Discharge Behavior and Dielectric Breakdown of Oxide Films during Single Pulse Anodizing of Aluminum Micro-Electrodes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Pre-Treatment
2.2. Single Pulse Anodizing
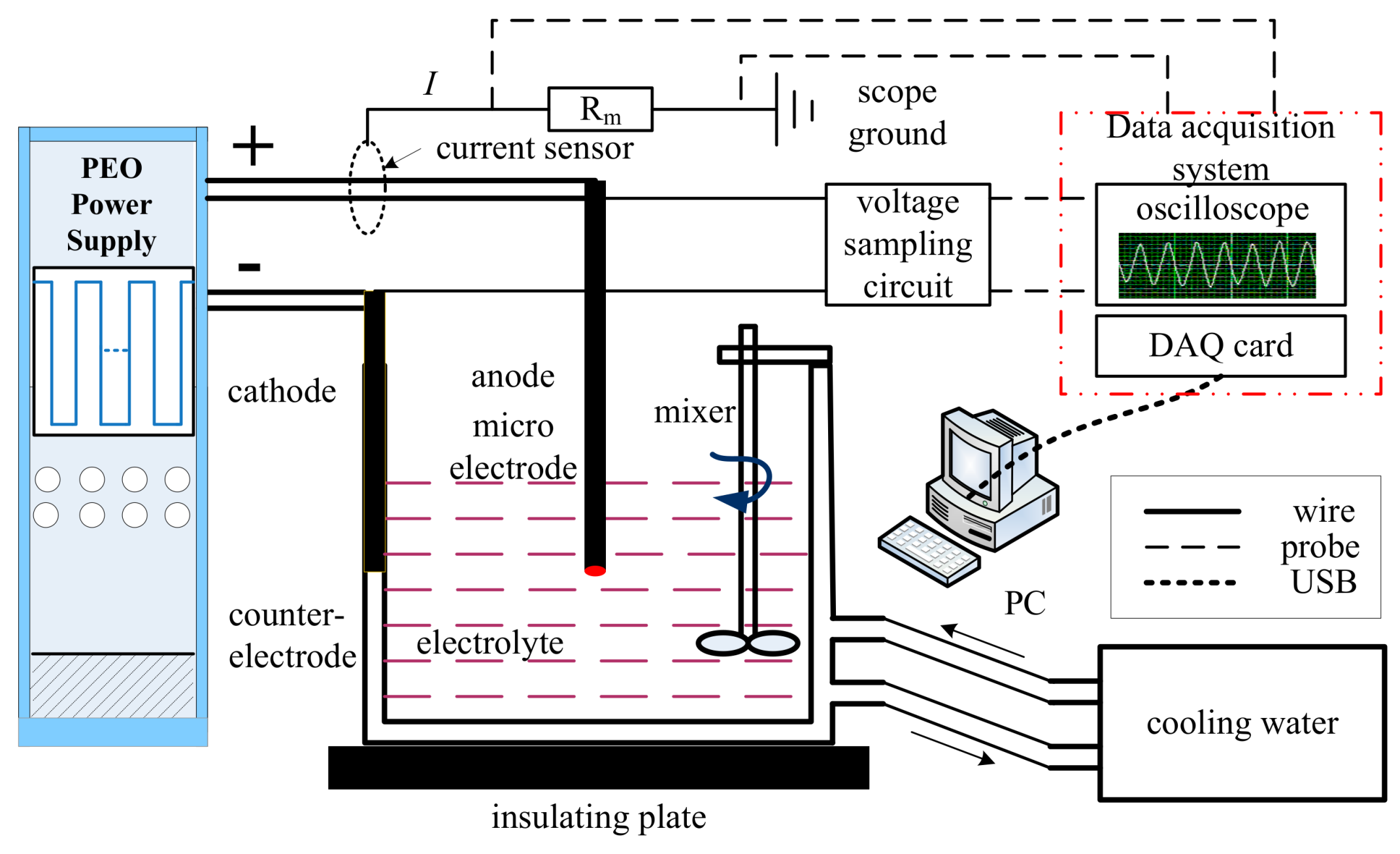
2.3. Data Monitoring and Post-Processing
3. Results
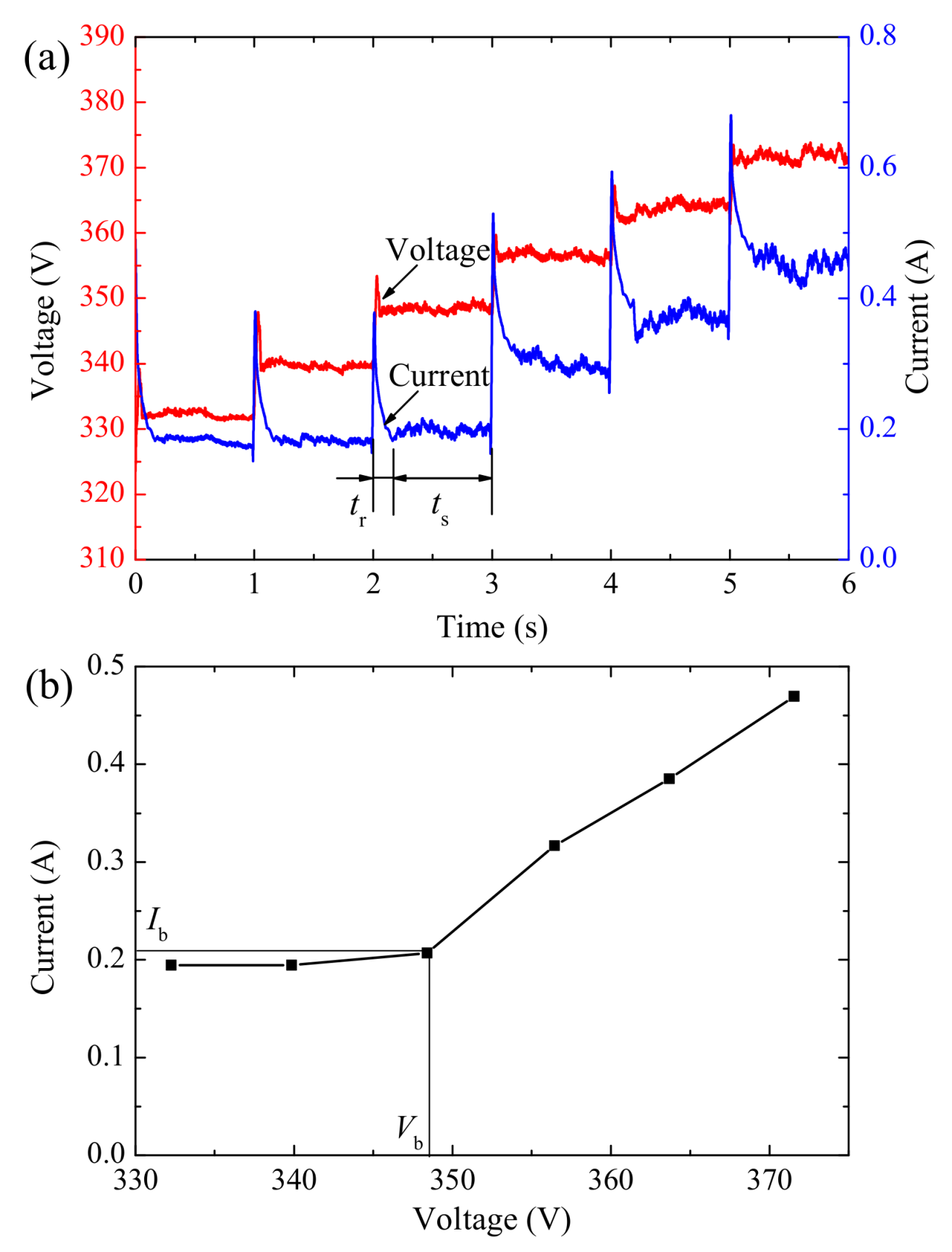
