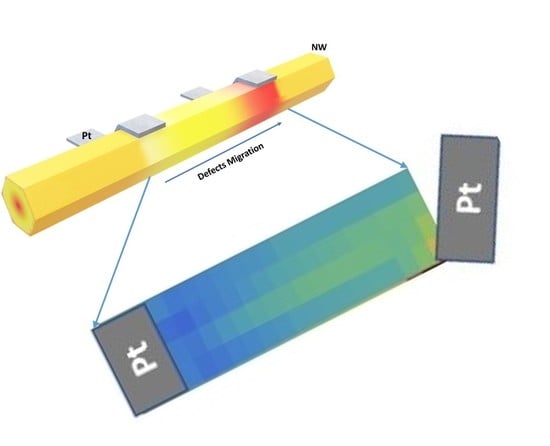
Native Point Defect Measurement and Manipulation in ZnO Nanostructures
Abstract
:1. Introduction
2. Electronic Properties of ZnO Nanostructures
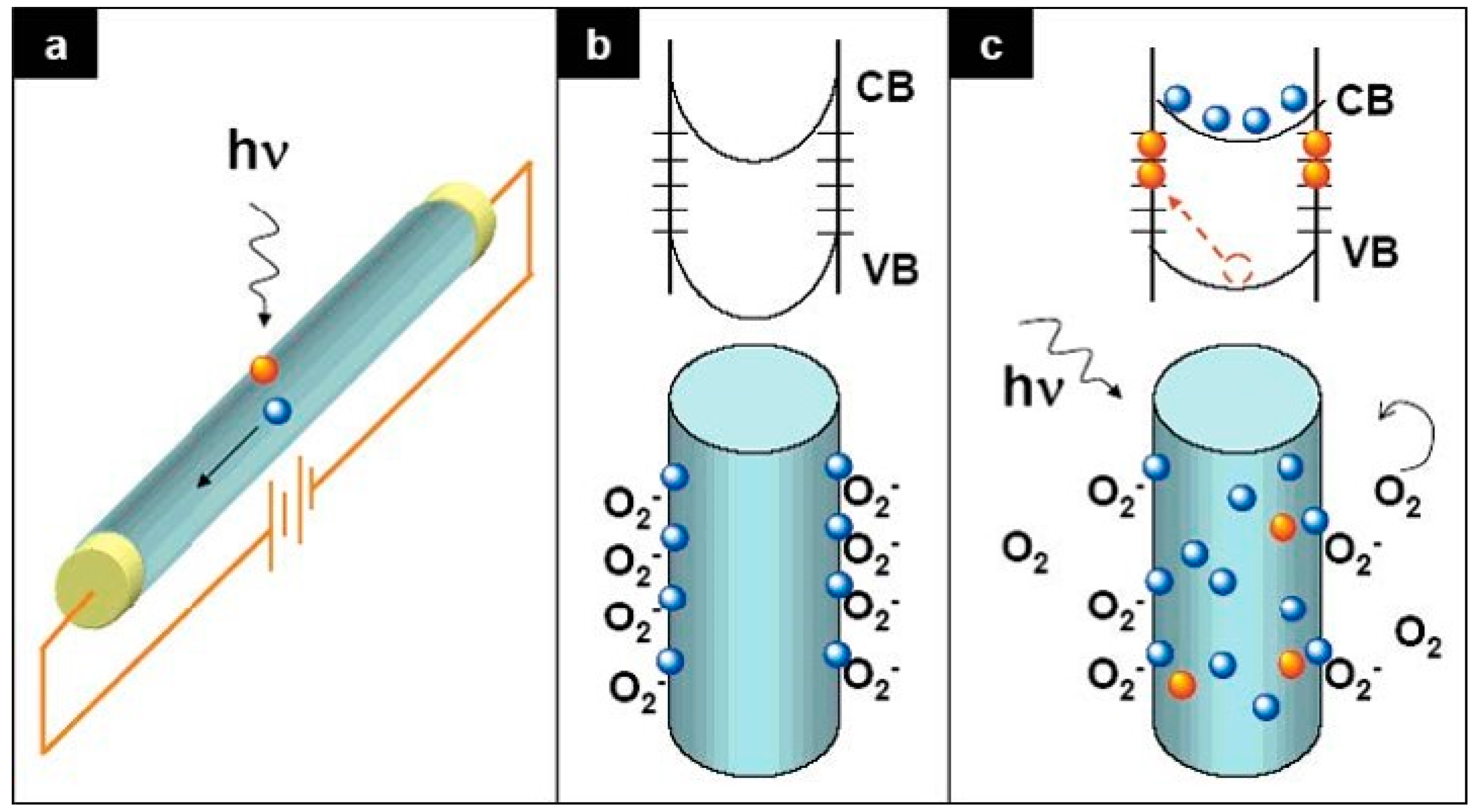
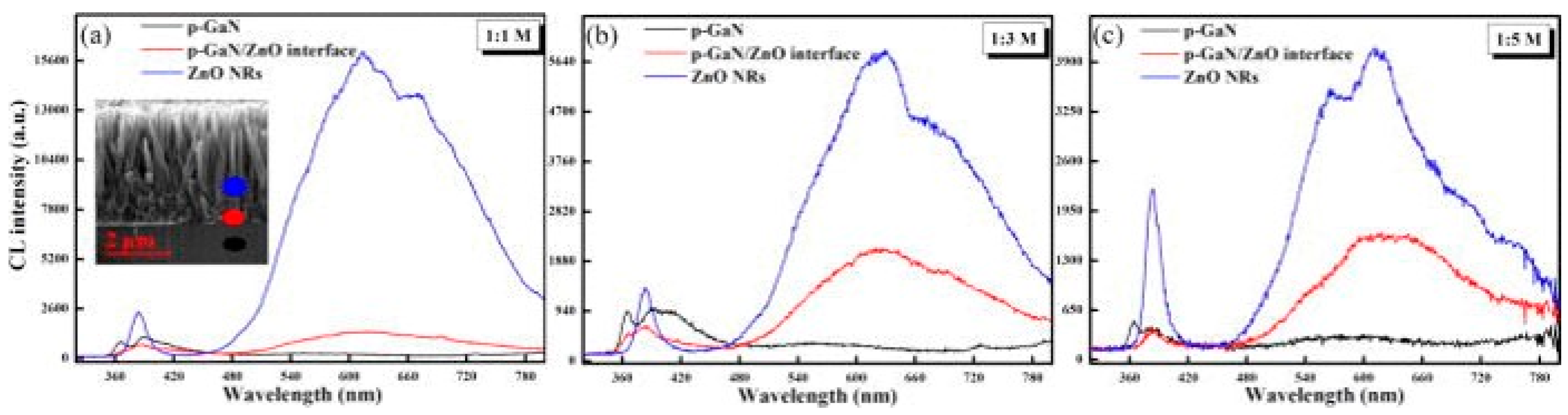
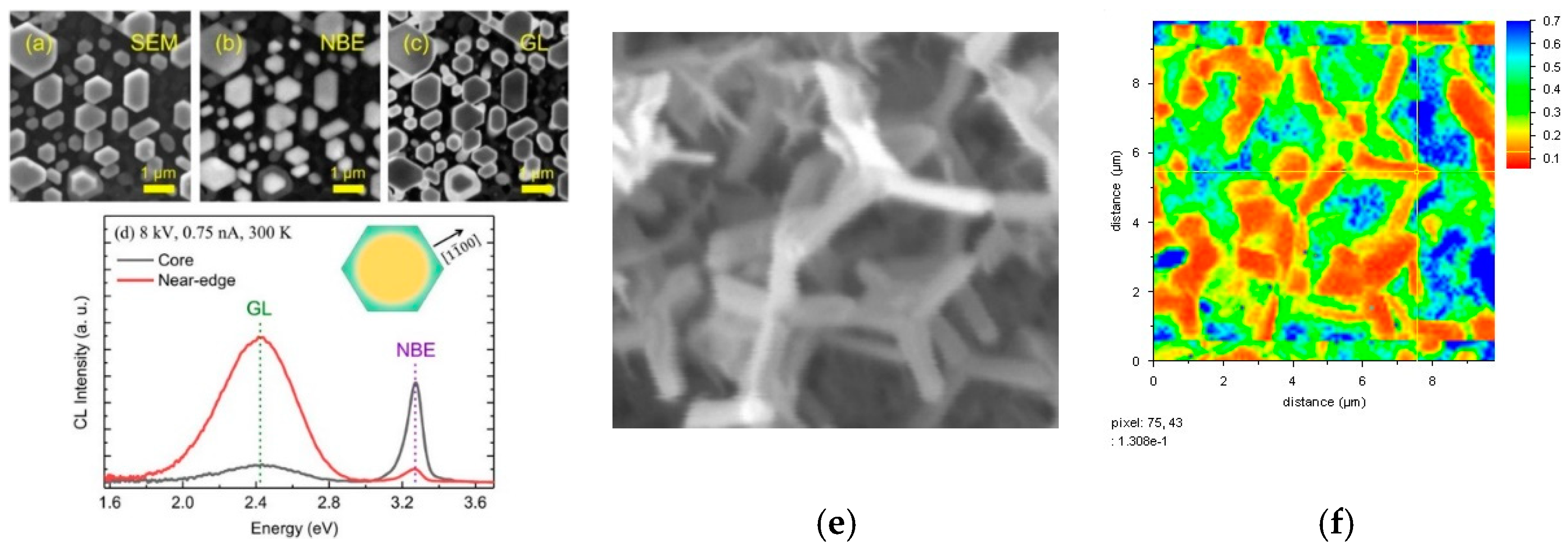
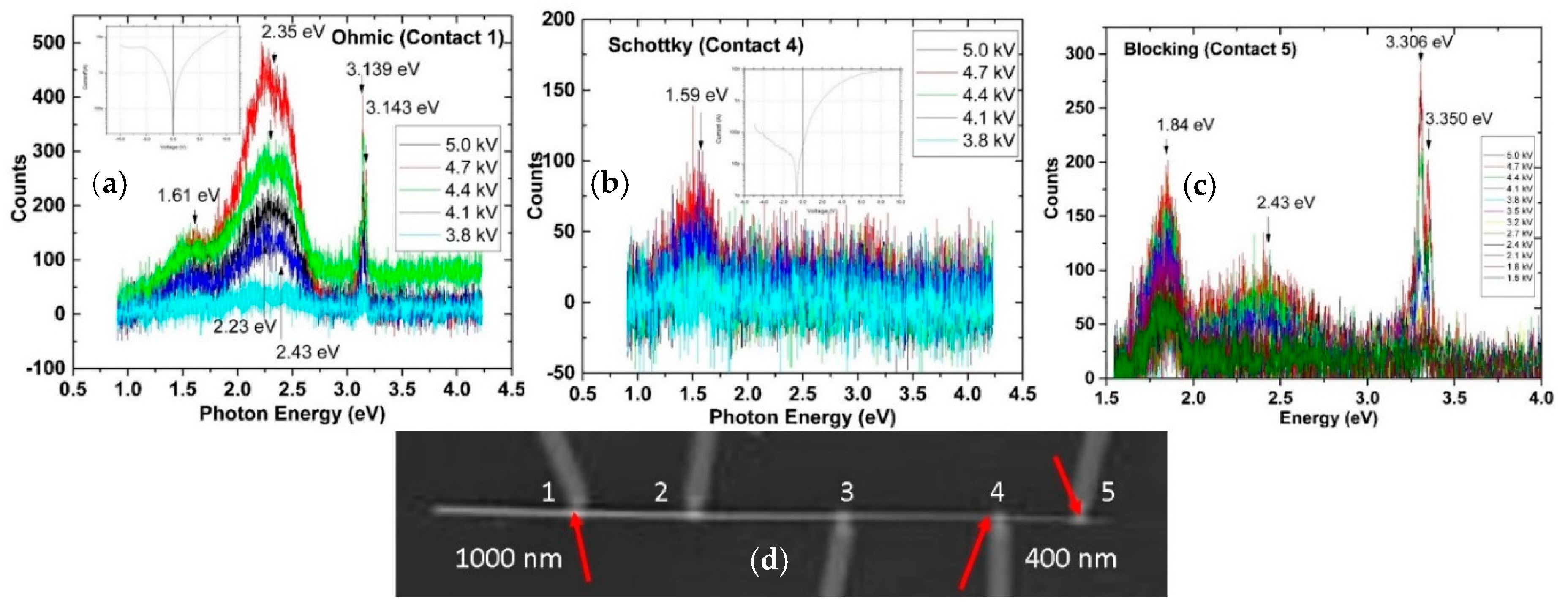
3. Defect Distributions in ZnO
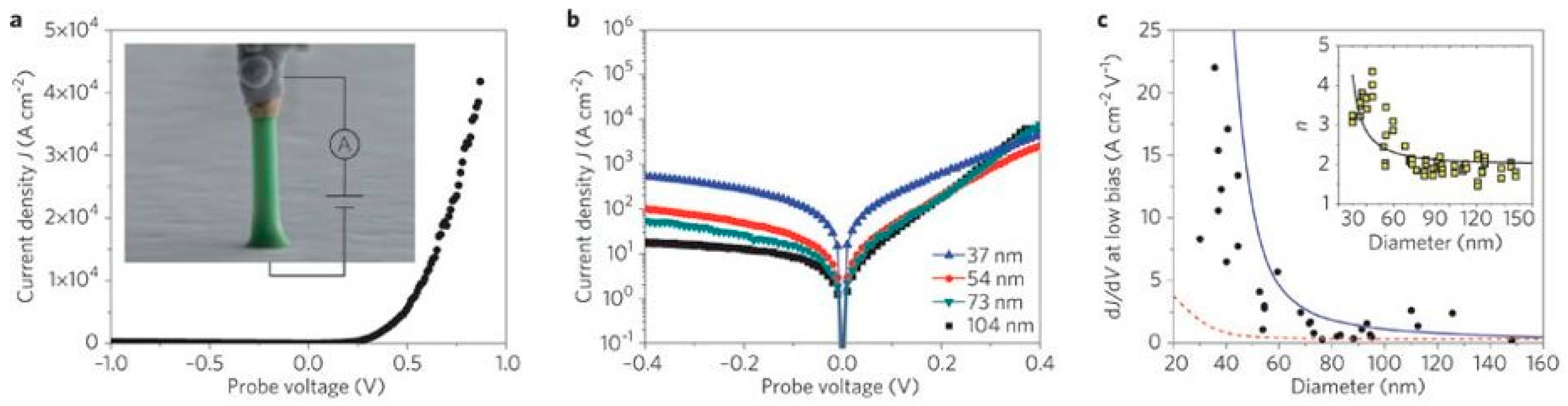
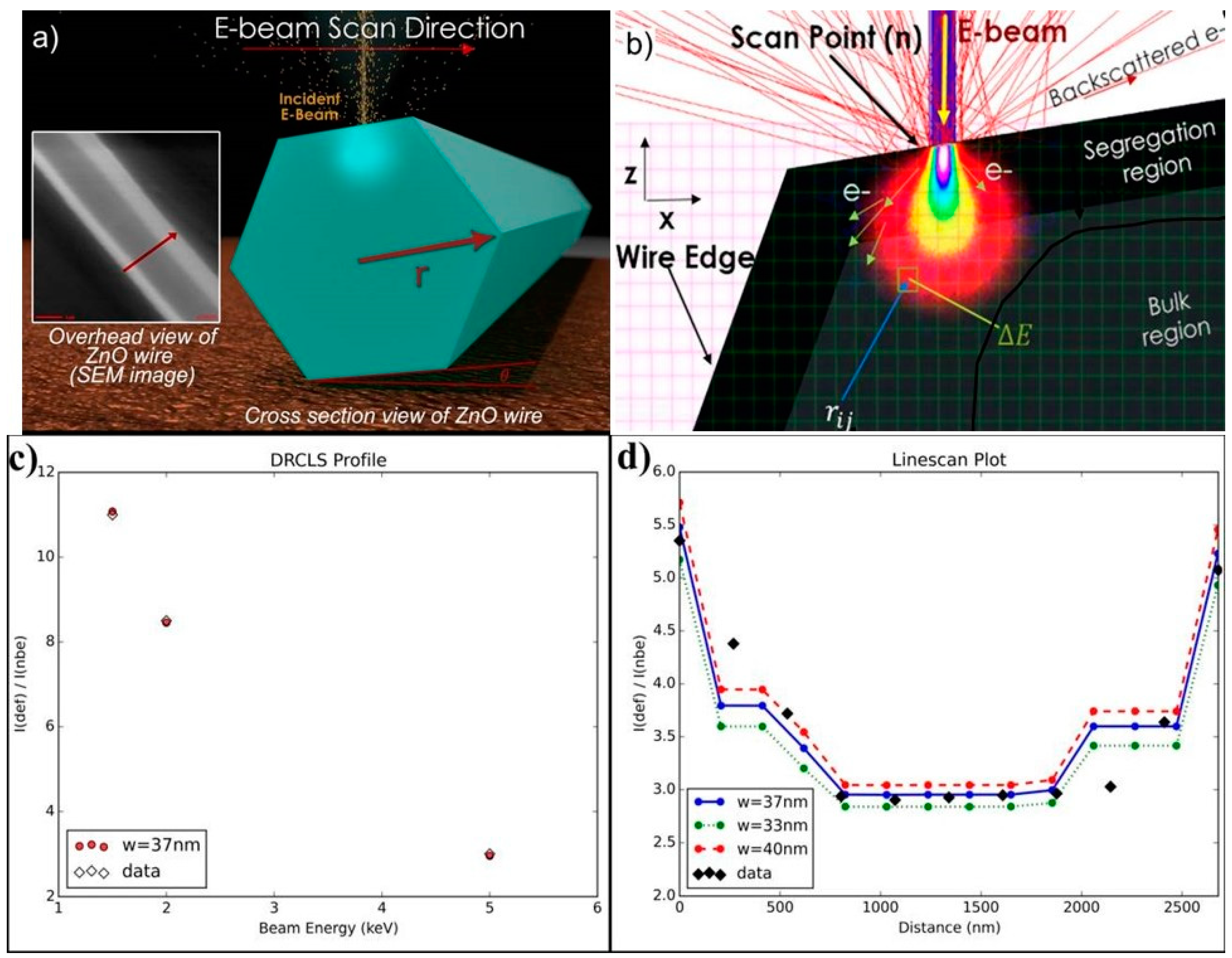
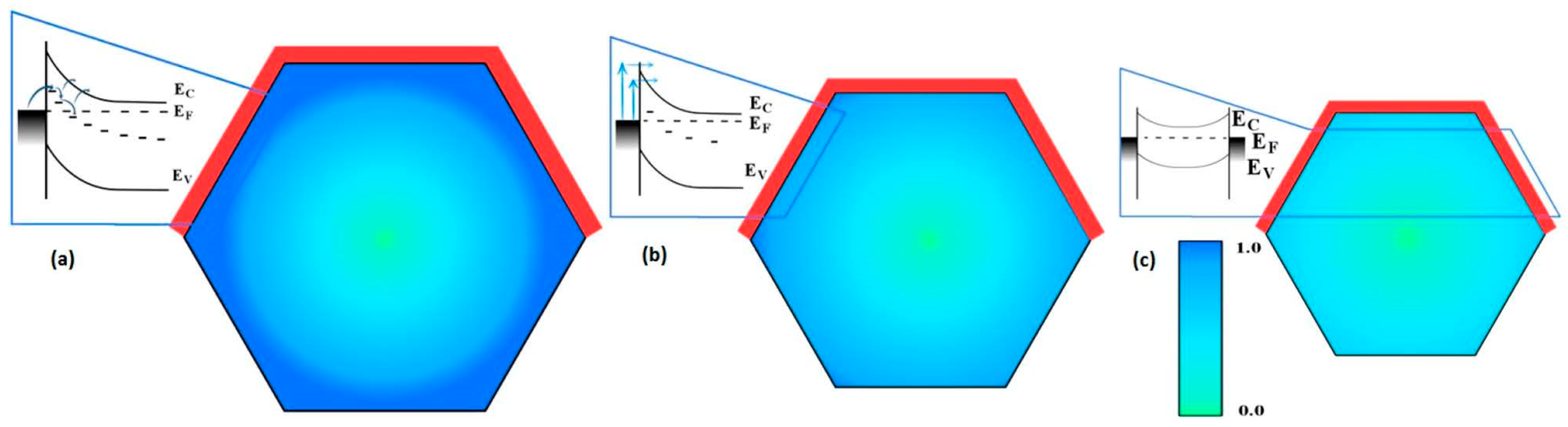
4. Impact of ZnO Defect Distributions on Electronic Measurements
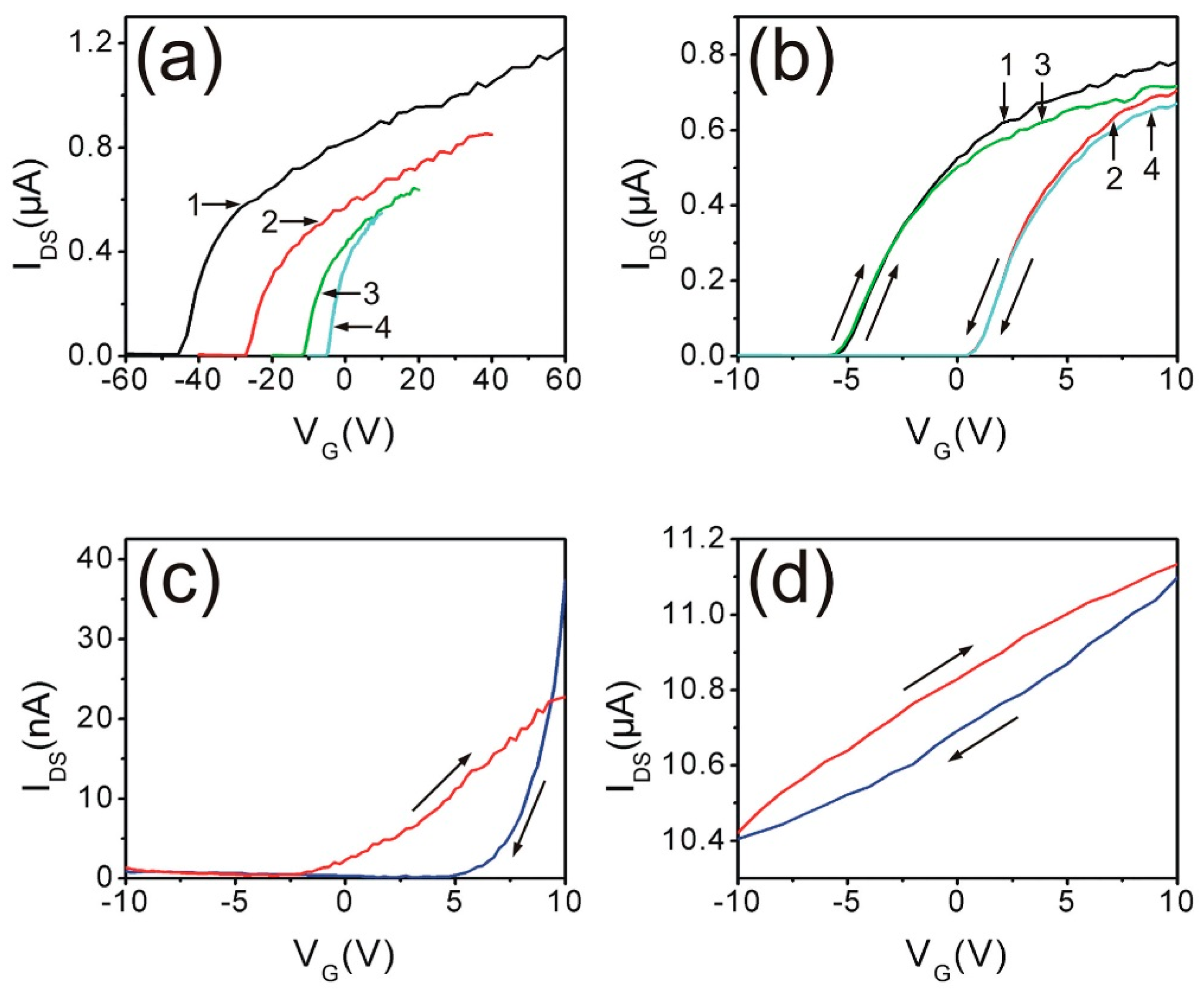
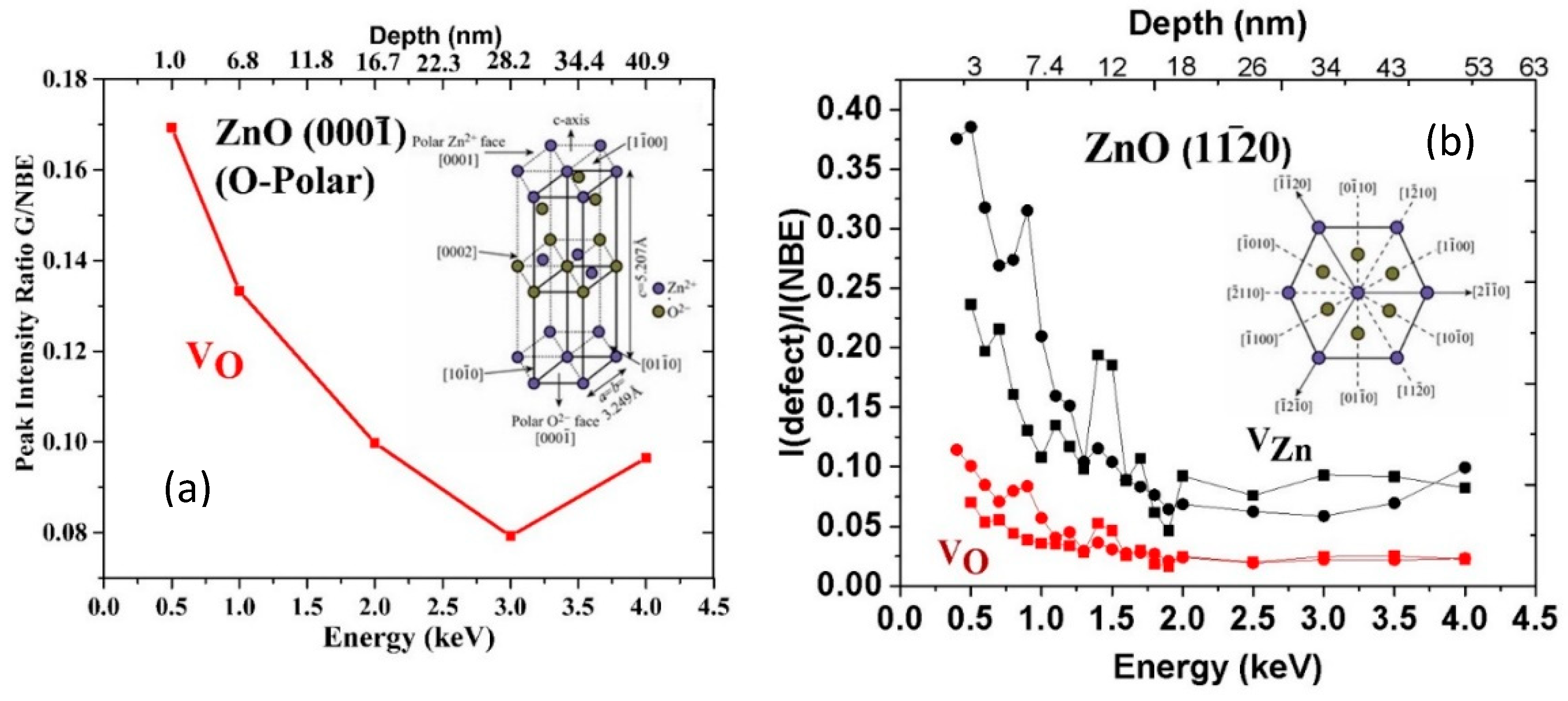
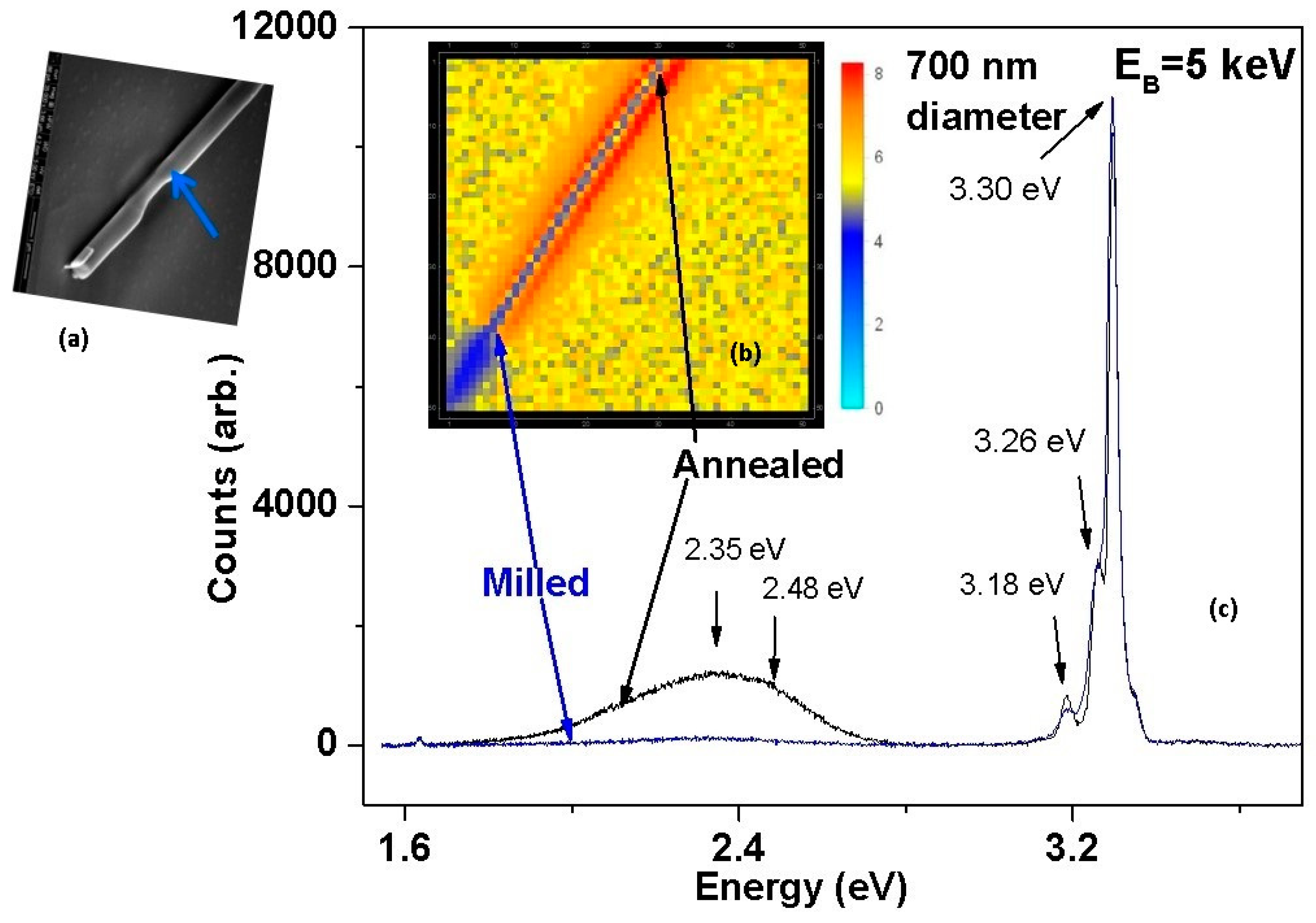
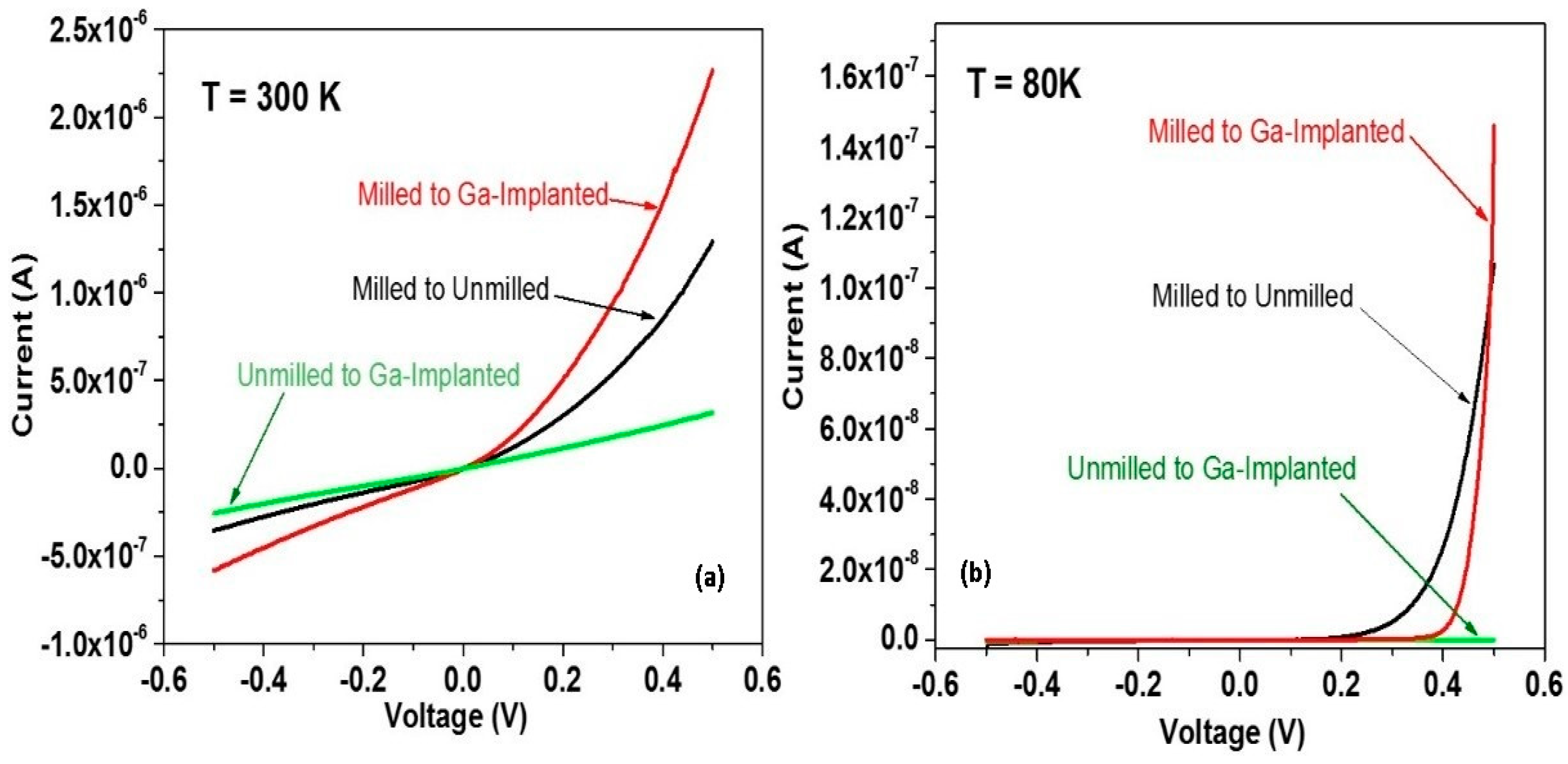
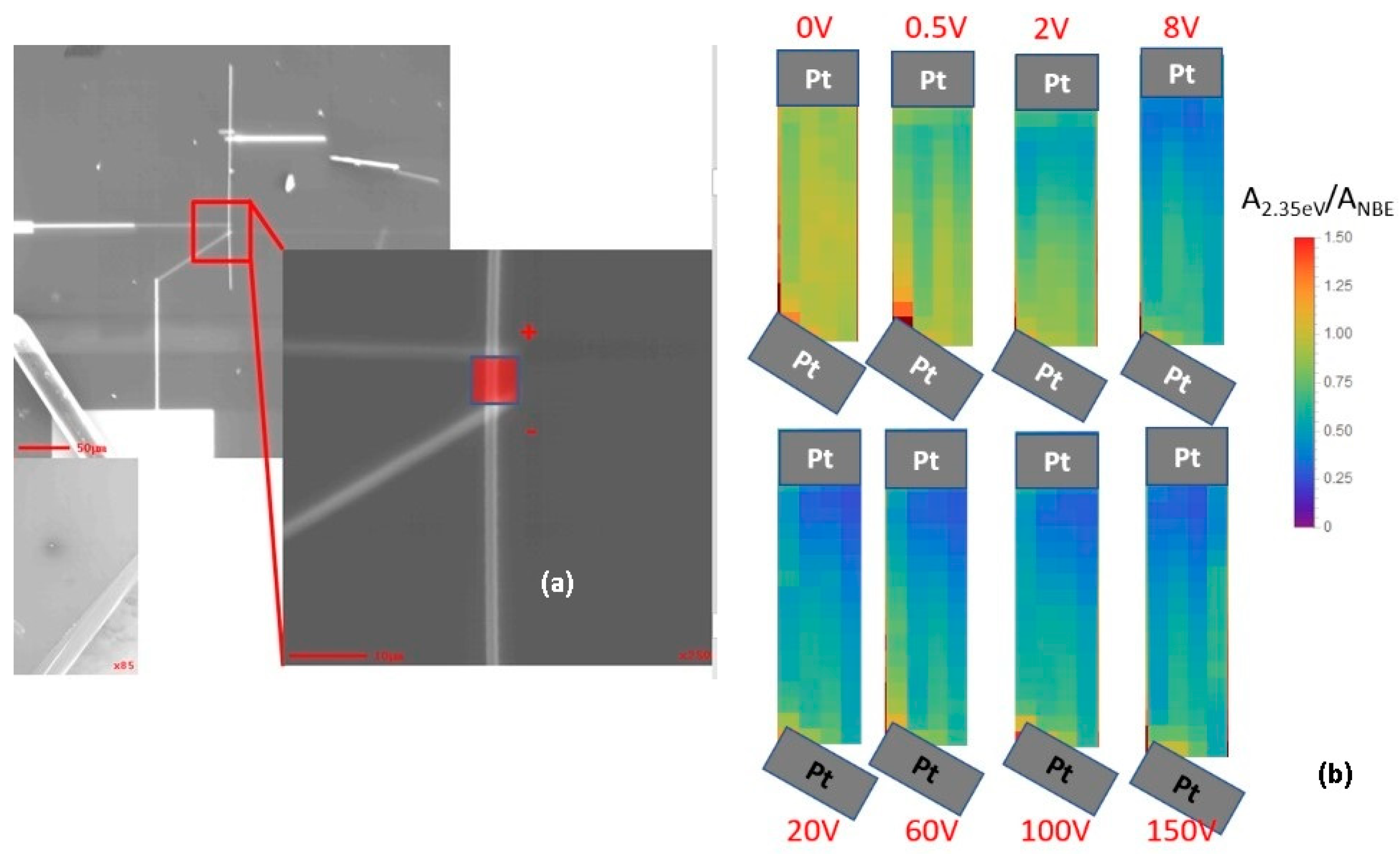
5. Manipulating Native Point Defects and Controlling Electronic Properties
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Look, D.C. Recent Advances in ZnO Materials and Devices. Mater. Sci. Eng. B 2001, 80, 383–387. [Google Scholar] [CrossRef]
- Tsukazaki, A.; Ohtomo, A.; Kita, T.; Ohno, Y.; Ohno, H.; Kawasaki, M. Quantum Hall Effect in Polar Oxide Heterostructures. Science 2007, 315, 1388–1391. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.L.; Song, J.H. Piezoelectric Nanogenerators Based on Zinc Oxide Nanowire Arrays. Science 2006, 312, 2420246. [Google Scholar] [CrossRef] [PubMed]
- Look, D.C.; Claflin, B. Electrical Optical Properties of ZnO Surfaces. In Zinc Oxide the Future Material for Electronics: A Comprehensive Review on ZnO Physics and Defects; Selim, F., Ed.; Research Signpost: Kerala, India, 2011; p. 61. [Google Scholar]
- Look, D.C. New Developments in ZnO Materials and Devices. Proc. SPIE 2007, 6474, 647402–647409. [Google Scholar]
- Pearton, S.J.; Norton, D.P.; Ip, K.; Heo, Y.W.; Steiner, T. Recent Progress in Processing and Properties of ZnO. Prog. Mater. Sci. 2005, 50, 293–340. [Google Scholar] [CrossRef]
