Preparation and Microwave Absorption Properties of C@Fe3O4 Magnetic Composite Microspheres
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Monodisperse P(MAA/St) Microspheres
2.3. Preparation of P(MAA/St)@Fe3O4 Magnetic Composite Microspheres
2.4. Preparation of C@Fe3O4 Magnetic Composite Microspheres
2.5. Characterization
3. Results and Discussion
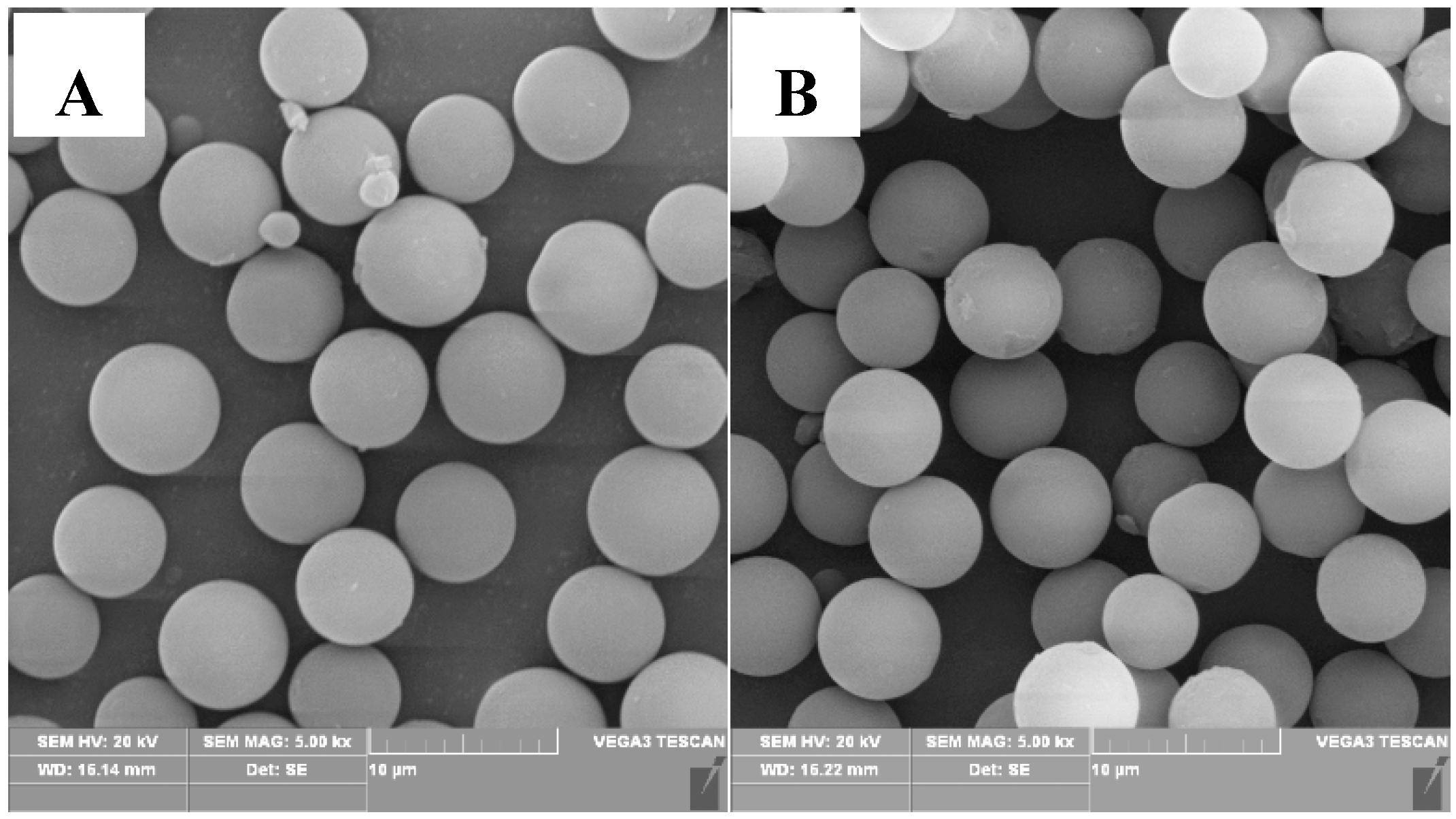
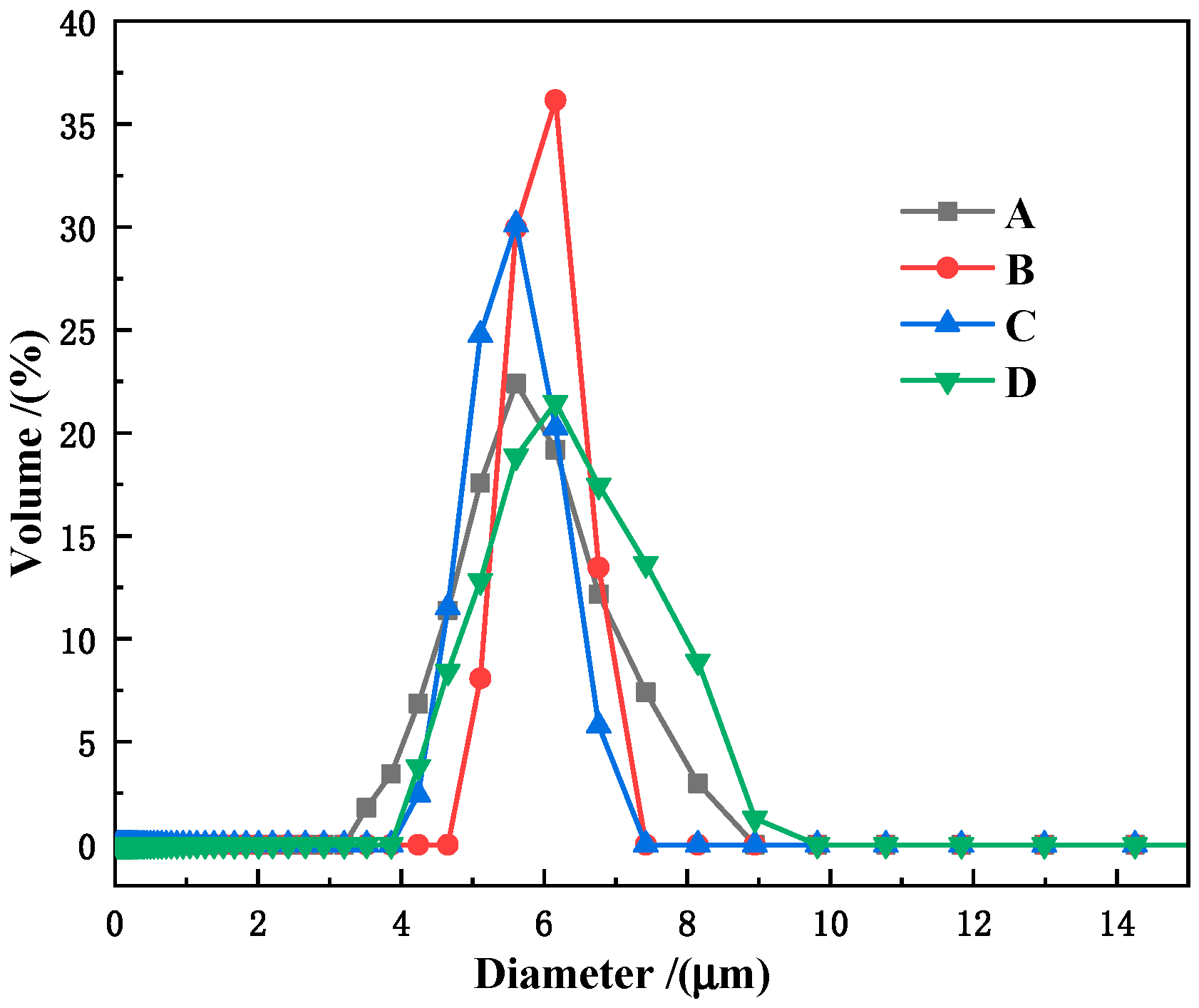
3.1. Morphology and Composition Analysis of the Synthesized P(MAA/St) Microspheres
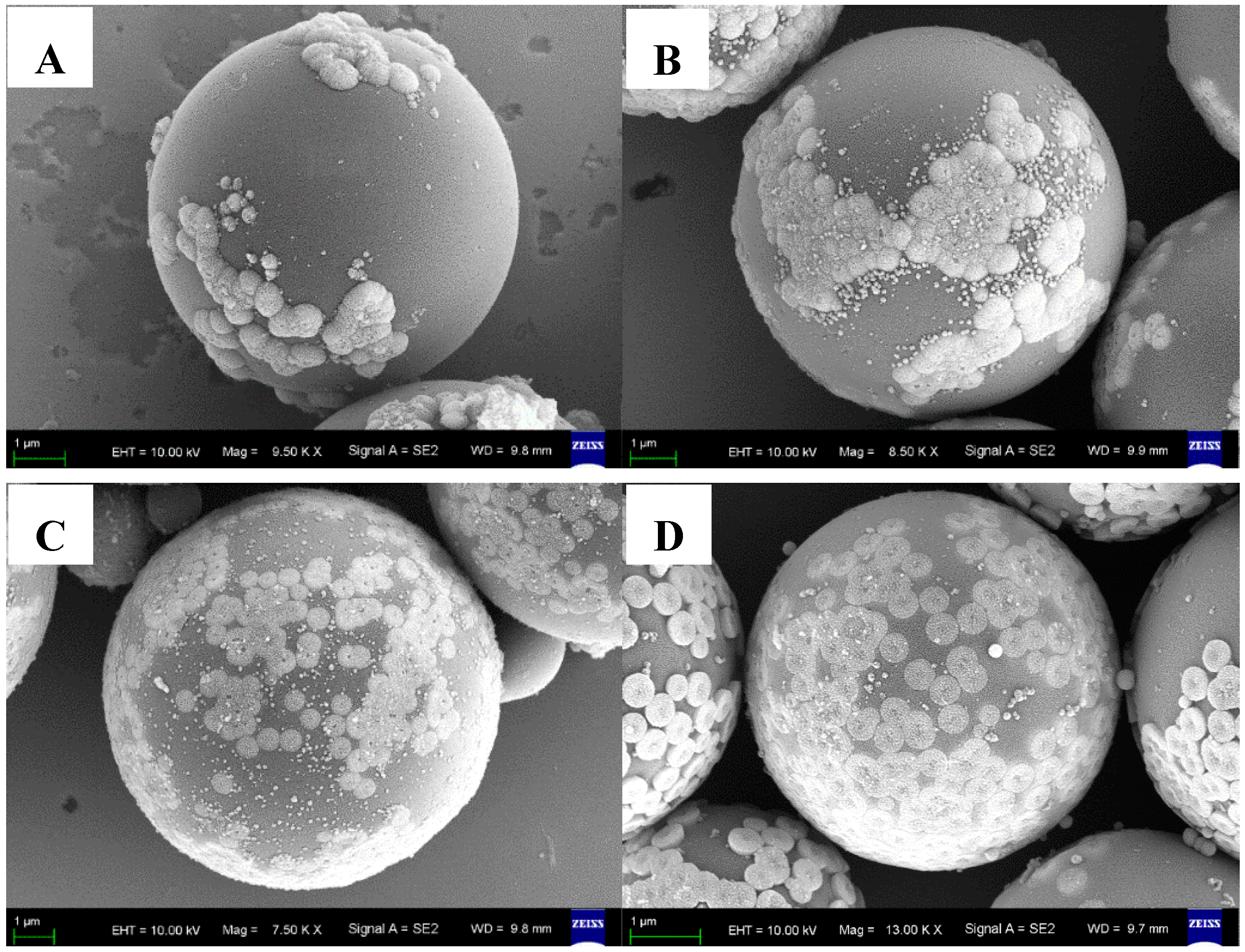
3.2. Characterization of the P(MAA/St)@Fe3O4 Magnetic Composite Microsphere
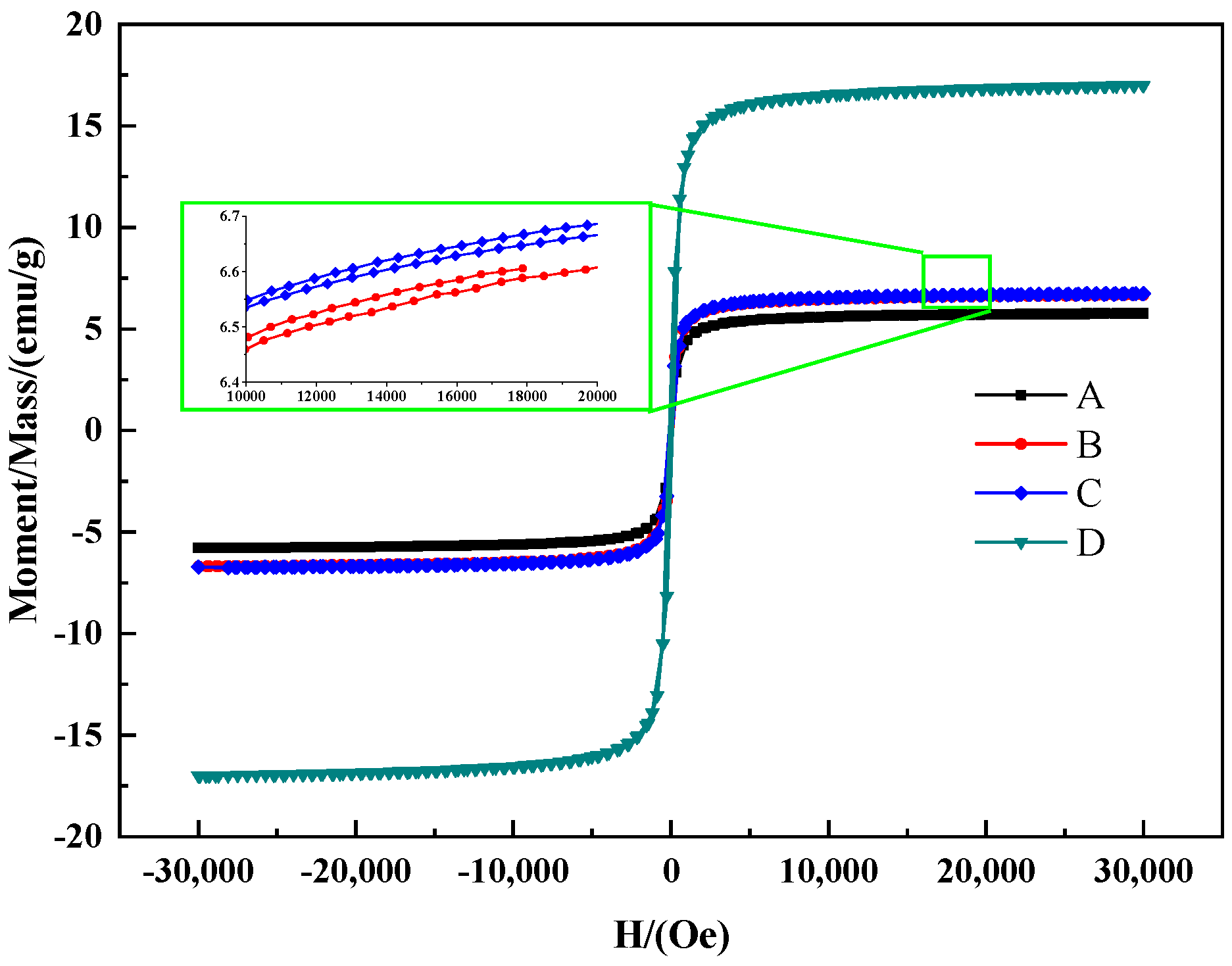
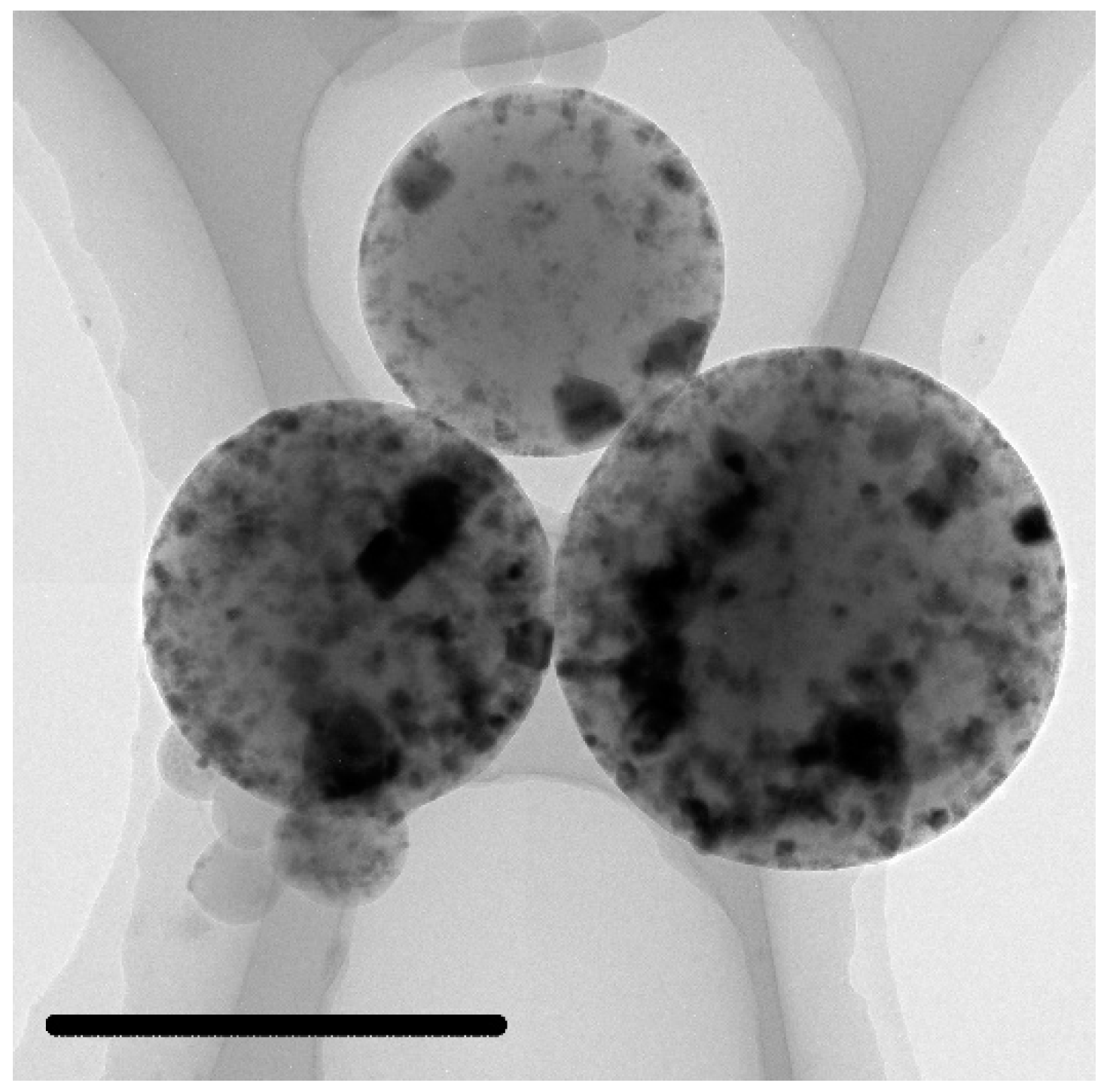
3.3. Characterization of the C@Fe3O4 Magnetic Composite Microsphere
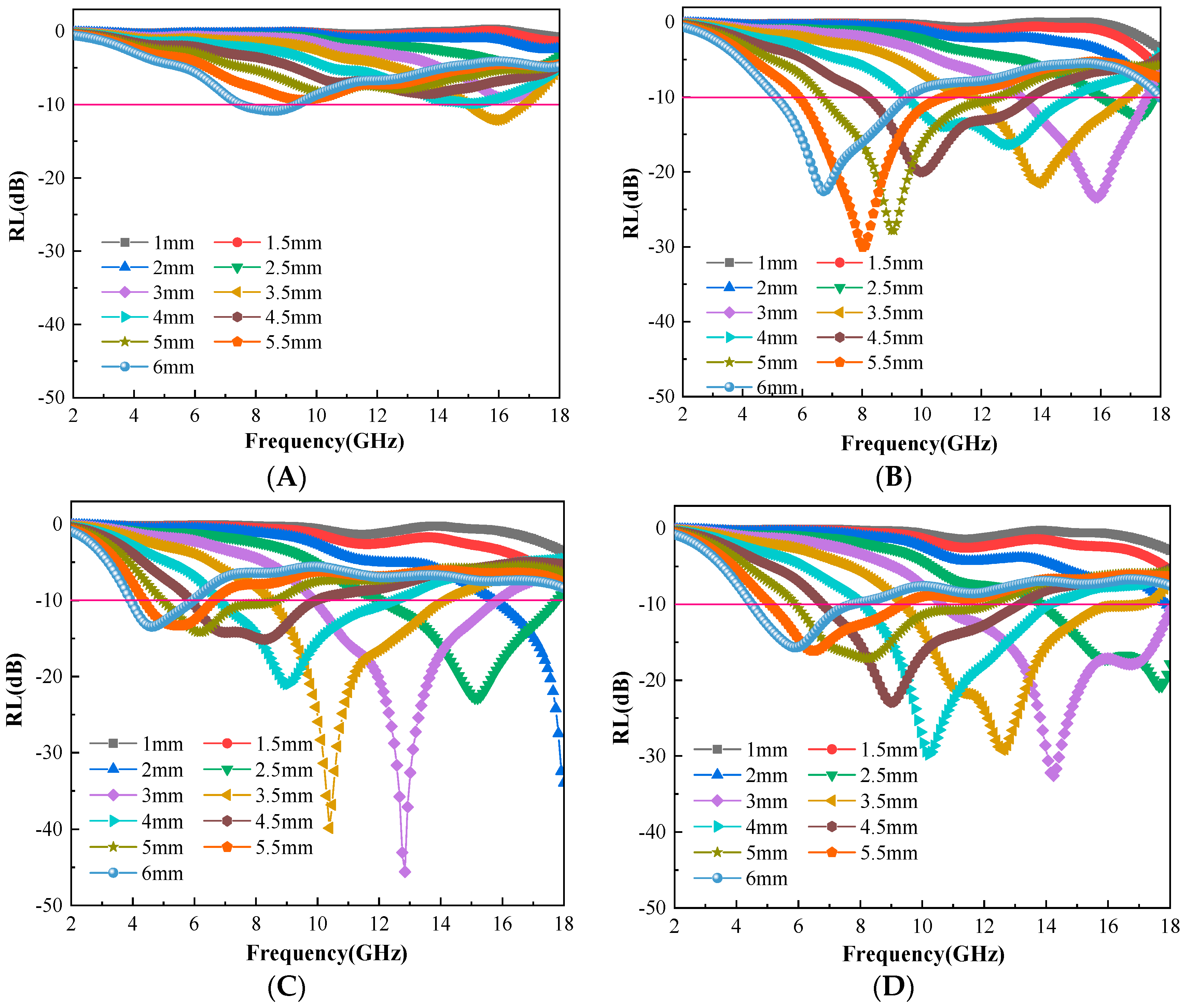
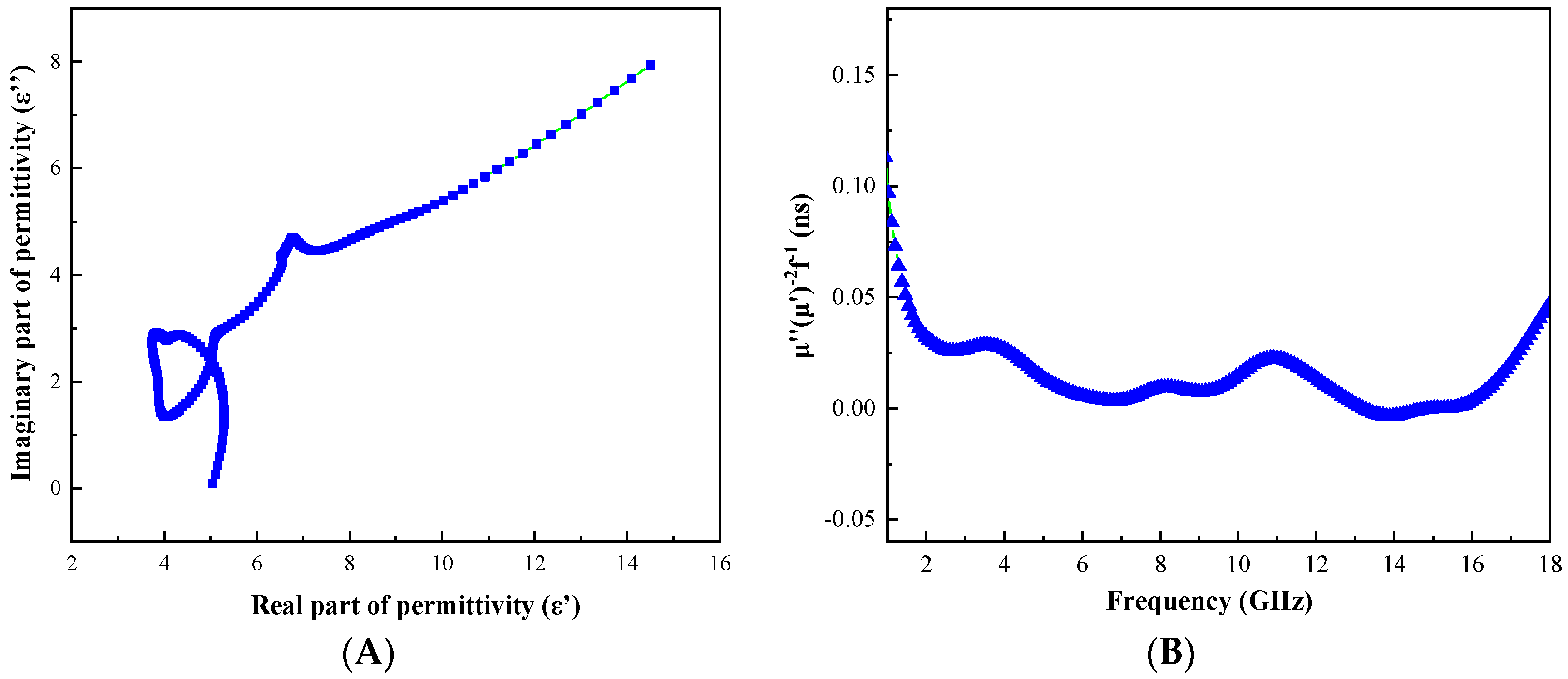
3.4. Evaluation of Microwave Absorbing Properties of C@Fe3O4
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chu, W.; Wang, Y.; Du, Y.; Qiang, R.; Tian, C.; Han, X. FeCo alloy nanoparticles supported on ordered mesoporous carbon for enhanced microwave absorption. J. Mater. Sci. 2017, 52, 13636–13649. [Google Scholar] [CrossRef]
