Effect of Composition on the Growth of Single Crystals of (1−x)(Na1/2Bi1/2)TiO3-xSrTiO3 by Solid State Crystal Growth
Abstract
1. Introduction
2. Materials and Methods
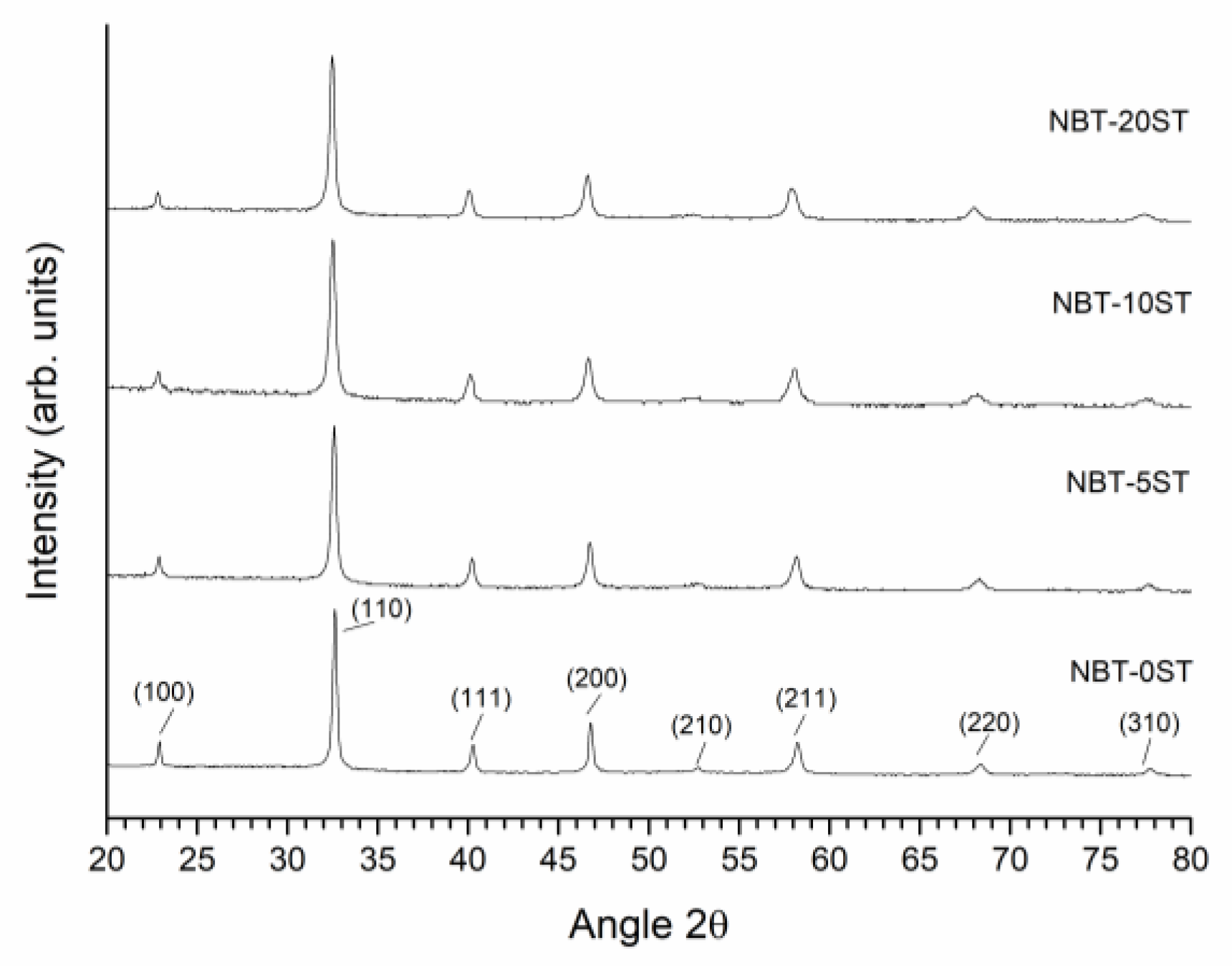
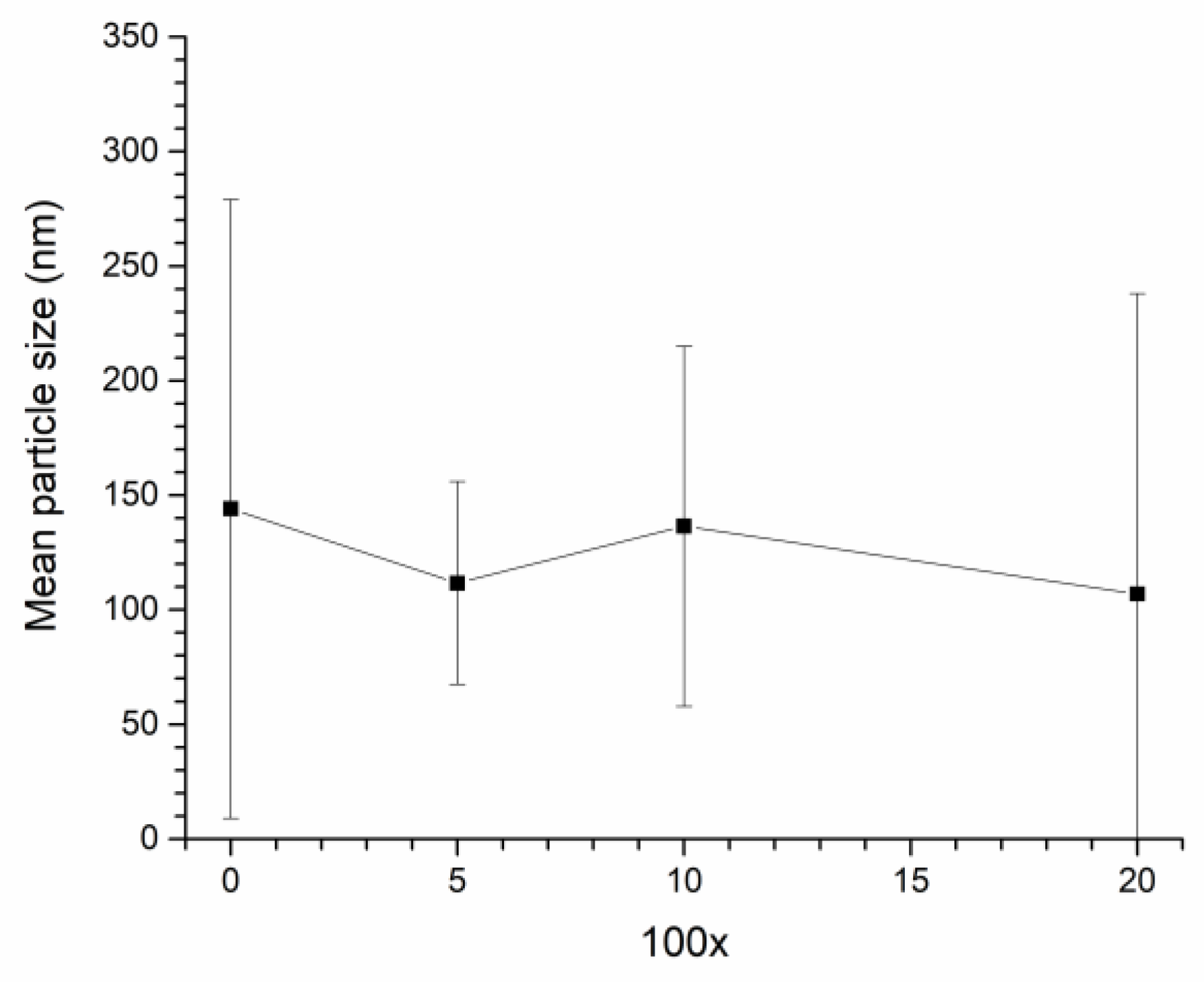
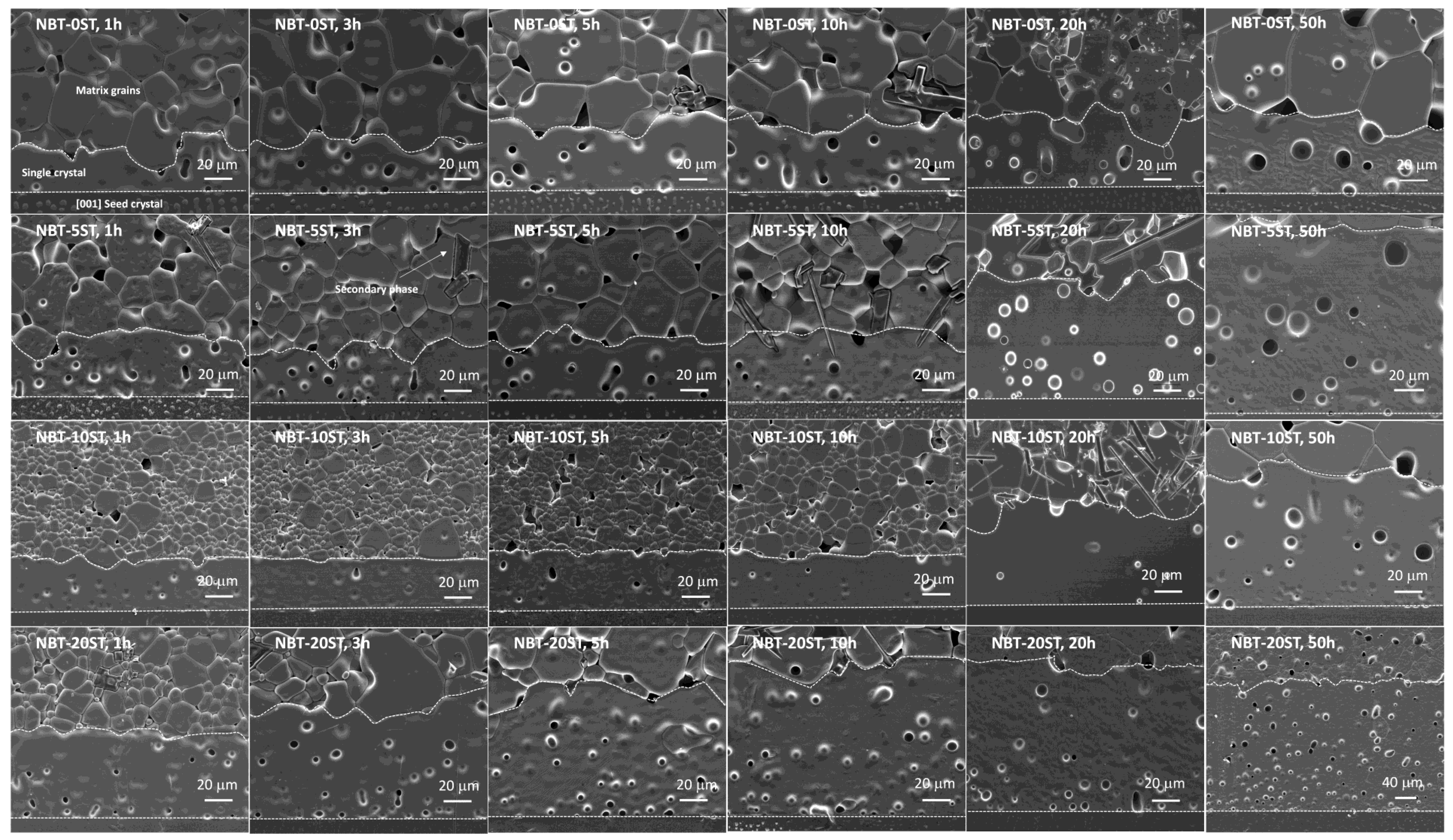
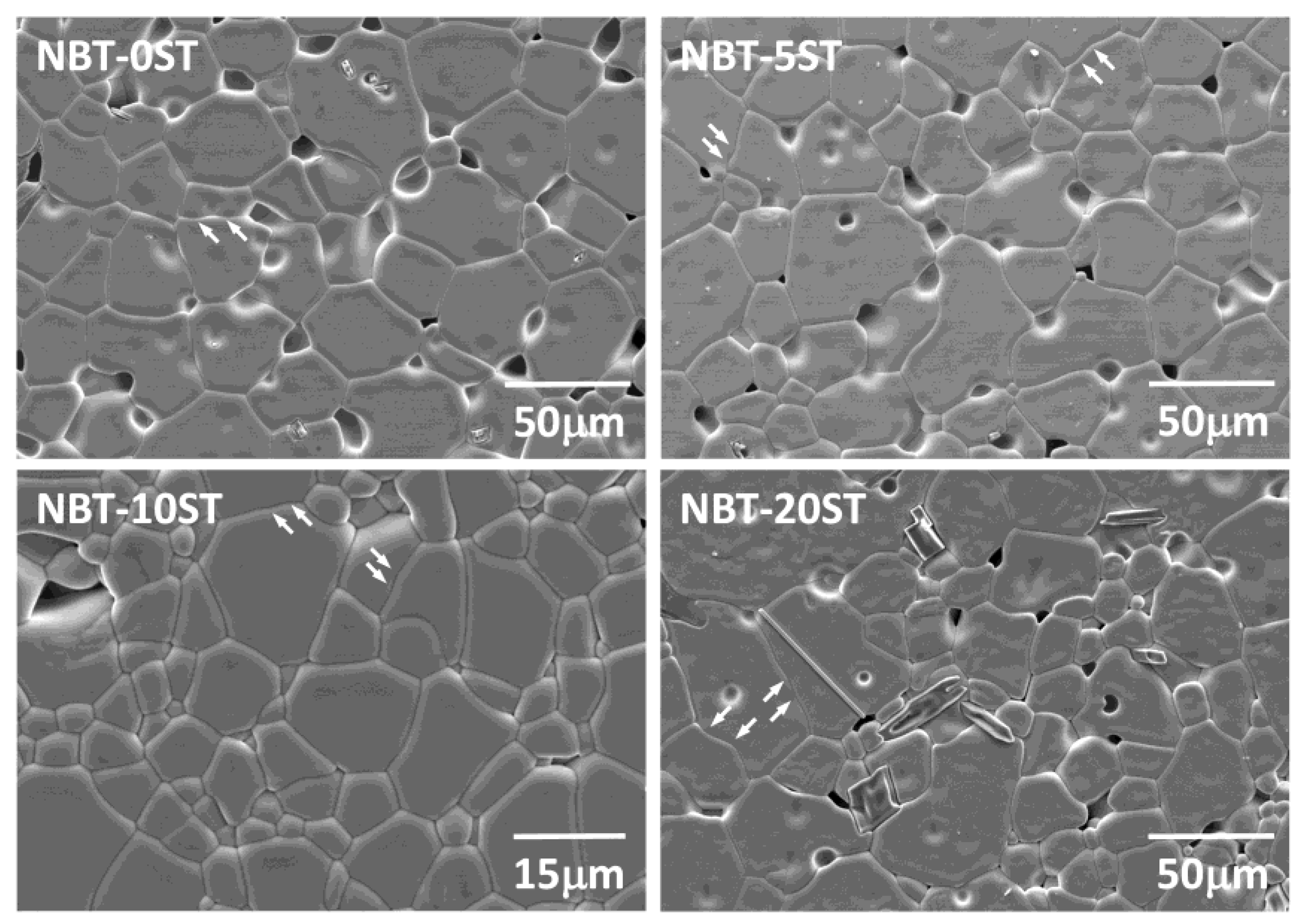
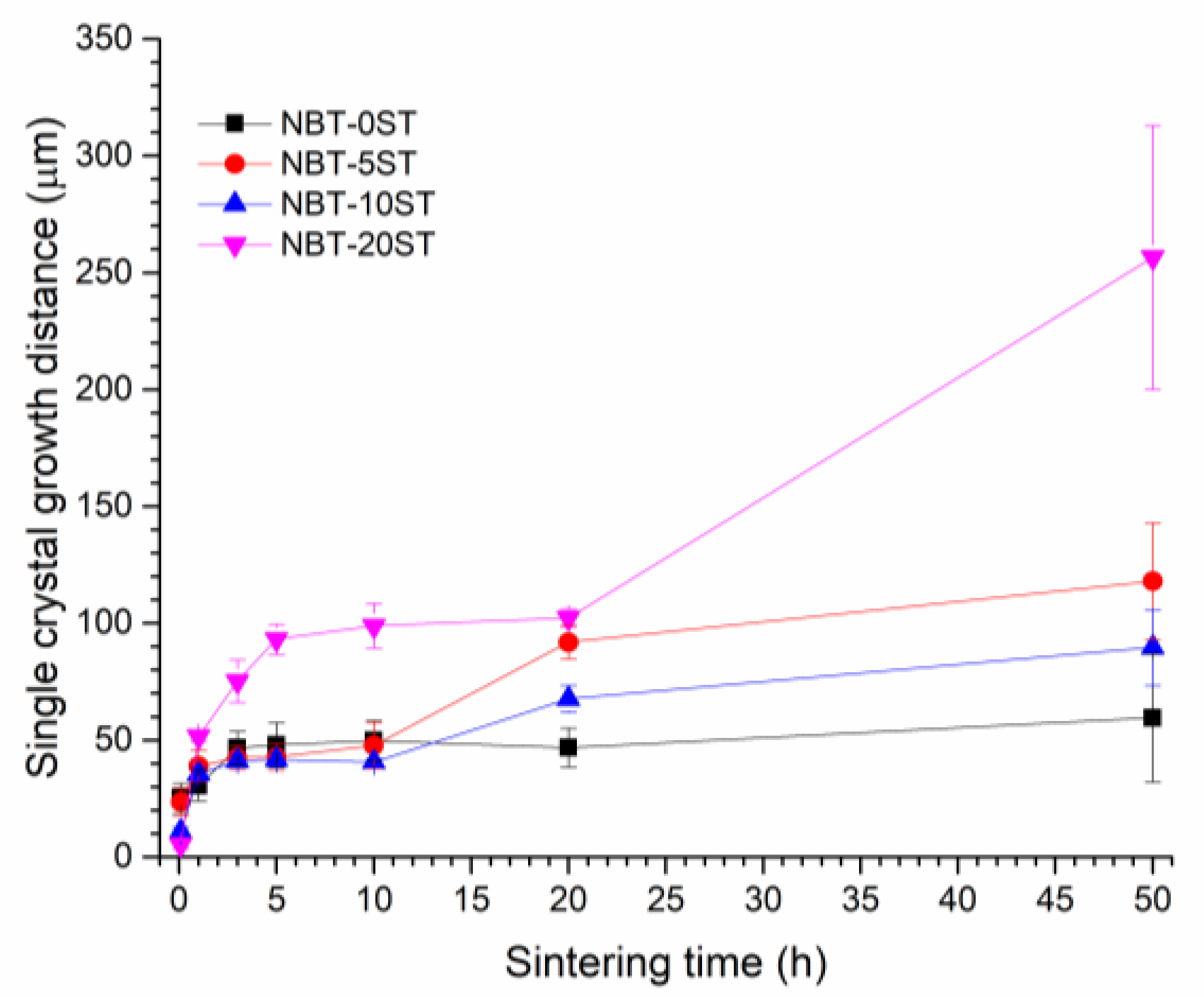
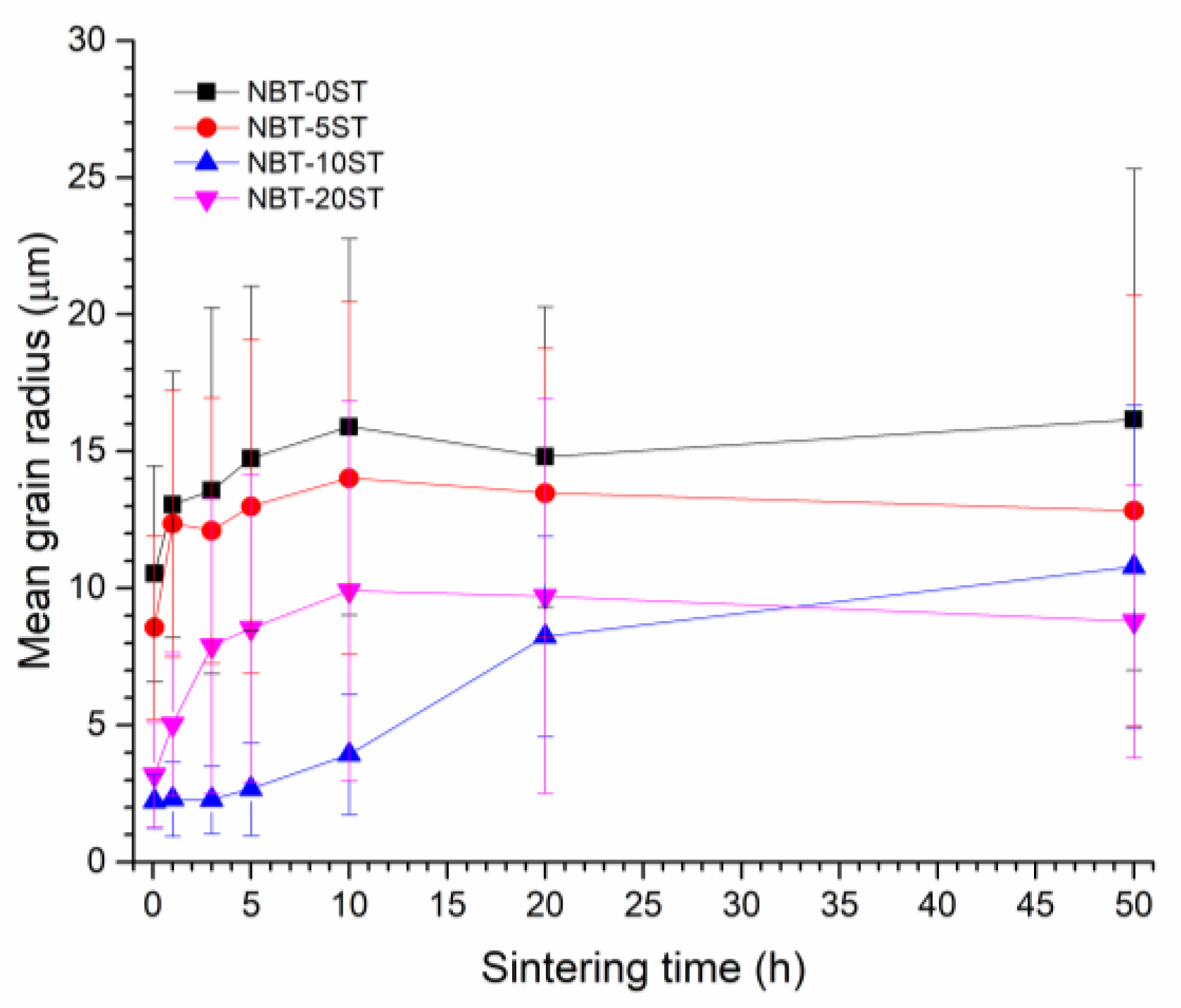
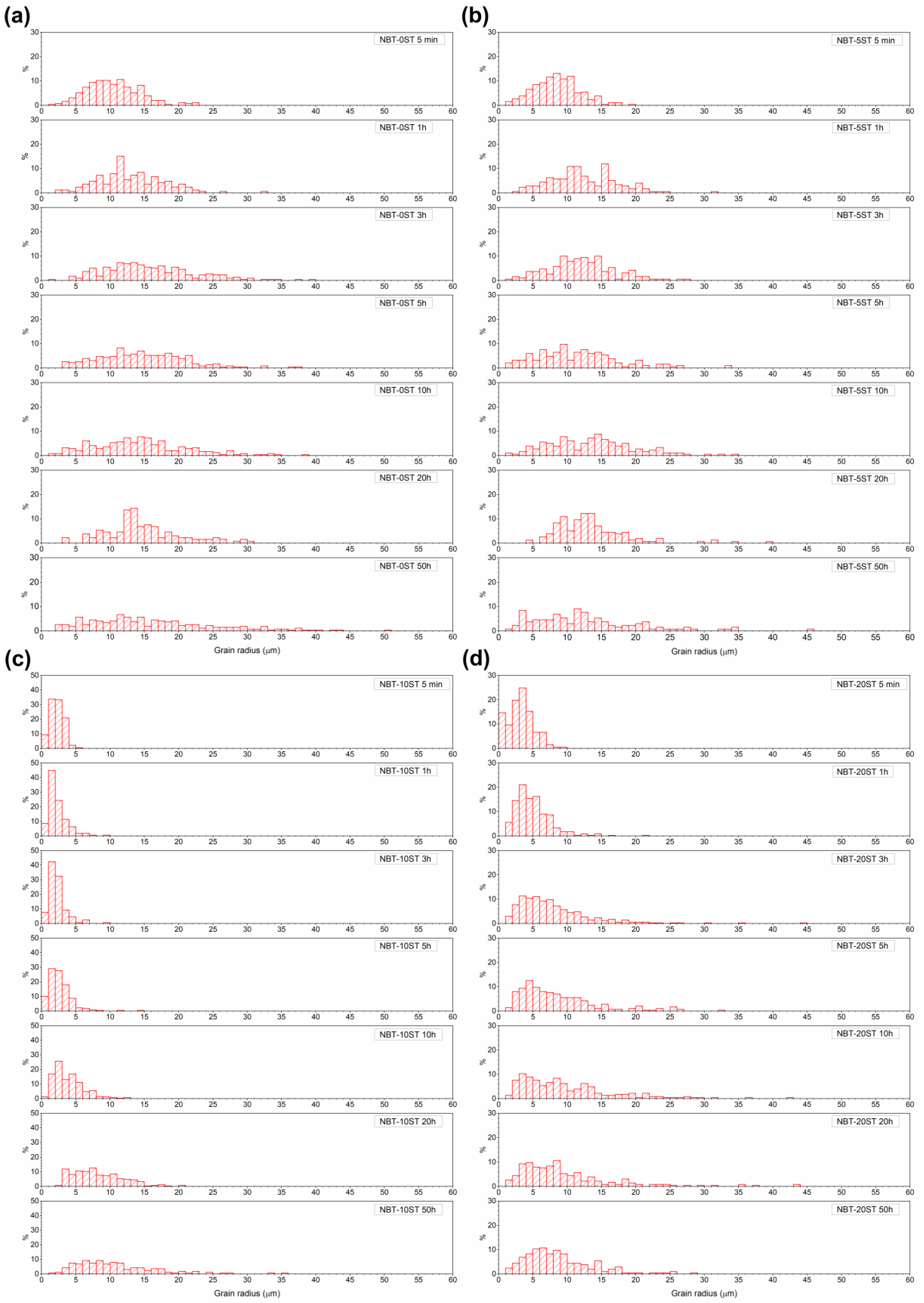
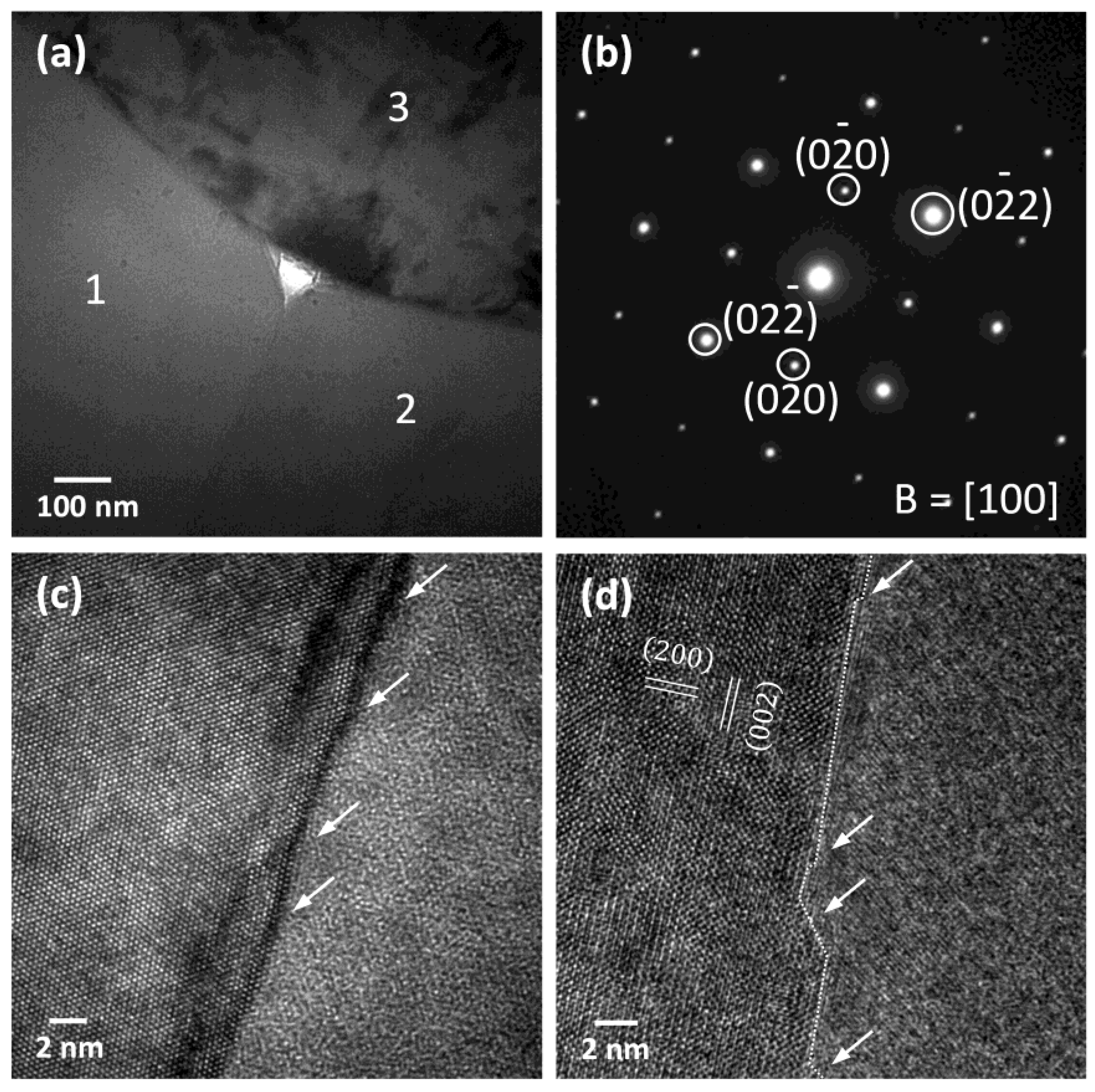
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rödel, J.; Jo, W.; Seifert, K.T.P.; Anton, E.-M.; Granzow, T.; Damjanovic, D. Perspective on the Development of Lead-free Piezoceramics. J. Am. Ceram. Soc. 2009, 92, 1153–1177. [Google Scholar] [CrossRef]
- Reichmann, K.; Feteira, A.; Li, M. Bismuth sodium titanate based materials for piezoelectric actuators. Materials 2015, 8, 5469. [Google Scholar] [CrossRef]
- Smolenskii, G.A.; Isupov, V.A.; Agranovskaya, A.I.; Krainik, N.N. New ferroelectrics of complex composition. Sov. Phys. Solid State 1961, 2, 2651–2654. [Google Scholar]
- Jones, G.O.; Thomas, P.A. The tetragonal phase of Na0.5Bi0.5TiO3—A new variant of the perovskite structure. Acta Crystallogr. Sect. B 2000, 56, 426–430. [Google Scholar] [CrossRef]
- Jones, G.O.; Thomas, P.A. Investigation of the structure and phase transitions in the novel A-site substituted distorted perovskite compound Na0.5Bi0.5TiO3. Acta Crystallogr. Sect. B 2002, 58, 168–178. [Google Scholar] [CrossRef]
- Pronin, I.P.; Syrnikov, P.P.; Isupov, V.A.; Egorov, V.M.; Zaitseva, N.V. Peculiarities of phase transitions in sodium-bismuth titanate. Ferroelectrics 1980, 25, 395–397. [Google Scholar] [CrossRef]
- Zvirgzds, J.A.; Kapostin, P.P.; Zvirgzde, J.V.; Kruzina, T.V. X-ray study of phase transitions in ferroelectric Na0.5Bi0.5TiO3. Ferroelectrics 1982, 40, 75–77. [Google Scholar] [CrossRef]
- Hiruma, Y.; Nagata, H.; Takenaka, T. Phase diagrams and electrical properties of (Bi1/2Na1/2)TiO3-based solid solutions. J. Appl. Phys. 2008, 104, 124106. [Google Scholar] [CrossRef]
