Dual Doping of Silicon and Manganese in Hydroxyapatites: Physicochemical Properties and Preliminary Biological Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Samples
2.2. Sample Characterization
- d—crystallite size (nm)
- λ—radiation wavelength (nm)
- β—the peak full width at half maximum intensity (radians)
- θ—the diffraction angle of the corresponding reflex (°).
3. Results and Discussion
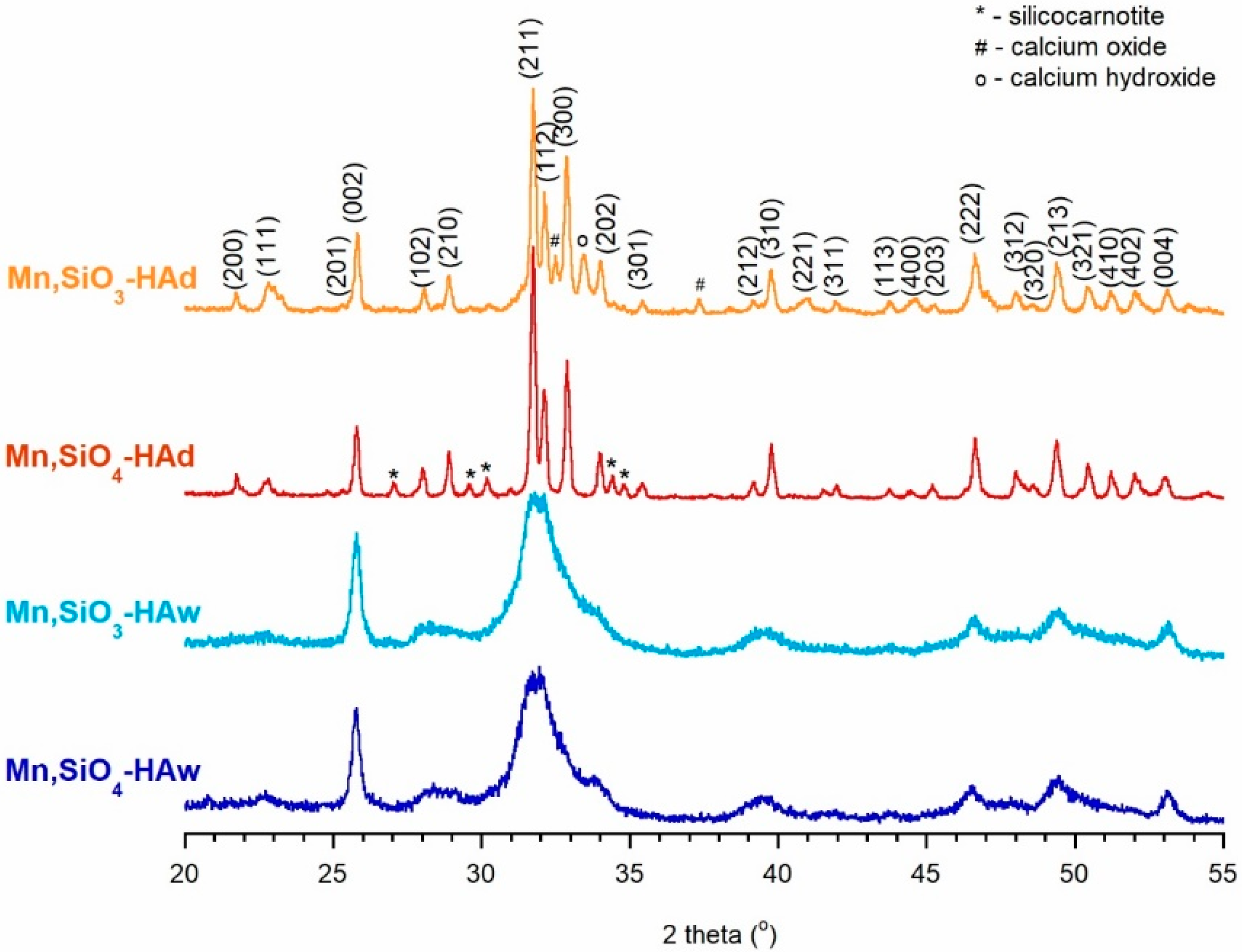
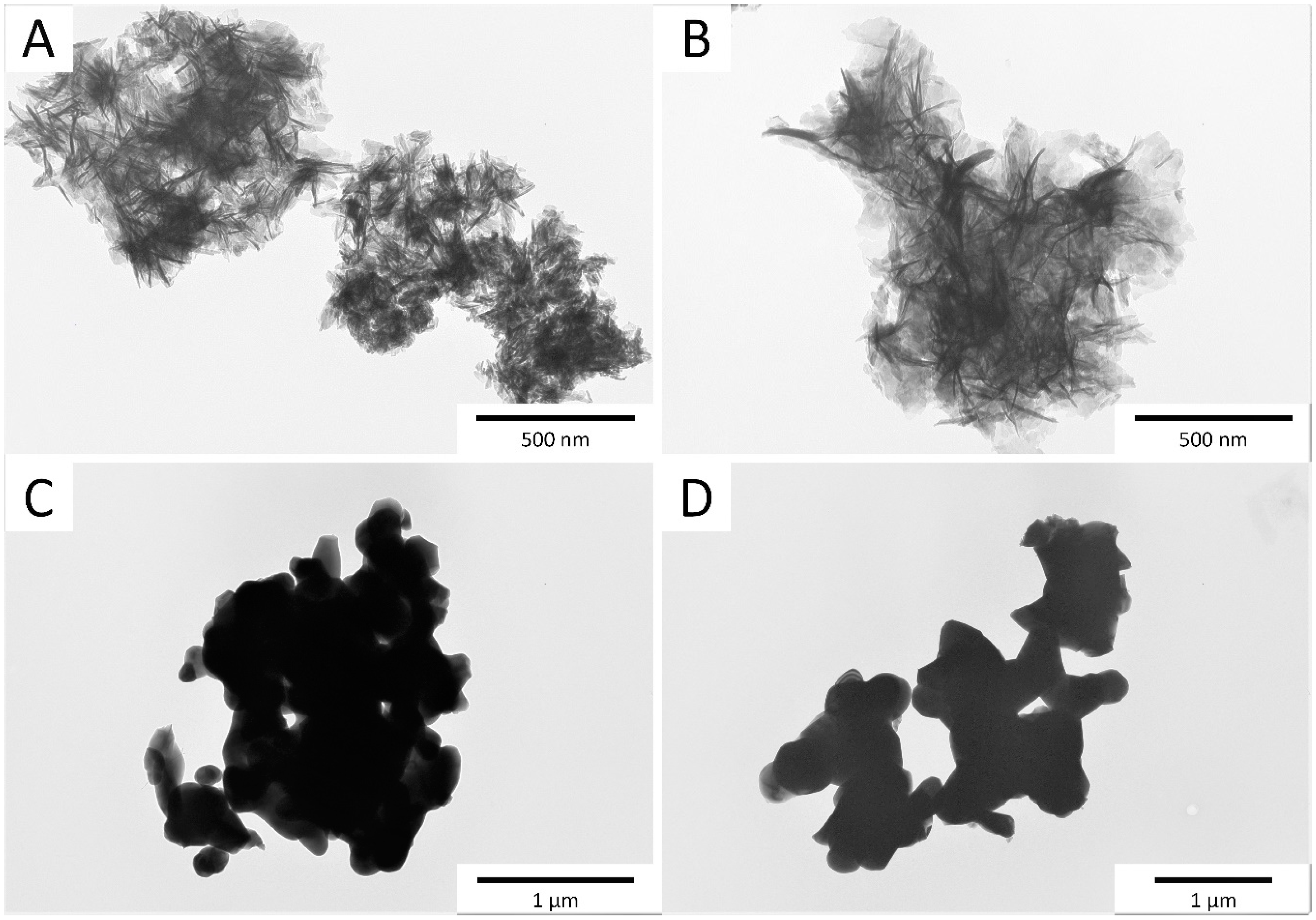
3.1. PXRD, TEM, and Elemental Analysis Results
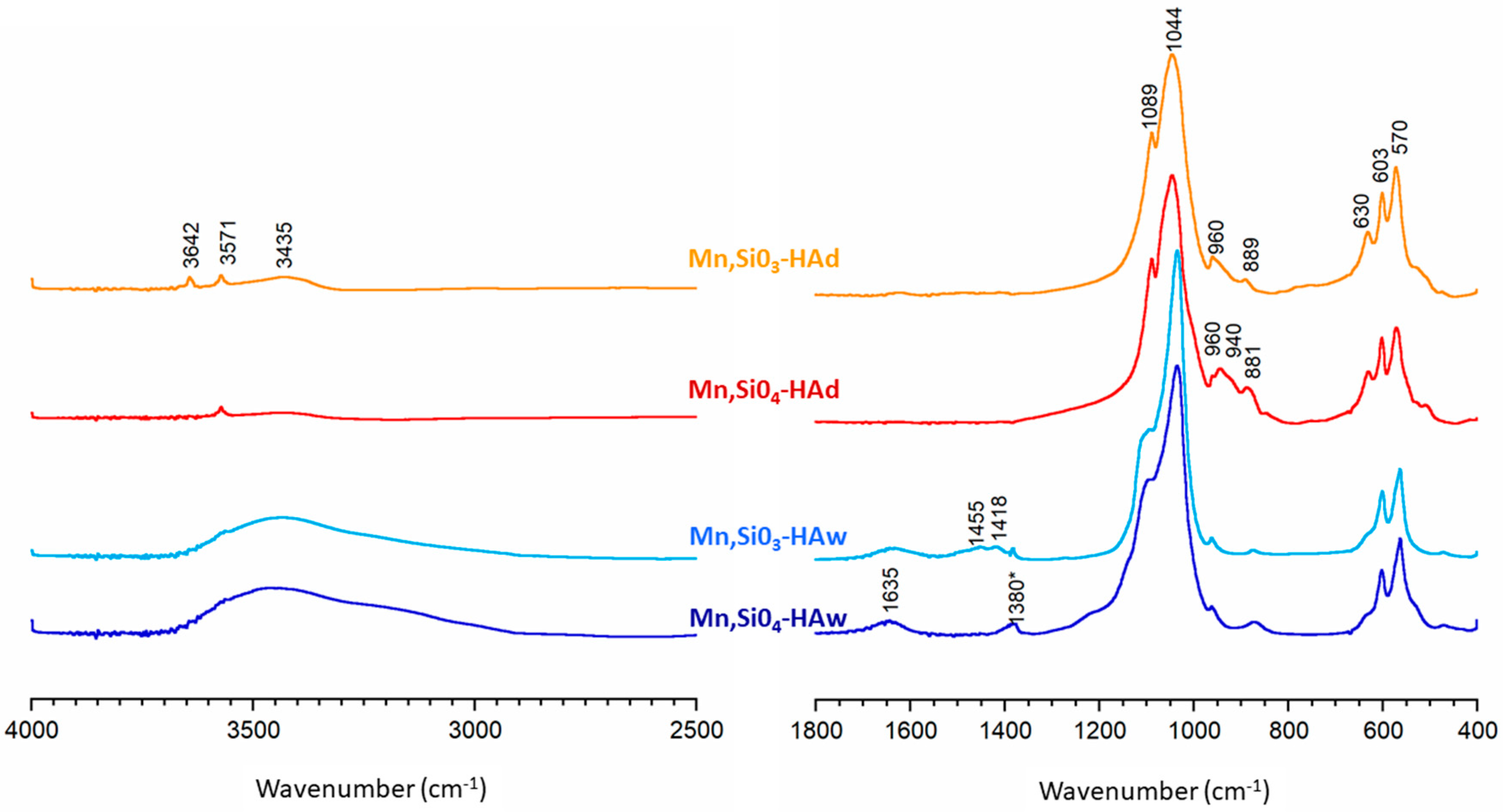