- Őzgür, Ü.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.A.; Doğan, S.; Avrutin, V.; Cho, S.J.; Morkoc, H. A Comprehensive Review of ZnO Materials and Devices. J. Appl. Phys. 2005, 98, 041301. [Google Scholar] [CrossRef]
- Ryu, Y.R.; Lee, T.S.; Lubguban, J.A.; White, H.W.; Kim, B.J.; Park, Y.S.; Youn, C.J. Next Generation of Oxide Photonic Devices: ZnO-Based Ultraviolet Light Emitting Diodes. Appl. Phys. Lett. 2006, 88, 241108. [Google Scholar] [CrossRef]
- Leong, E.S.P.; Yu, S.F.; Lau, S.P. Directional Edge-Emitting UV Random Laser Diodes. Appl. Phys. Lett. 2006, 89, 221109. [Google Scholar] [CrossRef]
- Barquinha, P.; Fortunato, E.; Goncalves, A.; Pimentel, A.; Marques, A.; Pereira, L.; Martins, R. A Study on the Electrical Properties of ZnO Based Transparent TFTs. Adv. Mater. Forum 2006, 514–516, 68–72. [Google Scholar] [CrossRef]
- Schmidt-Mende, L.; MacManus-Driscoll, J.L. ZnO-nanostructures, defects, and devices. Mater. Today 2007, 10, 40. [Google Scholar] [CrossRef]
- Djurišić, A.B.; Chen, X.; Leung, Y.H.; Ng, A.M.C. ZnO nanostructures: Growth, properties and applications. J. Mater. Chem. 2012, 22, 6526. [Google Scholar] [CrossRef]
- Chu, S.; Wang, G.; Zhou, W.; Lin, Y.; Chernyak, L.; Zhao, J.; Kong, J.; Li, L.; Ren, J.; Liu, J. Electrically pumped waveguide lasing from ZnO nanowires. Nat. Nanotechnol. 2011, 6, 506. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Yang, R.; Wang, S.; Wang, Z.L. Flexible High-Output Nanogenerators Based on Lateral ZnO Nanowire Array. Nano Lett. 2010, 10, 3151. [Google Scholar] [CrossRef] [PubMed]
- Ruane, W.T.; Leedy, K.; Look, D.C.; Farlow, G.; von Wenckstern, H.; Grundmann, M.; Brillson, L.J. Defect Segregation and Optical Emission in ZnO Nanowires and Microwires. Nanoscale 2016, 8, 7631–7637. [Google Scholar] [CrossRef] [PubMed]
- Jarjour, A.; Cox, J.W.; Ruane, W.T.; von Wenckstern, H.; Grundmann, M.; Brillson, L.J. Single Metal Ohmic and Rectifying Contacts to ZnO Nanowires: A Defect Based Approach. Ann. Phys. 2018, 530, 1700335. [Google Scholar] [CrossRef]