- Hiruma, Y.; Imai, Y.; Watanabe, Y.; Nagata, H.; Takenaka, T. Large electrostrain near the phase transition temperature of (Bi0.5Na0.5)TiO3-SrTiO3 ferroelectric ceramics. Appl. Phys. Lett. 2008, 92, 262904. [Google Scholar] [CrossRef]
- Hiruma, Y.; Nagata, H.; Takenaka, T. Formation of Morphotropic Phase Boundary and Electrical Properties of (Bi1/2Na1/2)TiO3-Ba(Al1/2Nb1/2)O3 Solid Solution Ceramics. Jpn. J. Appl. Phys. 2009, 48, 09KC08. [Google Scholar]
- Watanabe, Y.; Hiruma, Y.; Nagata, H.; Takenaka, T. Phase transition temperatures and electrical properties of divalent ions (Ca2+, Sr2+ and Ba2+) substituted (Bi1/2Na1/2)TiO3 ceramics. Ceram. Int. 2008, 34, 761–764. [Google Scholar] [CrossRef]
- Krauss, W.; Schütz, D.; Mautner, F.A.; Feteira, A.; Reichmann, K. Piezoelectric properties and phase transition temperatures of the solid solution of (1−x)(Bi0.5Na0.5)TiO3-xSrTiO3. J. Eur. Ceram. Soc. 2010, 30, 1827–1832. [Google Scholar] [CrossRef]
- Takenaka, T.; Maruyama, K.; Sakata, K. (Bi1/2Na1/2)TiO3-BaTiO3 system for lead-free piezoelectric ceramics. Jpn. J. Appl. Phys. 1991, 30, 2236–2239. [Google Scholar]
- Sayyed, S.; Acharya, S.A.; Kautkar, P.; Sathe, V. Structural and dielectric anomalies near the MPB region of Na0.5Bi0.5TiO3-SrTiO3 solid solution. RSC Adv. 2015, 5, 50644–50654. [Google Scholar] [CrossRef]
- Ma, C.; Tan, X.; Dul’kin, E.; Roth, M. Domain structure-dielectric property relationship in lead-free (1−x)(Bi1/2Na1/2)TiO3-xBaTiO3 ceramics. J. Appl. Phys. 2010, 108, 104105. [Google Scholar] [CrossRef]
- Elkechai, O.; Manier, M.; Mercurio, J.P. Na0.5Bi0.5TiO3-K0.5Bi0.5TiO3 (NBT-KBT) system: A structural and electrical study. Phys. Status Solidi (a) 1996, 157, 499–506. [Google Scholar] [CrossRef]
- Acosta, M.; Jo, W.; Rödel, J. Temperature- and Frequency-Dependent Properties of the 0.75Bi1/2Na1/2TiO3-0.25SrTiO3 Lead-Free Incipient Piezoceramic. J. Am. Ceram. Soc. 2014, 97, 1937–1943. [Google Scholar]
- Sun, R.; Zhang, Q.; Fang, B.; Jiao, J.; Li, X.; Zhao, X.; Lin, D.; Wang, D.; Luo, H. Dielectric, electromechanical coupling properties of Mn-doped Na0.5Bi0.5TiO3-BaTiO3 lead-free single crystal. Appl. Phys. A Mater. Sci. Process. 2011, 103, 199–205. [Google Scholar]
