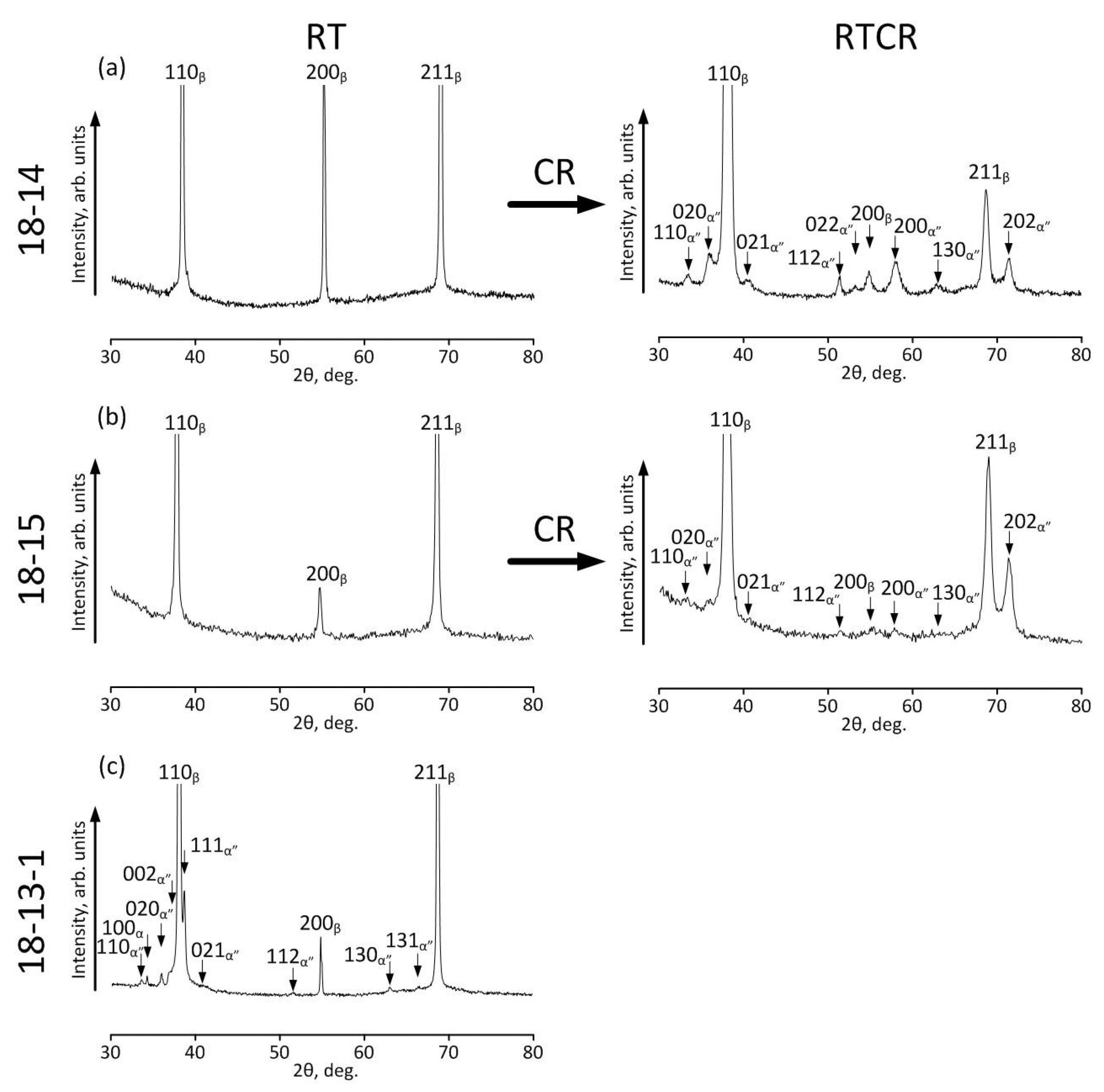
4.3. Surface Film Characterization
In the framework of AES elemental analysis of the surface oxide films on the 18-14, 18-15, and 18-13-1 alloys before and after exposure to Hanks’ solution for 604.8 ks, attention is drawn to the relative concentration of the most reactive elements (Ti and Zr) in the uppermost layer (up to 3 nm) as well as in the near-surface area (about 100 nm), i.e., before and after ion sputtering.