- Foster, G.M.; Gao, H.; Mackessy, G.; Hyland, A.M.; Allen, M.W.; Wang, B.; Look, D.C.; Brillson, L.J. Impact of Defect Distribution on IrOx/ZnO Interface Doping and Schottky Barriers. Appl. Phys. Lett. 2017, 111, 101604. [Google Scholar] [CrossRef]
- Cox, J.W.; Foster, G.M.; Jarjour, A.; von Wenckstern, H.; Grundmann, M.; Brillson, L.J. Defect Manipulation to Control ZnO Micro-/Nanowire-Metal Contacts. Nano Lett. 2018, 18, 6974. [Google Scholar] [CrossRef] [PubMed]
- Brillson, L.J.; Dong, Y.; Doutt, D.; Look, D.C.; Fang, Z.Q. Massive Point Defect Redistribution Near Semiconductor Surfaces and Interfaces and Its Impact on Schottky Barrier Formation. Physica B 2009, 404, 4768–4773. [Google Scholar] [CrossRef]
- Léonard, F.; Talin, A.A.; Swartzentruber, B.S.; Picraux, S.T. Diameter-Dependent Electronic Transport Properties of Au-Catalyst/Ge-Nanowire Schottky diodes. Phys. Rev. Lett. 2009, 102, 106805. [Google Scholar] [CrossRef] [PubMed]
- Weissenberger, D.; Gerthsen, D.; Reiser, A.; Prinz, G.M.; Feneberg, M.; Thonke, K.; Zhou, H.; Sartor, J.; Fallert, J.; Klingshirn, C.; et al. Influence of the measurement procedure on the field-effect dependent conductivity of ZnO nanorods. Appl. Phys. Lett. 2009, 94, 042107. [Google Scholar] [CrossRef]
- Fan, Z.; Lu, J.G. Gate-refreshable nanowire chemical sensors. Appl. Phys. Lett. 2005, 86, 123510. [Google Scholar] [CrossRef]
- Frenzel, H.; Lajn, A.; von Wenckstern, H.; Lorenz, M.; Schein, F.; Zhang, Z.; Grundmann, M. Recent Progress on ZnO-Based Metal-Semiconductor Field-Effect Transistors and Their Application in Transparent Integrated Circuits. Adv. Mater. 2010, 22, 5332–5349. [Google Scholar] [CrossRef] [PubMed]
- Schlenker, E.; Bakin, A.; Weimann, T.; Hinze, P.; Weber, D.H.; Gölzhäuser, A.; Wehmann, H.H.; Waag, A. On the difficulties in characterizing ZnO nanowires. Nanotechnology 2008, 19, 365707. [Google Scholar] [CrossRef] [PubMed]
- McCluskey, M.D.; Jokela, S.J. Defects in ZnO. Appl. Phys. Rev. 2009, 106, 071101. [Google Scholar] [CrossRef] [Green Version]