3.2. FTIR Spectroscopy
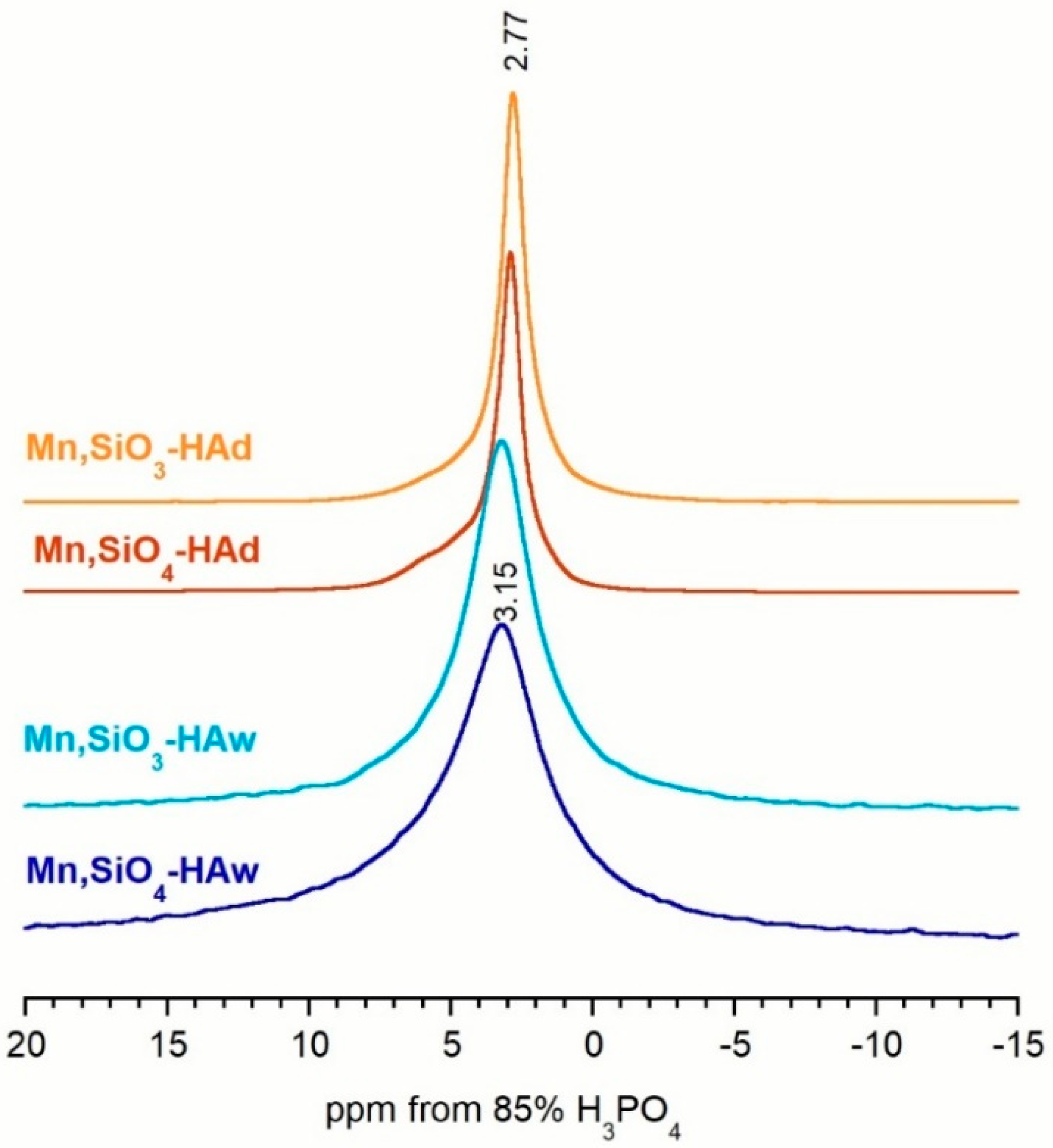
3.3. 31P and 29Si MAS NMR Spectroscopy
3.4. Preliminary Biological Tests
4. Conclusions
- Samples obtained using precipitation were nanocrystalline and monophasic. Solid-state samples were microcrystalline and contained secondary phases (impurities), i.e., calcium oxide, calcium hydroxide, and silicocarnotite, which were produced during the sintering process.
- Manganese and silicon ions were successfully incorporated, as confirmed using WD-XRF and spectroscopic measurements. The substitution efficiency was between 45% and 95%, depending on the synthesis method and the reagents.
- A higher efficiency of Mn2+ substitution occurred in the case of precipitation synthesis, while the substitution of silicon was favoured in the solid-state synthesis. In both cases, the yield was better when using silicon acetate instead of sodium metasilicate as a silicon source.
- The introduction of ions did not significantly affect the crystallinity and unit cell parameters. The degree of crystallinity, as well as the size and shape of the crystals, depended mainly on the synthesis method.
