Corrosion Resistance to Chloride of a Novel Stainless Steel: The Threshold Chloride Value and Effect of Surface State
Abstract
:1. Introduction
2. Experimental Program
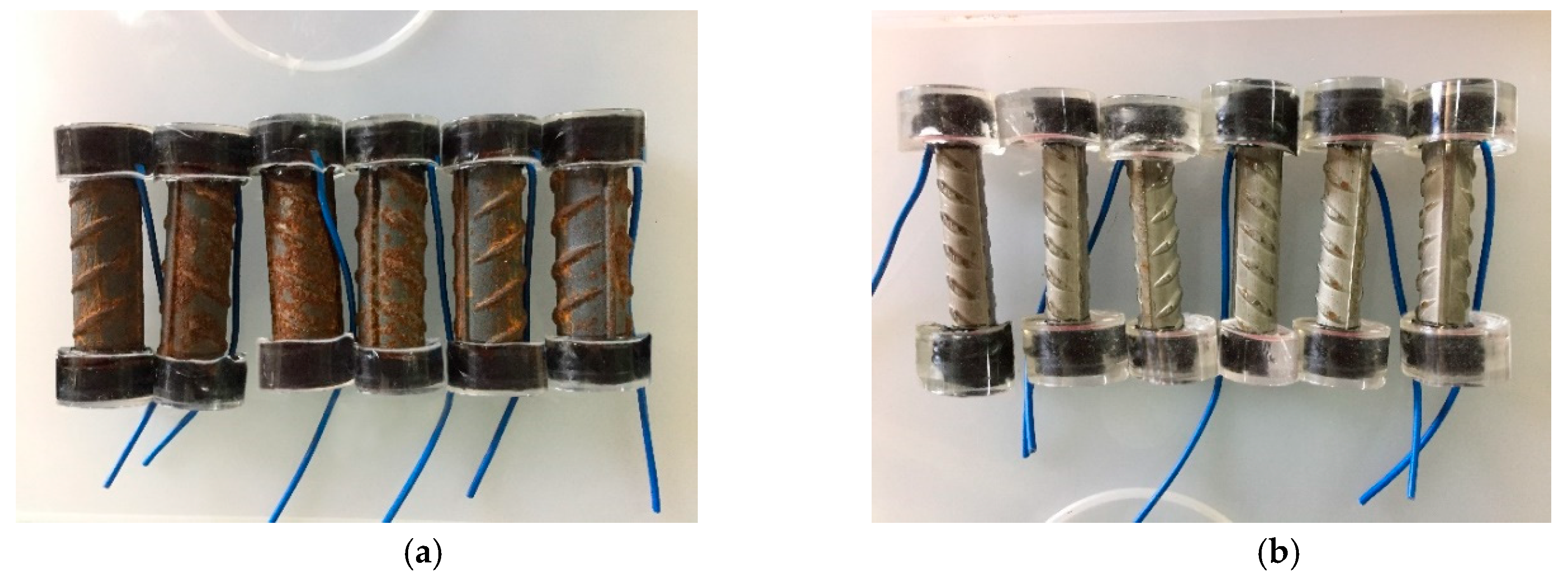
2.1. Materials, Specimens and Solutions
2.2. Surface Treatment
2.3. Electrochemical Tests
3. Results and discussion
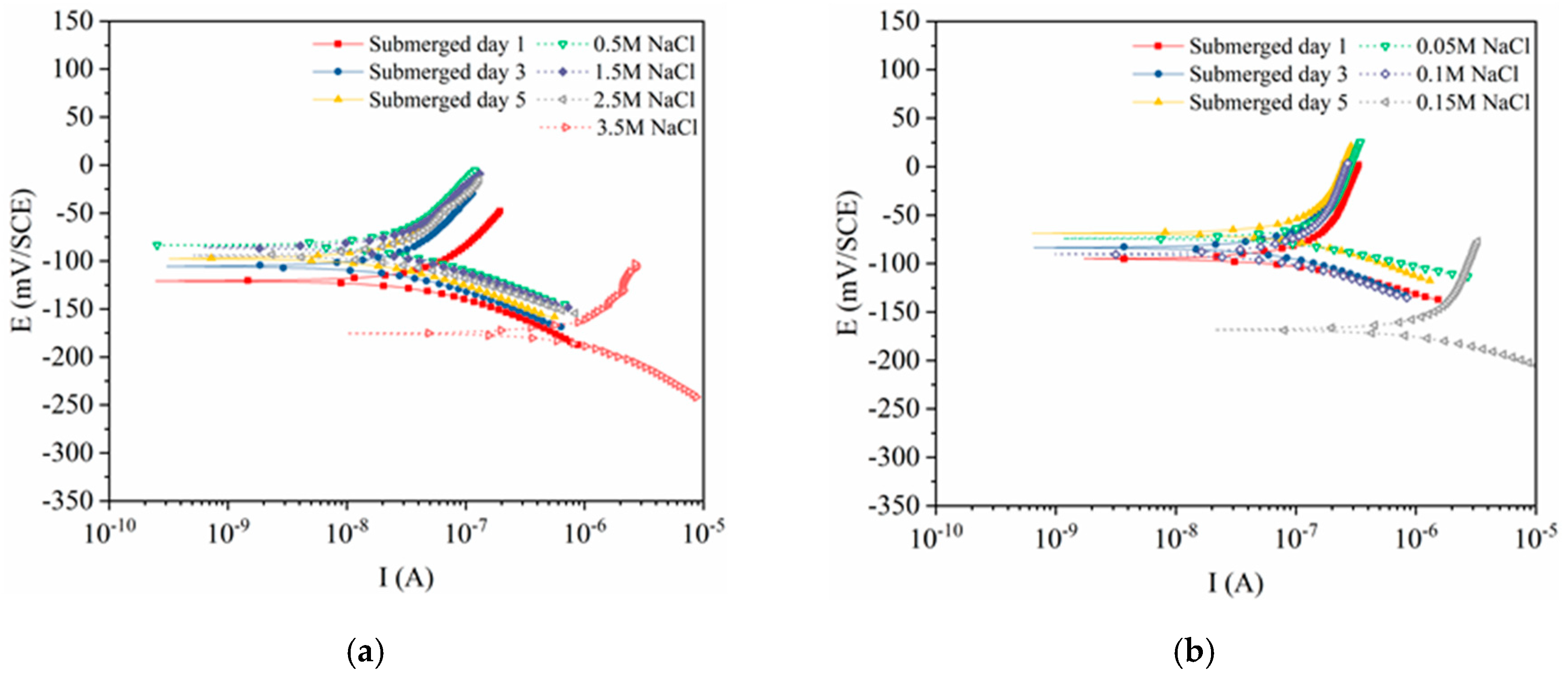
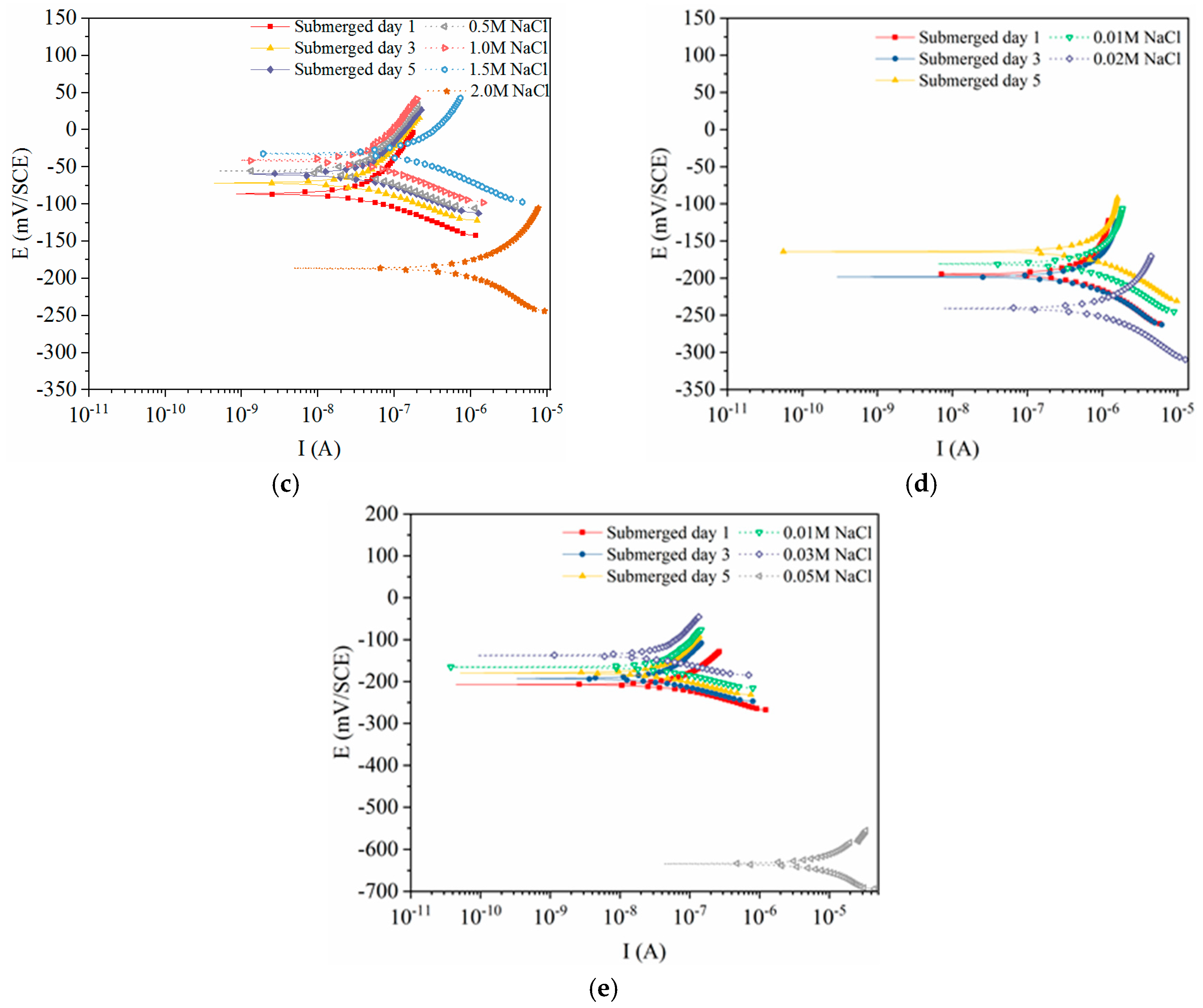