- Soci, C.; Zhang, A.; Xiang, B.; Dayeh, S.A.; Aplin, D.P.R.; Bao, J.P.X.Y.; Lo, Y.H.; Wang, D. ZnO Nanowire UV Photodetectors with High Internal Gain. Nano Lett. 2007, 7, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Okur, S.; Metzner, S.; Izyumskaya, N.; Zhang, F.; Avrutin, V.; Karbaum, C.; Bertram, F.; Christen, J.; Morkoç, H.; Özgür, Ü. Microscopic distribution of extended defects and blockage of threading dislocations by stacking faults in semipolar (1ī01) GaN revealed from spatially resolved luminescence. Appl. Phys. Lett. 2013, 103, 211908. [Google Scholar] [CrossRef]
- Furusawa, K.; Ishikawa, Y.; Tashiro, M.; Hazu, K.; Nagao, S.; Ikeda, H.; Fujito, K.; Chichibu, S.F. Local carrier dynamics around the sub-surface basal-plane stacking faults of GaN studied by spatio-time-resolved cathodoluminescence using a front-excitation-type photoelectron-gun. Appl. Phys. Lett. 2013, 103, 052108. [Google Scholar] [CrossRef]
- Koch, H.; Bertram, F.; Pietzonka, I.; Ahl, J.P.; Strassburg, M.; August, O.; Christen, J.; Kalisch, H.; Vescan, A.; Lugauer, H.J. InGaN: Direct correlation of nanoscopic morphology features with optical and structural properties. Appl. Phys. Lett. 2014, 105, 072108. [Google Scholar] [CrossRef]
- Toth, M.; Zachreson, C.; Aharonovich, I. Role of recombination pathway competition in spatially resolved cathodoluminescence spectroscopy. Appl. Phys. Lett. 2014, 105, 241112. [Google Scholar] [CrossRef] [Green Version]
- Christen, J.; Grundmann, M.; Bimberg, D. Scanning cathodoluminescence microscopy: A unique approach to atomic scale characterization of heterointerfaces and imaging of semiconductor inhomogeneities. J. Vac. Sci. Technol. B 1991, 9, 2358. [Google Scholar] [CrossRef]
- Kennedy, O.W.; White, E.R.; Howkins, A.; Williams, C.K.; Boyd, I.W.; Warburton, P.A.; Shaffer, M.S.P. Mapping the Origins of Luminescence in ZnO Nanowires by STEM-CL. J. Phys. Chem. Lett. 2019, 10, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Djurišić, A.B.; Ng, A.M.C.; Chen, X.Y. ZnO nanostructures for optoelectronics: Material properties and device applications. Prog. Quantum Electron. 2010, 34, 191. [Google Scholar] [CrossRef]