- Liu, X.; Cui, X.; Chen, Y.; Zhang, X.; Yu, R.; Wang, G.; Ma, H. Modulation of electromagnetic wave absorption by carbon shell thickness in carbon encapsulated magnetite nanospindles-poly(vinylidene fluoride) composites. Carbon 2015, 95, 870–878. [Google Scholar] [CrossRef]
- Kong, L.; Yin, X.; Yuan, X.; Zhang, Y.; Liu, X.; Cheng, L.; Zhang, L. Electromagnetic wave absorption properties of graphene modified with carbon nanotube/poly(dimethyl siloxane) composites. Carbon 2014, 73, 185–193. [Google Scholar] [CrossRef]
- Li, Z.; Han, X.; Ma, Y.; Liu, D.; Wang, Y.; Xu, P.; Du, Y. MOFs-Derived Hollow Co/C Microspheres with Enhanced Microwave Absorption Performance. ACS Sustain. Chem. Eng. 2018, 6, 8904–8913. [Google Scholar] [CrossRef]
- Yang, Z.; Xu, J.; Wang, J.; Zhang, Q.; Zhang, B. Design and preparation of self-driven BSA surface imprinted tubular carbonnanofibers and their specific adsorption performance. Chem. Eng. J. 2019, 373, 923–934. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Z.; Xu, J.; Ahmad, M.; Zhang, H.; Zhang, A.; Zhang, B. Surface Microstructure Regulation of Porous PolymerMicrospheres by Volume Contraction of Phase SeparationProcess in Traditional Suspension Polymerization System. Macromol. Rapid Commun. 2019, 1800768, 1–6. [Google Scholar]