- Park, J.H.; Lee, H.Y.; Kang, S.J.L. Solid-state conversion of (Na1/2Bi1/2)TiO3-BaTiO3-(K1/2Na1/2)NbO3 single crystals and their piezoelectric properties. Appl. Phys. Lett. 2014, 104, 222910. [Google Scholar] [CrossRef]
- Zhang, H.; Deng, H.; Chen, C.; Li, L.; Lin, D.; Li, X.; Zhao, X.; Luo, H.; Yan, J. Chemical nature of giant strain in Mn-doped 0.94(Na0.5Bi0.5)TiO3-0.06BaTiO3 lead-free ferroelectric single crystals. Scr. Mater. 2014, 75, 50–53. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, X.; Wang, Y.; Zhang, H.; Deng, H.; Li, X.; Jiang, X.; Jiang, X.; Luo, H. Giant strain and electric-field-induced phase transition in lead-free (Na0.5Bi0.5)TiO3-BaTiO3-(K0.5Na0.5)NbO3 single crystal. Appl. Phys. Lett. 2016, 108, 022903. [Google Scholar] [CrossRef]
- Lee, D.; Vu, H.; Sun, H.; Pham, T.L.; Nguyen, D.T.; Lee, J.-S.; Fisher, J.G. Growth of (Na0.5Bi0.5)TiO3-SrTiO3 single crystals by solid state crystal growth. Ceram. Int. 2016, 42, 18894–18901. [Google Scholar] [CrossRef]
- Le, P.G.; Jo, G.Y.; Ko, S.Y.; Fisher, J.G. The effect of sintering temperature and time on the growth of single crystals of 0.75 (Na0.5Bi0.5)TiO3-0.25 SrTiO3 by solid state crystal growth. J. Electroceram. 2018, 40, 122–137. [Google Scholar] [CrossRef]
- Kang, S.J.L.; Park, J.H.; Ko, S.Y.; Lee, H.Y. Solid-State Conversion of Single Crystals: The Principle and the State-of-the-Art. J. Am. Ceram. Soc. 2015, 98, 347–360. [Google Scholar] [CrossRef]
- Benčan, A.; Tchernychova, E.; Uršič, H.; Kosec, M.; Fisher, J. Growth and Characterization of Single Crystals of Potassium Sodium Niobate by Solid State Crystal Growth. In Ferroelectrics-Material Aspects; Lallart, M., Ed.; InTech: Rijeka, Croatia, 2011; pp. 87–108. [Google Scholar]
- HY, L. Solid-State Single CrystalGrowth (SSCG) Method: A Cost-effective Way of Growing Piezoelectric Single Crystals. In Piezoelectric Single Crystals and Their Application; Trolier-McKinstry, S., Cross, L.E., Yamashita, Y., Eds.; Pennsylvania State University: State College, PA, USA, 2004; pp. 160–177. [Google Scholar]
- Moon, K.S.; Rout, D.; Lee, H.Y.; Kang, S.J.L. Solid state growth of Na1/2Bi1/2TiO3–BaTiO3 single crystals and their enhanced piezoelectric properties. J. Cryst. Growth 2011, 317, 28–31. [Google Scholar] [CrossRef]