3.1. Polarization Curves of the Steels Exposed to Pore Solutions with Chloride Ions
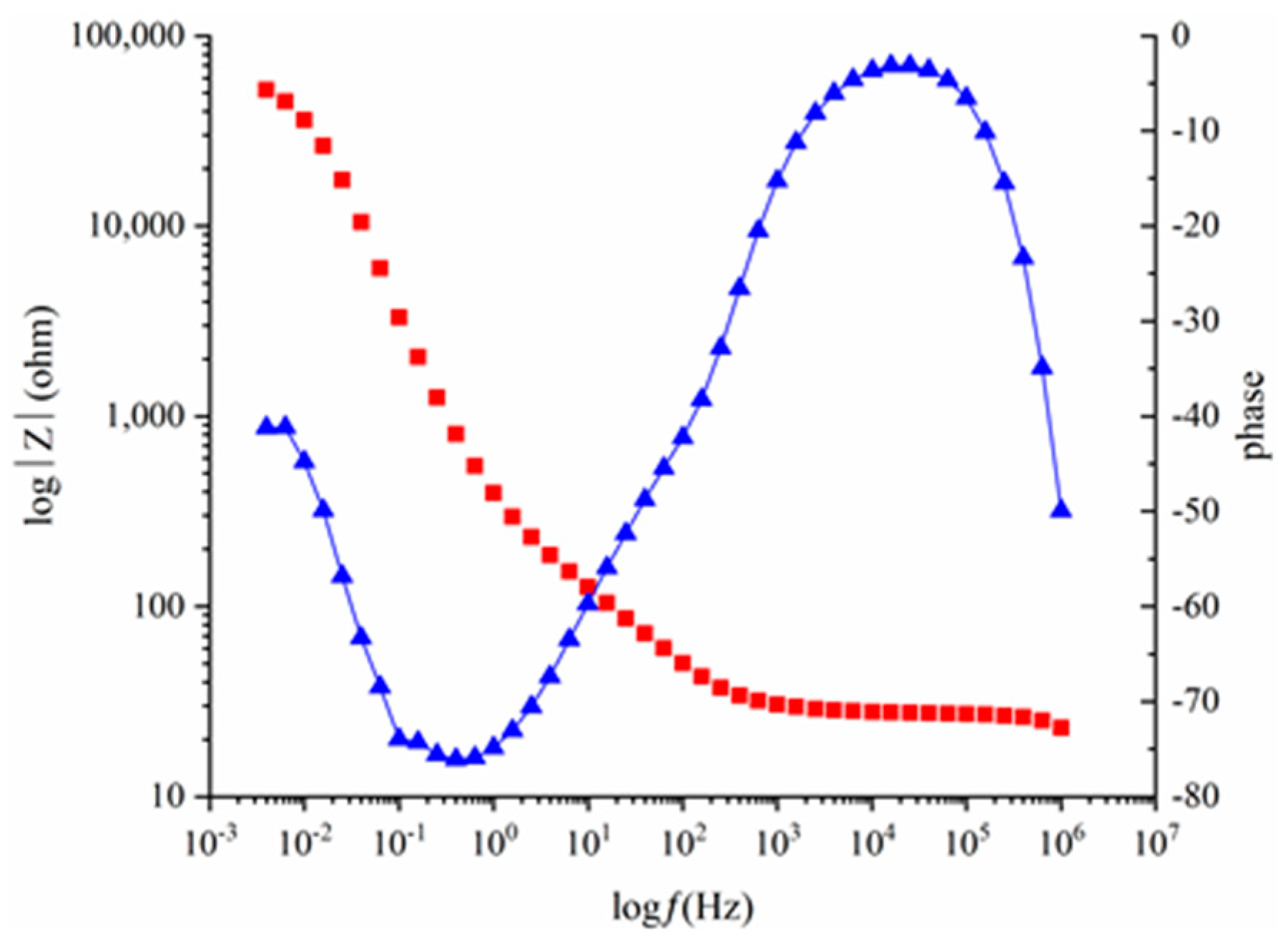
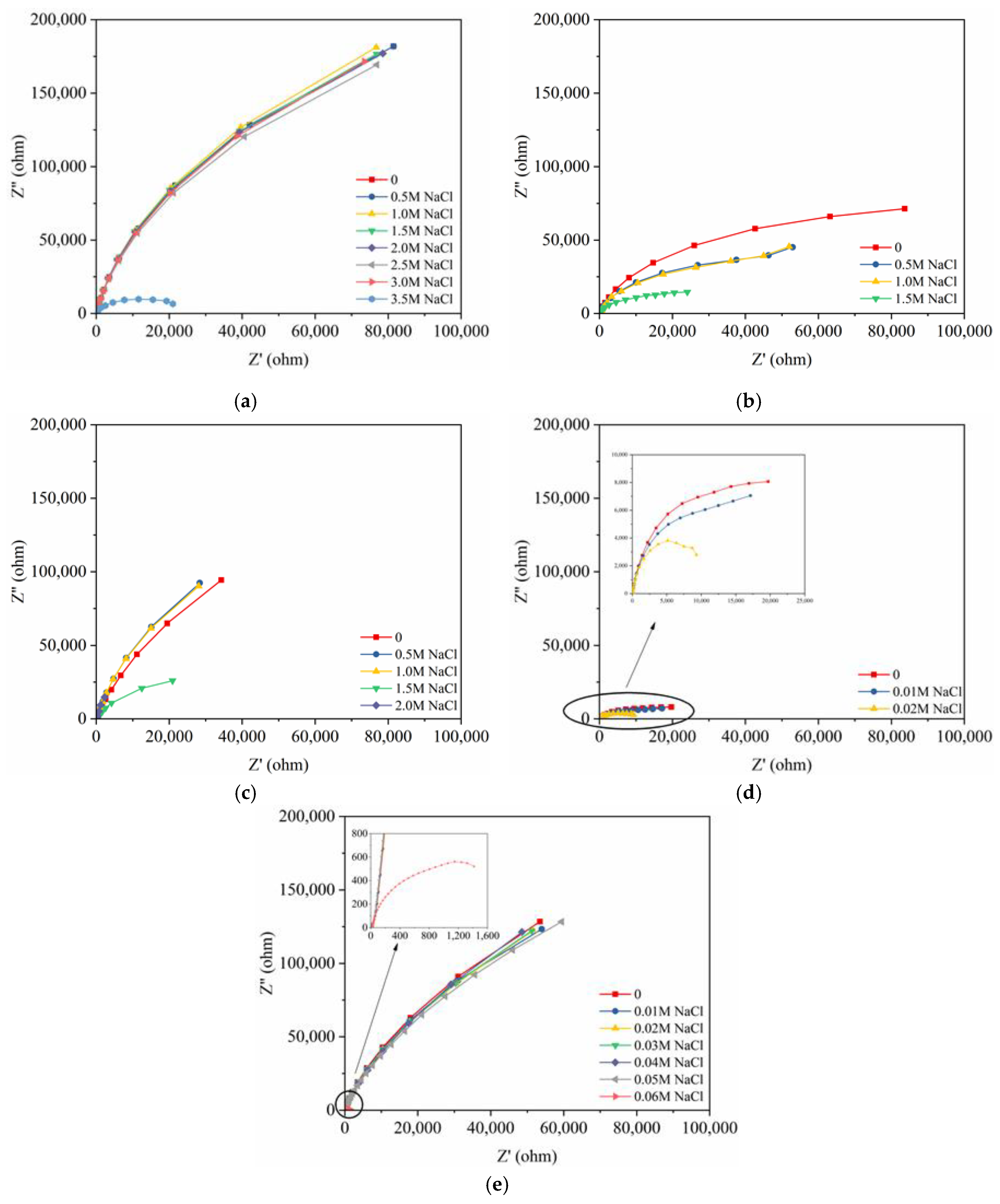
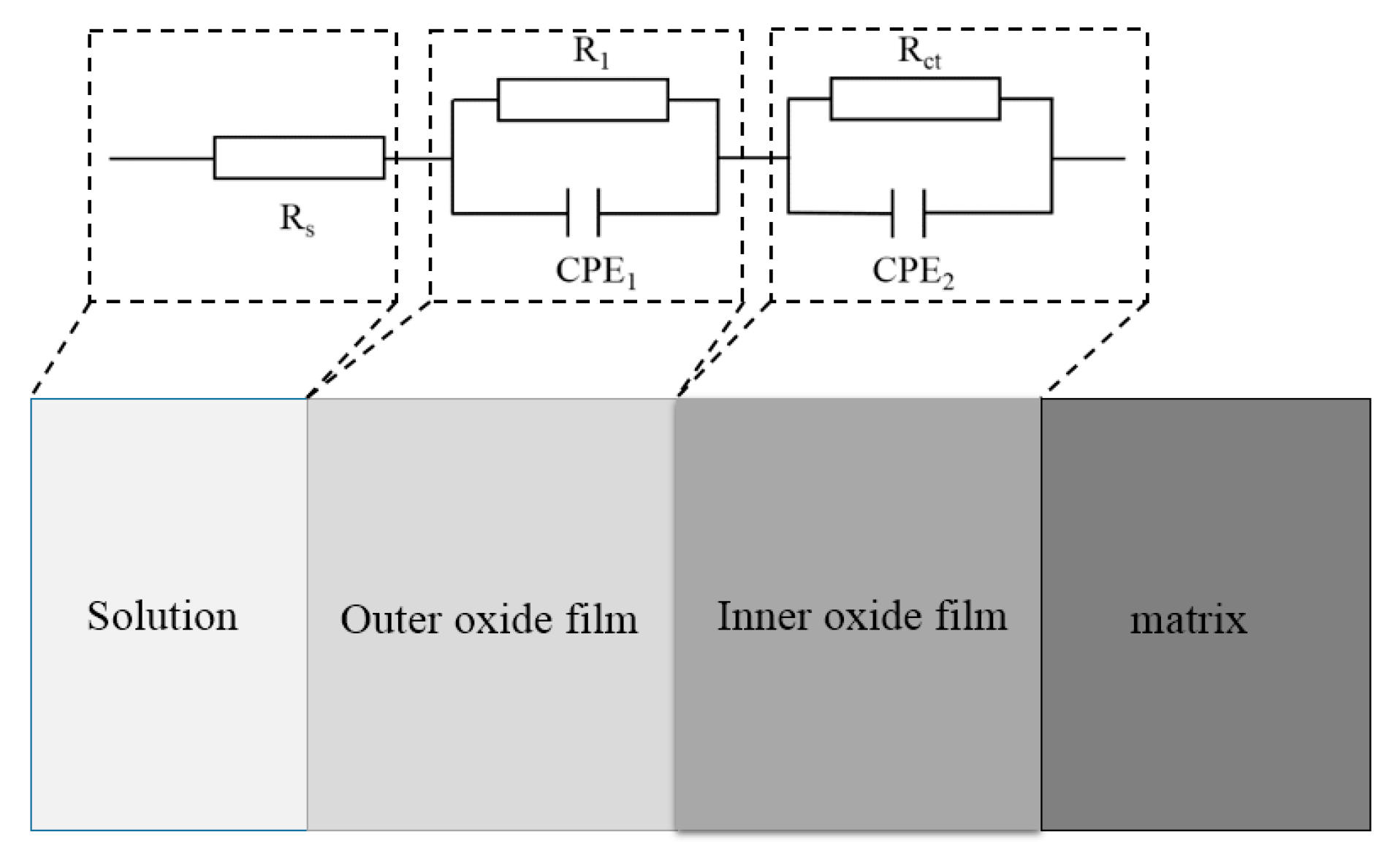
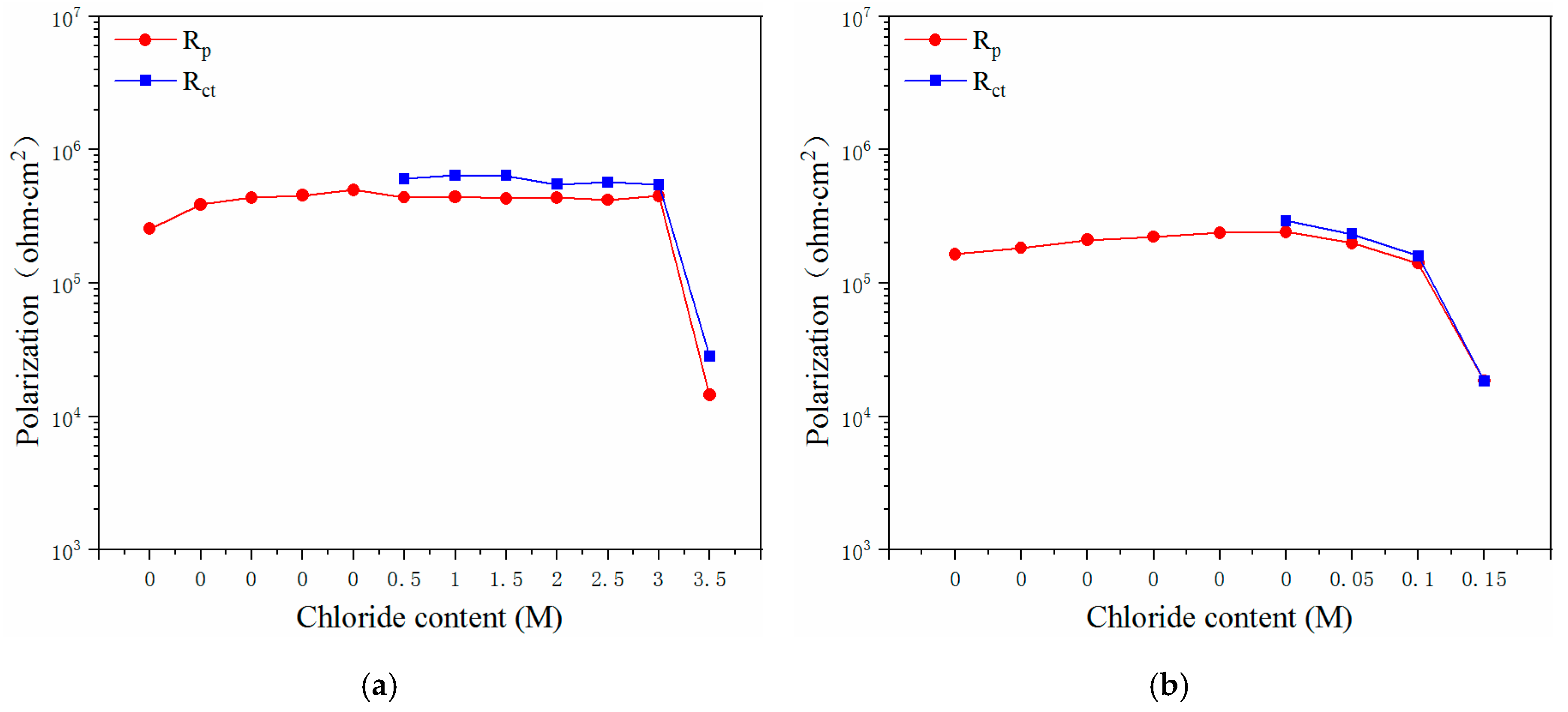
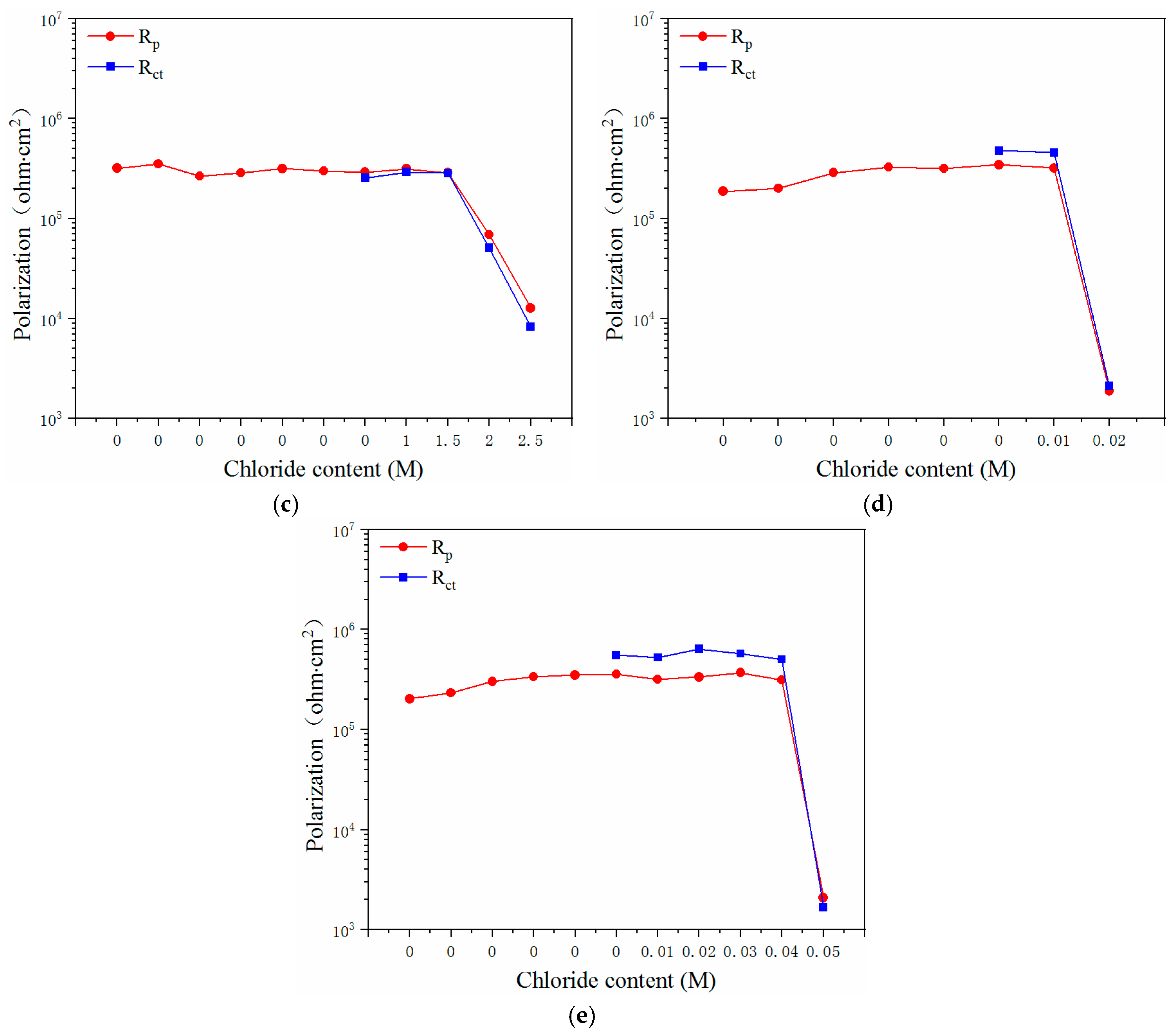
3.2. EIS Curves of the Steels Exposed to the Pore Solutions with Chloride Ions