- Wang, J.; Yang, Z.; Xu, J.; Ahmad, M.; Zhang, H.; Zhang, A.; Zhang, B. Preparation of surface protein imprinted thermosensitive polymermonolithic column and its specific adsorption for BSA. Talanta 2019, 200, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Liu, T.; Yu, B.; Gao, H.; Xu, P.; Wang, J.; Han, X. The electromagnetic properties and microwave absorption of mesoporous carbon. Mater. Chem. Phys. 2012, 135, 884–891. [Google Scholar] [CrossRef]
- Zhao, T.; Hou, C.; Zhang, H.; Zhu, R.; She, S.; Wang, J.; Wei, B. Electromagnetic wave absorbing properties of amorphous carbon nanotubes. Sci. Rep. 2014, 4, 5619. [Google Scholar] [CrossRef]
- Lv, H.; Liang, X.; Cheng, Y.; Zhang, H.; Tang, D.; Zhang, B.Y. Coin-like alpha-Fe2O3@CoFe2O4 core-shell composites with excellent electromagnetic absorption performance. ACS Appl. Mater. Interfaces 2015, 7, 4744–4750. [Google Scholar] [CrossRef]
- Lv, H.; Liang, X.; Ji, G.; Zhang, H.; Du, Y. Porous Three-Dimensional Flower-like Co/CoO and Its Excellent Electromagnetic Absorption Properties. ACS Appl. Mater. Interfaces 2015, 7, 9776–9783. [Google Scholar] [CrossRef]
- Zheng, J.; Lv, H.; Lin, X.; Ji, G.; Li, X.; Du, Y. Enhanced microwave electromagnetic properties of Fe3O4/graphene nanosheet composites. J. Alloys Compd. 2014, 589, 174–181. [Google Scholar] [CrossRef]