Table 9 shows Zr/Ti concentration ratio values for the studied alloys before and after exposure to Hanks’ solution, as calculated from
Table 1,
Table 3, and
Table 4.
The obtained results show that exposure to Hanks’ solution leads to significant surface enrichment with zirconium, which indicates the predominant oxidation of Zr, the latter being associated with higher oxygen affinity as compared to Ti.
Ion sputtering eliminates surface oxide layers; consequently, at a depth of about 100 nm, Zr/Ti ratio values are similar for all alloys and close to the nominal one. The observed mismatch between the near-surface layer composition and the nominal values may be caused by selective sputtering and slight deviations in the elements’ actual relative sensitivity factors from the standardized values.
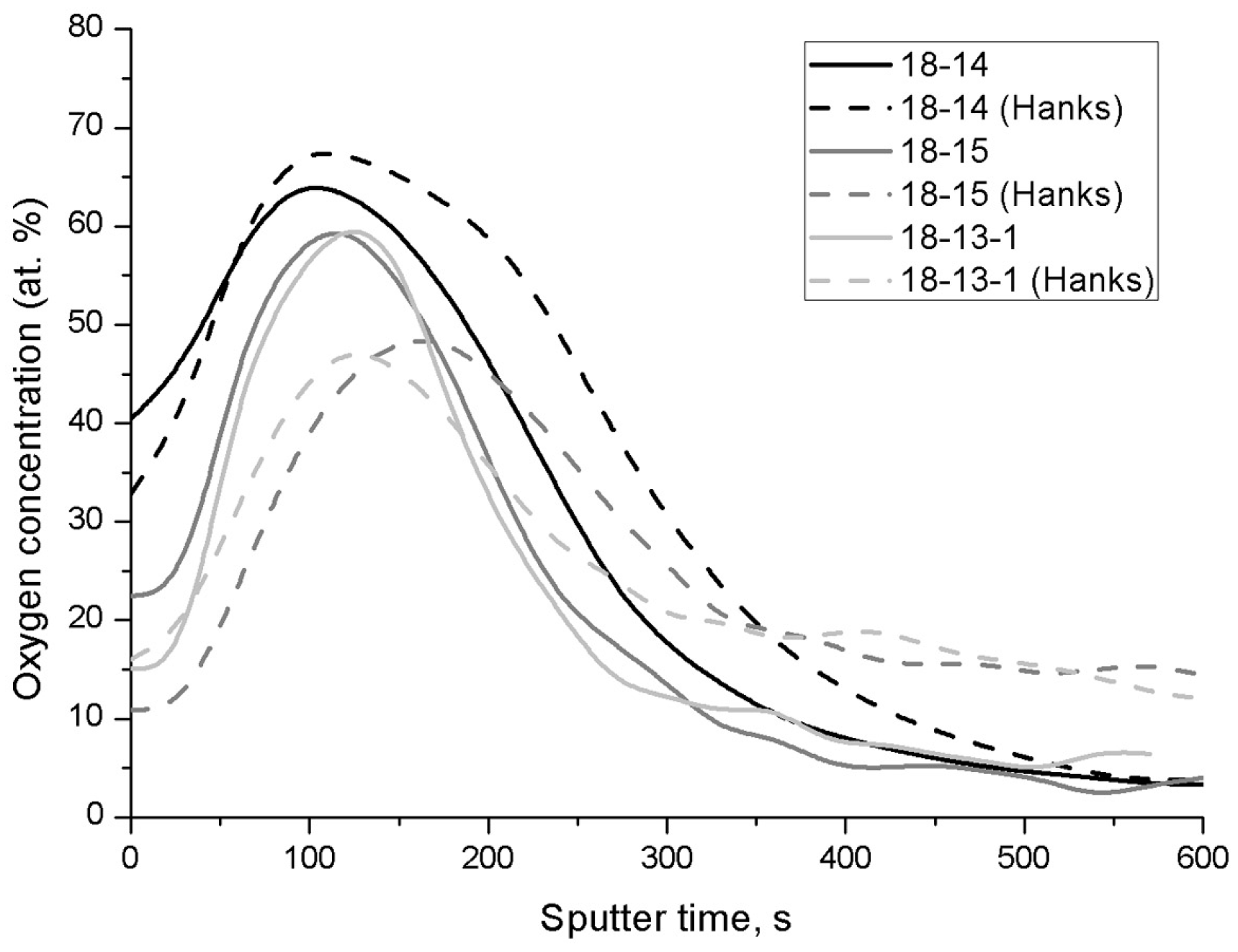
The oxide film thickness estimation carried out using AES oxygen distribution profiles (
Figure 3,
Table 5) reveals that the thinnest film, as well as the lowest oxide film thickening after exposure to Hanks’ solution, can be attributed to the 18-13-1 alloy, while the oxide layers on the 18-14 and 18-15 alloys are thicker and grow measurably in the solution environment.