- Lee, D.-K.; Vu, H.; Fisher, J.G. Growth of (Na0.5Bi0.5)TiO3-Ba(Ti1−xZrx)O3 single crystals by solid state single crystal growth. J. Electroceram. 2015, 34, 150–157. [Google Scholar] [CrossRef]
- Sun, H.; Fisher, J.G.; Moon, S.H.; Tran Tran, H.; Lee, J.S.; Han, H.S.; Kim, H.P.; Jo, W. Solid-state-growth of lead-free piezoelectric (Na1/2Bi1/2)TiO3-CaTiO3 single crystals and their characterization. Mater. Sci. Eng. B 2017, 223, 109–119. [Google Scholar] [CrossRef]
- Fisher, J.G.; Benčan, A.; Godnjavec, J.; Kosec, M. Growth behaviour of potassium sodium niobate single crystals grown by solid-state crystal growth using K4CuNb8O23 as a sintering aid. J. Eur. Ceram. Soc. 2008, 28, 1657–1663. [Google Scholar] [CrossRef]
- An, S.M.; Yoon, B.K.; Chung, S.Y.; Kang, S.J.L. Nonlinear driving force-velocity relationship for the migration of faceted boundaries. Acta Mater. 2012, 60, 4531–4539. [Google Scholar] [CrossRef]
- Jung, S.H.; Yoon, D.Y.; Kang, S.J.L. Mechanism of abnormal grain growth in ultrafine-grained nickel. Acta Mater. 2013, 61, 5685–5693. [Google Scholar] [CrossRef]
- Fisher, J.G.; Kang, S.-J.L. Effect of Interface Structure on Microstructural Evolution in Polycrystals. Trans. Mater. Res. Soc. Jpn. 2010, 35, 455–460. [Google Scholar] [CrossRef][Green Version]
- Chung, S.Y.; Yoon, D.Y.; Kang, S.J.L. Effects of donor concentration and oxygen partial pressure on interface morphology and grain growth behaviour in SrTiO3. Acta Mater. 2002, 50, 3361–3371. [Google Scholar]
- Yoon, B.K.; Lee, B.A.; Kang, S.J.L. Growth behaviour of rounded (Ti,W)C and faceted WC grains in a Co matrix during liquid phase sintering. Acta Mater. 2005, 53, 4677–4685. [Google Scholar] [CrossRef]
- Kang, S.J.L.; Lee, M.G.; An, S.M. Microstructural Evolution During Sintering with Control of the Interface Structure. J. Am. Ceram. Soc. 2009, 92, 1464–1471. [Google Scholar] [CrossRef]
- Jung, Y.I.; Yoon, D.Y.; Kang, S.J.L. Coarsening of polyhedral grains in a liquid matrix. J. Mater. Res. 2009, 24, 2949–2959. [Google Scholar] [CrossRef]
- Park, Y.J.; Hwang, N.M.; Yoon, D.Y. Abnormal growth of faceted (WC) grains in a (Co) liquid matrix. Metall. Mater. Trans. A 1996, 27, 2809–2819. [Google Scholar]
