (MPa) | Hardness (°Sh D) | Impact Resistance (J/m2) | S Crystal Content before Aging (wt.%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| virgin | after aging | virgin | after aging | virgin | after aging | virgin | after aging | virgin | after aging | |
| 1 control | 204 ± 57 | 109 ± 76 | 4.4 ± 3.5 | 1.0 ± 0.2 | 27.5 ± 0.3 | 27.6 ± 0.3 | 38.2 ± 6 | 91.3 ± 9 | 52.3 | 61.4 |
| 2 SDS | 383 ± 71 | 599 ± 46 | 4.4 ± 3.0 | 6.3 ± 4.0 | 27.0 ± 0.1 | 27.0 ± 0.1 | 63.4 ± 2 | 186.8 ± 16 | 50.0 | 57.4 |
| 3 SDT | 399 ± 51 | 188 ± 32 | 3.7 ± 2.6 | 2.5 ± 0.6 | 27.0 ± 0.1 | 27.5 ± 0.2 | 44.3 ± 2 | 131.1 ± 12 | 63.4 | 50.5 |
| 4 SDD | 234 ± 50 | 14 ± 15 | 5.6 ± 0.8 | 2.1 ± 0.7 | 26.9 ± 0.1 | 27.3 ± 0.2 | 35.8 ± 2 | 126.0 ± 10 | 59.5 | 48.4 |
| 5 SDF | 381 ± 58 | 256 ± 61 | 4.1 ± 0.5 | 1.2 ± 0.8 | 27.1 ± 0.2 | 27.4 ± 0.2 | 42.2 ± 6 | 129.3 ± 12 | 66.8 | 69.0 |
| Sample Number | Bulk Density kg/m3 (pcf) | E compressive Strength Cube Samples MPa (psi) | L Compressive Strength Cube Samples MPa (psi) | Compressive Strength Cuboid Samples MPa (psi) | Tensile Bending Strength Cuboid Samples MPa (psi) |
|---|---|---|---|---|---|
| 1 C | 2260 (141) | 46.5 (6744) | - * | 38.3 (5555) | 5.6 (812) |
| 2 | 2390 (149) | 57.0 (8267) | 51.7 (7498) | 66.1 (9587) | 7.3 (1059) |
| 3 | 2240 (140) | 50.6 (7339) | 68.7 (9964) | 58.3 (8456) | 13.3 (1929) |
| 4 | 2260 (141) | 49.6 (7194) | 50.5 (7324) | 51.6 (7484) | 4.5 (653) |
| Sample Number | Test | Requirements | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Freeze–Thaw (FT) Resistance | ||||||||||
| Compressive strength reference samples MPa (psi) | Compressive strength tested samples MPa (psi) | Strength loss in comparison to reference samples (%) | Number of performed cycles | Water absorption after 24 h (%) | ||||||
| 2 | 57.0 (8257) | 31.2 (4525) | 45 | 198 | 0.47 | Strength loss in comparison to reference samples max. 20% and no visible cracks | ||||
| 3 | 56.1 (8137) | 36.3 (5265) | 35 | 211 | 0.07 | |||||
| 4 | 49.6 (7194) | 26.9 (3902) | 46 | 100 | 0.35 | |||||
| Freeze–Thaw (FT) Resistance in 3% Salt Solution (Scaling) | ||||||||||
| Mass of scaled material after 28 cycles (m28) (g) | Mass of scaled material after 56 cycles (m56) (g) | Mass of scaled material after 28 cycles (m28) (kg/m2) | Mass of scaled material after 56 cycles (m56) (kg/m2) | |||||||
| 1A | 1.50 | 2.51 | 0.16 | 0.26 | FT2 m28 ≤ 0.5 m56 ≤ 1.0 (kg/m2) | |||||
| 2B | 0.01 | 0.13 | 0 | 0.01 | ||||||
| 3B | 0.03 | 0.11 | 0 | 0.01 | ||||||
| 4B | 0.07 | 0.36 | 0.01 | 0.04 | ||||||
| Böhme’s Plate Abrasion Tests | ||||||||||
| Sample mass loss (g) | Sample volume loss (g/mm3) | Results (mm3/5 000 mm2) | ||||||||
| 1 | 23.37 | 10 260 | 10 095 | Class 4 ≤ 18,000 | ||||||
| 2 | 30.35 | 13 239 | 13 276 | |||||||
| Wide Wheel Abrasion Tests—Width of Groove | ||||||||||
| Man value (mm) | Results correlated to calibration sample (mm) | |||||||||
| 2 | 15.1 | 20.0 | Class 4 ≤ 20 mm | |||||||
| 3 | 14.4 | 19.0 | ||||||||
| 4 | 15.0 | 19.5 | ||||||||