These observations can be explained by a lower imperfection level of the oxide film on the 18-13-1 alloy, which is achieved by substituting a certain number of defects in the lattices of prevailing ZrO
2/TiO
2 oxides by tantalum atoms; the total added, however, is small and cannot be reliably detected by AES. The ionic radius of Ta
5+ ion is comparable to that of Ti
4+ и Nb
5+ ions (0.66, 0.64, and 0.66 Å, respectively), which promotes its incorporation into the growing oxide layer. It is also worth noting that TiO
2 is an anion-deficient n-type semiconductor. According to the Wagner‒Hauffe theory, the doping of TiO
2 with higher valence ions leads to a decrease in the defect concentration and corrosion rate reduction [
47].
4.4. Electrochemical Characterization in Static Conditions
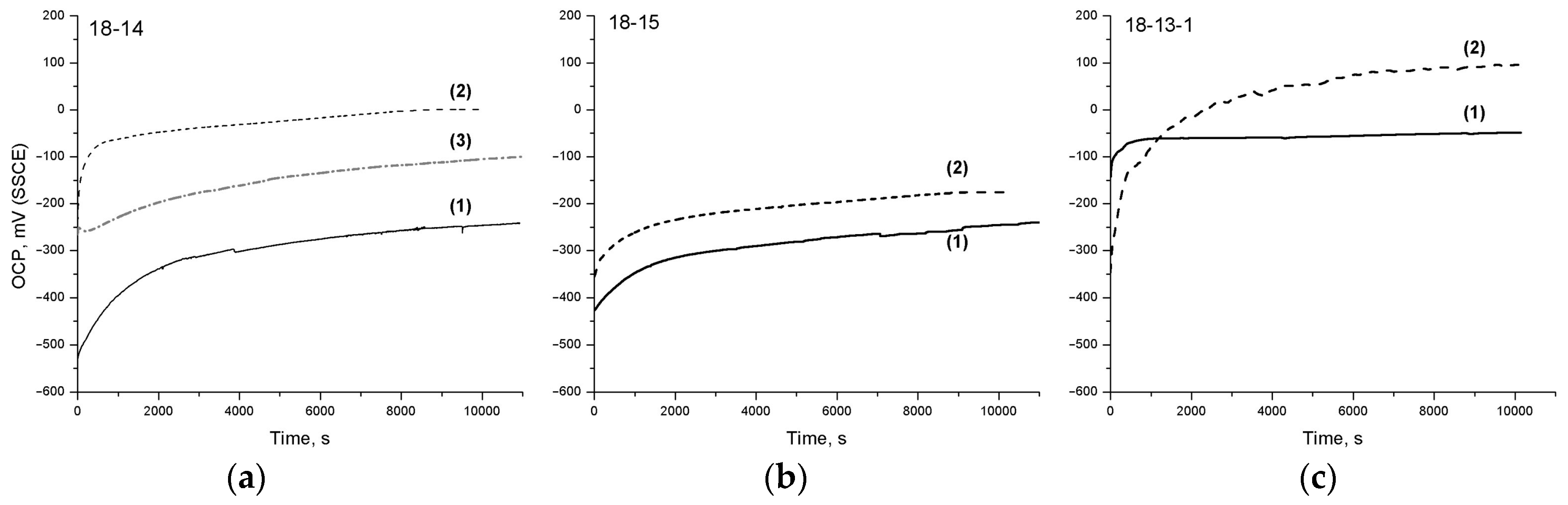
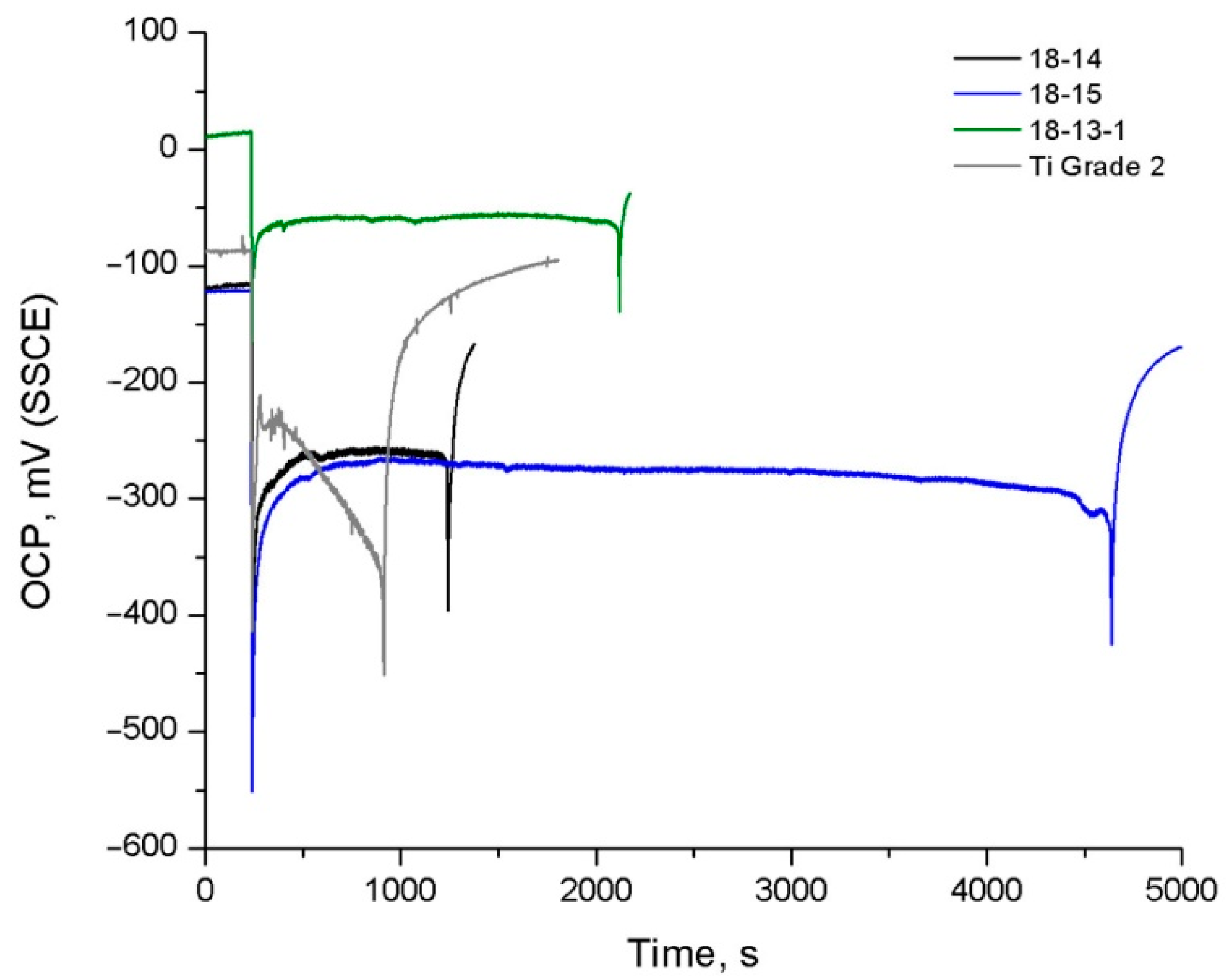
The analysis of the OCP curves obtained during the exposure to Hanks’ solution (
Figure 4 and
Table 6) shows that the highest steady-state potential values
Est before and after TMT are exhibited by the 18-13-1 alloy, which can be a consequence of its passive film’s advanced protective properties (see
Section 4.3). For the 18-14 and 18-15 alloys, the E
st values are comparable before TMT, while after TMT, the
Est of the 18-14 alloy is 170 mV higher.