- Jung, S.H.; Kang, S.J.L. An explanation for the formation of polyhedral abnormal grains in single-phase systems. Scr. Mater. 2014, 82, 49–52. [Google Scholar] [CrossRef]
- Ardell, A.J. The effect of volume fraction on particle coarsening: Theoretical considerations. Acta Metall. 1972, 20, 61–71. [Google Scholar] [CrossRef]
- Kang, S.J.L.; Jung, Y.I.; Jung, S.H.; Fisher, J.G. Interface Structure-Dependent Grain growth Behaviour in Polycrystals. In Microstructural Design of Advanced Engineering Materials; Molodov, D.A., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA.: Weinheim, Germany, 2013; pp. 299–322. [Google Scholar]
- Markov, I.V. Crystal growth. In Crystal Growth for Beginners: Fundamentals of Nucleation, Crystal Growth and Epitaxy, 2nd ed.; World Scientific: Singapore, 2003; pp. 181–351. [Google Scholar]
- Hirth, J.P.; Pound, G.M. Growth and Evaporation of Liquids and Dislocation-Free Crystals. In Condensation and Evaporation: Nucleation and Growth Kinetics; Pergamon Press: Oxford, UK, 1963; pp. 77–106. [Google Scholar]
- Peteves, S.D.; Abbaschian, R. Growth kinetics of solid-liquid Ga interfaces: Part I. Experimental. Metall. Trans. A 1991, 22, 1259–1270. [Google Scholar] [CrossRef]
- Markov, I.V. Chapter 2 Nucleation. In Crystal Growth for Beginners: Fundamentals of Nucleation, Crystal Growth and Epitaxy; World Scientific: Singapore, 2003. [Google Scholar]
- Markov, I.V. Chapter 1 Crystal-Ambient Phase Equilibrium. In Crystal Growth for Beginners: Fundamentals of Nucleation, Crystal Growth and Epitaxy; World Scientific: Singapore, 2003; p. 1. [Google Scholar]
- Peteves, S.D.; Abbaschian, R. Growth kinetics of solid-liquid Ga interfaces: Part II. Theoretical. Metall. Trans. A 1991, 22, 1271–1286. [Google Scholar] [CrossRef]
- Wynblatt, P.; Gjostein, N.A. Particle growth in model supported metal catalysts—I. Theory. Acta Metall. 1976, 24, 1165–1174. [Google Scholar] [CrossRef]
- Kang, S.J.L. Grain Shape and Grain Growth in a Liquid Matrix. In Sintering: Densification, Grain Growth & Microstructure; Elsevier Butterworth Heinemann: Oxford, UK, 2005; pp. 205–226. [Google Scholar]