- According to the Microtox® and Spirotox tests, none of the samples was considered to be toxic. Such promising results may constitute the starting point for further biological research.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vallet-Regi, M.; Gonzales-Calbet, J.M. Calcium phosphates as substitution of bone tissues. Prog. Solid State Chem. 2004, 32, 1–31. [Google Scholar] [CrossRef]
- Zhou, H.; Lee, J. Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomater. 2011, 7, 2769–2781. [Google Scholar] [CrossRef] [PubMed]
- Suchanek, W.; Yoshimura, M. Processing and properties of hydroxyapatite-based biomaterials for use as hard tissue replacement implants. J. Mater. Res. 1998, 13, 94–117. [Google Scholar] [CrossRef]
- Kolmas, J.; Krukowski, S.; Laskus, A.; Jurkitewicz, M. Synthetic hydroxyapatite in pharmaceutical applications. Ceram. Int. 2016, 42, 2472–2487. [Google Scholar] [CrossRef]
- Shepherd, J.H.; Shepherd, D.V.; Best, S.M. Substituted hydroxyapatites for bone repair. J. Mater. Sci. Mater. Med. 2012, 23, 2335–2347. [Google Scholar] [CrossRef] [PubMed]
- Ratnayake, J.T.B.; Mucalo, M.; Dias, G.J. Substituted hydroxyapatites for bone regeneration: A review of current trends. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 1285–1299. [Google Scholar] [CrossRef] [PubMed]
- Supova, M. Substituted hydroxyapatite for biomedical applications: A review. Ceram. Int. 2015, 41, 9203–9231. [Google Scholar] [CrossRef]
- Nassif, N.; Martineau, F.; Syzgantseva, O.; Gobeaux, F.; Willinger, M.; Coradin, T.; Cassaignon, S.; Azaïs, T.; Giraud-Guille, M.M. In vivo inspired conditions to synthesize biomimetic hydroxyapatite. Chem. Mater. 2010, 22, 3653–3663. [Google Scholar] [CrossRef]
- Roveri, N.; Iafisco, M. Evolving application of biomimetic nanostructured hydroxyapatite. Nanotechnol. Sci. Appl. 2010, 3, 107–125. [Google Scholar] [CrossRef]
- Fore, H.; Morton, R.A. Manganese in Bone. Biochem. J. 1952, 51, 598–600. [Google Scholar] [CrossRef]
- Jugdaohsingh, R. Silicon and bone health. J. Nutr. Health Aging 2007, 11, 99–110. [Google Scholar]
- Armulik, A.; Svineng, G.; Wennerberg, K.; Fassler, R.; Johansson, S. Expression of integrin subunit β1B in integrin β1-deficient GD25 cells does not interfere with αVβ3 functions. Exp. Cell Res. 2000, 254, 55–63. [Google Scholar] [CrossRef]
- Bracci, B.; Torricelli, T.; Panzavolta, S.; Boanini, E.; Giardino, R.; Bigi, A. Effect of Mg2+, Sr2+, and Mn2+ on the chemico-physical and in vitro biological properties of calcium phosphate biomimetic coatings. J. Inorg. Biochem. 2009, 103, 1666–1674. [Google Scholar] [CrossRef]
- Huang, Y.; Ding, Q.; Han, S.; Yan, Y.; Pang, X. Characterisation, corrosion resistance and in vitro bioactivity of manganese-doped hydroxyapatite films electrodeposited on titanium. J. Mater. Sci. Mater. Med. 2013, 24, 1853–1864. [Google Scholar] [CrossRef]
- Saltman, P.D.; Strause, L.G. The role of trace minerals in osteoporosis. J. Am. Coll. Nutr. 1993, 12, 384–389. [Google Scholar] [CrossRef]
- Torres, P.M.C.; Vieira, S.I.; Cerqueira, A.R.; Pina, S.; da Cruz Silva, O.A.B.; Abrantes, J.C.C.; Ferreira, J.M.F. Effects of Mn-doping on the structure and biological properties of β-tricalcium phosphate. J. Inorg. Biochem. 2014, 136, 57–66. [Google Scholar] [CrossRef]
- Rico, H.; Gomez-Raso, N.; Revilla, M.; Hernandez, E.R.; Seco, C.; Paez, E.; Crespo, E. Effects on bone loss of manganese alone or with copper supplement in ovariectomized rats. A morphometric and densitomeric study. Eur J. Obstet. Gynecol. Reprod. Biol. 2000, 90, 97–101. [Google Scholar] [CrossRef]