- Wang, G.; Gao, Z.; Tang, S.; Chen, C.; Duan, F.; Zhao, S.; Qin, Y. Microwave Absorption Properties of Carbon Nanocoils Coated with Highly Controlled Magnetic Materials by Atomic Layer Deposition. ACS Nano 2012, 6, 11009–11017. [Google Scholar] [CrossRef] [PubMed]
- Tong, G.; Liu, Y.; Cui, T.; Li, Y.; Zhao, Y.; Guan, J. Tunable dielectric properties and excellent microwave absorbing properties of elliptical Fe3O4nanorings. Appl. Phys. Lett. 2016, 108, 072905. [Google Scholar] [CrossRef]
- Ni, S.; Wang, X.; Zhou, G.; Yang, F.; Wang, J.; He, D. Designed synthesis of wide range microwave absorption Fe3O4-carbon sphere composite. J. Alloys Compd. 2010, 489, 252–256. [Google Scholar] [CrossRef]
- Ni, S.; Wang, X.; Zhou, G.; Yang, F.; Wang, J.; He, D. Low temperature synthesis of Fe3O4 micro-spheres and its microwave absorption properties. Mater. Chem. Phys. 2010, 124, 353–358. [Google Scholar] [CrossRef]
- Liu, X.; Cao, K.; Chen, Y.; Ma, Y.; Zhang, Q.; Zeng, D.; Peng, D.L. Shape-dependent magnetic and microwave absorption properties of iron oxide nanocrystals. Mater. Chem. Phys. 2017, 192, 339–348. [Google Scholar] [CrossRef]
- Liu, X.; Cao, K.; Chen, Y.; Ma, Y.; Zhang, Q.; Zeng, D.; Peng, D.L. Enhanced microwave absorption properties in GHz range of Fe3O4/C composite materials. J. Alloys Compd. 2015, 649, 537–543. [Google Scholar] [CrossRef]