3.1. Identification of Critical Breakdown Voltage
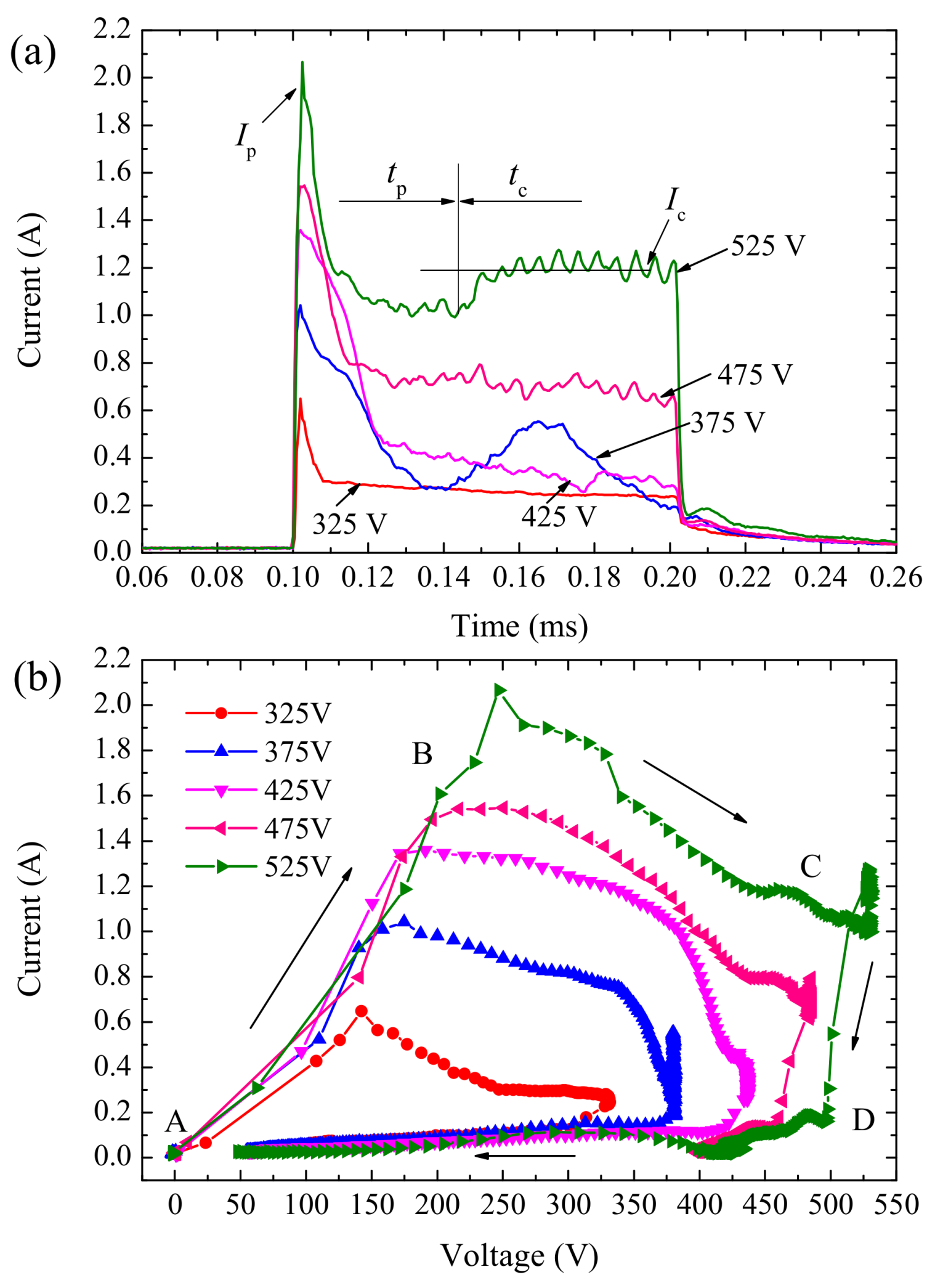
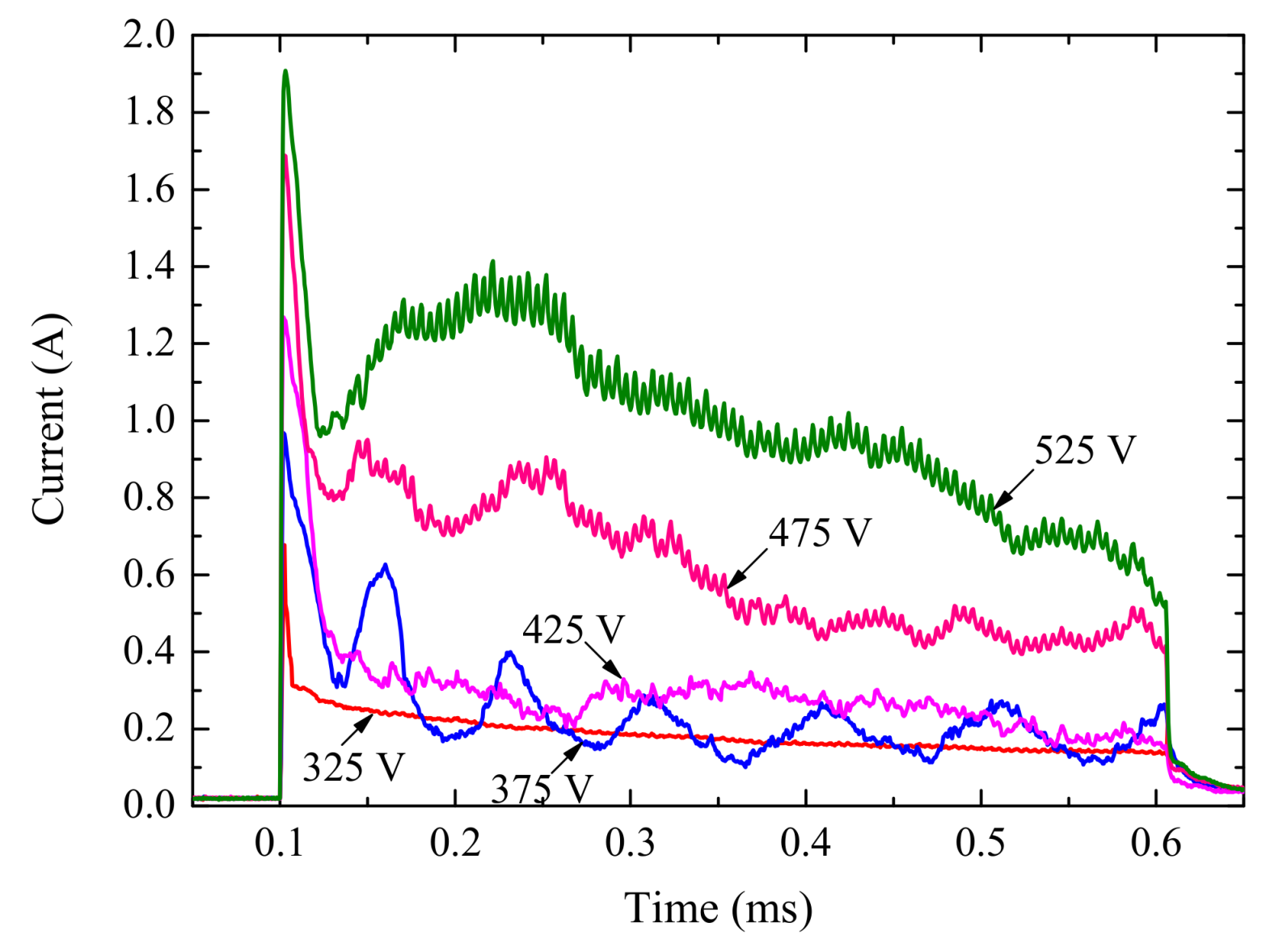
3.2. Electrical Characteristics of Single Pulse Anodizing
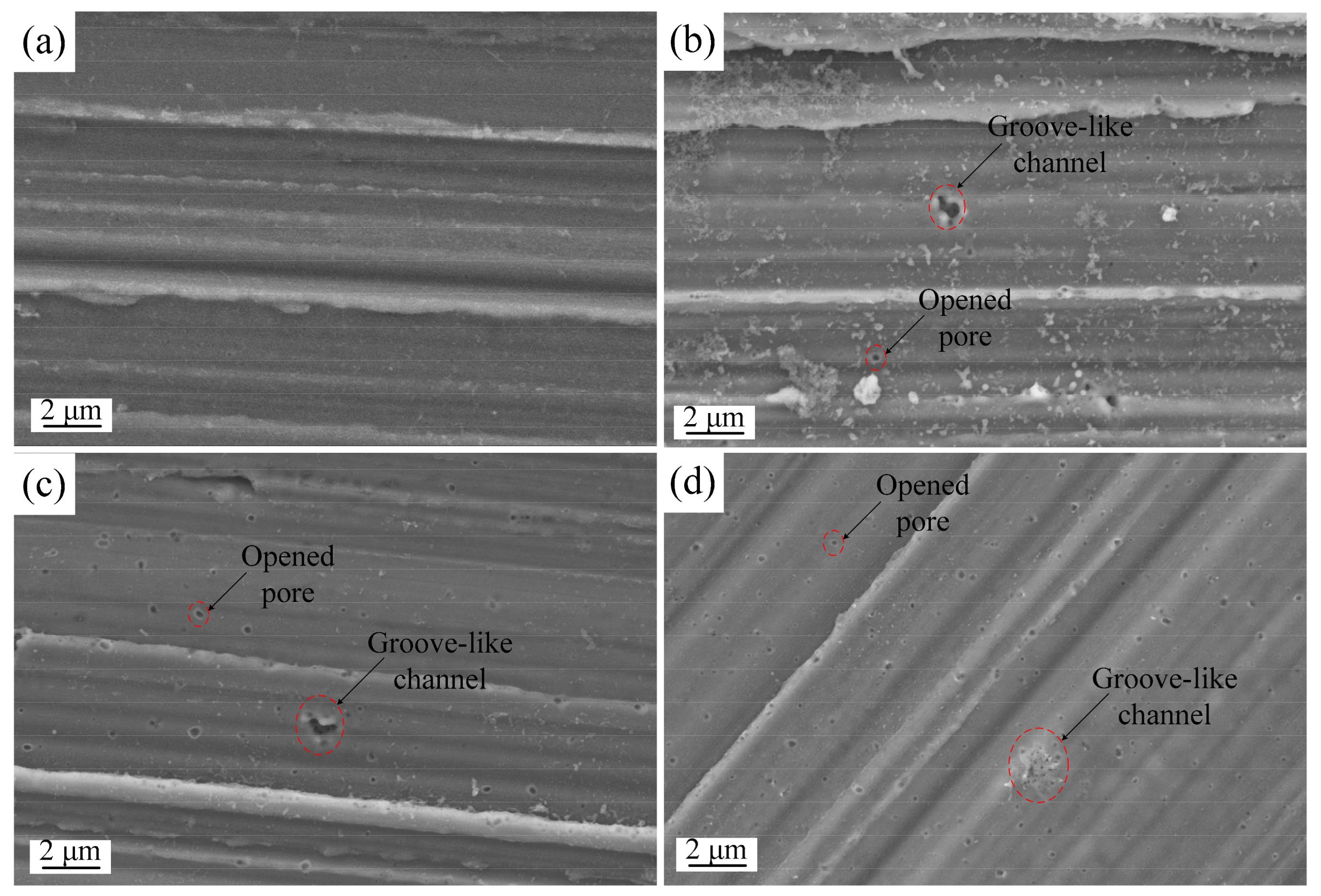
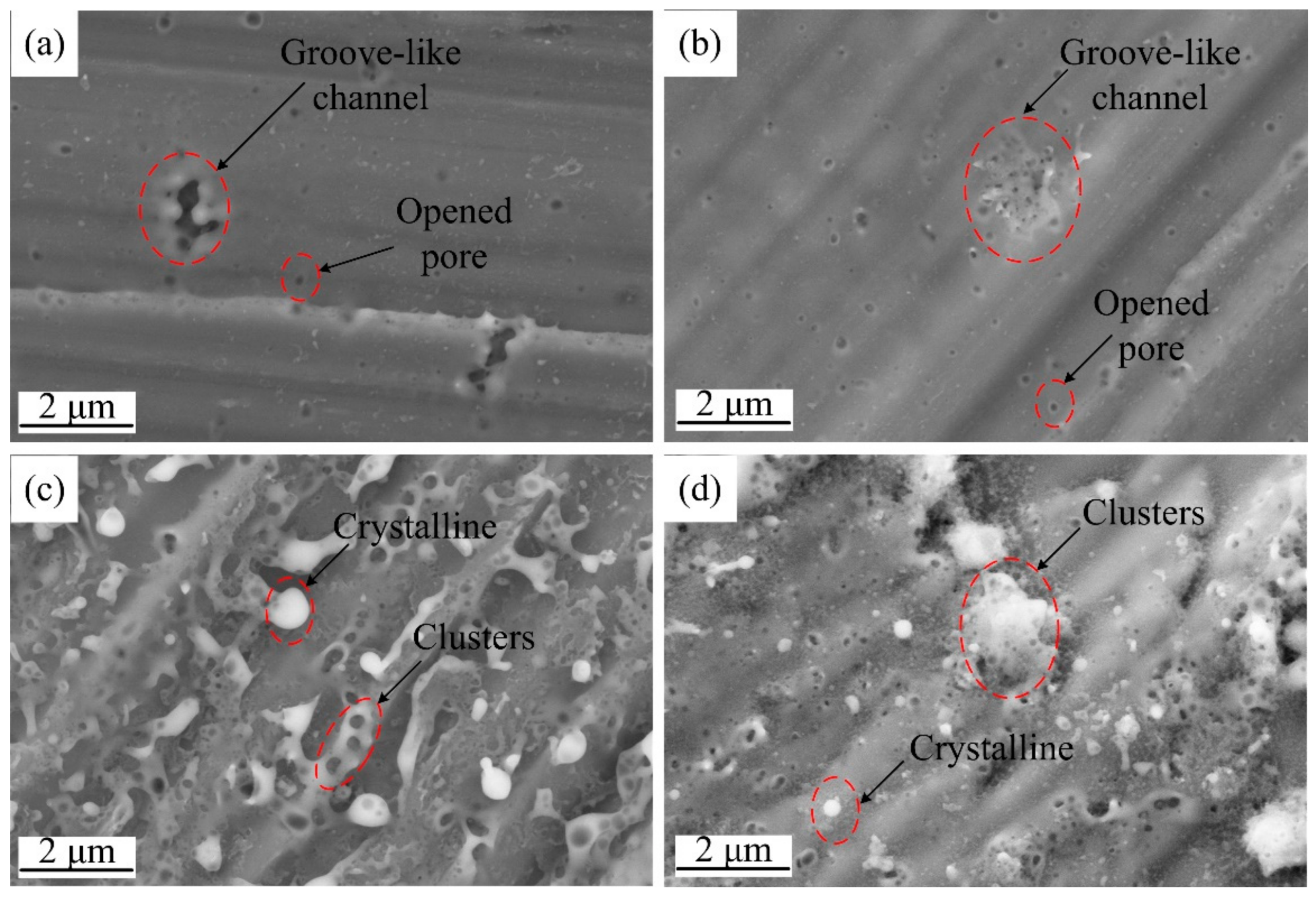
3.3. Effects of Pulse Parameters on Surface Morphology
3.4. Characteristic Parameters of Discharge Event
4. Discussion
5. Conclusions
- (1)
- Characteristic phenomenon, with the occurrence of an abrupt current increase when potential reaches a critical value, shown in the voltage-current characteristic curve, allows a feasible identification method for critical breakdown voltage of oxide film.
- (2)
- Magnitude of peak current (Ip), current transition time (tp), and constant current (Ic) of the single pulse anodizing process were determined by pulse potential. Dynamic V-I characteristic curves for single pulses were characterized by loop circle with the length and area of this loop circle being related to pulse potential. With the increase of pulse width, the effective value of the current becomes smaller.
- (3)
- Characteristic shapes of discharge channels were observed under single pulse anodizing and were correlated with the pulse parameters. Isolated circular opened pores were primarily found under shorter and lower voltage pulses. In contrast, groove-like discharge channels formed in addition to opened pores under longer and higher voltage pulses. Opened discharge pores were found to close within the groove-like discharge channels region with increasing pulse width.
- (4)
- Approximate characteristic parameters of individual discharge events were estimated for individual discharge events of short duration (~hundreds of μs), low current (~tens of mA), and small size (~hundreds of μm).
6. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Parfenov, E.V.; Yerokhin, A.; Nevyantseva, R.R.; Gorbatkov, M.V.; Liang, C.J.; Matthews, A. Towards smart electrolytic plasma technologies: An overview of methodological approaches to process modelling. Surf. Coat. Technol. 2015, 269, 2–22. [Google Scholar] [CrossRef]
- Rokosz, K.; Hryniewicz, T.; Gaiaschi, S.; Chapon, P.; Raaen, S.; Matýsek, D.; Dudek, L.; Pietrzak, K. Novel Porous Phosphorus–Calcium–Magnesium Coatings on Titanium with Copper or Zinc Obtained by DC Plasma Electrolytic Oxidation Fabrication and Characterization. Materials 2018, 11, 1680. [Google Scholar] [CrossRef] [PubMed]
- Hussein, R.O.; Nie, X.; Northwood, D.O. An investigation of ceramic coating growth mechanisms in plasma electrolytic oxidation (PEO) processing. Electrochim. Acta 2013, 112, 111–119. [Google Scholar] [CrossRef]
- Matykina, E.; Arrabal, R.; Scurr, D.J.; Baron, A.; Skeldon, P.; Thompson, G.E. Investigation of the mechanism of plasma electrolytic oxidation of aluminium using 18O tracer. Corros. Sci. 2010, 52, 1070–1076. [Google Scholar] [CrossRef]
- Liu, X.; Li, G.; Xia, Y. Investigation of the discharge mechanism of plasma electrolytic oxidation using Ti tracer. Surf. Coat. Technol. 2012, 206, 4462–4465. [Google Scholar] [CrossRef][Green Version]
- Liu, X.; Zhu, L.; Liu, H.; Li, W. Investigation of MAO coating growth mechanism on aluminum alloy by two-step oxidation method. Appl. Surf. Sci. 2014, 293, 12–17. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Yan, Z.; Fu, W. Optical emission spectroscopy studies of discharge mechanism and plasma characteristics during plasma electrolytic oxidation of magnesium in different electrolytes. Surf. Coat. Technol. 2010, 205, 1651–1658. [Google Scholar] [CrossRef]
- Matykina, E.; Berkani, A.; Skeldon, P.; Thompson, G.E. Real-time imaging of coating growth during plasma electrolytic oxidation of titanium. Electrochim. Acta 2007, 53, 1987–1994. [Google Scholar] [CrossRef]
- Cheng, Y.; Wu, F.; Matykina, E.; Skeldon, P.; Thompson, G.E. The influences of micro discharge types and silicate on the morphologies and phase compositions of plasma electrolytic oxidation coatings on Zircaloy-2. Corros. Sci. 2012, 59, 307–315. [Google Scholar] [CrossRef]
- Arrabal, R.; Matykina, E.; Hashimoto, T.; Skeldon, P.; Thompson, G.E. Characterization of AC PEO coatings on magnesium alloys. Surf. Coat. Technol. 2009, 203, 2207–2220. [Google Scholar] [CrossRef]
- Yerokhin, A.L.; Snizhko, L.O.; Gurevina, N.L.; Leyland, A.; Pilkington, A.; Matthews, A. Spatial characteristics of discharge phenomena in plasma electrolytic oxidation of aluminum alloy. Surf. Coat. Technol. 2004, 177–178, 779–783. [Google Scholar] [CrossRef]
- Stojadinović, S.; Jovović, J.; Petković, M.; Vasilić, R.; Konjević, N. Spectroscopic and real-time imaging investigation of tantalum plasma electrolytic oxidation (PEO). Surf. Coat. Technol. 2011, 205, 5406–5413. [Google Scholar] [CrossRef]
- Me´cuson, F.; Czerwiec, T.; Belmonte, T.; Dujardin, L.; Viola, A.; Henrion, G. Diagnostics of an electrolytic microarc process for aluminum alloy oxidation. Surf. Coat. Technol. 2005, 200, 804–808. [Google Scholar] [CrossRef]
- Hussein, R.O.; Nie, X.; Northwood, D.O. A spectroscopic and microstructural study of oxide coatings produced on a Ti-6Al-4V alloy by plasma electrolytic oxidation. Mater. Chem. Phys. 2012, 134, 484–492. [Google Scholar] [CrossRef]
- Liu, R.; Wu, J.; Xue, W.; Qu, Y.; Yang, C.; Wang, B.; Wu, X. Discharge behaviors during plasma electrolytic oxidation on aluminum alloy. Mater. Chem. Phys. 2014, 148, 284–292. [Google Scholar] [CrossRef]