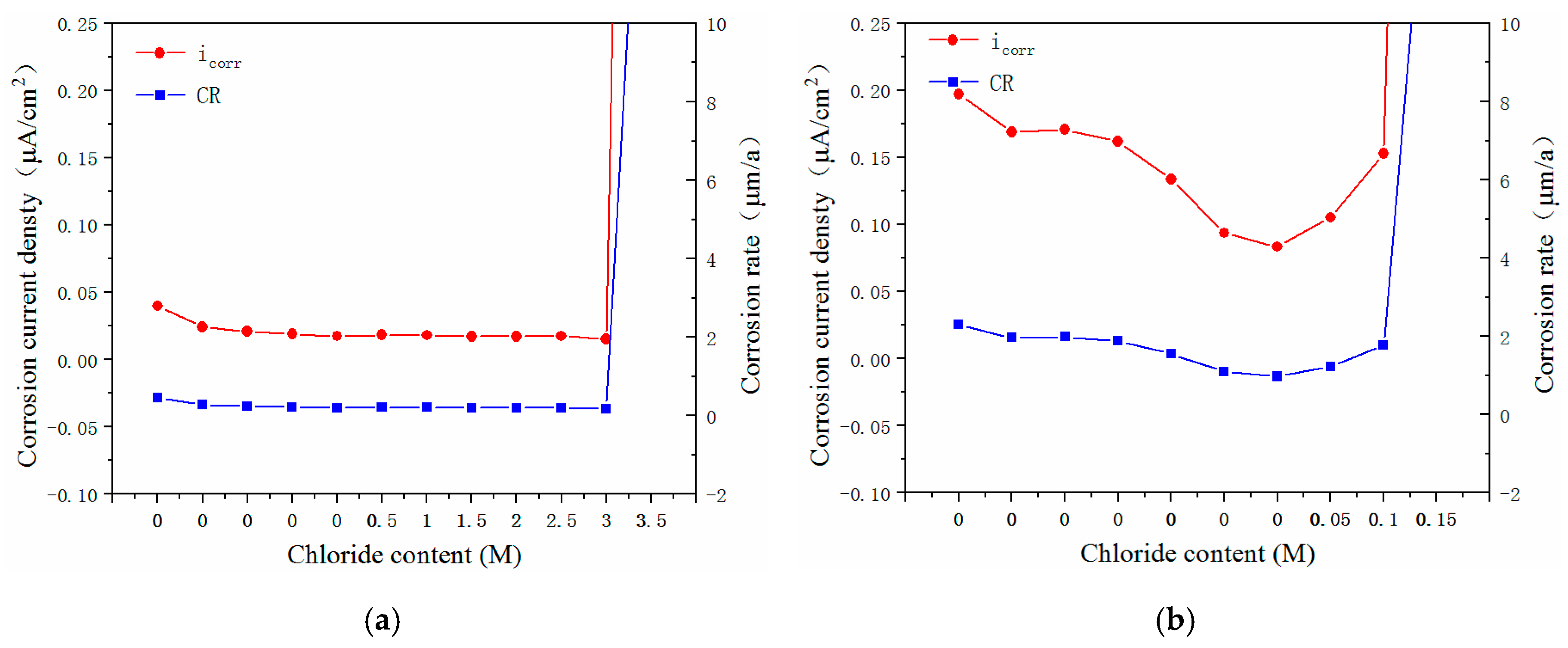
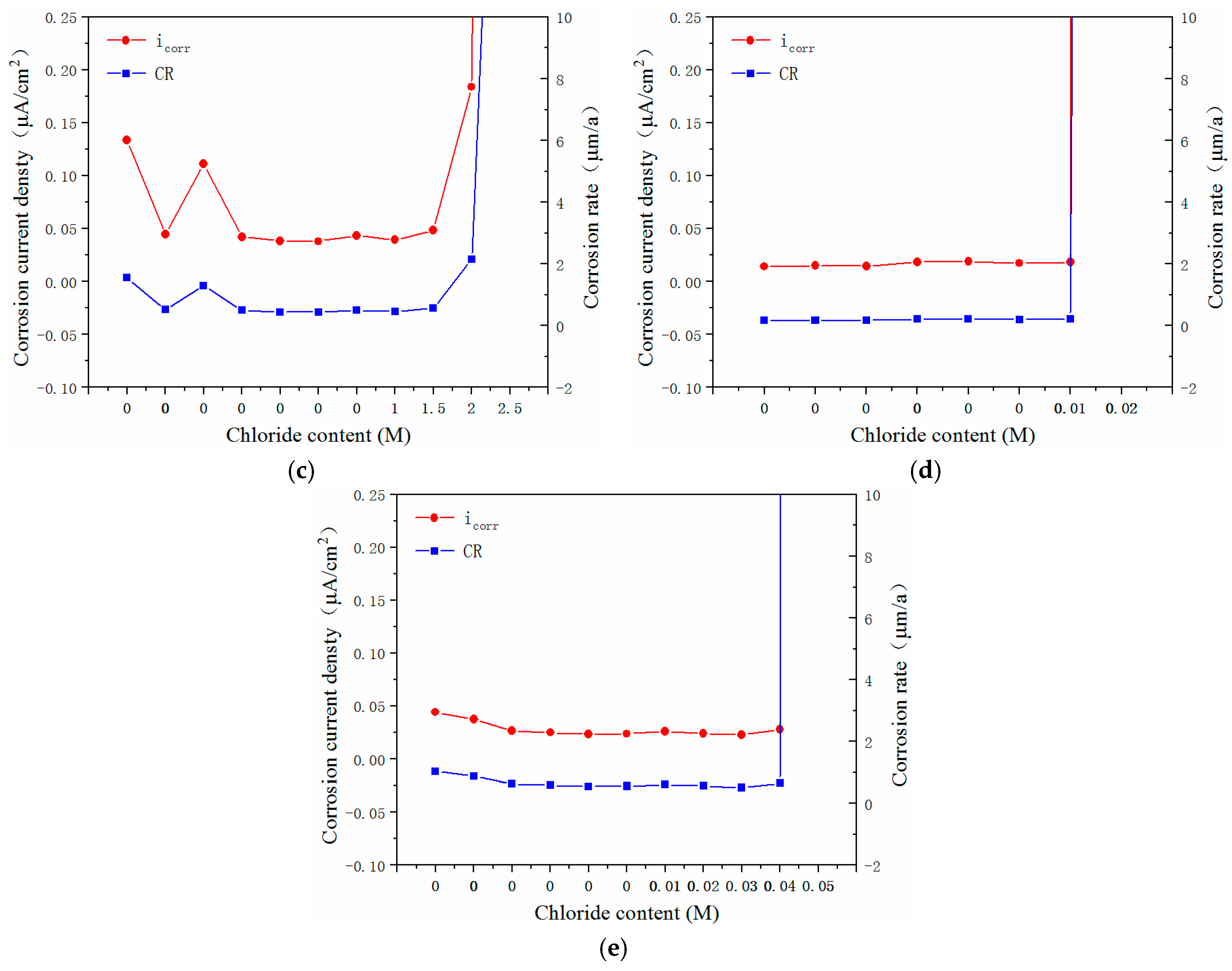
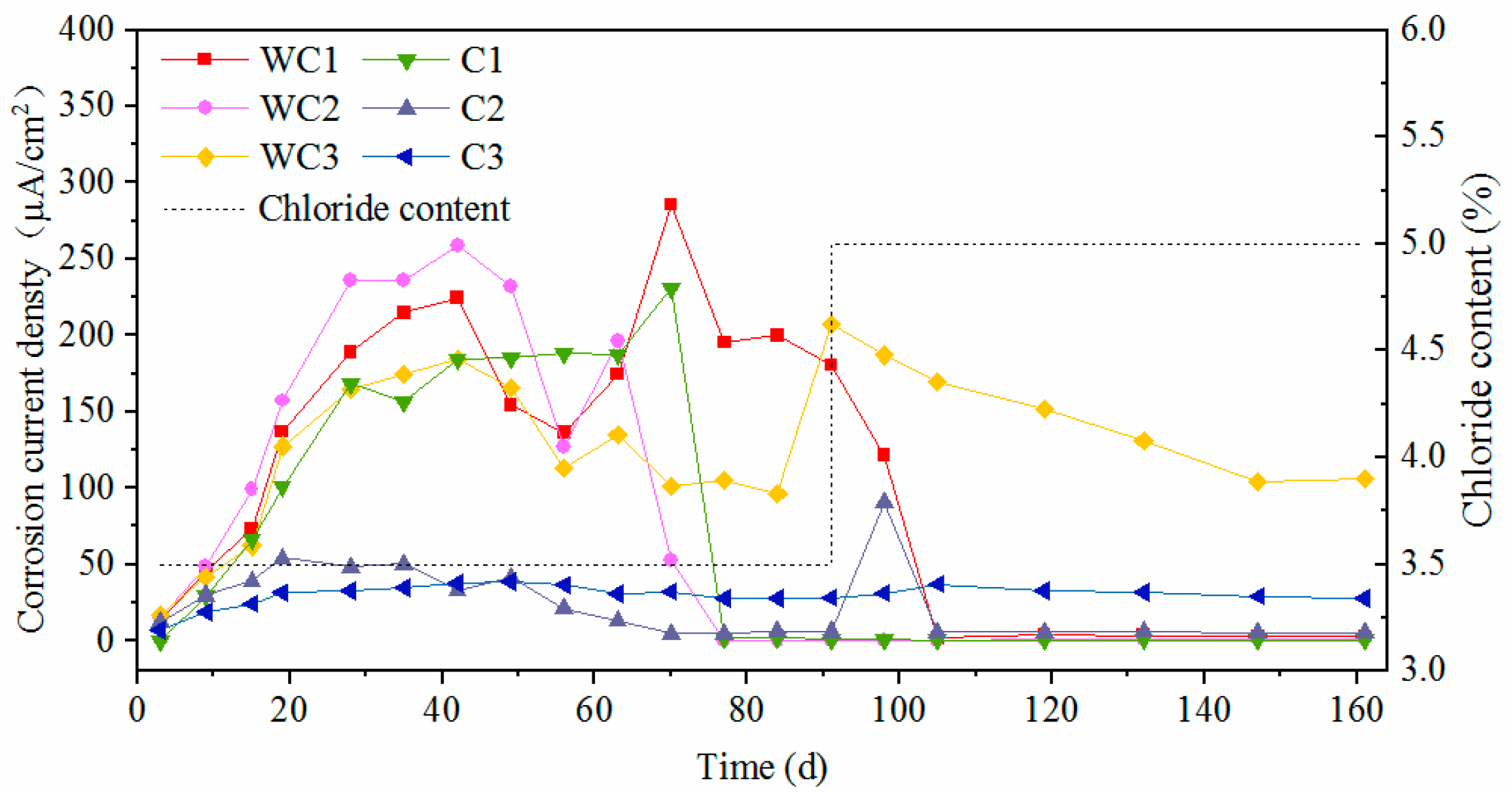
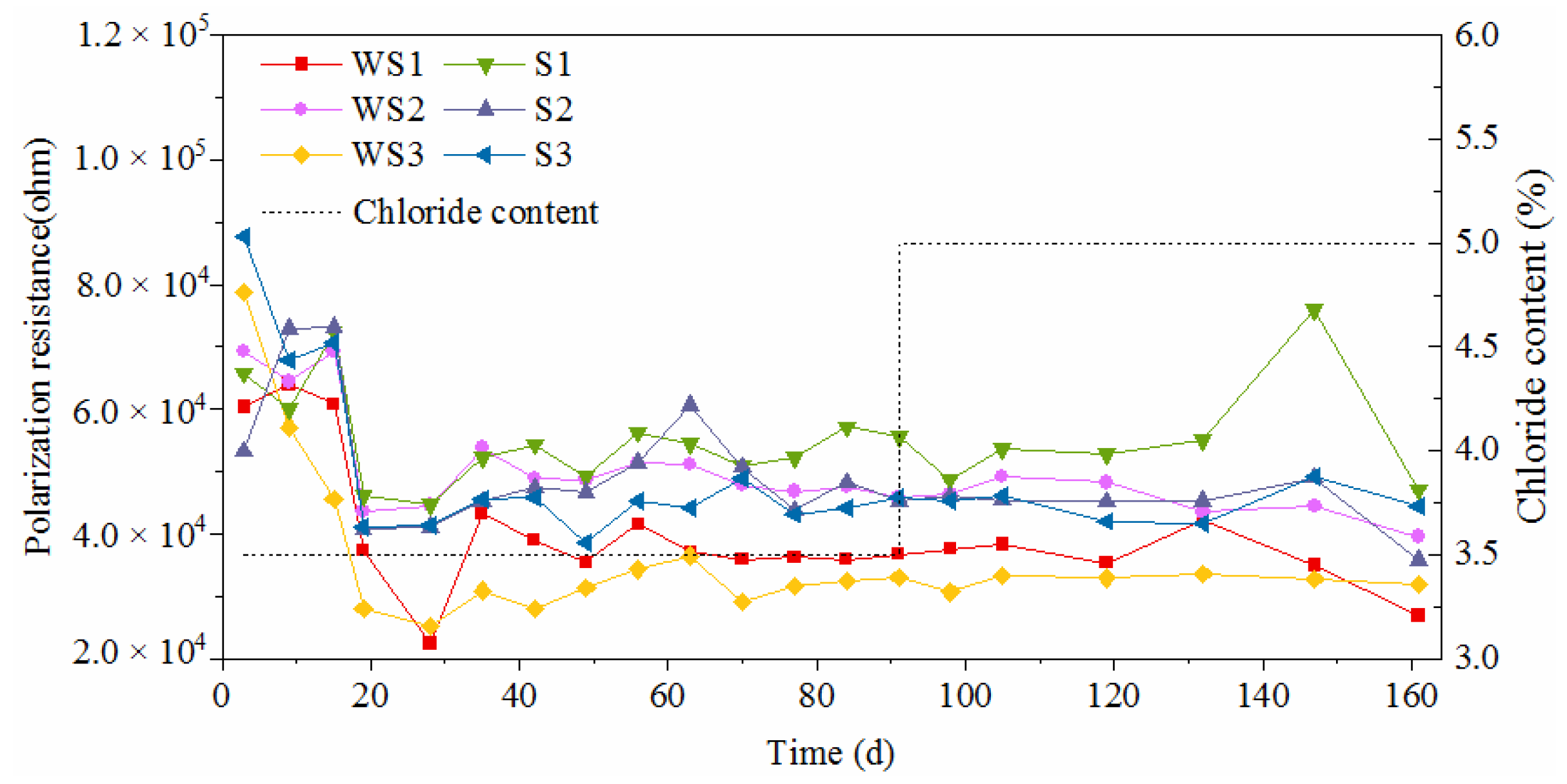
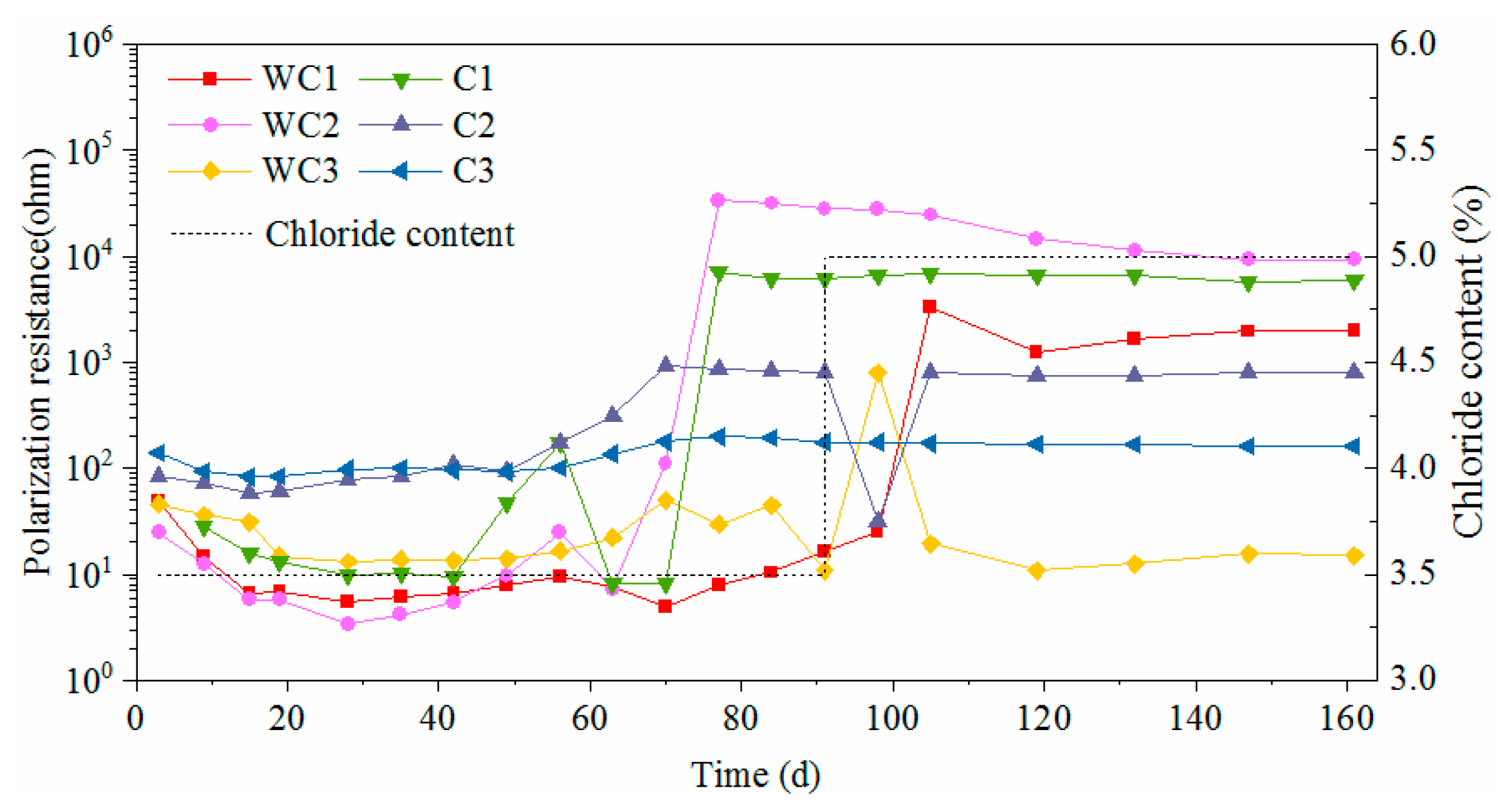
3.3. Electrochemical Characteristics and Threshold Chloride Concentrations
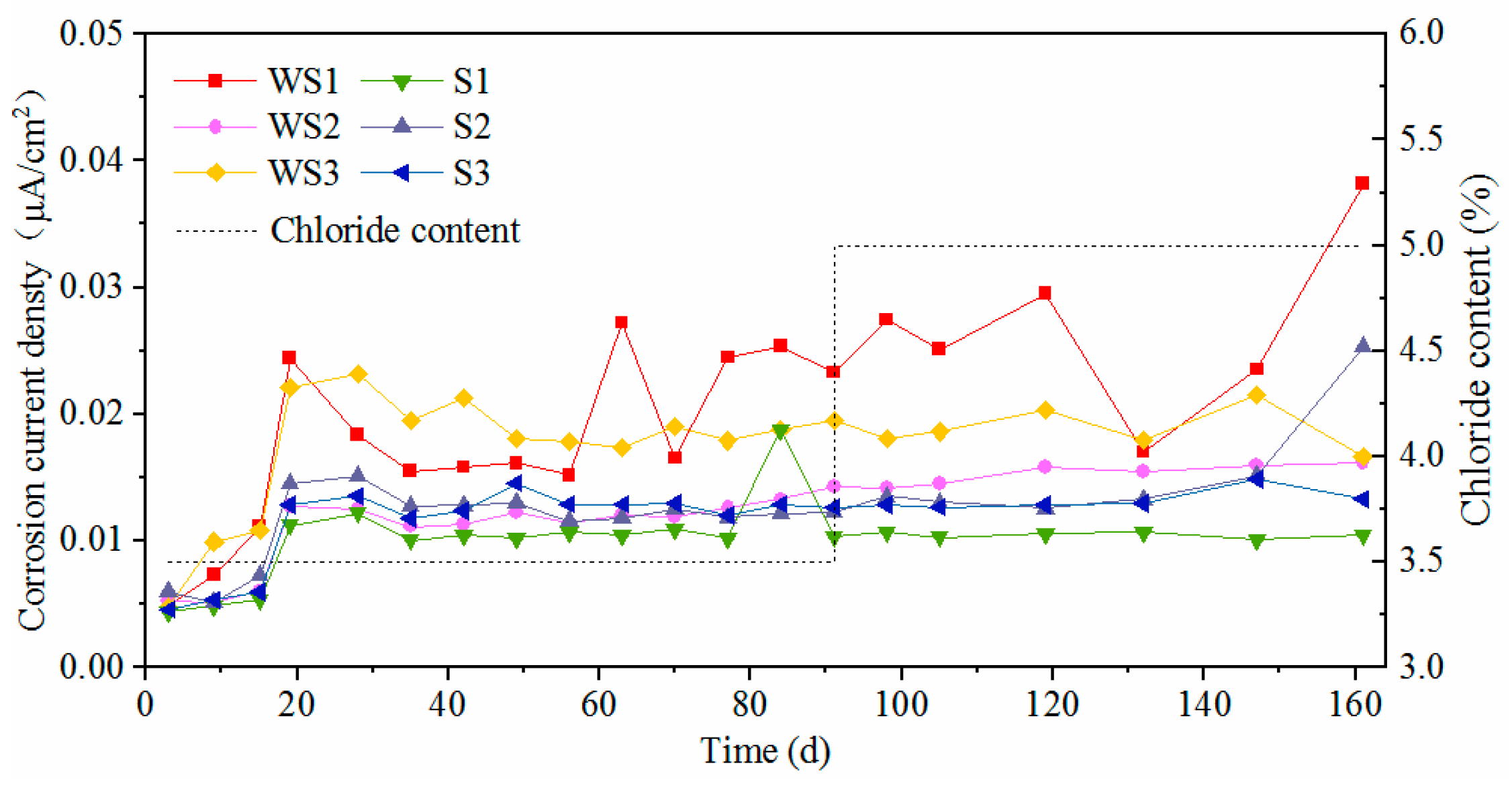
3.4. Effect of Surface State on Corrosion Characteristics