- Tong, G.; Hua, Q.; Wu, W.; Qin, M.; Li, L.; Gong, P. Effect of liquid-solid ratio on the morphology, structure, conductivity, and electromagnetic characteristics of iron particles. Sci. China Technol. Sci. 2011, 54, 484–489. [Google Scholar] [CrossRef]
- Zhang, K.; Gao, X.; Zhang, Q.; Chen, H.; Chen, X. Fe3O4 nanoparticles decorated MWCNTs@C ferrite nanocomposites and their enhanced microwave absorption properties. J. Magn. Magn. Mater. 2018, 452, 55–63. [Google Scholar] [CrossRef]
- Zhang, B.; Huyan, Y.; Wang, J.; Chen, X.; Zhang, H.; Zhang, Q. Fe3O4@SiO2@CCS porous magnetic microspheres as adsorbent for removal of organic dyes in aqueous phase. J. Alloys Compd. 2018, 735, 1986–1996. [Google Scholar] [CrossRef]
- Wu, T.; Liu, Y.; Zeng, X.; Cui, T.; Zhao, Y.; Li, Y.; Tong, G. Facile Hydrothermal Synthesis of Fe3O4/C Core-Shell Nanorings for Efficient Low-Frequency Microwave Absorption. ACS Appl. Mater. Interfaces 2016, 8, 7370–7380. [Google Scholar] [CrossRef]
- Li, Y.; Wu, T.; Jin, K.; Qian, Y.; Qian, N.; Jiang, K.; Tong, G. Controllable synthesis and enhanced microwave absorbing properties of Fe3O4/NiFe2O4/Ni heterostructure porous rods. Appl. Surf. Sci. 2016, 387, 190–201. [Google Scholar] [CrossRef]