- Fisher, J.G.; Kang, S.-J.L. Strategies and practices for suppressing abnormal grain growth during liquid phase sintering. J. Am. Ceram. Soc. 2019, 102, 717–735. [Google Scholar] [CrossRef]
- Farooq, M.U.; Fisher, J.G. Growth of (Na0.5K0.5)NbO3-SrTiO3 lead-free piezoelectric single crystals by the solid state crystal growth method and their characterization. Ceram. Int. 2014, 40, 3199–3207. [Google Scholar] [CrossRef]
- Choi, S.-Y.; Kang, S.J.L. Sintering kinetics by structural transition at grain boundaries in barium titanate. Acta Mater. 2004, 52, 2937–2943. [Google Scholar] [CrossRef]
- Zandvliet, H.J.W.; Gurlu, O.; Poelsema, B. Temperature dependence of the step free energy. Phys. Rev. B 2001, 64, 073402. [Google Scholar]
- Yang, J.; Yang, Q.; Li, Y.; Liu, Y. Growth mechanism and enhanced electrical properties of K0.5Na0.5NbO3-based lead-free piezoelectric single crystals grown by a solid-state crystal growth method. J. Eur. Ceram. Soc. 2016, 36, 541–550. [Google Scholar] [CrossRef]
- Van Beijeren, H. Exactly Solvable Model for the Roughening Transition of a Crystal Surface. Phys. Rev. Lett. 1977, 38, 993–996. [Google Scholar]
- Kizuka, T. Atomic processes of grain-boundary migration and phase transformation in zinc oxide nanocrystallites. Philos. Mag. Lett. 1999, 79, 417–422. [Google Scholar] [CrossRef]
- Lee, B.K.; Chung, S.Y.; Kang, S.J.L. Grain boundary faceting and abnormal grain growth in BaTiO3. Acta Mater. 2000, 48, 1575–1580. [Google Scholar]
- Koo, J.B.; Yoon, D.Y. Abnormal grain growth in bulk Cu—The dependence on initial grain size and annealing temperature. Metall. Mater. Trans. A 2001, 32, 1911–1926. [Google Scholar] [CrossRef]
- Merkle, K.L.; Thompson, L.J. Atomic-scale observation of grain boundary motion. Mater. Lett. 2001, 48, 188–193. [Google Scholar] [CrossRef]
- Merkle, K.L.; Thompson, L.J.; Phillipp, F. Collective effects in grain boundary migration. Phys. Rev. Lett. 2002, 88, 1–4. [Google Scholar]
- Lee, S.B.; Choi, S.-Y.; Kang, S.-J.L.; Yoon, D.Y. TEM Observations of Singular Grain Boundaries and their Roughening Transition in TiO2-Excess BaTiO3. Z. Metallkunde 2003, 94, 193–199. [Google Scholar] [CrossRef]
- Lee, S.B.; Kim, Y.-M. Kinetic roughening of a Σ5 tilt grain boundary in SrTiO3. Acta Mater. 2009, 57, 5264–5269. [Google Scholar] [CrossRef]