- Bae, Y.J.; Kim, M.H. Manganese supplementation improves mineral density of the spine and femur and serum osteocalcin in rats. Biol. Trace Elem. Res. 2008, 124, 28–34. [Google Scholar] [CrossRef]
- Medvecky, L.; Stulajterova, R.; Parilak, L.; Trpcevska, J.; Durisin, J.; Barinov, S.M. Influence of manganese on stability and particle growth of hydroxyapatite in simulated body fluid. Colloids Surf. A Physicochem. Eng. Asp. 2006, 281, 221–229. [Google Scholar] [CrossRef]
- Bigi, A.; Bracci, B.; Cuisinier, F.; Elkaim, R.; Fini, M.; Mayer, I.; Mihailescu, I.N.; Socol, G.; Sturba, L.; Torricelli, P. Human osteoblast response to pulsed laser deposited calcium phosphate coatings. Biomaterials 2005, 26, 2381–2389. [Google Scholar] [CrossRef]
- Kolmas, J.; Jabłoński, M.; Ślósarczyk, A.; Kołodziejski, W. Solid-state NMR study of Mn2+ for Ca2+ substitution in thermally processed hydroxyapatites. J. Am. Ceram. Soc. 2015, 98, 1265–1274. [Google Scholar] [CrossRef]
- Mayer, I.; Peto, G.; Karacs, A.; Molnar, G.; Popov, I. Divalent Mn in calcium hydroxyapatite by pulse laser deposition. J. Inorg. Biochem. 2010, 104, 1107–1111. [Google Scholar] [CrossRef]
- Sopyan, I.; Ramesh, S.; Nawawi, N.A.; Tampieri, A.; Sprio, S. Effects of manganese doping on properties of sol-gel derived biphasic calcium phosphate ceramics. Ceram. Int. 2011, 37, 3703–3715. [Google Scholar] [CrossRef]
- Paluszkiewicz, C.; Ślósarczyk, A.; Pijocha, D.; Sitarz, M.; Bućko, M.; Zima, A.; Chróścicka, A.; Lewandowska-Szumieł, M. Synthesis, structural properties and thermal stability of Mn-doped hydroxyapatite. J. Mol. Struct. 2010, 976, 301–309. [Google Scholar] [CrossRef]
- Henstock, J.R.; Canham, L.T.; Anderson, S.I. Silicon: The evolution of its use in biomaterials. Acta Biomater. 2015, 11, 17–26. [Google Scholar] [CrossRef]
- Szurkowska, K.; Kolmas, J. Hydroxyapatites enriched in silicon – Bioceramic materials for biomedical and pharmaceutical applications. Prog. Nat. Sci. Mater. 2017, 27, 401–409. [Google Scholar] [CrossRef]
- Carlisle, E.M. Silicon: A possible factor in bone calcification. Science 1970, 167, 279–280. [Google Scholar] [CrossRef]
- Carlisle, E.M. Silicon: An essential element for the chick. Science 1972, 178, 619–621. [Google Scholar] [CrossRef]
- Reffitt, D.M.; Ogston, N.; Jugdaohsingh, R.; Cheung, H.F.J.; Evans, B.A.J.; Thompson, R.P.H.; Powell, J.J.; Hampson, G.N. Orthosilicic acid stimulates collagen type 1 synthesis and osteoblastic differentiation in human osteoblast-like cells in vitro. Bone 2003, 32, 127–135. [Google Scholar] [CrossRef]
- Spector, T.D.; Calomme, M.R.; Anderson, S.H.; Clement, G.; Bevan, L.; Demeester, N.; Swaminathan, R.; Jugdaohsingh, R.; Vanden Berghe, D.A.; Powell, J.J. Choline-stabilized orthosilicic acid supplementation as an adjunct to calcium/vitamin D3 stimulates markers of bone formation in osteopenic females: A randomized, placebo-controlled trial. BMC Musculoskelet. Disord. 2008, 9, 85. [Google Scholar] [CrossRef]
- Zhou, S.; Ireland, D.; Brooks, R.A.; Rushton, N.; Best, S. The effects of silicate ions on human osteoblast adhesion, proliferation, and differentiation. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 90, 123–130. [Google Scholar]
- Zhao, X.; Wang, T.; Qian, S.; Liu, X.; Sun, J.; Li, B. Silicon-doped titanium dioxide nanotubes promoted bone formation on titanium implants. Int J. Mol. Sci. 2016, 17, 292. [Google Scholar] [CrossRef]
- Wang, Q.; Hu, H.; Qiao, Y.; Zhang, Z.; Sun, J. Enhanced performance of osteoblasts by silicon incorporated porous TiO2 coating. J. Mater. Sci. Technol. 2012, 28, 109–117. [Google Scholar] [CrossRef]