4. Conclusions
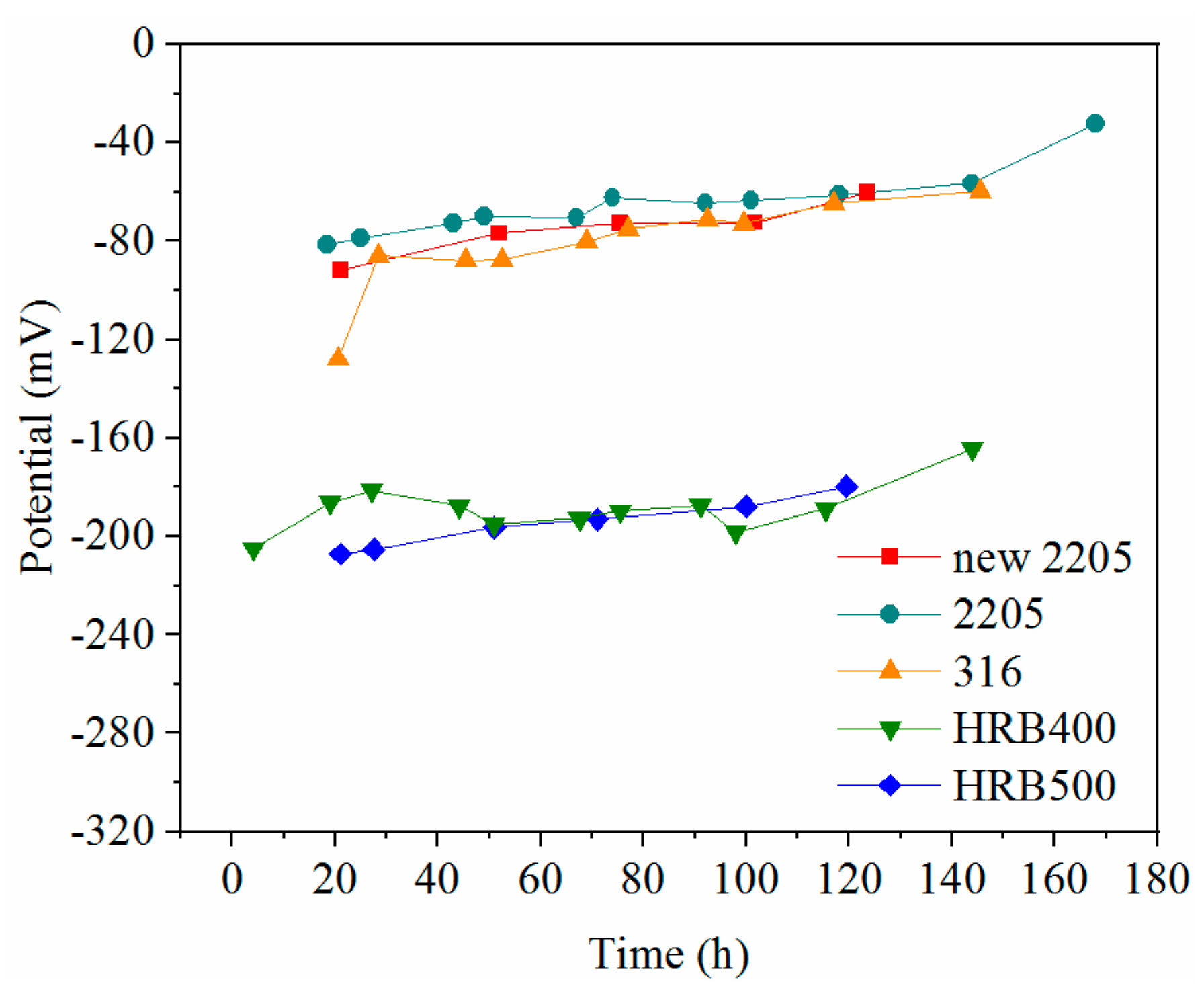
- The potentials of the stainless steels were 110 mV greater than those of the carbon steels, indicating that they were less likely to corrode when exposed to a chloride-containing environment.
- The critical chloride concentration of the novel type-2205 duplex stainless steel was at least 20 times greater than that of the type-2205 duplex stainless steel, 1.5 times greater than that of the type-316 austenitic stainless steel, and was significantly greater than that of the carbon steels. This indicated that, compared with the other steels, the novel type-2205 duplex stainless steel offered superior resistance to chloride ions and could remain passivated for a longer period of time.
- The element content of the steel bar had a significant influence on its corrosion resistance. Higher chromium and molybdenum content in this novel type-2205 duplex stainless steel offered greater corrosion resistance. Simultaneously, the low nickel content reduced the initial cost associated with its use.
- The presence of surface damage led to significant decrease of corrosion resistance for carbon steel. Although the oxide layer was destroyed, the novel type-2205 stainless steel still showed a great corrosion resistance.
- The corrosion can be inhibited to a certain degree with the accumulation of rust on the steel surface for carbon steel. However, the rust cannot be accumulated on the surface of novel type-2205 duplex stainless steel in a short period of time, due to its low corrosion rate.