- Dunleavy, C.S.; Golosnoy, I.O.; Curran, J.A.; Clyne, T.W. Characterisation of discharge events during plasma electrolytic oxidation. Surf. Coat. Technol. 2009, 203, 3410–3419. [Google Scholar] [CrossRef]
- Dunleavy, C.S.; Curran, J.A.; Clyne, T.W. Time dependent statistics of plasma discharge parameters during bulk AC plasma electrolytic oxidation of aluminium. Appl. Surf. Sci. 2013, 268, 397–409. [Google Scholar] [CrossRef]
- Parfenov, E.V.; Yerokhin, A.L.; Matthews, A. Frequency response studies for the plasma electrolytic oxidation process. Surf. Coat. Technol. 2007, 201, 8661–8670. [Google Scholar] [CrossRef]
- Yerokhin, A.; Parfenov, E.V.; Liang, C.J.; Mukaeva, V.R.; Matthews, A. System linearity quantification for in-situ impedance spectroscopy of plasma electrolytic oxidation. Electrochem. Commun. 2013, 27, 137–140. [Google Scholar] [CrossRef]
- Boinet, M.; Verdier, S.; Maximovitch, S.; Dalard, F. Plasma electrolytic oxidation of AM60 magnesium alloy: Monitoring by acoustic emission technique. Electrochemical properties of coatings. Surf. Coat. Technol. 2005, 199, 141–149. [Google Scholar] [CrossRef]
- Clyne, T.W.; Troughton, S.C. A review of recent work on discharge characteristics during plasma electrolytic oxidation of various metals. Int. Mater. Rev. 2019, 64, 127–162. [Google Scholar] [CrossRef]
- Sah, S.P.; Tatsuno, Y.; Aoki, Y.; Habazaki, H. Dielectric breakdown and healing of anodic oxide films on aluminum under single pulse anodizing. Corros. Sci. 2011, 53, 1838–1844. [Google Scholar] [CrossRef]
- Aliofkhazraei, M.; Rouhaghdam, A.S. Study of nanoparticle adsorption in single discharge of plasma electrolysis. Electrochem. Commun. 2012, 20, 88–91. [Google Scholar] [CrossRef]
- Zhang, X.; Yao, Z.; Jiang, Z.; Zhang, Y.; Liu, X. Investigation of the plasma electrolytic oxidation of Ti6Al4V under single-pulse power supply. Corros. Sci. 2011, 53, 2253–2262. [Google Scholar] [CrossRef]
- Yang, K.; Cao, B.; Ding, L.; Yang, G.; Huang, Z.H. Design of High Frequency Narrow Pulsed Inverter Power Supply for Micro Arc Oxidation. J. Sou. Chi. Univer. Technol. 2014, 42, 18–23. [Google Scholar]
- Yang, K.; Cao, B. Electrical characteristics identification of dielectric film breakdown during plasma electrolytic oxidation process. Mater. Lett. 2015, 143, 177–180. [Google Scholar] [CrossRef]
- Mécuson, F.J.; Czerwiec, T.; Henrion, G.; Belmonte, T.; Dujardin, L.; Viola, A.; Beauvir, J. Tailored aluminum oxide layers by bipolar current adjustment in the Plasma Electrolytic Oxidation (PEO) process. Surf. Coat. Technol. 2007, 201, 8677–8682. [Google Scholar] [CrossRef]

| Current Level (Ievent, mA) | Duration (td, ms) | Spatial Distribution Ratio (Dn, mm−2·ms−1) | Diameter (φ, μm) |
|---|---|---|---|
| 97.1 | 0.94 | 4.69 | 369.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, K.; Huang, H.; Chen, J.; Cao, B. Discharge Behavior and Dielectric Breakdown of Oxide Films during Single Pulse Anodizing of Aluminum Micro-Electrodes. Materials 2019, 12, 2286. https://doi.org/10.3390/ma12142286
Yang K, Huang H, Chen J, Cao B. Discharge Behavior and Dielectric Breakdown of Oxide Films during Single Pulse Anodizing of Aluminum Micro-Electrodes. Materials. 2019; 12(14):2286. https://doi.org/10.3390/ma12142286
Chicago/Turabian StyleYang, Kai, Haisong Huang, Jiadui Chen, and Biao Cao. 2019. "Discharge Behavior and Dielectric Breakdown of Oxide Films during Single Pulse Anodizing of Aluminum Micro-Electrodes" Materials 12, no. 14: 2286. https://doi.org/10.3390/ma12142286
APA StyleYang, K., Huang, H., Chen, J., & Cao, B. (2019). Discharge Behavior and Dielectric Breakdown of Oxide Films during Single Pulse Anodizing of Aluminum Micro-Electrodes. Materials, 12(14), 2286. https://doi.org/10.3390/ma12142286