- Li, L.; Chen, K.; Liu, H.; Tong, G.; Qian, H.; Hao, B. Attractive microwave-absorbing properties of M-BaFe12O19 ferrite. J. Alloys Compd. 2013, 557, 11–17. [Google Scholar] [CrossRef]
- Qiao, M.; Lei, X.; Ma, Y.; Tian, L.; He, X.; Su, K.; Zhang, Q. Application of yolk-shell Fe3O4@N-doped carbon nanochains as highly effective microwave-absorption material. Nano Res. 2018, 11, 1500–1519. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Y.; Lu, X.; Zhu, Y.; Jiang, L. Self-healing superhydrophobicpolyvinylidene fluoride/Fe3O4@polypyrrole fiber with core–sheath structures for superior microwave absorption. Nano Res. 2016, 9, 2034–2045. [Google Scholar] [CrossRef]
- Min, D.; Zhou, W.; Qing, Y.; Luo, F.; Zhu, D. Highly oriented flake carbonyl iron/carbon fiber composite as thin-thickness and wide-bandwidth microwave absorber. J. Alloys Compd. 2018, 744, 629–636. [Google Scholar] [CrossRef]
- Zhang, X.J.; Wang, G.S.; Cao, W.Q.; Wei, Y.Z.; Liang, J.F.; Guo, L.; Cao, M.S. Enhanced microwave absorption property of reduced graphene oxide (RGO)-MnFe2O4 nanocomposites and polyvinylidene fluoride. ACS Appl. Mater. Interfaces 2014, 6, 7471–7478. [Google Scholar] [CrossRef]
- Ning, M.; Li, J.; Kuang, B.; Wang, C.; Su, D.; Zhao, Y.; Cao, M. One-step fabrication of N-doped CNTs encapsulating M nanoparticles (M = Fe, Co, Ni) for efficient microwave absorption. Appl. Surf. Sci. 2018, 447, 244–253. [Google Scholar] [CrossRef]