Author Contributions
Funding
Conflicts of Interest
References
- Gancedo, J.R.; Alonso, C.; Andrade, C.; Gracia, M. Technical Note: AES Study of the Passive Layer Formed on Iron in Saturated Ca(OH)2 Solutions. Corros. Houst. Tx 1989, 45, 976–977. [Google Scholar] [CrossRef]
- Castro-Borges, P.; Rincón OT, D.; Moreno, E.I.; Torres-Acosta, A.A. Performance of a 60-year-old concrete pier with stainless steel reinforcemen. Mater. Perform. 2002, 41, 50–55. [Google Scholar]
- Bautista, A.; Paredes, E.C.; Velasco, F.; Alvarez, S.M. Corrugated stainless steels embedded in mortar for 9 years: Corrosion results of non-carbonated, chloride-contaminated samples. Constr. Build. Mater. 2015, 93, 350–359. [Google Scholar] [CrossRef]
- Knudsen, A.; Jensen, F.M.; Klinghoffer, O.; Skovsgaard, T. Cost-effective enhancement of durability of concrete structures by intelligent use of stainless steel reinforcement. In Proceedings of the Conference on Corrosion and Rehabilitation of Reinforced Concrete Structures, Orlando, FL, USA, 7–11 December 1998. [Google Scholar]
- Gedge, G. Structural uses of stainless steel buildings and civil engineering. J. Constr. Steel Res. 2008, 64, 1194–1198. [Google Scholar] [CrossRef]
- Pérez-Quiroz, J.T.; Terán, J.; Herrera, M.J.; Martínez, M.; Genescá, J. Assessment of stainless steel reinforcement for concrete structures rehabilitation. J. Constr. Steel Res. 2008, 64, 1317–1324. [Google Scholar]
- Feng, X.; Lu, X.; Zuo, Y.; Zhuang, N.; Chen, D. The effect of deformation on metastable pitting of 304 stainless steel in chloride contaminated concrete pore solution. Corros. Sci. 2016, 103, 223–229. [Google Scholar] [CrossRef]
- Luo, H.; Su, H.; Dong, C.; Li, X. Passivation and electrochemical behavior of 316L stainless steel in chlorinated simulated concrete pore solution. Appl. Surf. Sci. 2016, 400, 38–48. [Google Scholar] [CrossRef]
- Fajardo, S.; Bastidas, D.M.; Criado, M.; Romero, M.; Bastidas, J.M. Corrosion behaviour of a new low-nickel stainless steel in saturated calcium hydroxide solution. Constr. Build. Mater. 2011, 25, 4190–4196. [Google Scholar] [CrossRef]
- Freire, L.; Carmezim, M.J.; Ferreira MG, S.; Montemor, M.F. The electrochemical behaviour of stainless steel AISI 304 in alkaline solutions with different pH in the presence of chlorides. Electrochim. Acta 2011, 56, 5280–5289. [Google Scholar] [CrossRef]
- Cabrini, M.; Cabrini, M.; Lorenzi, S.; Pastore, T. Cyclic voltammetry evaluation of inhibitors for localised corrosion in alkaline solutions. Electrochim. Acta 2014, 124, 156–164. [Google Scholar] [CrossRef]
- Marcelin, S.; Pébère, N.; Régnier, S. Electrochemical characterisation of a martensitic stainless steel in a neutral chloride solution. Electrochim. Acta 2013, 87, 32–40. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Li, B. Electrochemical and Corrosion Behavior of 2205 Duplex Stainless Steel in Simulated Concrete Pore Solution. Int. J. Electrochem. Sci. 2017, 12, 8432–8446. [Google Scholar] [CrossRef]
- Criado, M.; Fajardo, S.; Bastidas, J.M. Corrosion Behaviour of a New Low-Nickel Stainless Steel Reinforcement: A Study in Simulated Pore Solutions and in Fly Ash Mortars. Int. J. Corros. 2012, 2012, 847323. [Google Scholar] [CrossRef]
- Fajardo, S.; Bastidas, D.M.; Ryan, M.P.; Criado, M.; Mcphail, D.S.; Bastidas, J.M. Low-nickel stainless steel passive film in simulated concrete pore solution: A SIMS study. Appl. Surf. Sci. 2010, 256, 6139–6143. [Google Scholar] [CrossRef]
- García-Alonso, M.C.; González, J.A.; Miranda, J.; Escudero, M.L.; Correia, M.J.; Salta, M.; Bennani, A. Corrosion behaviour of innovative stainless steels in mortar. Cem. Concr. Res. 2007, 37, 1562–1569. [Google Scholar] [CrossRef] [Green Version]
- García-Alonso, M.C.; Escudero, M.L.; Miranda, J.M.; Vega, M.I.; Capilla, F.; Correia, M.J.; Salta, M.; Bennani, A.; González, J.A. Corrosion behaviour of new stainless steels reinforcing bars embedded in concrete. Cem. Concr. Res. 2007, 37, 1463–1471. [Google Scholar] [CrossRef] [Green Version]
- Ping, G.; Elliott, S.; Beaudoin, J.; Arsenault, B. Corrosion resistance of stainless steel in chloride contaminated concrete. Cem. Concr. Res. 1996, 26, 1151–1156. [Google Scholar] [CrossRef]
- Freire, L.; Carmezim, M.J.; Ferreira MG, S.; Montemor, M.F. The passive behaviour of AISI 316 in alkaline media and the effect of pH: A combined electrochemical and analytical study. Electrochim. Acta 2010, 55, 6174–6181. [Google Scholar] [CrossRef]
- Bertolini, L.; Bolzoni, F.; Pastore, T.; Pedeferri, P. Behaviour of stainless steel in simulated concrete pore solution. Br. Corros. J. 1996, 31, 218–222. [Google Scholar] [CrossRef]
- Blanco, G.; Bautista, A.; Takenouti, H. EIS study of passivation of austenitic and duplex stainless steels reinforcements in simulated pore solutions. Cem. Concr. Compos. 2006, 28, 212–219. [Google Scholar] [CrossRef]
- Moser, R.D.; Singh, P.M.; Kahn, L.F.; Kurtis, K.E. Chloride-induced corrosion resistance of high-strength stainless steels in simulated alkaline and carbonated concrete pore solutions. Corros. Sci. 2012, 57, 241–253. [Google Scholar] [CrossRef]
- Kouřil, M.; Novák, P.; Bojko, M. Threshold chloride concentration for stainless steels activation in concrete pore solutions. Cem. Concr. Res. 2010, 40, 431–436. [Google Scholar] [CrossRef]
- Angst, U.; Elsener, B.; Larsen, C.K.; Vennesland, Ø. Critical chloride content in reinforced concrete—A review. Cem. Concr. Res. 2009, 60, 1122–1138. [Google Scholar] [CrossRef]
- Figueira, R.B.; Sadovski, A.; Melo, A.P.; Pereira, E.V. Chloride threshold value to initiate reinforcement corrosion in simulated concrete pore solutions: The influence of surface finishing and pH. Constr. Build. Mater. 2017, 141, 183–200. [Google Scholar] [CrossRef]
- Cabrini, M.; Lorenzi, S.; Pastore, T. Steel damaging in flowing mortar. Corros. Eng. Sci. Technol. 2016, 51, 596–605. [Google Scholar] [CrossRef]
- Cabrini, M.; Cortellini, M.; Lorenzi, S.; Marcassoli, P.; Pastore, T. Erosion and corrosion of steel in fresh concrete. Metall. Ital. 2010, 102, 39–44. [Google Scholar]
- Luo, H.; Dong, C.F.; Li, X.G.; Xiao, K. The electrochemical behaviour of 2205 duplex stainless steel in alkaline solutions with different pH in the presence of chloride. Electrochim. Acta 2012, 64, 211–220. [Google Scholar] [CrossRef]
- Pan, J.; Leygraf, C.; Thierry, D.; Ektessabi, A.M. Corrosion resistance for biomaterial applications of TiO2 films deposited on titanium and stainless steel by ion-beam-assisted sputtering. J. Biomed. Mater. Res. 1997, 35, 309–318. [Google Scholar] [CrossRef]
- Clayton, C.R.; Olefjord, I. Passivity of austenitic stainless steels. In Corrosion Mechanism Theory and Practice; Marcus, P., Oudar, J., Eds.; Marcel Dekker: New York, NY, USA, 1995; pp. 175–199. [Google Scholar]
- Jin, W.L.; Zhao, Y.X. Durability of Concrete Structures; Science Press: Beijing, China, 2014. [Google Scholar]


| Composition | C | Mn | S | Si | Cr | Ni | Mo |
|---|---|---|---|---|---|---|---|
| HRB400 | 0.23 | 1.58 | – | 0.32 | 1.35 | – | – |
| HRB500 | 0.25 | 1.62 | 0.06 | 0.12 | 0.31 | – | – |
| 316 | 0.03 | 0.93 | 0.03 | 0.24 | 16.56 | 11.07 | 2.23 |
| 2205 | 0.03 | 0.82 | 0.01 | 0.23 | 20.95 | 5.84 | 2.57 |
| Novel 2205 | 0.02 | 1.36 | – | 0.14 | 22.83 | 6.15 | 3.21 |
| Type of Steel | New 2205 | 2205 | 316 | HRB400 | HRB500 |
|---|---|---|---|---|---|
| Critical value | 3.0–3.5 M | 0.1–0.15 M | 1.5–2.0 M | 0.01–0.02 M | 0.04–0.05 M |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Wu, Y.; Sun, X.; Ling, J.; Zou, D. Corrosion Resistance to Chloride of a Novel Stainless Steel: The Threshold Chloride Value and Effect of Surface State. Materials 2019, 12, 2235. https://doi.org/10.3390/ma12142235
Wang H, Wu Y, Sun X, Ling J, Zou D. Corrosion Resistance to Chloride of a Novel Stainless Steel: The Threshold Chloride Value and Effect of Surface State. Materials. 2019; 12(14):2235. https://doi.org/10.3390/ma12142235
Chicago/Turabian StyleWang, Hailong, Yuanjian Wu, Xiaoyan Sun, Jiayan Ling, and Daoqin Zou. 2019. "Corrosion Resistance to Chloride of a Novel Stainless Steel: The Threshold Chloride Value and Effect of Surface State" Materials 12, no. 14: 2235. https://doi.org/10.3390/ma12142235
APA StyleWang, H., Wu, Y., Sun, X., Ling, J., & Zou, D. (2019). Corrosion Resistance to Chloride of a Novel Stainless Steel: The Threshold Chloride Value and Effect of Surface State. Materials, 12(14), 2235. https://doi.org/10.3390/ma12142235