- Zhou, X.; Moussa, F.M.; Mankoci, S.; Ustriyana, P.; Zhang, N.; Abdelmagid, S.; Molenda, J.; Murphy, W.L.; Safadi, F.F.; Sahai, N. Orthosilicic acid, Si(OH)4, stimulates osteoblast differentiation in vitro by upregulating miR-146a to antagonize NF-κB activation. Acta Biomater. 2016, 39, 192–202. [Google Scholar] [CrossRef]
- Gibson, I.R.; Best, S.M.; Bonfield, W. Chemical characterization of silicon-substituted hydroxyapatite. J. Biomed. Mater. Res. 1999, 44, 422–428. [Google Scholar] [CrossRef]
- Marchat, D.; Zymelka, M.; Coelho, C.; Gremillard, L.; Joly-Pottuz, L.; Babonneau, F.; Esnouf, C.; Chevalier, J.; Bernache-Assollant, D. Accurate characterization of pure silicon-substituted hydroxyapatite powders synthesized by a new precipitation route. Acta Biomater. 2013, 9, 6992–7004. [Google Scholar] [CrossRef]
- Palard, M.; Champion, E.; Foucaud, S. Synthesis of silicated hydroxyapatite Ca10(PO4)6−x(SiO4)x(OH)2−x. J. Solid State Chem. 2008, 181, 1950–1960. [Google Scholar] [CrossRef]
- Aminian, A.; Solati-Hashjin, M.; Samadikuchaksaraei, A.; Bakhshi, F.; Gorjipour, F.; Farzadi, A.; Moztarzadeh, F.; Schmucker, M. Synthesis of silicon-substituted hydroxyapatite by a hydrothermal method with two different phosphorus sources. Ceram. Int. 2011, 37, 1219–1229. [Google Scholar] [CrossRef]
- Botelho, C.M.; Brooks, R.A.; Best, S.M.; Lopes, M.A.; Santos, J.D.; Rushton, N.; Bonfield, W. Human osteoblast response to silicon-substituted hydroxyapatite. J. Biomed. Mater. Res. A 2006, 79, 723–730. [Google Scholar] [CrossRef]
- Honda, M.; Kikushima, K.; Kawanobe, Y.; Konishim, T.; Mizumoto, M.; Aizawa, M. Enhanced early osteogenic differentiation by silicon-substituted hydroxyapatite ceramics fabricated via ultrasonic spray pyrolysis route. J. Mater. Sci. Mater. Med. 2012, 23, 2923–2932. [Google Scholar] [CrossRef]
- Thian, E.S.; Huang, J.; Best, S.M.; Barber, Z.H.; Brooks, R.A.; Rushton, N.; Bonfield, W. The response of osteoblasts to nanocrystalline silicon-substituted hydroxyapatite thin films. Biomaterials 2006, 27, 2692–2698. [Google Scholar] [CrossRef]
- Patel, N.; Best, S.M.; Bonfield, W.; Gibson, I.R.; Hing, K.A.; Damien, E.; Revell, P.A. A comparative study on the in vivo behavior of hydroxyapatite and silicon substituted hydroxyapatite granules. J. Mater. Sci. Mater. Med. 2002, 13, 1199–1206. [Google Scholar] [CrossRef]
- Patel, N.; Brooks, R.A.; Clarke, M.T.; Lee, P.M.T.; Rushton, N.; Gibson, I.R.; Best, S.M.; Bonfield, W. In vivo assessment of hydroxyapatite and silicate-substituted hydroxyapatite granules using an ovine defect model. J. Mater. Sci. Mater. Med. 2005, 16, 429–440. [Google Scholar] [CrossRef]
- Porter, A.E.; Patel, N.; Skepper, J.N.; Best, S.M.; Bonfield, W. Comparison of in vivo dissolution processes in hydroxyapatite and silicon-substituted hydroxyapatite bioceramics. Biomaterials 2003, 24, 4609–4620. [Google Scholar] [CrossRef]
- Porter, A.E.; Patel, N.; Skepper, J.N.; Best, S.M.; Bonfield, W. Effect of sintered silicate-substituted hydroxyapatite on remodelling processes at the bone–implant interface. Biomaterials 2004, 25, 3303–3314. [Google Scholar] [CrossRef]
- Hing, K.A.; Revell, P.A.; Smith, N.; Buckland, T. Effect of silicon level on rate, quality and progression of bone healing within silicate-substituted porous hydroxyapatite scaffolds. Biomaterials 2006, 27, 5014–5026. [Google Scholar] [CrossRef]
- Zhang, E.; Zou, C. Porous titanium and silicon-substituted hydroxyapatite biomodification prepared by a biomimetic process: Characterization and in vivo evaluation. Acta Biomater. 2009, 5, 1732–1741. [Google Scholar] [CrossRef]