- Xu, P.; Han, X.; Wang, C.; Zhou, D.; Lv, Z.; Wen, A.; Zhang, B. Synthesis of Electromagnetic Functionalized Nickel/Polypyrrole Core/Shell Composites. J. Phys. Chem. B 2008, 112, 10443–10448. [Google Scholar] [CrossRef]
- Shu, R.; Li, W.; Zhou, X.; Tian, D.; Zhang, G.; Gan, Y.; He, J. Facile preparation and microwave absorption properties of RGO/MWCNTs/ZnFe2O4 hybrid nanocomposites. J. Alloys Compd. 2018, 743, 163–174. [Google Scholar] [CrossRef]
- Liu, Q.; Cao, Q.; Bi, H.; Liang, C.; Yuan, K.; She, W.; Che, R. CoNi@SiO2@TiO2 and CoNi@Air@TiO2 Microspheres with Strong Wideband Microwave Absorption. Adv. Mater. 2016, 28, 486–490. [Google Scholar] [CrossRef]
- Chen, Y.J.; Xiao, G.; Wang, T.S.; Ouyang, Q.Y.; Qi, L.H.; Ma, Y.; Jin, H.B. Porous Fe3O4/Carbon Core/Shell Nanorods: Synthesis and Electromagnetic Properties. J. Phys. Chem. C 2011, 115, 10061–10064. [Google Scholar] [CrossRef]
- Zhang, Y.; Kang, Z.; Ding, C. Synthesis and microwave absorbing properties of Mn-Zn nanoferrite produced by microwave assisted ball milling. J. Mater. Sci. Mater. Electron. 2014, 25, 4246–4251. [Google Scholar] [CrossRef]

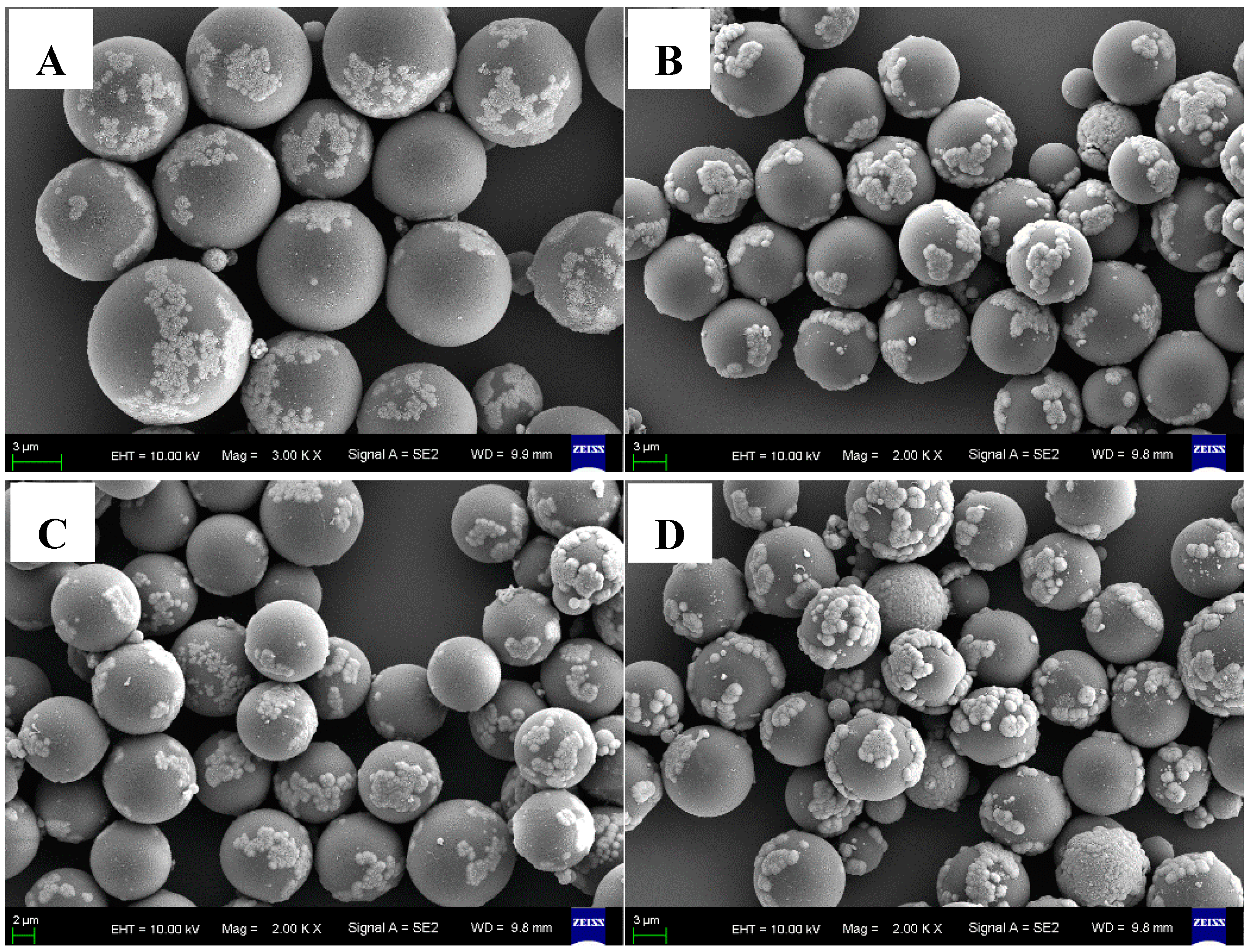
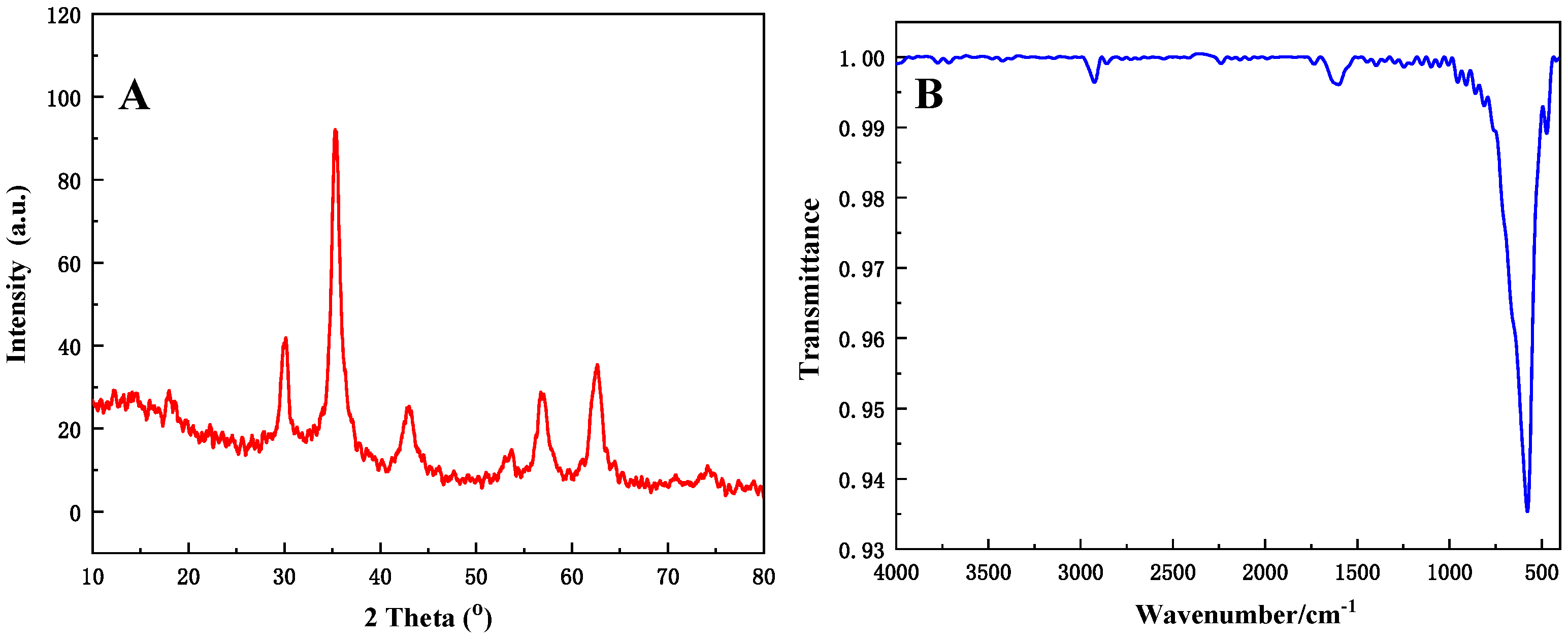
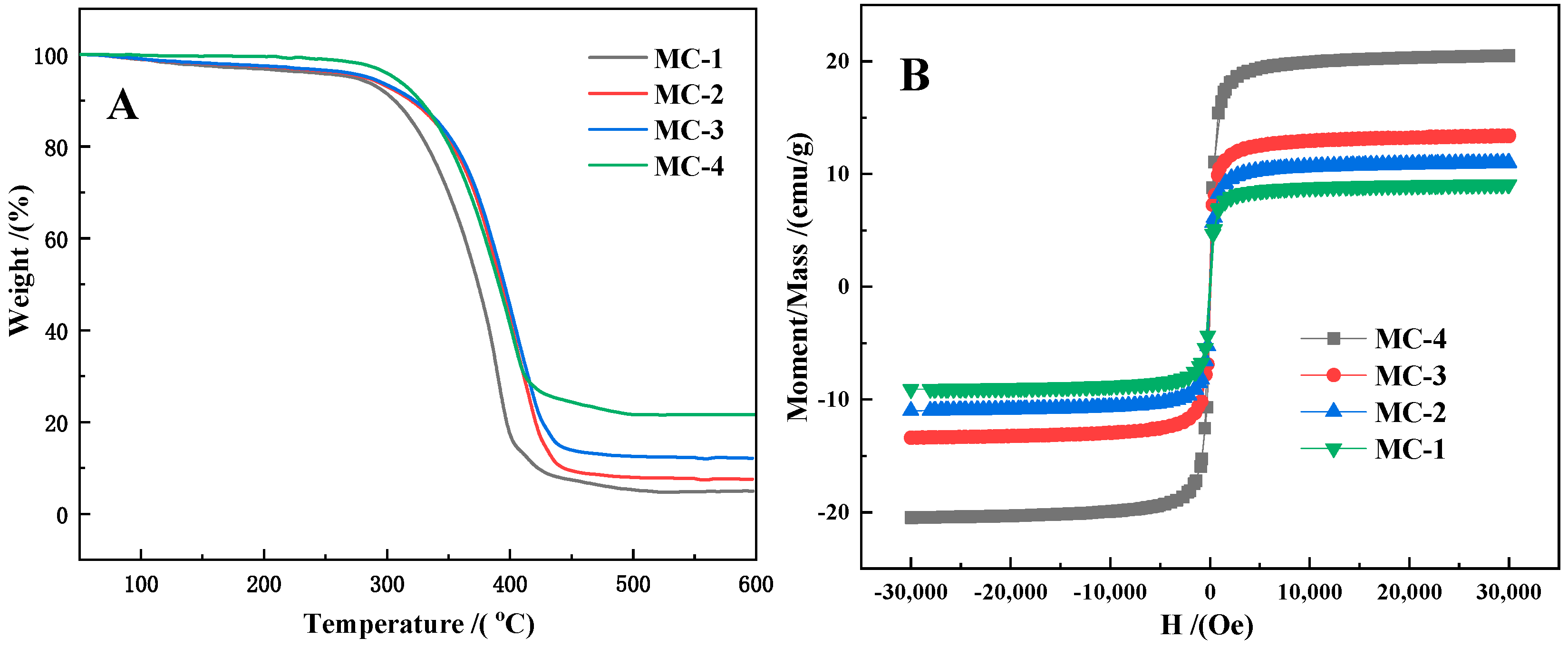