- Lee, S.B.; Kim, Y.-M.; Ko, D.-S.; Ahn, T.-Y.; Kim, Y.-W.; Park, J. Kinetic roughening of a ZnO grain boundary. Appl. Phys. Lett. 2010, 96, 191906. [Google Scholar] [CrossRef]
- Fisher, J.G.; Choi, S.Y.; Kang, S.J.L. Influence of Sintering Atmosphere on Abnormal Grain Growth Behaviour in Potassium Sodium Niobate Ceramics Sintered at Low Temperature. J. Korean Ceram. Soc. 2011, 48, 641–647. [Google Scholar] [CrossRef]
- Sung Bo, L.; Seung Jo, Y.; Peter, A.v.A. Roughening of a stepped GaN grain boundary with increasing driving force for migration. EPL (Europhys. Lett.) 2017, 120, 16002. [Google Scholar]
- Jo, W.; Hwang, N.M.; Kim, D.Y. Effect of crystal shape on the grain growth during liquid phase sintering of ceramics. J. Korean Ceram. Soc. 2006, 43, 728–733. [Google Scholar]
- Jo, W.; Kim, D.Y.; Hwang, N.M. Effect of Interface Structure on the microstructural evolution of ceramics. J. Am. Ceram. Soc. 2006, 89, 2369–2380. [Google Scholar] [CrossRef]
- Rottman, C.; Wortis, M. Equilibrium crystal shapes for lattice models with nearest-and next-nearest-neighbor interactions. Phys. Rev. B 1984, 29, 328–339. [Google Scholar]
- Rottman, C.; Wortis, M. Statistical mechanics of equilibrium crystal shapes: Interfacial phase diagrams and phase transitions. Phys. Rep. 1984, 103, 59–79. [Google Scholar] [CrossRef]
- Wortis, M. Equilibrium Crystal Shapes and Interfacial Phase Transitions. In Chemistry and Physics of Solid Surfaces VII; Vanselow, R., Howe, R., Eds.; Springer: Berlin/Heidelberg, Germany, 1988; pp. 367–405. [Google Scholar]
- Moon, K.S.; Kang, S.J.L. Coarsening Behaviour of Round-Edged Cubic Grains in the Na1/2Bi1/2TiO3-BaTiO3 System. J. Am. Ceram. Soc. 2008, 91, 3191–3196. [Google Scholar] [CrossRef]
- Moon, K.S.; Rout, D.; Lee, H.Y.; Kang, S.J.L. Effect of TiO2 addition on grain shape and grain coarsening behaviour in 95Na1/2Bi1/2TiO3-5BaTiO3. J. Eur. Ceram. Soc. 2011, 31, 1915–1920. [Google Scholar] [CrossRef]
- Williams, E.D. Surface steps and surface morphology: Understanding macroscopic phenomena from atomic observations. Surf. Sci. 1994, 299–300, 502–524. [Google Scholar] [CrossRef]