- Szurkowska, K.; Zgadzaj, A.; Kuras, M.; Kolmas, J. Novel hybrid material based on Mg2+ and SiO44- co-substituted nano-hydroxyapatite, alginate and chondroitin sulphate for potential use in biomaterials engineering. Ceram. Int. 2018, 44, 18551–18559. [Google Scholar] [CrossRef]
- Nałęcz-Jawecki, G. Spirotox—Spirostomum ambiguum acute toxicity test—10 years of experience. Environ. Toxicol. 2004, 19, 359–364. [Google Scholar] [CrossRef]
- Lafon, J.P.; Champion, E.; Bernache-Assollant, D.; Gibert, R.; Danna, A.M. Thermal decomposition of carbonated calcium phosphate apatites. J. Therm. Anal. Calorim. 2003, 72, 1127–1134. [Google Scholar] [CrossRef]
- Andreev, A.S.; Bulina, N.V.; Chaikina, M.V.; Prosanov, I.Y.; Terskikh, V.V.; Lapina, O.B. Solid-state NMR and computational insights into the crystal structure of silicocarnotite-based bioceramic materials synthesized mechanochemically. Solid State Nucl. Magn. Reson. 2017, 84, 151–157. [Google Scholar] [CrossRef]
- Hayakawa, S.; Kanaya, T.; Tsuru, K.; Shirosaki, Y.; Osaka, A.; Fujii, E.; Kawabata, K.; Gasqueres, G.; Bonhomme, C.; Babonneau, F.; et al. Heterogeneous structure and in vitro degradation behavior of wet-chemically derived nanocrystalline silicon-containing hydroxyapatite particles. Acta Biomater. 2013, 9, 4856–4867. [Google Scholar] [CrossRef]
- Chang, M.C.; Tanaka, J. FT-IR study for hydroxyapatite/collagen nanocomposite cross-linked by glutaraldehyde. Biomaterials 2002, 23, 4811–4818. [Google Scholar] [CrossRef]
- Merry, J.C.; Gibson, I.R.; Best, S.M.; Bonfield, W. Synthesis and characterization of carbonate hydroxyapatite. J. Mater. Sci. Mater. Med. 1998, 9, 779–783. [Google Scholar] [CrossRef]
- Ye, H.; Liu, X.Y.; Hong, H. Characterization of sintered titanium/hydroxyapatite biocomposite using FTIR spectroscopy. J. Mater. Sci. Mater. Med. 2009, 20, 843–850. [Google Scholar] [CrossRef]
- Shaltout, A.A.; Allam, M.A.; Moharram, M.A. FTIR spectroscopic, thermal and XRD characterization of hydroxyapatite from new natural sources. Spectrochim. Acta A Mol. Biol. Spectrosc. 2011, 83, 56–60. [Google Scholar] [CrossRef]
- Cengiz, B.; Gokce, Y.; Yildiz, N.; Aktas, Z.; Calimli, A. Synthesis and characterization of hydroxyapatite nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2008, 322, 29–33. [Google Scholar] [CrossRef]
- Tian, T.; Jiang, D.; Zhang, J.; Lin, Q. Synthesis of Si-substituted hydroxyapatite by a wet mechanochemical method. Mater. Sci. Eng. C 2008, 28, 57–63. [Google Scholar] [CrossRef]
- Padmanabhan, S.K.; Haq, E.U.; Licciulli, A. Rapid synthesis and characterization of silicon substituted nano hydroxyapatite using microwave irradiation. Curr. Appl. Phys. 2004, 14, 87–92. [Google Scholar] [CrossRef]
- Jager, C.; Welzel, T.; Meyer-Zaika, W.; Epple, M. A solid-state NMR investigation of the structure of nanocrystalline hydroxyapatite. Magn. Reson. Chem. 2006, 44, 573–580. [Google Scholar] [CrossRef]
- Pourpoint, F.; Gervais, C.; Bonhomme-Coury, L.; Azaïs, T.; Coelho, C.; Mauri, F.; Alonso, B.; Babonneau, F.; Bonhomme, C. Calcium phosphates and hydroxyapatite: Solid-state NMR experiments and first-principles calculations. Appl. Magn. Reson. 2007, 32, 435–457. [Google Scholar] [CrossRef]
- Panda, R.N.; Hsieh, M.F.; Chung, R.J.; Chin, T.S. FTIR, XRD, SEM and solid state NMR investigations of carbonate-containing hydroxyapatite nano-particles synthesized by hydroxide-gel technique. J. Phys. Chem. Solids 2003, 64, 193–199. [Google Scholar] [CrossRef]