| Sample Number | Acidithiobacillus ferroxidans1 | Acidithiobacillus thiooxidans2 | Thiobacillus thioparus3 | |||
|---|---|---|---|---|---|---|
| after 30 days | after 60 days | after 30 days | after 60 days | after 30 days | after 60 days | |
| 2 | X: 5.5 | X: 4.0 | X: 2603.5 | X: 1402.0 | X: 2653.5 | X: 715.0 |
| SD: 0.7 | SD: 1.4 | SD: 3672.0 | SD: 1977.1 | SD:3742.7 | SD: 148.9 | |
| 3 | X: 6.0 | X: 5.5 | X: 126.0 | X: 6650.0 | X: 5.0 | X: 13500.0 |
| SD: 0.0 | SD: 2.1 | SD: 175.4 | SD: 7566.0 | SD: 1.4 | SD: 2121.3 | |
| 4 | X:5.0 | X: 4.0 | X: 32.0 | X: 6300.0 | X: 840.0 | X: 4600.0 |
| SD: 0.0 | SD: 0.0 | SD: 0.0 | SD: 424.3 | SD: 0.0 | SD: 3676.9 | |
| Sample Number | Ergosterol Content (μg/sample) | |
|---|---|---|
| ater 30 days | after 60 days | |
| 2 | X: 502.6 | X: 16.64 |
| SD: 45.26 | SD: 0.83 | |
| 3 | X: 662.36 | X: 12.43 |
| SD: 72.86 | SD: 1.24 | |
| 4 | X: 797.98 | X: 10.92 |
| SD: 39.90 | SD: 0.87 | |
| Sample Number | Acidithiobacillus ferroxidans1 | Acidithiobacillus thiooxidans2 | Thiobacillus thioparus3 | Penicillium chrysogenum, Aspergillus versicolor, Cladosporium herbarum | ||||
|---|---|---|---|---|---|---|---|---|
| after 30 days | after 60 days | after 30 days | after 60 days | after 30 days | after 60 days | after 30 days | after 60 days | |
| 2 | 1.34 ± 0.02 | 1.43 ± 0.01 | 4.72 ± 0.11 | 4.74 ± 0.07 | 6.38 ± 0.33 | 6.50 ± 1.15 | 5.40 ± 0.30 | 2.09 ± 0.01 |
| 3 | 1.35 ± 0.18 | 1.52 ± 0.21 | 4.08 ± 0.11 | 4.20 ± 0.12 | 6.23 ± 1.44 | 5.28 ± 2.14 | 4.56 ± 0.19 | 2.71 ± 1.06 |
| 4 | 1.41 ± 0.01 | 1.57 ± 0.01 | 4.88 ± 0.01 | 5.08 ± 0.01 | 6.31 ± 0.01 | 6.35 ± 0.01 | 4.90 ± 0.10 | 3.57 ± 0.10 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutarowska, B.; Kotynia, R.; Bieliński, D.; Anyszka, R.; Wręczycki, J.; Piotrowska, M.; Koziróg, A.; Berłowska, J.; Dziugan, P. New Sulfur Organic Polymer-Concrete Composites Containing Waste Materials: Mechanical Characteristics and Resistance to Biocorrosion. Materials 2019, 12, 2602. https://doi.org/10.3390/ma12162602
Gutarowska B, Kotynia R, Bieliński D, Anyszka R, Wręczycki J, Piotrowska M, Koziróg A, Berłowska J, Dziugan P. New Sulfur Organic Polymer-Concrete Composites Containing Waste Materials: Mechanical Characteristics and Resistance to Biocorrosion. Materials. 2019; 12(16):2602. https://doi.org/10.3390/ma12162602
Chicago/Turabian StyleGutarowska, Beata, Renata Kotynia, Dariusz Bieliński, Rafał Anyszka, Jakub Wręczycki, Małgorzata Piotrowska, Anna Koziróg, Joanna Berłowska, and Piotr Dziugan. 2019. "New Sulfur Organic Polymer-Concrete Composites Containing Waste Materials: Mechanical Characteristics and Resistance to Biocorrosion" Materials 12, no. 16: 2602. https://doi.org/10.3390/ma12162602
APA StyleGutarowska, B., Kotynia, R., Bieliński, D., Anyszka, R., Wręczycki, J., Piotrowska, M., Koziróg, A., Berłowska, J., & Dziugan, P. (2019). New Sulfur Organic Polymer-Concrete Composites Containing Waste Materials: Mechanical Characteristics and Resistance to Biocorrosion. Materials, 12(16), 2602. https://doi.org/10.3390/ma12162602