The 18-14-based foam material exhibits a significantly higher OCP than the bulk 18-14 alloy, which can be related to the residual partially oxidized and more developed surface that could not be removed by polishing prior to OCP measurements. This corresponds to a relatively small ΔE shift during exposure to the solution.
Generally, a positive effect of TMT on the OCP behavior of the studied alloys is revealed. In particular, steady-state potential values
Est increase; the 18-15 alloy, after TMT, additionally features a larger
ΔE shift, which indicates an improvement in the surface’s protective ability upon exposure to Hanks’ solution. The initially small
ΔE value for the 18-13-1 alloy before TMT correlates with the insignificant change in its oxide layer thickness during the exposure (
Table 5).
In order to reveal the mechanisms of the passivating film formation and the kinetic regularities of their growth during the exposure to Hanks’ solution, the OCP curves were processed, taking into account the proportional dependence between the OCP values and the passive layer thicknesses. The approach is described in more detail in [
48].
For each time moment
t and corresponding OCP value
Et, the normalized potential change was calculated via Equation (1):
The analysis shows that all normalized OCP curves can be approximated with a high confidence level (
R2 > 0,99,
Table 10) with two logarithmic dependences describing the variations of
et in two time periods—the initial period (A) and the residual period (B) corresponding to Equations (2) and (3), respectively. The equations are as follows:
where
a,
k,
c are fitting parameters.
The time moment where the A-region changes to the B-region (tx) was adjusted to obtain the best reliability in OCP curve fitting (highest R2 values) using Origin 8.0 software (OriginLab Corp., Northampton, MA, USA). These regions correspond to different mechanisms that define the growth rate of surface oxide films.
The kinetic regularities corresponding to Equations (2) and (3) were introduced by Cabrera and Mott [
49] and Hauffe and Ilschner [
50], respectively, to describe the growth of thin oxide films (less than 5 nm) at low temperatures. According to their concerns, the formation of the thinnest layers at the initial stage should follow the direct logarithmic time dependence (A) with a subsequent change to the reciprocal logarithmic one corresponding to different mechanisms that limit the film growth and, therefore, define the process rate—electron tunneling through the thinner oxide film and ion transfer through the thicker oxide film.
The parameter
k in Equations (2) and (3) can be considered rate constants of oxide film formation. The OCP curves processing results are summarized in
Table 10.
It follows from
Table 10 that the oxide film growth rate increases significantly after TMT, which is indicated by the decrease in
tx values (the thinnest film formation stage (A) terminates earlier). This trend can be explained by the formation of a more developed surface substructure leading to an increase in surface reactivity and an intensification of the film’s growth.
The highest tx value is observed for the 18-14-based foam material, which can be related to the hindered inlet of oxygen to some parts of the developed surface, resulting in the inhibited oxide layer growth.
Before TMT, the 18-14 alloy demonstrates the highest inclination to self-passivation, considering the highest k value in period (A). It can be noted that the most considerable influence of TMT on the film growth kinetics is evident for the 18-14 alloy—parameter c in region (A) in the case of the sample after TMT is two orders of magnitude greater than that of the sample before TMT, while for 18-15 and 18-13-1 the difference is not so sharp.