- Magi, M.; Lippmaa, E.; Samoson, A.; Engelhardt, G.; Grimmer, A.R. Solid-state high-resolution silicon-29 chemical shifts in silicates. J. Phys. Chem. 1984, 88, 1518–1522. [Google Scholar] [CrossRef]
- Gasqueres, G.; Bonhomme, C.; Maquet, J.; Babonneau, F.; Hayakawa, S.; Kanayab, T.; Osaka, A. Revisiting silicate substituted hydroxyapatite by solid-state NMR. Magn. Reson. Chem. 2008, 46, 342–346. [Google Scholar] [CrossRef]
- Gomes, S.; Renaudin, G.; Mesbah, A.; Jallot, E.; Bonhomme, C.; Babonneau, F.; Nedelec, J.M. Thorough analysis of silicon substitution in biphasic calcium phosphate bioceramics: A multi-technique study. Acta Biomater. 2010, 6, 3264–3274. [Google Scholar] [CrossRef]
- Schneider, J.; Mastelaro, V.R.; Panepucci, H.; Zanotto, E.D. 29Si MAS-NMR studies of Qn structural units in metasilicate glasses and their nucleating ability. J. Non-Cryst. Solids 2000, 273, 8–18. [Google Scholar] [CrossRef]
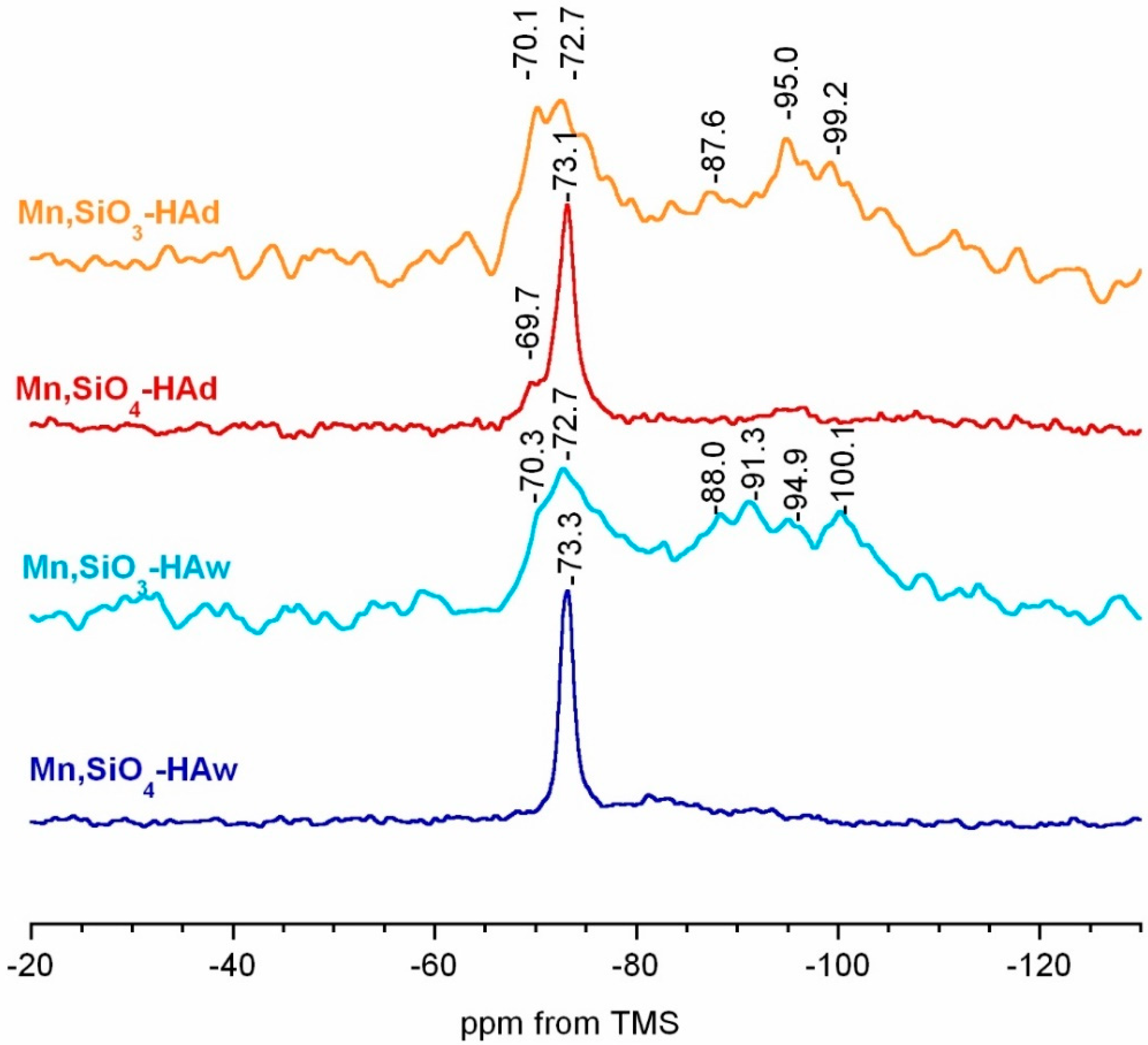
| Mn,SiO3-HAw | Mn,SiO4-HAw | Mn,SiO3-HAd | Mn,SiO4-HAd | |
|---|---|---|---|---|
| Synthesis method | Precipitation | Solid-state | ||
| Silicon source | Na2SiO3 | Si(CH3COO)4 | Na2SiO3 | Si(CH3COO)4 |
| Parameter a (Å) a | 9.424 | 9.433 | 9.418 | 9.415 |
| Parameter c (Å) a | 6.882 | 6.875 | 6.886 | 6.896 |
| Mn content (wt%) | 0.0086 ± 0.0004 | 0.0110 ± 0.0004 | 0.0076 ± 0.0003 | 0.0080 ± 0.0004 |
| Si content (wt%) | 1.217 ± 0.005 | 1.512 ± 0.003 | 1.819 ± 0.003 | 1.879 ± 0.004 |
| Mn substitution efficiency (%) | 50.59 | 64.71 | 44.71 | 47.06 |
| Si substitution efficiency (%) | 61.48 | 76.34 | 91.88 | 94.90 |
| (Ca + Mn)/(P + Si) molar ratio | 1.54 ± 0.05 | 1.59 ± 0.04 | 1.61 ± 0.03 | 1.62 ± 0.05 |
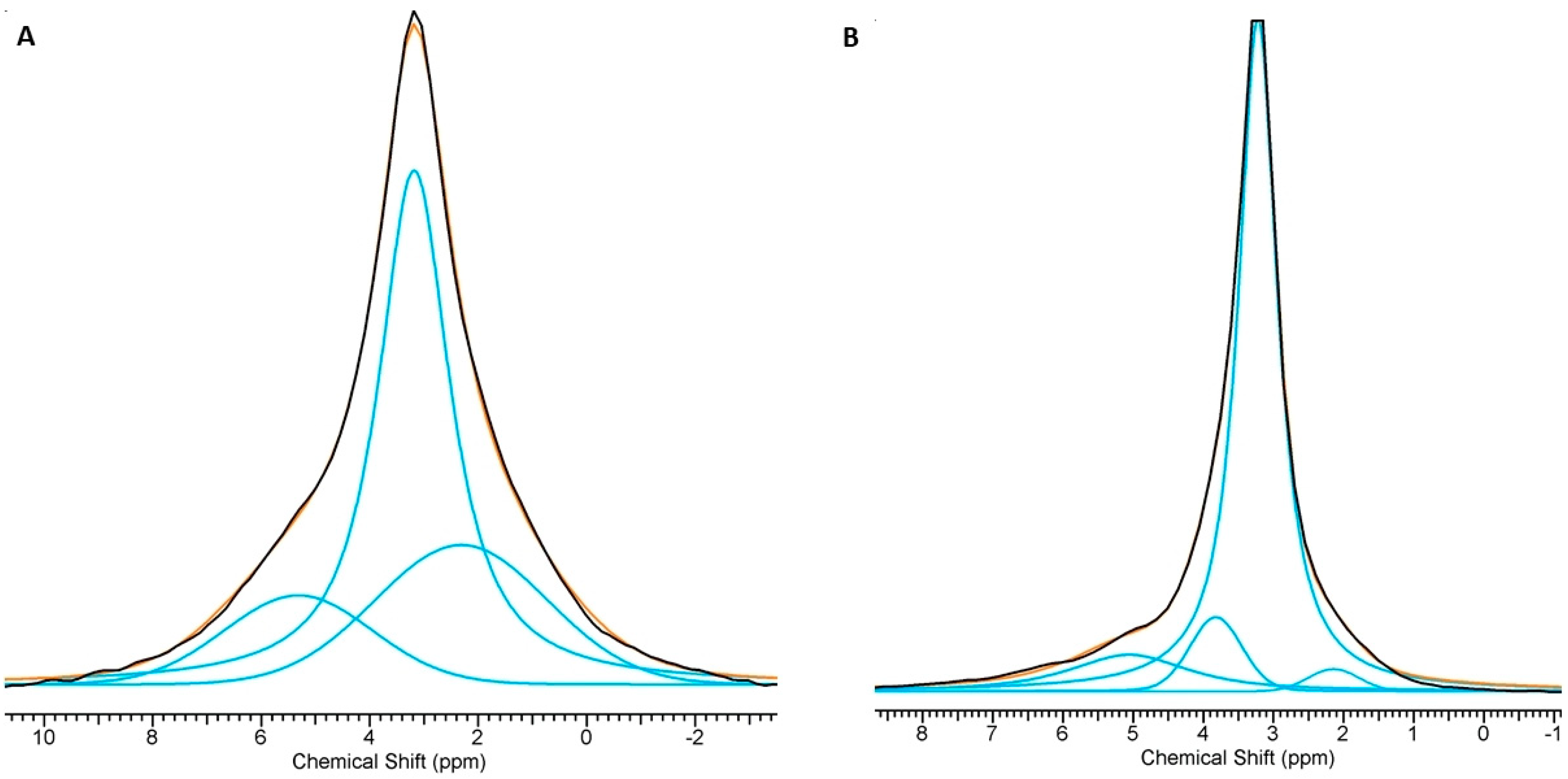
| Assignments | Chemical Shift (ppm) | FWHM (Hz) | Percentage of Total Area (%) | |
|---|---|---|---|---|
| Mn,SiO4-HAw | ≡POxH | 2.31 | 606 | 27.4 |
| main | 3.18 | 256 | 57.4 | |
| ≡POx | 5.31 | 523 | 15.1 | |
| Mn,SiO3-HAw | ≡POxH | 2.25 | 640 | 22.3 |
| main | 3.22 | 248 | 54.5 | |
| ≡POx | 5.28 | 551 | 23.2 | |
| Mn,SiO4-HAd | ≡POxH | 2.14 | 138 | 2.4 |
| main | 3.22 | 108 | 77.0 | |
| ≡POx | 5.10 | 329 | 12.1 | |
| silicocarnotite | 3.85 | 142 | 7.5 | |
| Mn,SiO3-HAd | ≡POxH | 2.33 | 145 | 4.2 |
| main | 3.15 | 111 | 78.5 | |
| ≡POx | 5.35 | 316 | 15.2 | |
| silicocarnotite | 4.12 | 156 | 2.1 |
| Sample | Microtox® (15 min-PE) 1 | Spirotox 2 | ||
|---|---|---|---|---|
| 1.0 mg/mL | 2.0 mg/mL | 1.0 mg/mL | 2.0 mg/mL | |
| Mn,SiO4-HAw | 0 | 0 | NT | NT |
| Mn,SiO3-HAw | 0 | 0 | NT | NT |
| Mn,SiO4-HAd | 0 | 0 | NT | NT |
| Mn,SiO3-HAd | 0 | 0 | NT | NT |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szurkowska, K.; Drobniewska, A.; Kolmas, J. Dual Doping of Silicon and Manganese in Hydroxyapatites: Physicochemical Properties and Preliminary Biological Studies. Materials 2019, 12, 2566. https://doi.org/10.3390/ma12162566
Szurkowska K, Drobniewska A, Kolmas J. Dual Doping of Silicon and Manganese in Hydroxyapatites: Physicochemical Properties and Preliminary Biological Studies. Materials. 2019; 12(16):2566. https://doi.org/10.3390/ma12162566
Chicago/Turabian StyleSzurkowska, Katarzyna, Agata Drobniewska, and Joanna Kolmas. 2019. "Dual Doping of Silicon and Manganese in Hydroxyapatites: Physicochemical Properties and Preliminary Biological Studies" Materials 12, no. 16: 2566. https://doi.org/10.3390/ma12162566
APA StyleSzurkowska, K., Drobniewska, A., & Kolmas, J. (2019). Dual Doping of Silicon and Manganese in Hydroxyapatites: Physicochemical Properties and Preliminary Biological Studies. Materials, 12(16), 2566. https://doi.org/10.3390/ma12162566