- Dong, Y.; Tuomisto, F.; Svensson, B.G.; Kuznetsov, A.Y.; Brillson, L.J. Vacancy Defect and Defect Cluster Energetics in Ion-implanted ZnO. Phys. Rev. B Rapid Commun. 2010, 81, 081201(R). [Google Scholar] [CrossRef]
- Ton-That, C.; Phillips, M.R. Cathodoluminescence microanalysis of ZnO microwires in semiconductor nanowires: Materials. In Synthesis, Characterization and Applications; Arbiol, J., Xiong, Q., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; Chapter 14. [Google Scholar]
- Dierre, B.; Yuan, X.; Sekiguchi, T. Low-energy cathodoluminescence microscopy for the characterization of nanostructures. Sci. Technol. Adv. Mater. 2010, 11, 043001. [Google Scholar] [CrossRef] [PubMed]
- Stowe, D. Assessing the capabilities and limitations of cathodoluminescence microscopy to investigate the optical properties of individual nanostructures. Phys. Status Solidi 2011, 8, 1423. [Google Scholar] [CrossRef]
- Willander, M.; Israr, M.Q.; Sadaf, J.R.; Nur, O. Progress on one-dimensional zinc oxide nanomaterials based photonic devices. Nanophotonics 2012, 1, 99. [Google Scholar] [CrossRef]
- Willander, M.; Nur, O.; Zhao, Q.X.; Yang, L.L.; Lorenz, M.; Cao, B.Q.; Pérez, J.Z.; Czekalla, C.; Zimmermann, G.; Grundmann, M.; et al. Zinc oxide nanorod based photonic devices: Recent progress in growth, light emitting diodes and lasers. Nanotechnology 2009, 20, 33201. [Google Scholar] [CrossRef]
- Brillson, L.J.; Ruane, W.T.; Gao, H.; Zhang, Y.; Luo, J.; von Wenckstern, H.; Grundmann, M. Spatially-Resolved Cathodoluminescence Spectroscopy of ZnO Defects. Mater. Sci. Semicond. Process. 2017, 57, 197. [Google Scholar] [CrossRef]
- Brillson, L.J.; Foster, G.M.; Cox, J.; Ruane, W.T.; Jarjour, A.B.; Gao, H.; von Wenckstern, H.; Grundmann, M.; Hyland, I.; Allen, M.W. Defect Characterization, Imaging, and Control in Wide Band Gap Semiconductors and Devices. J. Electron. Mater. 2018, 47, 4980. [Google Scholar] [CrossRef]