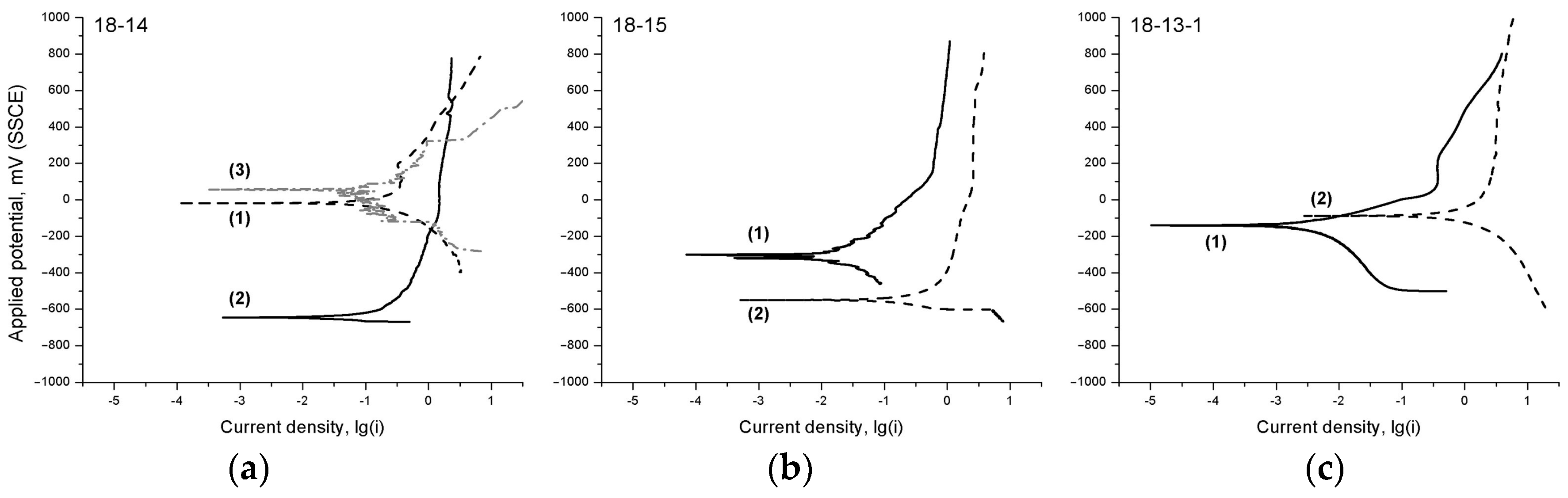
The polarization diagrams of the studied alloys (
Figure 5) feature a change in the cathodic (lower) part of the curve, which is associated with the overvoltage decrease (release) of the rate-controlling cathodic process; in the case of Hanks’ solution, this is oxygen depolarization reaction O
2 + 2H
2O + 4ē = 4OH¯.
It is known that the indirect comparative aspect of the electrode process’ inhibition is the slope of the polarization curve. It can be seen that Ti‒Zr-based materials before TMT are characterized by a much steeper slope of the cathodic part of the polarization diagrams compared with after TMT. The reason for this cathodic process release after TMT may be an increase in the amount of orthorhombic face-centered α’’-martensite acting as a cathode, i.e., the surface regions where the cathodic process can take place.
In addition, it is reasonable to assume that, during TMT, the redistribution of interstitial impurities can take place, with their segregation regions being the additional local zones favoring the cathodic reaction. It is also probable that the oxide film forming on the surface after TMT is more heterogeneous than in the case with no TMT [
51], with more oxidized surface areas acting as the cathodic regions.
In the case of the 18-14-based foam material, the increase in corrosion rate, as well as the current density fluctuations observed in the polarization curve, may be related to the developed surface of the sample and the local passivation instability caused by the deficiency in oxygen required to form a highly protective oxide film.
4.5. Electrochemical Characterization under Cyclic Loading
For all alloys, the cycling start is accompanied by a drastic drop of OCP towards more negative values. This shift is associated with the cathodic polarization resulting from the local passive film destruction.
At the initial stage of cycling, the OCP drop
ΔE is bigger for the 18-15 samples than for other alloys (
Table 7), which indicates higher oxide film damageability under mechanical loading.