- West, A.R. Solid State Chemistry and its Applications Second Edition; John Wiley & Sons Ltd.: Chichester, UK, 2014; pp. 87–124. [Google Scholar]
- Luo, Y.R. Comprehensive Handbook of Chemical Bond Energies; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- An, S.M.; Kang, S.J.L. Boundary structural transition and grain growth behaviour in BaTiO3 with Nd2O3 doping and oxygen partial pressure change. Acta Mater. 2011, 59, 1964–1973. [Google Scholar] [CrossRef]
- Choi, K.; Hwang, N.M.; Kim, D.Y. Effect of grain shape on abnormal grain growth in liquid-phase-sintered Nb1−xTixC-Co alloys. J. Am. Ceram. Soc. 2002, 85, 2313–2318. [Google Scholar] [CrossRef]
- Rheinheimer, W.; Altermann, F.J.; Hoffmann, M.J. The equilibrium crystal shape of strontium titanate: Impact of donor doping. Scr. Mater. 2017, 127, 118–121. [Google Scholar] [CrossRef]

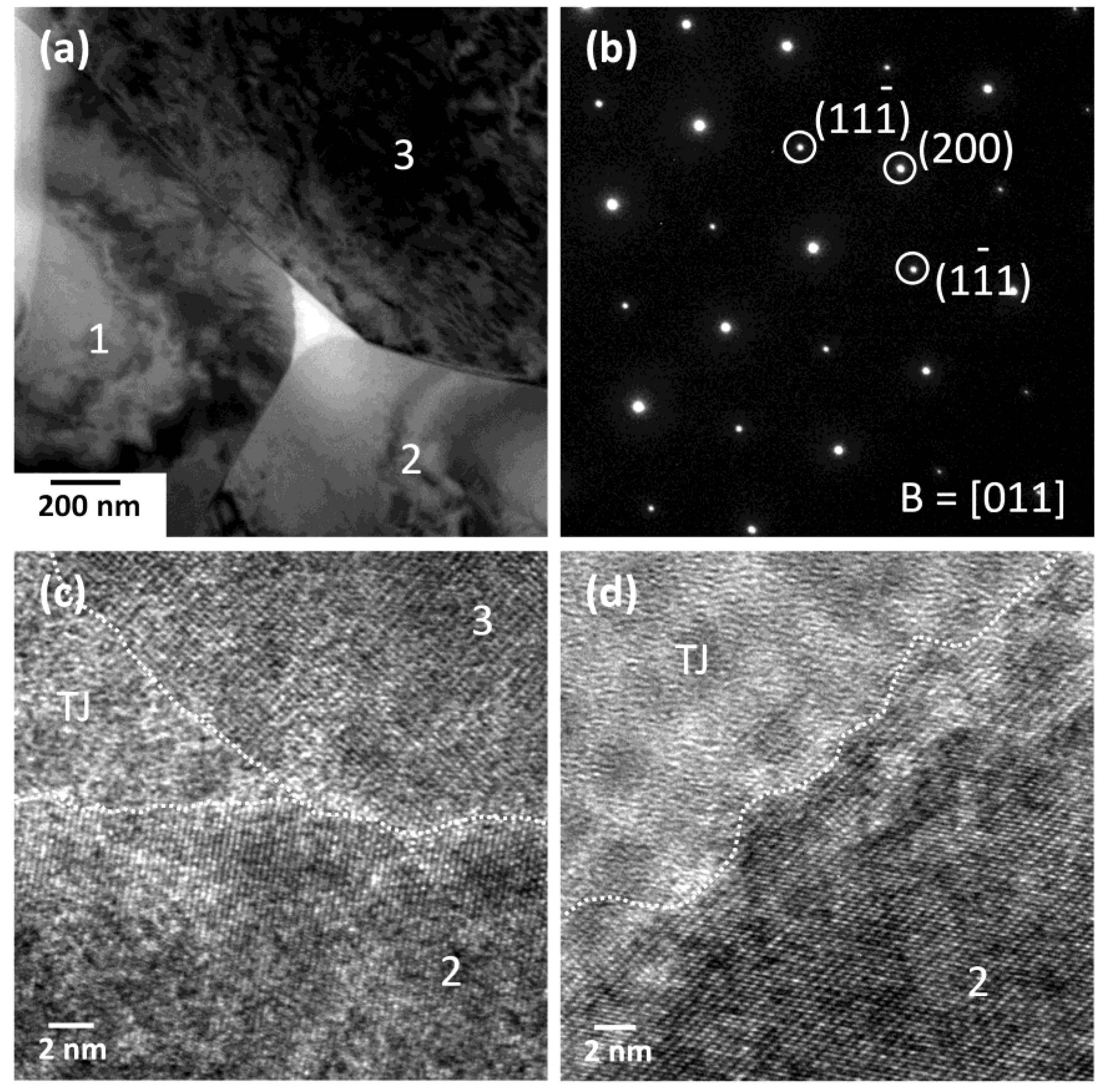
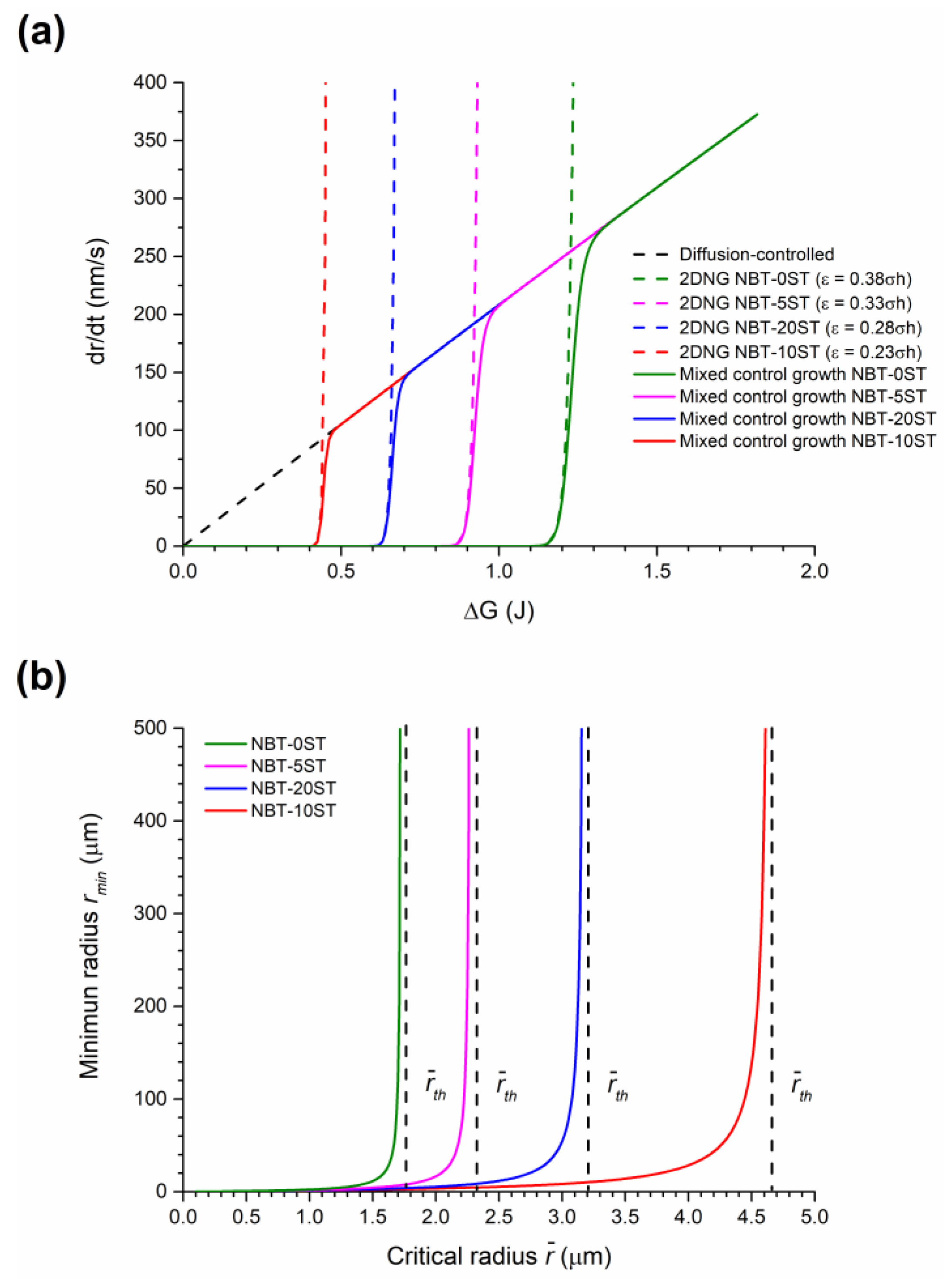
| Element | Single Crystal (at. %) | Matrix (at. %) | Nominal (at. %) |
|---|---|---|---|
| Na | 5.3 ± 1.7 | 5.6 ± 1.5 | 8.0 |
| Bi | 7.9 ± 0.7 | 8.5 ± 1.5 | 8.0 |
| Ti | 20 ± 0.0 | 20 ± 0.0 | 20.0 |
| Sr | 3.7 ± 0.7 | 3.7 ± 0.4 | 4.0 |
| O | 46.3 ± 5.8 | 43.3 ± 5.0 | 60.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, P.G.; Fisher, J.G.; Moon, W.-J. Effect of Composition on the Growth of Single Crystals of (1−x)(Na1/2Bi1/2)TiO3-xSrTiO3 by Solid State Crystal Growth. Materials 2019, 12, 2357. https://doi.org/10.3390/ma12152357
Le PG, Fisher JG, Moon W-J. Effect of Composition on the Growth of Single Crystals of (1−x)(Na1/2Bi1/2)TiO3-xSrTiO3 by Solid State Crystal Growth. Materials. 2019; 12(15):2357. https://doi.org/10.3390/ma12152357
Chicago/Turabian StyleLe, Phan Gia, John G. Fisher, and Won-Jin Moon. 2019. "Effect of Composition on the Growth of Single Crystals of (1−x)(Na1/2Bi1/2)TiO3-xSrTiO3 by Solid State Crystal Growth" Materials 12, no. 15: 2357. https://doi.org/10.3390/ma12152357
APA StyleLe, P. G., Fisher, J. G., & Moon, W.-J. (2019). Effect of Composition on the Growth of Single Crystals of (1−x)(Na1/2Bi1/2)TiO3-xSrTiO3 by Solid State Crystal Growth. Materials, 12(15), 2357. https://doi.org/10.3390/ma12152357