- Nobis, T.; Kaidashev, E.M.; Rahm, A.; Lorenz, M.; Lenzner, J.; Grundmann, M. Spatially inhomogeneous impurity distribution in ZnO micropillars. Nano Lett. 2004, 4, 797. [Google Scholar] [CrossRef]
- Schirra, M.; Reiser, A.; Prinz, G.M.; Ladenburger, A.; Thonke, K.; Sauer, R. Cathodoluminescence study of single zinc oxide nanopillars with high spatial and spectral resolution. J. Appl. Phys. 2007, 101, 113509. [Google Scholar] [CrossRef]
- Gautam, U.K.; Imura, M.; Rout, C.S.; Bando, Y.; Fang, X.; Dierre, B.; Sakharov, L.; Govindaraj, A.; Sekiguchi, T.; Goldberg, D.; et al. Unipolar assembly of zinc oxide rods manifesting polarity-driven collective luminescence. Proc. Natl. Acad. Sci. USA 2010, 107, 13588. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, F.; Villani, M.; Catellani, A.; Calzolari, A.; Cicero, G.; Calestani, D.; Calestani, G.; Zappettini, A.; Dierre, B.; Sekiguchi, T.; et al. Zn vacancy induced green luminescence on non-polar surfaces in ZnO nanostructures. Sci. Rep. 2014, 4, 5158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, W.W.; Kim, S.B.; Yi, J.; Nichols, W.T.; Park, W.I. Surface polarity-dependent cathodoluminescence in hydrothermally grown ZnO hexagonal rods. J. Phys. Chem. C 2011, 116, 456. [Google Scholar] [CrossRef]
- Alnoor, H.; Pozina, G.; Khranovskyy, V.; Liu, X.; Iandolo, D.; Willander, M.; Nur, O. Influence of ZnO seed layer precursor molar ratio on the density of interface defects in low temperature aqueous chemically synthesized ZnO nanorods/GaN light emitting diodes. J. Appl. Phys. 2016, 119, 165702. [Google Scholar] [CrossRef]
- Zhu, L.; Lockrey, M.; Phillips, M.R.; Ton-That, C. Spatial Distribution of Defect Luminescence in ZnO Nanorod: An Investigation by Spectral Cathodoluminescence Imaging. Phys. State. Sol. A 2018, 215, 1800389. [Google Scholar] [CrossRef]
- Bernardini, F.; Fiorentini, V.; Vanderbilt, D. Spontaneous polarization and piezoelectric constants of III-V nitrides. Phys. Rev. B 1997, 56, R10024. [Google Scholar] [CrossRef]