It must be noted that the OCP behavior during the cycling of superelastic Ti‒Zr-based alloys and non-superelastic Ti Grade 2 is different. For superelastic alloys, during cycling, an increase in OCP is observed, which is related to the plastifying impact of the chemomechanical effect, which takes place at the sample/electrolyte interface [
52,
53,
54], with the subsequent establishment of a constant OCP value during cycling,
Ec. As follows from
Table 7, the highest
Ec values are observed for the 18-13-1 alloy, which can be explained by the higher permeability of thinner oxide films, which does not facilitate the accumulation of dislocations in the near-surface area.
In the case of Ti Grade 2, during about the first 50 cycles, recovery of the potential values is observed, related to the reconstruction of the damaged oxide layer and chemomechanical effect. Further OCP decrease indicates the impossibility of restoring the passive state during the unloading half-cycle. After about 900 cycles, the sample failure takes place (
Table 7).
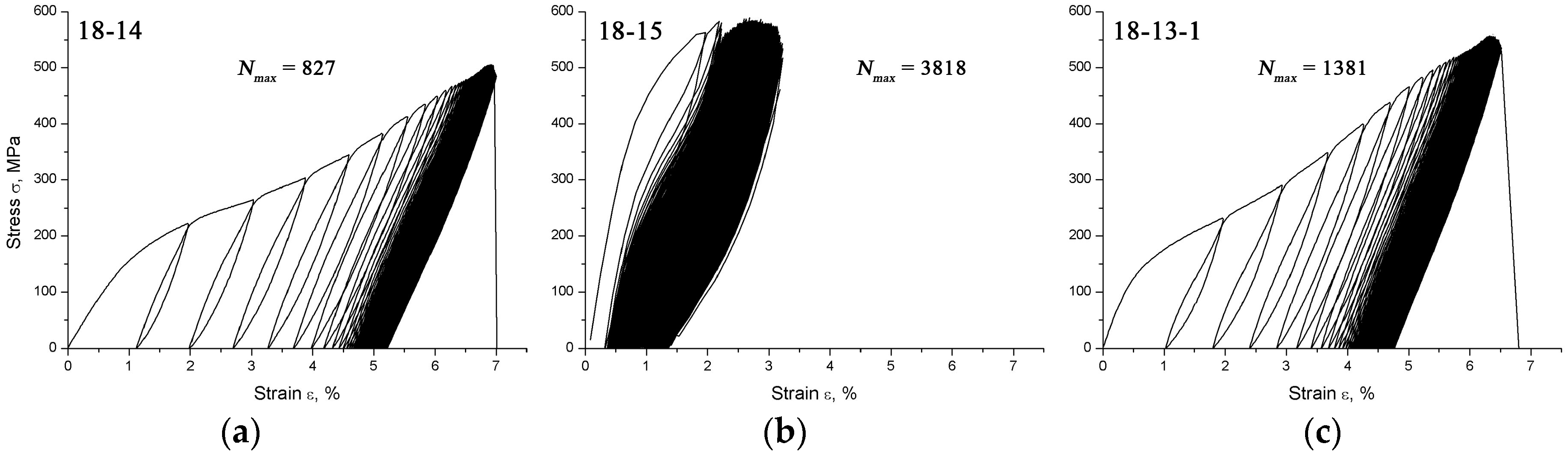
The highest number of cycles to failure is provided by the 18-15 alloy (
Nmax = 4225 ± 677) and this gain is caused, to a great extent, by the favorable
Ms temperature point resulting in an equally prolonged fatigue life in air (see
Section 4.2).