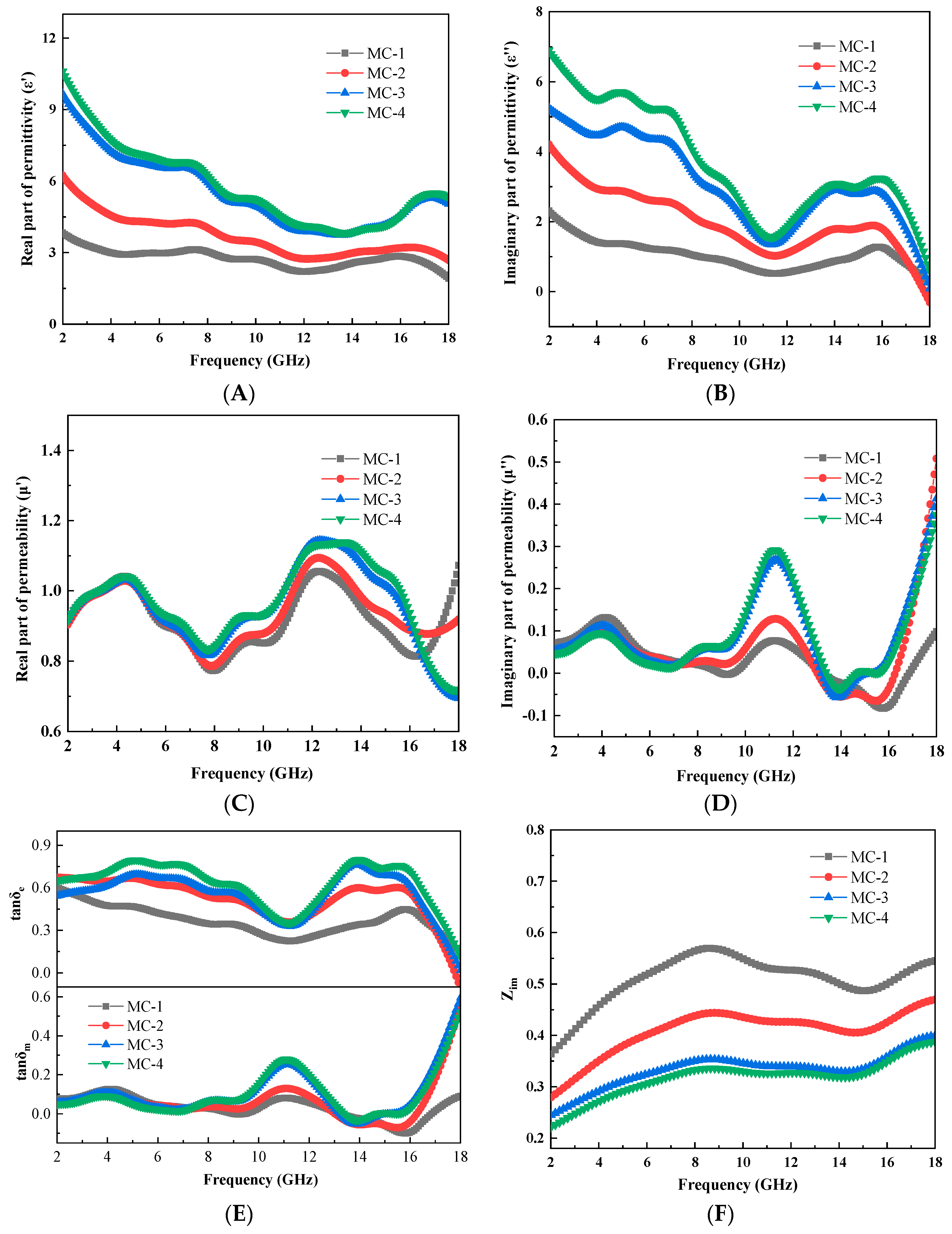
| Sample No. | P-1 | P-2 | P-3 | P-4 |
|---|---|---|---|---|
| MAA (g) | 0.05 | 0.10 | 0.15 | 0.20 |
| St (g) | 6.30 | 6.25 | 6.20 | 6.15 |
| Sample No. | P-1 | P-2 | P-3 | P-4 |
|---|---|---|---|---|
| Carboxyl content (mmol/g) | 0.07 | 0.15 | 0.23 | 0.40 |
| Surface density of carboxyl groups (μmol/m2) | 23.14 | 88.03 | 92.64 | 142.80 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.; Yin, Y.; Zhang, Y.; Hu, Y.; Liu, W. Preparation and Microwave Absorption Properties of C@Fe3O4 Magnetic Composite Microspheres. Materials 2019, 12, 2404. https://doi.org/10.3390/ma12152404
Shi Y, Yin Y, Zhang Y, Hu Y, Liu W. Preparation and Microwave Absorption Properties of C@Fe3O4 Magnetic Composite Microspheres. Materials. 2019; 12(15):2404. https://doi.org/10.3390/ma12152404
Chicago/Turabian StyleShi, Youqiang, Yanan Yin, Yi Zhang, Yue Hu, and Weifeng Liu. 2019. "Preparation and Microwave Absorption Properties of C@Fe3O4 Magnetic Composite Microspheres" Materials 12, no. 15: 2404. https://doi.org/10.3390/ma12152404
APA StyleShi, Y., Yin, Y., Zhang, Y., Hu, Y., & Liu, W. (2019). Preparation and Microwave Absorption Properties of C@Fe3O4 Magnetic Composite Microspheres. Materials, 12(15), 2404. https://doi.org/10.3390/ma12152404