- Mosbacker, H.L.; Strzhemechny, Y.M.; White, B.D.; Smith, P.E.; Look, D.C.; Look, D.C.; Reynolds, D.C.; Litton, C.W.; Brillson, L.J. Role of near-surface states in ohmic-Schottky conversion of Au contacts to ZnO. Appl. Phys. Lett. 2005, 87, 012102. [Google Scholar] [CrossRef] [Green Version]
- Perkins, J.; Foster, G.M.; Myer, M.; Mehra, S.; Chauveau, J.M.; Hierro, A.; Redondo-Cubero, A.; Brillson, W.W.J. Impact of Mg content on native point defects in MgxZn1-xO (0 ≤ x ≤ 0.56). APL Mater. 2015, 3, 062801. [Google Scholar] [CrossRef]
- Dong, Y.; Fang, Z.; Look, D.C.; Cantwell, G.; Zhang, J.; Song, J.J.; Brillson, L.J. Zn and O-face effects at ZnO surfaces and metal interfaces. Appl. Phys. Lett. 2008, 93, 072111. [Google Scholar] [CrossRef]
- Foster, G.M.; Perkins, J.; Myer, M.; Mehra, S.; Chauveau, J.M.; Hierro, A.; Redondo-Cubero, A.; Brillson, W.W.J. Native point defect energies, densities, and electrostatic repulsion across (Mg,Zn) O alloys. Phys. Status Solidi A 2015, 212, 1448. [Google Scholar] [CrossRef]
- Foster, G.M.; Faber, G.; Yao, Y.F.; Yang, C.C.; Heller, E.R.; Look, D.C.; Brillson, L.J. Direct Measurement of Defect and Dopant Abruptness at High Electron Mobility ZnO Homojunctions. Appl. Phys. Lett. 2016, 109, 143506. [Google Scholar] [CrossRef]
- Gao, H.; Asel, T.J.; Noesges, B.; Brillson, L.J. Electric field-induced migration of native point defects in SrTiO3. 2019; Unpublished. [Google Scholar]
- Gao, H.; Muralidharan, S.; Pronin, N.; Karim, M.R.; White, S.M.; Asel, T.; Foster, G.; Krishnamoorthy, S.; Rajan, S.; Cao, L.R.; et al. Optical Signatures of Deep Level Defects in Ga2O3. Appl. Phys. Lett. 2018, 112, 242102. [Google Scholar] [CrossRef]
- Janotti, A.; van de Walle, C.G. Hydrogen multicenter bonds. Nat. Mater. 2007, 6, 44. [Google Scholar] [CrossRef]
- Nakamura, S.; Mukai, T.; Senoh, M.; Iwasa, N. Thermal Annealing Effects on P-Type Mg-Doped GaN Films. Jpn. J. Appl. Phys. 1992, 31, L139. [Google Scholar] [CrossRef]