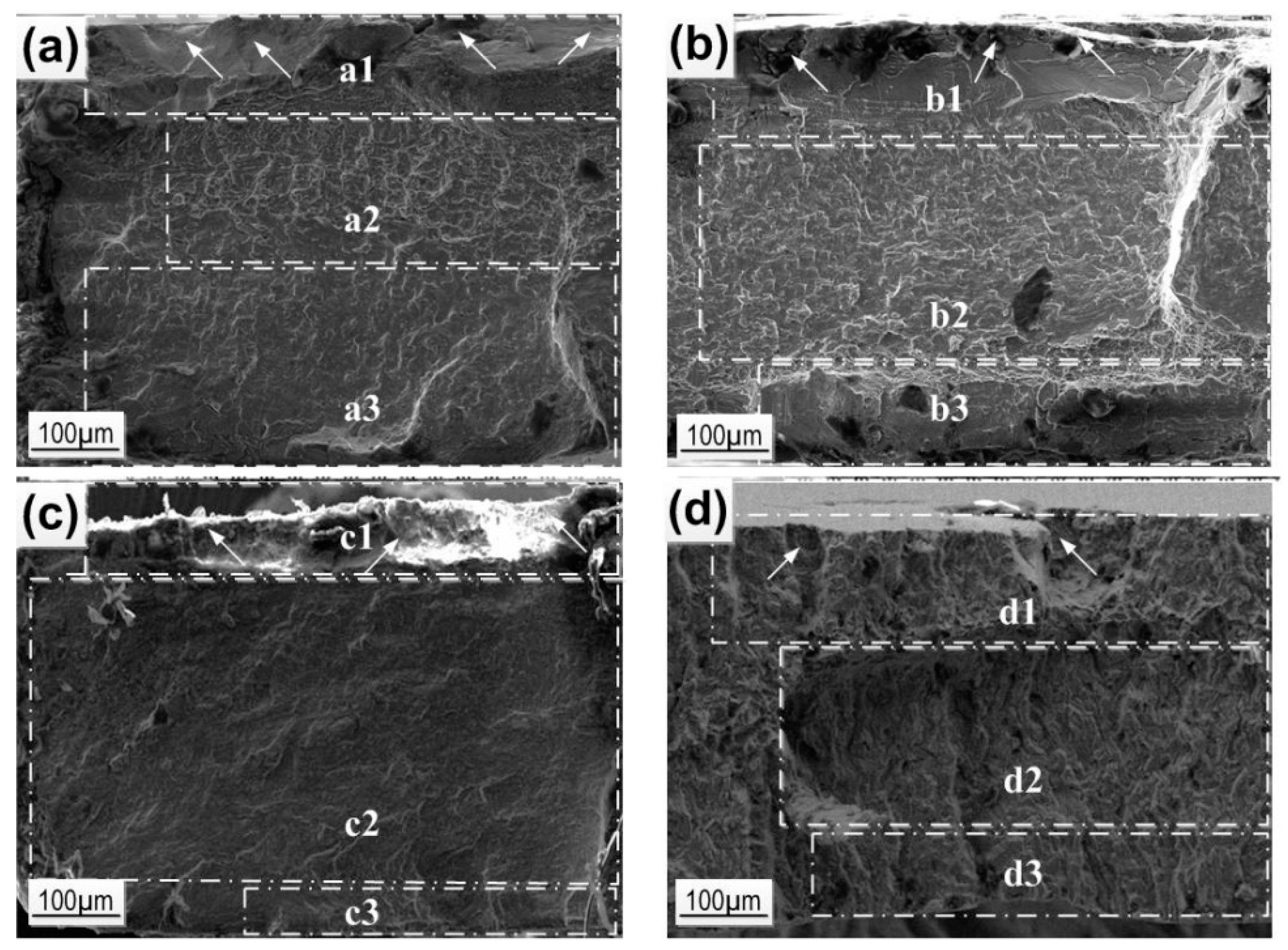
Fracture images analysis (
Figure 7) shows that the largest area of the fatigue crack propagation and the smallest mechanical rupture zone are observed for the 18-15 alloy and correspond to its outstanding fatigue life. The delayed failure of this alloy is evidently connected with the effective slowdown of the crack propagation due to the formation of martensite crystals at its tip as well as the stress relaxation via the martensitic transformation mechanisms [
53,
54].
For the 18-13-1 alloy, the crack propagation area is closer in size to the rupture zone; for the 18-14 alloy, it exceeds it, which corresponds to fewer cycles to failure.
For Ti Grade 2, the crack propagation zone is significant, but due to the absence of a reversible martensitic transformation, it shows the worst functional fatigue behavior in Hanks’ solution.