- Savoyant, A.; Alnoor, H.; Pilone, O.; Nur, O.; Willander, M. Core-defect reduction in ZnO nanorods by cobalt incorporation. Nanotechnology 2017, 28, 285705. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.; Haseman, M.; von Wenckstern, H.; Grundmann, M.; Brillson, L.J. Electric field-induced native defect movement in ZnO nanowires. 2019; Unpublished. [Google Scholar]
- Ip, K.; Gila, B.P.; Onstine, A.H.; Lambers, E.S.; Heo, Y.W.; Baik, K.H.; Norton, D.P.; Pearton, S.J. Improved Pt/Au and W/Pt/Au Schottky contacts on n-type ZnO using ozone cleaning. Appl. Phys. Lett. 2004, 84, 5133. [Google Scholar] [CrossRef]
- Coppa, B.J.; Fulton, C.C.; Hartlieb, P.J.; Davis, R.F.; Rodriguez, B.J.; Shields, B.J.; Nemanich, R.J. In situ cleaning and characterization of oxygen and zinc-terminated, n-type, ZnO{0001} surfaces. J. Appl. Phys. 2004, 95, 5856. [Google Scholar] [CrossRef]
- Balaz, S.; Singh, J.; Brillson, L.J. Orientation-dependent native point defect segregation and dipole formation in SrTiO3. 2019; Unpublished. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brillson, L.; Cox, J.; Gao, H.; Foster, G.; Ruane, W.; Jarjour, A.; Allen, M.; Look, D.; von Wenckstern, H.; Grundmann, M. Native Point Defect Measurement and Manipulation in ZnO Nanostructures. Materials 2019, 12, 2242. https://doi.org/10.3390/ma12142242
Brillson L, Cox J, Gao H, Foster G, Ruane W, Jarjour A, Allen M, Look D, von Wenckstern H, Grundmann M. Native Point Defect Measurement and Manipulation in ZnO Nanostructures. Materials. 2019; 12(14):2242. https://doi.org/10.3390/ma12142242
Chicago/Turabian StyleBrillson, Leonard, Jonathan Cox, Hantian Gao, Geoffrey Foster, William Ruane, Alexander Jarjour, Martin Allen, David Look, Holger von Wenckstern, and Marius Grundmann. 2019. "Native Point Defect Measurement and Manipulation in ZnO Nanostructures" Materials 12, no. 14: 2242. https://doi.org/10.3390/ma12142242
APA StyleBrillson, L., Cox, J., Gao, H., Foster, G., Ruane, W., Jarjour, A., Allen, M., Look, D., von Wenckstern, H., & Grundmann, M. (2019). Native Point Defect Measurement and Manipulation in ZnO Nanostructures. Materials, 12(14), 2242. https://doi.org/10.3390/ma12142242